Abstract
Introduction
The cavernous nerve (CN) is commonly injured during prostatectomy, resulting in erectile dysfunction (ED). Although peripheral nerves have a limited ability to regenerate, a return of function typically does not occur due to irreversible down stream morphological changes in the penis that result from CN injury. We have shown in previous studies that sonic hedgehog (SHH) is critical for CN regeneration and improves erectile function after crush injury.
Aims
Examine a new direction, to determine if SHH is neuroprotective to the pelvic ganglia (PG)/CN after crush injury. A secondary focus is to examine if SHH signaling decreases with age in the PG/CN.
Methods
Sprague Dawley rats underwent bilateral CN crush and SHH and glial fibrillary acidic protein were quantified by western analysis of the PG/CN (n=6 rats at each time point) at 1, 2, 4, 7 and 14 days, and the apoptotic index was measured in the penis. SHH was quantified by western in the PG/CN with blockade of anterograde transport (n=4 rats) in comparison to mouse IgG (n=4 rats). If SHH is neuroprotective was examined at 4 (n=14 rats) and 7 days (n=16 rats) of treatment after CN crush. SHH protein was quantified in aging (P200-300, n=5 rats) PG/CN in comparison to normal adult (P115-120, n=3 rats) PG/CN.
Main Outcome Measures
SHH pathway was examined in PG via immunohistochemistry, in situ, western and TUNEL.
Results
SHH is neuroprotective in the PG/CN with injury. SHH localization in the PG/CN suggests SHH interaction in neuronal/glial signaling. SHH protein is significantly decreased in the PG/CN after crush injury and in the aged PG/CN. Signals from the PG are required to maintain SHH in the CN.
Conclusions
There is a window of opportunity immediately after nerve insult in which manipulation of SHH signaling in the nerve microenvironment can affect long-term regeneration outcome.
Keywords: Sonic hedgehog, cavernous nerve, neuroprotective, regeneration, transport, aging, neuroprotective
Introduction
The cavernous nerve (CN) is a peripheral nerve that provides innervation to the penis. The CN commonly undergoes resection, crush or tension injuries during prostatectomy, which results in erectile dysfunction (ED). Neuropathy of the CN also frequently occurs in diabetic patients, and in aging patients, which results in ED. Our previous studies show that the secreted protein sonic hedgehog (SHH) is essential to maintain and regenerate the CN since: SHH is abundant in Schwann cells of the CN, SHH is necessary for maintenance of CN morphology, SHH inhibition causes demyelination and axonal degeneration of CN fibers, and SHH protein treatment promotes CN regeneration [1, 2]. In this study we propose to examine a new direction of research, to determine if SHH is neuroprotective to the PG/CN in the first weeks after crush injury. A secondary hypothesis is that SHH signaling is decreased in the PG/CN with age, thus proposing a potential mechanism of how aging related ED may develop. A neuroprotective role for SHH is supported by observations in the literature that delivery of SHH to the facial nerve after axotomy promoted motor neuron survival for 3-5 days [3,4], Shh mRNA was elevated and SHH protein had prominent localization within the regenerating axons 24 hours after sciatic nerve crush [5,6], injured sciatic nerves had enhanced recovery in the presence of SHH protein [7], and Shh expression was significantly lower in mice with impaired Wallerian degeneration [8]. If SHH is neuroprotective in addition to promoting CN regeneration, as shown in past studies [2], is unknown, but a better understanding of SHH signaling in the PG/CN is critical to manipulate the nerve microenvironment to induce regeneration more quickly.
CN injury has a significant impact on quality of life of ED patients and their partners. ED affects 61% of men between the ages of 40-69, 77% of men over 70 [9] and has been shown to be an early warning sign for cardiovascular disease [10]. ED occurs in 16-82% of patients treated by prostatectomy [11], which results from injury to the CN. Tissues innervated by the damaged nerve have deteriorating function, morphological remodeling including induction of smooth muscle apoptosis [12] and fibrosis [13], which affect the responsiveness of penile smooth muscle. A recent study showed that only 36% of prostatectomy patients recover erectile function without intervention [14] and PDE5 inhibitors improve erectile function in only ~31% of prostatectomy patients [15]. Thus new therapies that address both the down stream morphological changes in the penis and the underlying cause of the dysfunction, injury to the CN, are needed. As is the case with other peripheral nerves, efforts to regenerate the CN have so far had limited success in animal models, and these treatments have not yet translated into clinical therapies. Since our previous studies show that SHH treatment is effective in promoting CN regeneration [2], a better understanding of SHH signaling in the PG/CN is critical for development of new treatments. In this study we examine if SHH is neuroprotective to the PG/CN in the first weeks after injury and if SHH signaling is decreased in the aged PG/CN, suggesting a potential mechanism of how aging related ED may develop.
Materials and Methods
Animals
Male Sprague Dawley rats postnatal day 115-120 (P115-P120) and retired male Sprague Dawley breeder rats (P200-P300) were obtained from Charles River.
Ethics statement
All animals were cared for in accordance with institutional IACUC approval and the National Research Council publication Guide for Care and Use of Laboratory Animals.
In vivo SHH protein delivery by peptide amphiphile (PA)
PAs were synthesized at the Northwestern Institute for BioNanotechnology in Medicine Chemistry Core Facility as described previously [16]. The PA used in this study had the structure (C16)-V2A2E2-(NH2). PA was prepared [2] by adding 20mM CaCl2 to a glass slide. 8μl of 100mM PA plus either 2.27μg SHH or BSA (control) proteins were pipetted onto the slide to form the linear PA.
CN crush time course
PG/CN were exposed in rats. Microforceps (size 0.02 × 0.06mm) were used to crush the CN bilaterally for 30 seconds [17-19]. Sham surgery was performed by exposing, but not crushing the CN. The reproducibility of the crush injury was previously verified [2]. Rats were sacrificed 1, 2, 4, 7, and 14 days after surgery. Six PG/CN (from six rats) for each time point were divided into three groups and were homogenized for western analysis. Pooling of tissue was required for homogenization because of the small size of the PG/CN. Samples were run in duplicate and the results were averaged for each time point. Penis tissue was fixed in 4% paraformaldehyde and embedded in paraffin for TUNEL.
Interruption of anterograde transport in vivo by anti-kinesin treatment of the PG
Affi-Gel beads (100-200 mesh, Bio-Rad) were equilibrated with mouse anti-kinesin (0.9 mg/mL, Sigma, St. Louis, MO, USA, K1005) or mouse IgG (control). Anti-kinesin disrupts anterograde nerve transport, however disruption of transport in either direction affects transport to a small degree (14%) in the other direction since it is not possible to completely uncouple these processes. Approximately 10-20 beads were implanted under the PG bilaterally. Rats were sacrificed after 2 days and PG/CNs were frozen for western. Eight anti-kinesin (4 rats) and eight IgG control (4 rats) PG/CN were divided into three groups and were homogenized for western. Samples were run in duplicate and the results averaged.
CN crush and SHH protein treatment of the CN in vivo via PA or pipette
The PG/CN were exposed in rats and bilateral CN crush was performed as described above. For rats receiving PA, 8μL of 100mM PA plus 2.27μg SHH protein dissolved in 1.5μL water (R&D Systems) were combined and pipetted onto a glass slide containing CaCl2, to form the linear PA. PA was transferred on top of crushed CNs bilaterally so that each rat received a total of 4.54μg SHH protein. The release rate of SHH protein from the PA was previously determined [2]. Ninety percent of SHH protein was released within 75 hours. For rats receiving a bolus delivery, SHH protein (2.27μg in 3μL H2O) was pipetted/dripped on top of the CN. Control rats received bilateral CN crush only or sham surgery. PG/CN were harvested after 4 and 7 days and were frozen for western. Six sham, six crush, eight SHH PA treated/CN crush, and eight SHH drip/CN crush PG/CN were used for the four day experiment (from 3, 3, 4 and 4 rats respectively). PG/CN were divided into two groups and were homogenized for western. For the seven day group, six sham, six crush, six SHH PA treated/CN crush, and six SHH drip/CN crush PG/CN were divided into three groups and were homogenized for western (from 4, 4, 4, and 4 rats). For both the four and seven day groups, samples were run in duplicate and the results were averaged.
Western of SHH in P115-120/P200-300 PG/CN
Six PG/CN (from 3 rats) were isolated from P120 and ten PG/CN (from 5 rats) were isolated from P200-300 rats. P120 and P200 PG/CN were divided into two groups and were homogenized for western. Samples were run in duplicate and the results averaged.
Western
Western was performed on protein samples isolated from PG/CN as previously described [20]. Membranes were blocked for 1 hour with 5% nonfat skim milk and were incubated with either 1/50 goat SHH (N-19, Santa Cruz, sc-1194), 1/3,000 rabbit glial fibrillary acidic protein (GFAP, Dako), or 1/50,000 mouse β-ACTIN (Sigma) overnight at 4°C. Secondary antibodies were horseradish peroxidase-conjugated 1/40,000 donkey anti-goat, 1/5,000 chicken anti-rabbit, or 1/80,000 chicken anti-mouse (Santa Cruz). Protein bands were visualized using HRP-conjugated anti-biotin (ECL, GE Healthcare) and were exposed to Hyperfilm (GE Healthcare). Bands were quantified by densitometry using Kodak ID software (Rochester, NY). Quantification of bands was performed by determining the ratio of the density of SHH/β-ACTIN and GFAP/β-ACTIN. Samples were run in duplicate, and the ratios for each sample were averaged and reported ± the standard error of the mean (SEM).
Immunohistochemical analysis (IHC)
IHC was performed as previously described [20] on PG/CN tissue assaying for goat polyclonal SHH (SC-1194), patched (PTCH1), and hedgehog interacting protein (HIP, 1/100, Santa Cruz, n=7, 9, and 6 respectively), rabbit smoothened (SMO, 1/50, MBL International, Woburn, MA, n=5) and GFAP (1/100, DAKO, n=5). Secondary antibodies used were Alexa Fluor rabbit anti-goat (1/300), and chicken anti-rabbit (1/600, Molecular Probes, Carlsbad, CA). S100 (1/100, NeoMarkers, Fremont, CA) IHC analysis was performed on the PG/CN using the DAKO LSAB+ kit (K0679, Carpinteria, CA).
In situ hybridization
In situ was performed as previously described [21] to examine Shh and Hip mRNA synthesis in the PG (n=11). A Shh RNA probe [22] and a Hip RNA probe [23] were obtained from Andrew McMahon.
Apoptotic index
TUNEL was performed using the Apoptag Kit (Chemicon International) on penis tissue as described previously [20]. All cells were stained for comparison using DAPI (0.005 mg/ml). The total number of cells and the number of apoptotic cells were counted in a given field selected at random by visual observation. The number of apoptotic cells/all cells in five fields from each section and five sections for each penis were counted and reported ± the standard error of the mean (SEM).
Statistics
Statistics were performed using the Excel program and a t-test was used to determine significant differences. P-values ≤0.05 were considered significant.
Results
Localization of SHH pathway in PG neurons
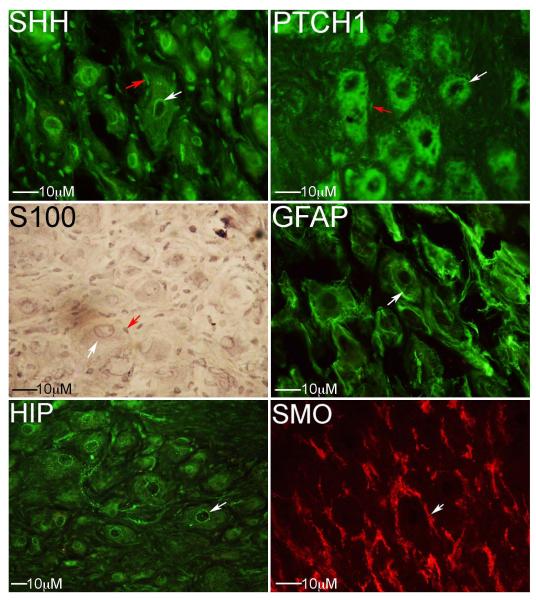
IHC and insitu were performed on adult rat PG to determine SHH pathway localization. In situ showed that Shh and Hip (SHH target that limits Shh expression) mRNA were synthesized in PG neurons but not in glia (Figure 1). IHC shows that SHH protein was abundant in the perinuclear region of PG neurons and at a lower level in the cytoplasm (Figure 2). PTCH1, the SHH receptor, was abundant in the cytoplasm of PG neurons (Figure 2). HIP was identified in the perinuclear region of the cytoplasm (Figure 2). SMO, the other part of the SHH receptor, was localized in between PG neurons (Figure 2). SHH and PTCH1 proteins also localized in glial cells of the PG (Figure 2). Satellite glial cells were identified based on positive S100 staining but negative GFAP (Figure 2). The presence of SHH and PTCH1 proteins in glial cells is significant because extensive signaling between glial cells and neurons occurs in peripheral nerves after injury [24], suggesting a potential mechanism of how SHH promotes CN regeneration.
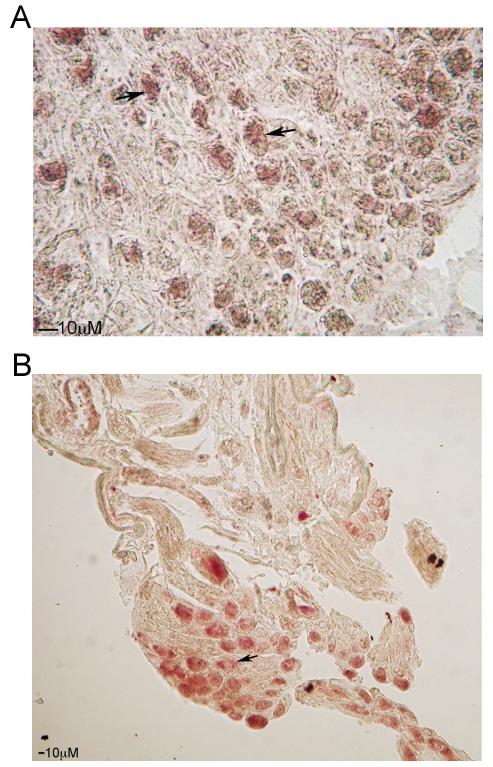
Figure 1.
In situ hybridization showing the localization of Shh (A) and Hip (B) RNA synthesis in the PG/CN. Shh and Hip are localized in PG neurons. 400X and 100X magnification, respectively. Arrows indicate Shh and Hip staining (pink).
Figure 2.
Immunohistochemical analysis showing the localization of SHH, PTCH1, HIP and SMO in PG neurons. SHH is abundant in the perinuclear region of PG neurons and satellite glial cells as confirmed by positive staining for S100 (red arrow) and negative staining for GFAP (white arrow indicates neuronal staining but not glial cells). SHH protein was also identified to a lesser extent in neuronal cytoplasm. PTCH1 is abundant in the cytoplasm of PG neurons and is identifiable in nearby glial cells. HIP is present as a thin layer surrounding the nucleus of PG neurons. SMO is abundant in between PG neurons. White arrows indicate staining in and around PG neurons. Red arrows indicate satellite glial cells. 250-400X magnification.
Time course of SHH and GFAP proteins in PG/CN after bilateral CN crush
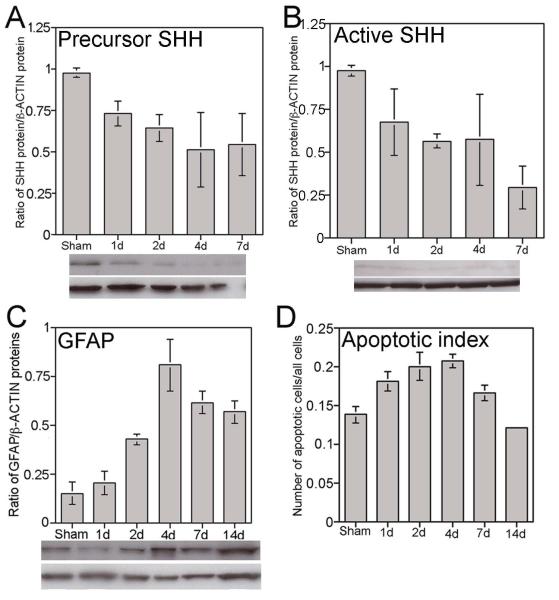
The precursor and active form of SHH protein were quantified in PG/CN 1-14 days after bilateral CN crush in comparison to sham controls. While SHH protein decreases in the penis with CN injury [20] its abundance in the PG/CN has not previously been quantified. Precursor SHH protein was significantly decreased the first day after injury (p=0.02) and remained decreased in the first week after crush (Figure 3). Active SHH protein was also significantly decreased in the first week after injury. These results suggest that SHH may be effective in promoting CN regeneration because its abundance is decreased in the PG/CN with injury.
Figure 3.
Graph of western analysis showing the abundance of precursor (A) and active (B) SHH protein from 1-7 days after bilateral CN crush in the PG/CN, in comparison to sham controls. SHH decreases in the PG/CN with crush injury. Graph of western analysis showing the abundance of GFAP protein (C) and the apoptotic index (D) from 1-14 days after bilateral CN crush in the PG/CN, in comparison to sham controls. GFAP and the apoptotic index increase in the PG/CN with crush injury.
We quantified GFAP in the PG/CN after crush injury. GFAP is an intermediate filament protein that is involved in maintaining structure and function of the cytoskeleton. GFAP increases in peripheral nerves with injury and decreases to normal levels as regeneration occurs [25]. GFAP is useful as a marker of the glial response, with suppressed GFAP after injury being suggestive of decreased injury. Thus quantification of GFAP protein may be useful as a tool to examine CN status. In other peripheral nerves such as the sciatic nerve, GFAP increases within 24-48 hours after nerve injury due to proliferation of GFAP positive Schwann cells [26]. We quantified the abundance of GFAP in comparison to sham control PG/CN from 1-14 days after bilateral CN crush. GFAP was significantly increased by 65% at 48 hours after crush (Figure 3, p=0.007). GFAP was most abundant at 4 days after crush (Figure 3, 82% increase, p=0.005) when the precursor form of SHH protein was least abundant. GFAP protein remained elevated ~75% at 7 and 14 days after CN crush (Figure 3, p= 0.002 and 0.003, respectively).
We examined the time course of apoptosis induction from 1-14 days after injury in penis tissue of rats that had their CNs crushed bilaterally. The apoptotic index increased 24% (p=0.05) the first day after injury and continued to rise until day 4 where it peaked at 33% (p=0.02, Figure 3). The apoptotic index was indistinguishable from sham levels at 7 (p=.095) and 14 days (p=0.13).
Blockade of anterograde transport decreased SHH protein in the PG/CN
We examined a potential mechanism of how SHH is regulated in the PG/CN by interrupting anterograde transport using anti-kinesin antibody and quantified SHH protein by western in comparison to IgG controls. There was a decreasing trend (23%) in precursor SHH protein that did not reach significance (p=0.12) however active SHH significantly decreased 45% (p=0.006) with two days of anti-kinesin treatment (Figure 4). Since SHH protein does not undergo anterograde transport [1], these results indicate that a factor(s) from the PG is essential to maintain SHH signaling in the CN.
Figure 4.
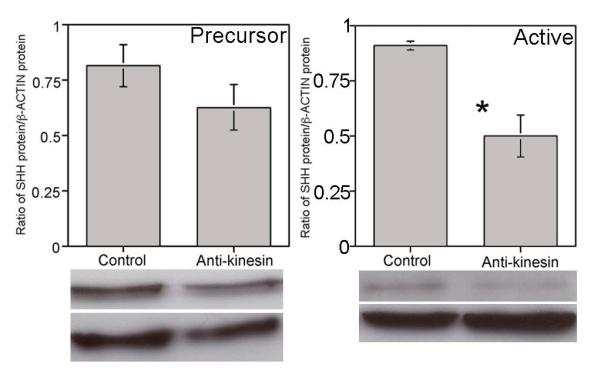
Western analysis of precursor and active SHH protein in anti-kinesin treated PG/CN (interrupts anterograde transport). There was a decreasing trend (23%) in precursor SHH protein that did not reach significance (p=0.12) however active SHH significantly decreased 45% (p=0.006) with two days of anti-kinesin treatment.
SHH protein treatment suppressed GFAP in the CN
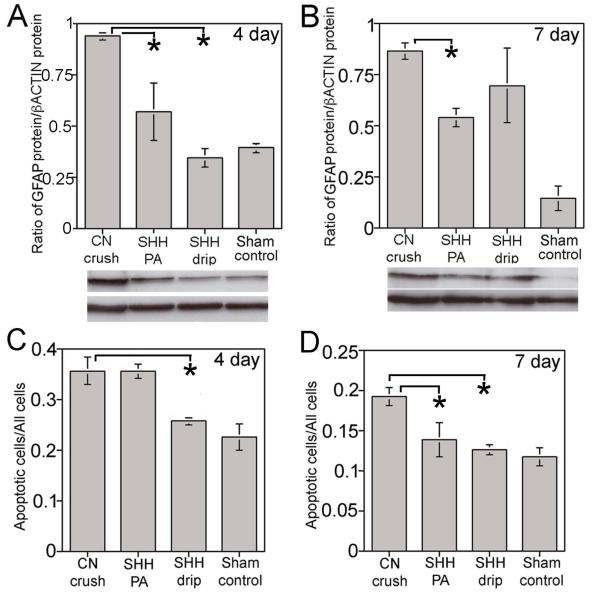
Preservation of the CN by SHH was quantified by examining GFAP protein by western in sham, CN crushed, CN crushed with SHH treatment by PA and CN crushed with SHH dripped on the CN. Rats were examined at 4 and 7 days after crush. GFAP protein was significantly increased in the crushed PG/CN by 58% (p=0.001) at 4 days after injury (Figure 5). In response to SHH PA treatment, GFAP was significantly suppressed 40% (p=0.05) in comparison to the CN crushed group and was indistinguishable from the sham (p=0.171, Figure 5). In the SHH dripped on the CN group, GFAP was decreased by 63% in comparison to the crushed group (p=0.004) and was indistinguishable from the sham (p=0.218, Figure 5). At 7 days after injury, GFAP protein was significantly increased in the crushed PG/CN by 83% in comparison to the sham (p=0.0003, Figure 5). In response to SHH treatment by PA, GFAP decreased 38% in comparison to the CN crushed group (p=0.003, Figure 5). In the SHH dripped on the CN group, there was a trend towards decreased GFAP (20%) that did not reach significance (p=0.209, Figure 5), most likely due to high standard deviation caused by variability of the drip delivery. These results show a decreased glial response in the SHH PA treated CNs, thus suggesting decreased injury.
Figure 5.
Western analysis of GFAP protein in PG/CN after 4 (A) and 7 (B) days following treatment (sham surgery, CN crushed, CN crushed with SHH PA treatment and CN crushed with SHH dripped on the CN). GFAP protein was significantly increased in the crushed PG/CN by 58% (p=0.001) at 4 days after injury. In response to SHH PA treatment, GFAP was significantly suppressed 40% (p=0.05) in comparison to the CN crushed group and was indistinguishable from the sham (p=0.171). In the SHH dripped on the CN group, GFAP was decreased by 63% in comparison to the crushed group (p=0.004) and was indistinguishable from the sham (p=0.218). At 7 days after injury, GFAP protein was significantly increased in the crushed PG/CN by 83% in comparison to the sham (p=0.0003). In response to SHH treatment by PA, GFAP decreased 38% in comparison to the CN crushed group (p=0.003). In the SHH dripped on the CN group, there was a trend towards decreased GFAP (20%) that did not reach significance (p=0.209). (C,D) Apoptotic index in PG/CN after 4 (C) and 7 (D) days following treatment. GFAP and the apoptotic index were decreased in the presence of SHH treatment.
The apoptotic index was examined in penis tissue of the groups listed above to see if SHH suppressed apoptosis. In comparison to sham control, the apoptotic index was significantly increased 37% at 4 days after crush (p=0.01, Figure 5). There was no difference in the apoptotic index of penis from rats that had under gone CN crush and CN crush plus SHH treatment by PA (p=0.50, Figure 5). However the apoptotic index was significantly decreased 28% in penis tissue of rats that had SHH dripped on the CN (p=0.004, Figure 5). At 7 days after crush the apoptotic index was significantly decreased 28% in rats treated with SHH by PA in the CN (p=0.04, Figure 5) and was indistinguishable from the apoptotic index of the sham (p=0.21). The apoptotic index was decreased 34% in penis of rats that had been treated with SHH dripped on the CN (p=0.003) and was indistinguishable from the apoptotic index in the sham (p=0.26). These results show that SHH treatment of the CN significantly decreased the apoptotic index in the penis after crush injury.
SHH protein was decreased in the PG/CN with age
SHH protein was quantified in P120 and P200-P300 PG/CN by western to examine if similar changes in SHH signaling take place in the aged rat as in prostatectomy [2] and diabetic models [27]. Precursor SHH protein was decreased 37% in aged rats in the PG/CN (p=0.001, Figure 6), while active SHH protein was decreased 77% in aged rats (p=0.044, Figure 6). Based on the neuroprotective and regenerative [2] effect that SHH has when the CN is injured, we propose that decreased SHH in the PG/CN of the aged rat may play a role in the development of age related ED. Past studies have shown that morphological remodeling caused by SHH inhibition is reversible [20].
Figure 6.
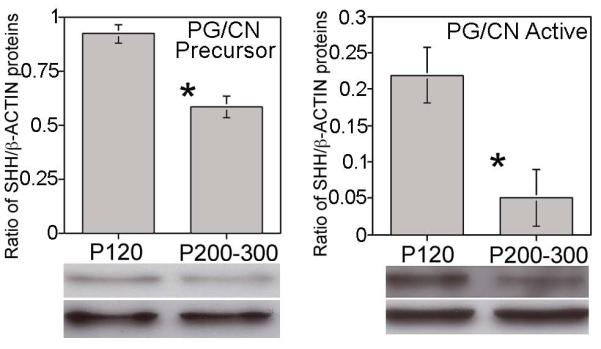
Western analysis for precursor and active SHH protein in PG/CN of adult (P120) and aged (P200-300) rats. The precursor form of SHH protein decreased 37% (p=0.001) and the active form decreased 77% (p=0.044) in the PG/CN of aged rats.
Discussion
How SHH signaling occurs in the PG/CN is an important question in order to understand the role of the SHH pathway in CN homeostasis, neuroprotection and in regeneration. Since Shh RNA is synthesized in PG neurons and both SHH and PTCH1 proteins are localized in PG neurons and satellite glia, this suggests that the glial cells are a SHH target and that the SHH pathway plays a role in signaling between PG neurons and glia. Glial cells control the neuronal microenvironment and can maintain chemical communications among themselves and with neurons and blood vessels [28-31]. They carry receptors for many neuroactive agents and can receive signals from other cells and respond to changes in their environment. Striking morphological changes occur in glial cells after nerve injury including hypertrophy and formation of bridges with other glial cells, which contain numerous newly formed gap junctions, suggesting that glial cells can sense injury-related changes in neurons. This is supported by extensive signaling between glial cells and neurons after injury in peripheral nerves [24]. These findings indicate paracrine signaling from the neurons to glia since SHH protein is present in both neurons and glia while Shh RNA is only present in neurons. These results also suggest that SHH pathway signaling between neurons and glia may be a critical component of how SHH facilitates neuroprotection and regeneration.
PTCH1 is abundant in the cytoplasm of PG neurons and in glia (Figure 2). Since Shh is not synthesized in glia, this suggests autocrine signaling of SHH and PTCH1 in PG neurons. Why this may occur is interesting and requires further study but likely involves regulation of the neuronal microenvironment.
Hip RNA is localized in the cytoplasm of PG/CN neurons (Figure 1) but is not present in glia or schwann cells of the CN. This indicates that the source of Hip is in the cytoplasm of PG neurons. HIP protein, which has both a membrane associated and soluble form [32], is abundant in a thin layer surrounding the nucleus of PG neurons. HIP is believed to inhibit expression of SHH signaling in other tissues, thus HIP protein surrounding the nucleus may restrict SHH distribution within the cytoplasm. Our past studies suggest a more complex role for HIP in the CN since we have shown that a soluble form of HIP undergoes anterograde transport down the CN and that CN tie causes HIP protein to build up in neuronal cytoplasm [33]. HIP transport in neuronal axons has not been observed in other tissues and its function in this capacity is unclear but interesting. The target of HIP is likely the nerve terminals in the CN where HIP may upregulate other signals or HIP may be transported to the penis as suggested by HIP protein localization in the nerve bundle of penis sections [33]. Since HIP undergoes anterograde transport it is likely that HIP is relaying signals from the PG to the penis. Inhibiting HIP in the PG disrupts both CN and penile architecture [33] and thus highlights the importance of maintaining crosstalk between the two organs.
SMO protein appears in between PG neurons. From the position of SMO it is possible that it is localized in nerve terminals originating from the spinal cord and thus may relay signals from the PG upstream to the spinal cord. Thus the SHH pathway may coordinate signaling between the PG/CN/penis and the central nervous system/spinal cord.
Little is known about how signaling in the PG/CN is maintained. Interruption of anterograde transport decreases SHH protein in the CN. This shows that in order to maintain SHH signaling in the CN and thus maintain CN homeostasis and architecture in the normal nerve, input from the PG is required. When the CN is injured either via crush or resection, as may occur during prostatectomy surgery, interruption of transport occurs in both directions resulting in decreased SHH protein in the CN and penis, and thus alters CN and penile morphology. Addition of SHH protein to the CN at the time of nerve injury can prevent the deleterious morphological changes that occur in both tissues [2]. This concept of neuronal signaling being required to regulate down stream tissue homeostasis is a novel way to think about how tissue morphology develops and is maintained and may profoundly influence development of future ED treatments.
SHH protein treatment in the first few days after injury prevents the rise in GFAP that occurs with injury, and suppresses apoptosis in the penis, suggesting that SHH functions to protect the CN from the effects of crush injury. SHH treatment is more effective by drip delivery at 4 days however PA delivery is more effective in suppressing GFAP at 7 days. This most likely results due to a difference in concentration. While both methods deliver the same total amount of protein, the bolus delivery occurs all at once while the PA delivers half of the protein in the first 5 hours and the remainder slowly over several days. It may be that a higher initial concentration of SHH is more effective in suppressing injury while a sustained release suppresses injury more effectively over a longer period of time. Despite the short window of SHH treatment, our results show a 30% reduction in apoptosis in the penis after 7 days. This suggests that the CN is injured less in response to crush injury when it is treated with SHH.
The timecourse of apoptosis induction in response to CN resection has been reported by members of our group [12]. Typically it is assumed that the CN responds in a similar manner to cutting, crushing, and freezing injuries, however this has not been tested. Our results show that the apoptotic index increased 24% the first day after crush injury, peaked at 33% at day 4, and returned to baseline by 7 days. When the CN was previously cut, apoptosis peaked between 2-4 days, was reduced by ~50% at 7 days, and continued to be slightly elevated at 14 and 28 days after resection. These results suggest that the penile response to crush and resection injury is similar, however after the initial intense apoptotic response in the first week, the resection injury has a low sustained level of apoptosis over a longer period of time than the crush injury.
Aging is also a significant cause of ED [34]. Our results show that SHH protein is decreased in the PG/CN of aged rats. Very little is known about what happens to the CN with aging. A study by Lue’s group showed that NOSI positive neurons decrease in the CN with age, suggesting impaired function of the CN [35]. This is in keeping with literature reports of increasing ED with age [34]. Since SHH and NOSI co-localize in neurons of the PG that innervate the penis [1], and previous studies show that SHH regulates NOSI and –III in the penis [36], the authors speculate that decreased SHH in the aged PG/CN may result in loss of NOSI positive neurons. With deteriorating CN function ED may develop. Thus the mechanism of how ED develops with aging, prostatectomy and diabetic models may result from similar signaling changes in the PG/CN.
In conclusion, our results suggest that SHH is neuroprotective in the PG/CN with injury and that neuronal-glial signaling may play a role in the neuroprotective response. SHH protein is significantly decreased in the CN with age in a similar manner to decreased SHH after crush injury, thus suggesting that decreased SHH may also play a role in aging related ED development. These findings suggest that there is a window of opportunity immediately after nerve insult in which manipulation of the nerve microenvironment with SHH can affect long-term regeneration outcome.
Summary sentence.
Sonic hedgehog is decreased in the pelvic ganglia/cavernous nerve with injury and in aged rats, is neuroprotective after crush injury, is regulated by signals from the pelvic ganglia and plays a role in neuronal-glial interactions which are critical to maintain nerve homeostasis and for regeneration.
Acknowledgments
Grant Sponsor: National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, Grant numbers: DK079184.
References
- 1.Bond C, Tang Y, Podlasek CA. Neural Regulation of Sonic Hedgehog and Apoptosis in the Penis. Biol. Reprod. 2008;78:947–956. doi: 10.1095/biolreprod.107.064766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angeloni NL, Bond CW, Tang Y, Harrington DA, Zhang S, Stupp SI, McKenna KE, Podlasek CA. Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials. 2011;32:1091–1101. doi: 10.1016/j.biomaterials.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akazawa C, Tsuzuki H, Nakamura Y, Sasaki Y, Ohsaki K, Nakamura S, Arakawa Y, Kohsaka S. The Upregulated Expression of Sonic Hedgehog in Motor Neurons After Rat Facial Nerve Axotomy. J Neuroscience. 2004;24:7923–7930. doi: 10.1523/JNEUROSCI.1784-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akazawa C, Kohsaka S. In Vivo Characterization of Sonic Hedgehog in the Peripheral Nerve Regeneration. Brain Nerve. 2007;59:1341–1346. [PubMed] [Google Scholar]
- 5.Xu QG, Midha R, Martinez JA, Gui GF, Zochodne DW. Facilitated Sprouting in a Peripheral Nerve Injury. Neuroscience. 2008;152:877–887. doi: 10.1016/j.neuroscience.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto M, Ishii K, Nakamura Y, Watabe K, Kohsaka S, Akazawa C. Neuroprotective Effect of Sonic Hedgehog up-regulated in Schwann Cells Following Sciatic Nerve Injury. J Neurochem. 2008;107:918–927. doi: 10.1111/j.1471-4159.2008.05666.x. [DOI] [PubMed] [Google Scholar]
- 7.Pepinsky RB, Shapiro RI, Wang S, Chakraborty A, Lepage SJ, Wen D, Rayhorn P, Horan GSB, Taylor FR, Garber EA, Galdes A, Engber TM. Long-Acting Forms of Sonic Hedgehog with Improved Pharmacokinetic and Pharmacodynamic Properties are Efficacious in a Nerve Injury Model. J Pharmaceutical Sciences. 2002;91:371–387. doi: 10.1002/jps.10052. [DOI] [PubMed] [Google Scholar]
- 8.Barrette B, Calvo E, Vallieres N, Lacroix S. Transcriptional profiling of the injured sciatic nerve of mice carrying the Wld(S) mutant gene: Identification of genes involved in neuroprotection,neuroinflammation, and nerve regeneration. Brain, Behavior, and Immunity. 2010;24:1254–1267. doi: 10.1016/j.bbi.2010.07.249. [DOI] [PubMed] [Google Scholar]
- 9.Kamal WC, Carrejo MH, Tan RS. The implications of increasing age on erectile dysfunction. American Journal of Men’s Health. 2012;6:273–279. doi: 10.1177/1557988311431629. [DOI] [PubMed] [Google Scholar]
- 10.Montorsi P, Ravagnani PM, Galli S, Salonia A, Briganti A, Werba JP, Montorsi F. Association between erectile dysfunction and coronary artery disease: matching the right target with the right test in the right patient. Eur Urol. 2006;50:721–731. doi: 10.1016/j.eururo.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Kendirci M, Hellstrom WJ. Current concepts in the management of erectile dysfunction in men with prostate cancer. Clin Prostate Cancer. 2004;3:87–92. doi: 10.3816/cgc.2004.n.017. [DOI] [PubMed] [Google Scholar]
- 12.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile weight and cell subtype specific changes in a post-radical prostatectomy model of erectile dysfunction. J Urology. 2003;169:1175–1179. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 13.Leungwattanakij S, Bivalacqua TJ, Usta MF, Yang D-Y, Hyun J-S, Champion HC, Abdel-Madeed AB, Hellstrom WJG. Cavernous neruotomy causes hypoxia and fibrosis in rat corpus cavernosum. J Andrology. 2003;24:239–245. doi: 10.1002/j.1939-4640.2003.tb02668.x. [DOI] [PubMed] [Google Scholar]
- 14.Gallina A, Ferrari M, Suardi N, Capitanio U, Abdollah F, Tutolo M, Bianchi M, Sacca A, Salonia A, Rigatti P, Montorsi F, Briganti A. erectile function outcome after bilateral nerve sparing radical prostatectomy: which patients may be left untreated? J Sex Med. 2012;9:903–908. doi: 10.1111/j.1743-6109.2011.02622.x. [DOI] [PubMed] [Google Scholar]
- 15.Pace G, Del Rosso A, Vicentini C. Penile rehabilitation therapy following radical prostatectomy. Disability and Rehabilitation. 2010;32:1204–1208. doi: 10.3109/09638280903511594. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Greenfield MA, Mata A, Palmer LC, Bitton R, Mantei JR, Aparicio C, de la Cruz MO, Stupp SI. A self assembly pathway to aligned monodomain gels. Nat Mater. 2010;9:594–601. doi: 10.1038/nmat2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullerad M, Donohue JF, Li PS, Scardino PT, Mulhall JP. Functional Sequelae of Cavernous Nerve Injury in the Rat: is There Model Dependency. J Sex Med. 2006;3:77–83. doi: 10.1111/j.1743-6109.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi N, Minor TX, Carrion R, Price R, Nunes L, Lue TF. The Effect of FK1706 on Erectile Function Following Bilateral Cavernous Nerve Crush Injury in a Rat Model. J Urology. 2006;176:824–829. doi: 10.1016/j.juro.2006.03.071. [DOI] [PubMed] [Google Scholar]
- 19.Nangle MR, Keast JR. Reduced Efficacy of Nitrergic Neurotransmission Exacerbates Erectile Dysfunction After Penile Nerve Injury Despite Axonal Regeneration. Exp Neurol. 2007;207:30–41. doi: 10.1016/j.expneurol.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Podlasek CA, Meroz CL, Tang Y, McKenna KE, McVary KT. Regulation of cavernous nerve injury-induced apoptosis by sonic hedgehog. Biol Reprod. 2007;76:19–28. doi: 10.1095/biolreprod.106.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podlasek CA, Zelner DJ, Bervig TR, Gonzalez CM, McKenna KE, McVary KT. Characterization and localization of nitric oxide synthase isoforms in the BB/WOR diabetic rat. J Urol. 2001;166:746–755. [PubMed] [Google Scholar]
- 22.Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 23.Chuang P, McMahon AP. Vertebrate Hedgehog signaling modulated by induction of a Hedgehog binding protein. Lett Nat. 1999;397:617–621. doi: 10.1038/17611. [DOI] [PubMed] [Google Scholar]
- 24.Bradman MJG, Arora DK, Morris R, Thippeswamy T. How do the satellite glia cells of the dorsal root ganglia respond to stressed neurons?-nitric oxide saga from embryonic development to axonal injury in adulthood. Neuron Glia Biology. 2010;6:11–17. doi: 10.1017/S1740925X09990494. [DOI] [PubMed] [Google Scholar]
- 25.Triolo D, Dina G, Lorenzetti I, Malaguti MC, Morana P, Del Carro U, Comi G, Messing A, Quattrini A, Previtali SC. Loss of Glial Fibrillary Acidic Protein (GFAP) Impairs Schwann Cell Proliferation and Delays Nerve regeneration After Damage. J Cell Science. 2006;119:3981–3993. doi: 10.1242/jcs.03168. [DOI] [PubMed] [Google Scholar]
- 26.Cheng C, Zochodne DW. In vivo proliferation, migration and phenotypic changes of Schwann cells in the presence of myelinated fibers. Neuroscience. 2002;115:321–329. doi: 10.1016/s0306-4522(02)00291-9. [DOI] [PubMed] [Google Scholar]
- 27.Podlasek CA, Zelner DJ, Harris JD, Meroz CL, Tang Y, McKenna KE, McVary KT. Altered Sonic Hedgehog Signaling is Associated with Morphological Abnormalities in the BB/WOR Diabetic Penis. Biol. Reprod. 2003;69:816–827. doi: 10.1095/biolreprod.102.013508. [DOI] [PubMed] [Google Scholar]
- 28.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 29.Perea G, Navarette M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 31.Hanani M. Satellite glial cells in sympathetic and parasympathetic ganglia: In search of function. Brain Research Reviews. 2010;64:304–327. doi: 10.1016/j.brainresrev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Coulombe J, Traiffort E, Loulier K, Faure H, Ruat M. Hedgehog interacting protein in the mature brain: membrane-associated and soluble forms. Mol Cell Neurosci. 2004;25:323–33. doi: 10.1016/j.mcn.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 33.Angeloni NL, Bond CW, Monsivais D, Tang Y, Podlasek CA. The role of hedgehog-interacting protein in maintaining cavernous nerve integrity and adult penile morphology. J Sex Med. 2009;6:2480–2493. doi: 10.1111/j.1743-6109.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Araujo AB, Mohr BA, McKinlay JB. Changes in sexual function in middle-aged and older men: longitudinal data from the Massachusetts male aging study. J Am Geriatr Soc. 2004;52:1502–1509. doi: 10.1111/j.0002-8614.2004.52413.x. [DOI] [PubMed] [Google Scholar]
- 35.Carrier S, Nagaraju P, Morgan DM, Baba K, Nunes L, Lue TF. Age decreases nitric oxide synthase-containing nerve fibers in the rat penis. Journal of Urology. 1997;157:1088–1092. [PubMed] [Google Scholar]
- 36.Podlasek CA, Meroz CL, Korolis H, Tang Y, McKenna KE, McVary KT. Sonic hedgehog, the penis and erectile dysfunction: A review of sonic hedgehog signaling in the penis. Current Pharmaceutical Design. 2005;11:4011–4027. doi: 10.2174/138161205774913408. [DOI] [PubMed] [Google Scholar]