Abstract
Objective
The purpose of this study was to evaluate an intervention to improve staff offers of choice to nursing home (NH) residents during morning care.
Design
A controlled trial with a delayed intervention design.
Setting
Four community, for-profit nursing homes.
Participants
A total of 169 long-stay NH residents who required staff assistance with morning care and were able to express their care preferences.
Intervention
Research staff held weekly training sessions with nurse aides (NAs) for 12 consecutive weeks focused on how to offer choice during four targeted morning care areas: when to get out of bed, when to get dressed/what to wear, incontinence care (changing and/or toileting), and where to dine. Training sessions consisted of brief video vignettes illustrating staff-resident interactions followed by weekly feedback about how often choice was being provided based on standardized observations of care conducted weekly by research staff.
Measurements
Research staff conducted standardized observations during a minimum of 4 consecutive morning hours per participant per week for 12-weeks of baseline and 12-weeks of intervention.
Results
There was a significant increase in the frequency that choice was offered for three of the four targeted morning care areas from baseline to intervention: (1) out of bed, 21% to 33% (p< .001); dressing, 20% to 32% (p< .001); incontinence care, 18% to 23%, (p< .014). Dining location (8% to 13%) was not significant. There was also a significant increase in the amount of NA staff time to provide care from baseline to intervention (8.01 ± 9.0 to 9.68 ± 9.9 minutes per person, p< .001).
Conclusion
A staff training intervention improved the frequency with which NAs offered choice during morning care but also required more time. Despite significant improvements, choice was still offered one-third or less of the time during morning care.
Keywords: culture change, resident-directed care, quality of life
INTRODUCTION
Considered a radical concept not long ago, resident-directed care in nursing homes (NHs) is now supported by multiple stakeholder groups.1 Federal guidelines for NHs, for example, now identify choice over daily schedules as a resident right.2 Similarly, many NHs, spurred by a culture change movement that promotes resident-directed care, report they have adopted policies and practices that emphasize resident choice.1 Despite growing support, however, resident-directed care represents a new practice in many NHs. According to a 2008 Commonwealth Fund survey, for example, only a third of NHs nationally allow residents to determine their own daily schedules.1 In many NHs, then, offering residents daily choices requires staff, in particular nurse aides (NAs), to implement new routines. This, in turn, can be challenging, as numerous studies to improve NH practices have shown. These studies, designed to improve care in areas ranging from incontinence management to pressure ulcer prevention, cite a myriad of barriers to practice change including insufficient staff time, inadequate training and weak management systems.4–7 While few studies have specifically examined whether direct care staff offer residents choice at the point of care, those that have identify problems. Two recent observational studies, for instance, revealed that NAs rarely offered residents choices during morning care routines.8,9 Other studies have also shown that residents are not encouraged to express choices about their daily care, even though both residents and staff agree that the ability to make choices about everyday activities is important for residents’ quality of life. 10,11 These findings suggest there may be a gap between the value placed on offering choice to residents and how this concept is translated into care practice.
Conceptual Framework for the Intervention
This study evaluated a staff training and management intervention designed to increase daily choices for residents during morning care. The intervention design is consistent with key elements of the widely advocated health-care quality improvement approach known as Quality Assessment and Performance Improvement (QAPI). 12 This approach combines performance improvement with data monitoring and a feedback system that actively incorporates input from staff and residents. Numerous studies across a range of fields—from organizational management 13,14 to health care 15,16 have shown that this quality improvement strategy is associated with measurable improvements in outcomes. As a result, the Centers for Medicare and Medicaid Services (CMS) will soon require NHs to adopt QAPI programs. 17
In keeping with the QAPI approach, the intervention combined brief staff training sessions designed to improve daily work performance with routine data monitoring and frequent feedback and discussion of the results with staff. With respect to data collection and monitoring, we first—prior to implementation of the intervention--developed a standardized observational protocol that supervisors could use to monitor whether NAs offered choice to residents at the point of care in four morning care areas: when to get out of bed, managing incontinence (changing and/or toileting), when to get dressed/what to wear, and deciding where to eat breakfast.9 Care areas were chosen that occur daily and typically occur together within a predictable time frame; these characteristics make these activities more conducive to observation.18 Additionally, morning care provides multiple opportunities for staff and residents to interact and, thus, for choice to be provided by staff.
During intervention, these standardized observations were conducted weekly by both research staff and trained supervisory NH staff. Staff education and feedback from the weekly observations was provided in 10-minute training sessions, with each session focused on a single morning care area. This approach, described in detail in the Methods section, reflects the QAPI-recommended Performance Improvement Project (PIP) strategy, whereby a concentrated effort is made to improve a well-defined problem or quality concern.19 Active learning was emphasized, such that NAs were expected to implement lessons learned between the feedback sessions. The primary research question addressed in this study was: What effect did the staff training and management intervention have on observations of how often NAs encouraged residents to make choices during morning care?
METHODS
Subjects and Setting
The intervention targeted NH staff, but outcomes were measured at the resident level. Residents were recruited from four proprietary NHs, which together housed 612 residents (average occupancy rate = 95%). Total nurse hours per resident day ranged from 3.23–4.38 across the four homes. None of the NHs used consistent NA staff-resident assignment. Administrators reported NA-turnover rates of 20%–24% during the study. None of the NHs had received survey citations related to resident choice. CMS-reported quality ratings ranged from two (below average) to five stars (high).
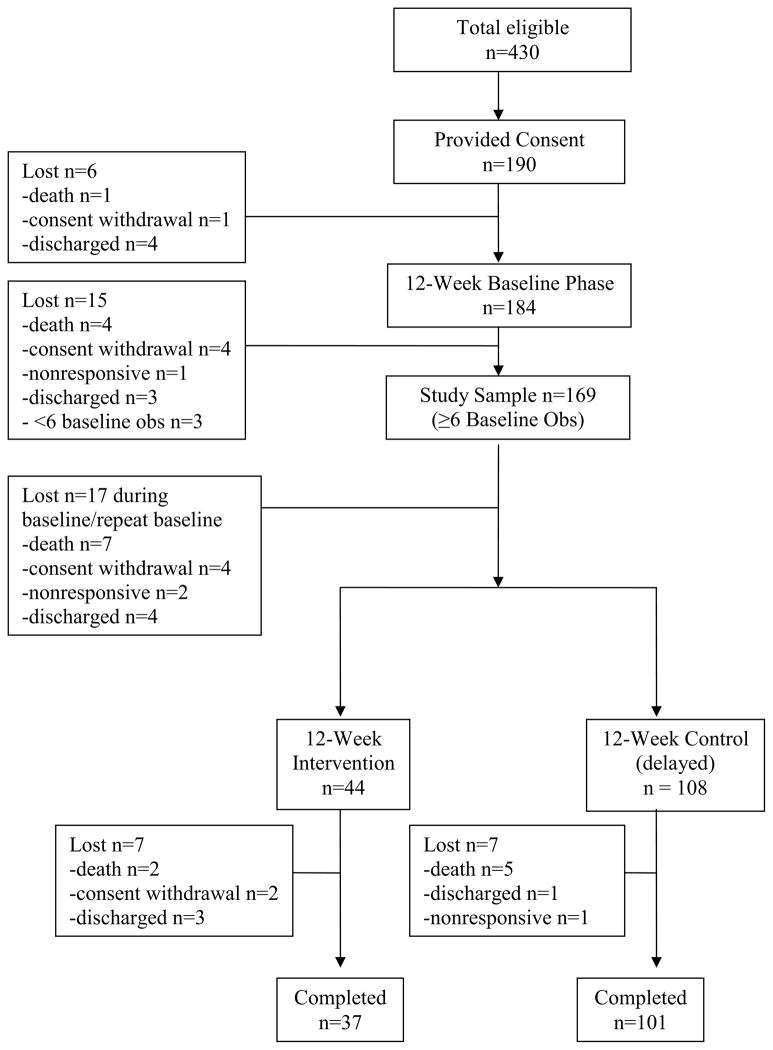
Figure 1 depicts the flow of participants through the trial. A total of 190 (44%) of 430 eligible residents provided consent. Study exclusion criteria included short-stay status, as defined by Medicare criteria, or rated by NH staff as nonresponsive or comatose in the medical records. Research staff confirmed each participant’s ability to respond to prompts via a structured interview, which resulted in additional participants being identified as nonresponsive. The rationale for this exclusion criterion was that care providers should not be expected to offer choice if residents are completely nonresponsive. In addition, study inclusion criteria required residents to be rated by NH staff on their most recent Minimum Data Set assessment as requiring staff assistance with transfer out of bed, dressing and/or incontinence care. Seventy-six percent of participants could not provide self-consent, but gave their assent, while consent was obtained from their designated proxies. The university-affiliated institutional review board approved all study procedures.
Figure 1.
Choice Study Participant Flow Chart
Homes were recruited in pairs and randomized into immediate or delayed intervention groups at the facility-level following a 12-week baseline phase. Six participants were lost prior to intervention (Figure 1) and 169 subjects completed at least 6 weeks of baseline observations, which was the criterion for inclusion in the data analyses. The immediate intervention group (n=44) received the intervention for 12 weeks, while the delayed group (n=108) remained in baseline. Seven residents were lost in the immediate intervention group. Finally, the intervention was replicated in the delayed group for 12 weeks, and seven residents were lost from this group. The remaining 37 residents in the immediate group and 101 residents in the delayed group completed all study phases (Figure 1).
Measures
Descriptive information was retrieved from each participant’s medical record along with the participant’s most recent Minimum Data Set assessment (MDS version 2.0 or 3.0). An MDS-derived measure of physical functioning (MDS-ADL) was calculated for each participant based on seven MDS items, yielding a total score ranging from 0 (rated by staff as independent) to 28 (rated by staff as completely dependent).21 MDS assessments and care plans were reviewed to determine whether NH staff had documented residents’ preferences for morning care activities and whether the resident was rated by staff as resistant to care or requiring physical assistance from two staff for transfer out of bed. Cognitive status was assessed with the Mini-Mental State Examination (MMSE), which yields a total score ranging from 0 (severely cognitively impaired) to 30 (cognitively intact).22
Observations of Morning Care – All Study Phases
Research staff conducted standardized observations of four morning care activities: transfer out of bed, incontinence care (changing and/or toileting), dressing (when to get dressed/what to wear), and breakfast. Observations of each participant were conducted for an average of 3.5 hours (up to four continuous hours) once per week during week days (Monday – Friday) during baseline, repeat baseline, and intervention conditions (12 consecutive weeks in each phase). The goal was to observe each participant at least once per week (minimum of 4 hours on one week day) throughout all study weeks, with the day of observation varying each week. The observation period was adjusted at each site (6:00 am-10:00 am or 7:00 am-11:00 am) based on the morning care routines at that facility.
Staff Communication Relevant to Choice
Standardized observations in previous studies led to the reliable coding of three mutually-exclusive types of staff prompts reflecting different levels of encouragement for residents to make a choice: (1) active choice, (2) passive choice, and (3) no choice.8,9 Active choice prompts (e.g., “Do you want to get up now or after breakfast?”) encouraged residents to make a decision. Passive choice prompts (e.g., “It’s time to get up now, okay?”) required residents to only assent to care.
There were three sub-categories within the “no choice” category: (a) staff did not provide care or speak to the resident (“no care or conversation”); (b) staff provided care without conversation; and (c) staff provided care with conversation. In the first sub-category, staff did not enter the resident’s room during the continuous 4-hour observation period to provide or even verbally offer care for the targeted areas. Thus, the resident had no opportunity to either receive care or express a choice. Staff members who entered the resident’s room during this period and provided or conversed with the resident about aspects of care that were not the focus of this study (e.g., medications or housekeeping) were not included in this category unless staff asked about the resident’s need for care in one or more of the targeted areas. In the second “no conversation” sub-category, staff provided care but said nothing to the resident about this care. In the third category, care was provided with general conversation (e.g., “How are you today?”) but no choices were offered pertaining to the care
Reliability of Coding
Research staff (n=5) were trained prior to data collection in NHs using real care situations until inter-rater reliability was achieved at a Kappa level of .80 or higher for each observation-based coding element listed above. The project coordinator or a research geriatric nurse practitioner continued to conduct inter-rater reliability checks twice monthly with each observer to prevent observer drift during all data collection phases. The Kappa values for the primary outcome of choice offered was .89-1.00 (P< .001) across the targeted care areas and study phases.
Other Observations
Residents spontaneously requested care or otherwise made their preferences known before NH staff prompted them or provided care on 3% to 9% of the observations. These occasions were coded as “spontaneous requests” and are not reported in this paper. In a few cases (6% to 9% overall) a resident performed a task independently even though all participants were rated by staff on their most recent MDS as requiring staff assistance for one or more of the morning care activities targeted in this study. In more cases (14% overall), care was provided, but a researcher was not present to determine if staff offered choice (e.g., care was provided before 6 am). Finally, researchers recorded the total time to provide care in the targeted areas during each observation period. If no conversation or care occurred for a resident, a “zero” time was entered for that care area.
Staff Training and Management Intervention Implementation
As noted earlier, the intervention combined performance improvement strategies (e.g., video presentations of how to offer choice) with data monitoring and feedback (e.g., feedback about choice based on observations of care) in keeping with a QAPI approach to quality improvement. Twelve consecutive weekly training sessions (3 weeks per care area), scheduled to last 10 minutes each (actual sessions ranged from 7.9–11.4 minutes), were held at the end of shifts at the administrators’ request. Sessions were led by the research team (principal investigator and/or a geriatric nurse practitioner). Each session focused on how to offer active choice in one care area, starting with “getting out of bed”. Each session featured two video vignettes that illustrated: (1) a communication that reflected no choice or passive choice (e.g., “Hi, Mrs. Smith, it’s time to get up” or “Hi, Mrs. Smith, it’s time to get up now, okay?”) and (2) a communication that reflected active choice (e.g., “Hi, Mrs. Smith, do you want to get up now?”). The videos showed an NA working with an older person in a variety of scenarios using scripts based on actual observational data collected in previous work. Three pairs of video vignettes were created for each care area. Prior to training, the video vignettes were presented to groups of families and NAs who rated the scenarios in which active choice was offered as reflecting significantly higher quality care than the scenarios in which no or passive choice was offered.23 These results suggest that the vignettes demonstrating active choice reflect care that caregivers regard as high quality. A sampling of these training videos can be viewed online at http://www.VanderbiltCQA.org/Choice.
As described earlier, between each weekly session researchers conducted a minimum of one 3–4 hour observation per participant of the targeted care areas to determine whether NAs offered choice in a manner similar to that modeled in the “active choice” video vignettes. At least one NH supervisor in each facility also was trained to conduct observations alongside the researchers on one day per week. NAs were given credit for offering either active or passive choice, although active choice was emphasized in the training sessions. Of importance was that the language used by NAs allowed residents to refuse or accept offers of care. Table 1 illustrates how choice was defined in each care domain and presents examples of staff prompts that were observed and counted as offering choice.
Table 1.
Care Domains and Examples of Active and Passive Choice Prompts
Out of Bed: Staff could either offer alternatives or an open-ended choice.
|
Incontinence: Staff could offer choice for checking soiled linens or garments, changing linens or garments or toileting in different receptacles.
|
Dressing: Staff could offer choice for what to wear and/or when to get dressed.
|
Dining location: Only staff offers of choice related to dining location were included.
|
Feedback in bar-graph form compared the baseline frequency for active choice in each week’s targeted care area as well as areas that had been previously targeted to that week’s observed frequency. Thus, the bar graphs presented “before” and “current” results for each week. Feedback was given for the first 3 weeks for out-of-bed choice. On the fourth week, the next care area, incontinence management, was targeted, and feedback was given about both out-of-bed and incontinence management for 3 weeks. Table 2 shows how the two intervention components (video-based performance improvement education and data monitoring and feedback) were implemented in sequential 3-week blocks for each of the four targeted care areas. Barriers to offering choice also were discussed in the weekly training sessions (e.g., staff time and routines, residents who resist care), and individualized solutions were identified to allow NAs to offer choice at least once. The training sessions repeated some material so that NAs would be exposed to some training even if they were newly hired or had missed earlier sessions.
Table 2.
Intervention Implementation Timeline and Results
| Targeted Care Area | 12-Weeks Baseline % Choice | Intervention Weeks 0–3 (% Choice) | Intervention Weeks 4–6 (% Choice) | Intervention Weeks 7–9 (% Choice) | Intervention Weeks 10–12 (% Choice) |
|---|---|---|---|---|---|
| Transfer Out of Bed | 21% | Video + Feedback (33%)* | Feedback Only (34%)* | Feedback Only (31%)* | Feedback Only (30%)* |
| Incontinence | 18% | -----(21%) | Video + Feedback (23%)* | Feedback Only (25%)* | Feedback Only (28%)* |
| Dressing | 20% | -----(26%)* | -----(30%)* | Video + Feedback (32%)* | Feedback Only (29%)* |
| Dining Location | 8% | -----(9%) | -----(7%) | -----(7%) | Video + Feedback (13%)* |
Shaded Cells indicates the 3-week period during which the intervention targeted the care area and provided both video training and feedback. Subsequent weeks following this period included “feedback only” related to the care area. Cells with “-----“ indicate weeks during which there was neither video training nor feedback provided related to the care area. Percentages calculated by dividing observations in which choice was offered by observations that care was provided with no choice + observations in which no care or conversation about care occurred.
Significant difference from baseline to each 3-week Intervention period based on paired-samples t tests (All P < .01).
Data Analyses
The primary outcome measure was defined as the total number of instances (counts) of “choice offered” for each participant within each care area. Again, this count combined both active and passive types of choice. The total number of counts of “choice offered” was aggregated for each participant from that participant’s binary outcome. The rate for each outcome was calculated for each participant by dividing by the corresponding total number of observations for that participant. Data were included for all participants who had at least six scheduled baseline observations (i.e., remained in the study for at least half of the baseline phase) for the primary outcome analysis, even though some of these participants were subsequently lost prior to completing the study (see Figure 1). The rationale for including all resident participants, irrespective of drop-out status, was that the primary outcome measure was related to staff behavior (offering choice) and was not resident-specific (i.e., staff were trained to offer choice to all participants). Hence, the likelihood-based method of analysis would provide valid estimates of the incidence rate ratio. The benefit of modeling the aggregated counts data is that the counts models, such as Poisson and negative binomial models, take into account a range of total number of observations that varied between subjects.
To examine intervention effects, a longitudinal analysis was conducted using negative binomial random-effects models to take into account extra-binomial variation and the correlation among repeated measurements.24 The incidence rate ratio (IRR) of intervention and baseline for each outcome (“choice offered” episodes per participant per care area) was estimated after adjusting for potential covariates, which included NH site and the natural logarithm of the total number of observations as well as the following a priori selected resident characteristics: the natural logarithm of length of stay, MMSE total score (0–30), MDS-ADL total score (0–28), rated by NH staff as requiring a two-person assist (yes/no), and rated by NH staff as resisting care (yes/no). The final statistical analysis included 161 of the 169 subjects scheduled for at least six baseline observations, due to missing covariate data.
RESULTS
Participants
Table 3 reports characteristics of the 169 participants who had at least six scheduled baseline observations. These characteristics are typical of long-stay NH residents. There was no medical record documentation of daily care preferences for any of the targeted care areas for any participant. There were no significant differences in characteristics between participants who completed the study (Figure 1. N=138) and those who dropped out.
Table 3.
Characteristics of Participants (N=169)
| Measures | Mean (± SD)a or Percent (n) |
|---|---|
| Age in Years | 80.5 (± 13.5) |
| Percent White | 74.0 (125) |
| Percent Female | 76.3 (129) |
| Length of Stay in Years | 3.4 (± 3.6) |
| MDS-ADL Dependency Score (0–28)b | 17.0 (± 6.2) |
| MMSE total score (0–30)c | 15.4 (± 8.4) |
| Percent probable chronic pain | 42.6 (95) |
| Percent Depression diagnosis | 81.1 (137) |
| Percent rated as resisting care at least once in last weekd | 20.1 (34) |
| Percent rated as 2-person physical assiste | 43.2 (73) |
SD = Standard Deviation
MDS-ADL Dependency Score = Minimum Data Set derived Activities of Daily Living score (total score range = 0, rated by staff as completely independent, to 28, rated by staff as completely dependent in all of 7 ADLs).
MMSE = Mini Mental State Examination (total score range = 0, severely cognitively impaired, to 30, cognitively intact).
Resisting care = MDS Behavioral Symptoms, proportion rated by staff as 1 (behavior occurred 1 to 3 days), 2 (behavior occurred 4 to 6 days) or 3 (behavior occurred daily) in last 7 days.
2-person physical assist = Care plan indication and/or MDS, Section G. Physical Functioning, ADL support provided, rating 3 (two+ person physical assist) for transfer.
Primary Outcome Measure: Rate of Choice Offered
Research staff completed 1,706 baseline observations for the 169 participants across the targeted care areas (average: 10.1 observations per participant). A total of 959 observations (average: 8.3 per participant) were completed during the repeat baseline (control) phase, and 1,956 observations (average: 12.95 per participant) were completed during the intervention phase. The most common reason for missed observations was that the participant was temporarily out of the facility (e.g., hospital stay), though in one facility observations were conducted less frequently during two weeks due to an influenza outbreak.
There was no difference in the incidence rate for “choice offered” from baseline to repeat baseline for any care area after adjusting for covariates (see Data Analysis). As a result, the primary analysis compared all participants using their first baseline phase to all participants in the intervention phase. Compared to baseline, the relative incidence rate ratios (IRR) for “choice offered” across all 12 intervention weeks significantly increased for all care areas, except dining (out of bed: IRR= 1.56, 95%; Confidence Intervals [CI]: 1.32–1.85, P < 0.001; incontinence: IRR= 1.26, 95% CI: 1.05–1.52, P = 0.014; and dressing: IRR= 1.71, 95% CI: 1.41–2.08, P < 0.001).
Choice in dining location did significantly increase from baseline to the 3-week intervention period during which this care area was targeted as a training topic (see Table 2. Baseline dining location = 8% choice offered relative to Video + Feedback Intervention weeks 10–12 = 13%, P<.01). Table 2 shows the percentage of “choice offered” for baseline and each 3-week intervention period along with a listing of the intervention component in effect during that period (none at all versus video + feedback versus feedback only). It is notable that the percentage of “choice offered” for every 3-week block subsequent to when a specific care area was the focus of intervention was significantly higher relative to baseline. In addition, both dressing and incontinence (but not dining) began to show improvements after the first 3 weeks when only out-of-bed was targeted, which suggests that initial staff training related to offering choice in one care area generalized, to some degree, to other care areas.
When facility effects were examined, there was a trend for one of the four sites to show less of an intervention effect in all care areas. Of the three “no choice” sub-categories (see Methods), the most frequent was “no care or conversation”. The percentage of baseline observations in this sub-category for out-of-bed, incontinence, and dressing ranged from 38% to 41%. There was a significant decrease in the frequency of “no care or conversation” episodes for out-of-bed and dressing from baseline to intervention of 8% and 9%, respectively (P < .02 and .01), but no change for incontinence care. A breakfast tray was almost always (95%) delivered to participants during the observation period; thus, there were few instances of “no care” related to dining.
The total time for all care provided per person per observation period was 8.01 (± 9.0) minutes during baseline and 7.96 (± 9.0) minutes during repeat baseline, a non-significant difference. Relative to baseline, care time increased significantly to 9.68 (± 9.9) minutes per person during intervention (t= 5.3, p<.001).
DISCUSSION
This study is the first controlled intervention trial designed to increase NH resident choice at the point of care, and results showed a significant increase in the frequency with which choice was offered by NA staff during targeted aspects of morning care. Moreover, these intervention effects were maintained over 12 weeks for three of the four care areas. The fact that dining was scheduled last for intervention and received only 3 weeks of targeted feedback may explain why there were only significant changes in this area at the end of the intervention.
Importantly, the intervention achieved its intended results using a QAPI framework designed to be feasible for NH staff. In this study, indigenous NAs altered their care routines in apparent response to regular but brief training and feedback sessions. No attempt was made to mitigate implementation barriers, such as staff turnover rates and understaffing, that other studies have identified as impeding quality improvement. Indeed, NAs in the participating NHs improved care quality--by offering more resident-directed care-- despite experiencing turnover rates of 20–24% during the study. Although, implementation barriers not addressed in this study may have contributed to the rather modest intervention effects. To spur replication, the intervention tools needed for staff training and monitoring (i.e., the video vignettes and the observational tool) have been rigorously evaluated and found reliable.8,9,23,28 These tools are now publicly available online at no charge (go to http://www.VanderbiltCQA.org/Choice). Nurse supervisors do not have to spend as much time observing care as research staff spent in this study; however, we do recommend weekly observations of care for a small sample of residents (3–6) identified by staff as requiring assistance with morning care. These observations could vary by day of the week, with each unit supervisor taking responsibility for observations, to yield accurate information about how often NAs are offering residents choices during daily care provision.
At the same time, we recognize that this trial used trained research staff to monitor care provision and conduct the feedback sessions, although NH supervisors worked alongside research staff for at least some of these tasks. To comply with the CMS QAPI initiative, NH staffs will have to undertake similarly proactive data collection and feedback tasks. To date, there is little evidence to suggest they routinely perform these quality improvement (QI) activities. More research is needed to evaluate and perhaps improve how NH supervisors achieve their new QI responsibilities under the QAPI initiative. This study was not designed to answer those questions; rather, it aimed to evaluate an intervention that, when implemented under qualified leadership, could be a successful QAPI project in an area—resident-directed care—of high value to NHs and residents alike.
Although the intervention effects were significant, they were also somewhat modest, a qualification worth exploring. Asked during each training session to identify barriers to offering choice, NAs most commonly reported lack of staff time (e.g., NAs often reported being in a “hurry”) and routine schedules that were difficult to change. Reports of insufficient staff time are supported by findings that the time spent providing morning care significantly increased from baseline to intervention. On average, the increase was small, but there was considerable variation, which reflects the variable nature of care when residents make choices that are time-consuming to honor. For example, assisting residents to the toilet can take five to ten minutes longer than simply changing them.25 Such variability challenges NAs to provide time-efficient care, as has been demonstrated in simulation models.26 Consequently, some NAs may forego offering choices to residents if they are working short-staffed or otherwise in a “hurry” to get care tasks done.27
Another reason why the incidence rate of choice did not increase more was due to the frequency of “no care and no conversation” episodes. This sub-category was the most frequent of the “no-choice” categories during both baseline and intervention. One positive outcome of the intervention was a statistically significant reduction in the frequency of “no care and no conversation” episodes for getting out of bed and dressing. Reducing this frequency further would require NAs to provide more frequent care to more residents as opposed to simply changing the way they communicate with residents for whom they are already providing care. To achieve this outcome, higher staffing ratios may be needed.
One could argue that residents who frequently received no care or choice did not want care and that NAs were aware of residents’ preferences such that they need not offer choice. This argument is weakened, however, by these facilities’ high staff turnover rates, inconsistent aide-to-resident assignment, and the absence of any written documentation of residents’ daily care preferences, in particular a desire to remain in bed until mid-day. In addition, we recently reported that requiring a two-person assist for transfer out of bed is the primary predictor of low-care occurrence among residents, which suggests that staff may limit care provision for these residents due more to labor resource issues than resident care preferences.28 Thus, a more tenable position is that residents should be offered at least the opportunity for choice, even if they elect to forego the offered care (e.g., to stay in bed all morning).
There are a few important study limitations. First, we did not assess quality of life outcomes, although such outcomes have been linked to an increased sense of control, which might be enhanced if choice is offered more frequently 29,30 Second, we do not report effects beyond the 12-week intervention period. As noted earlier, more research is needed to determine whether NH supervisors can initiate and maintain QAPI projects such as the intervention evaluated in this study. Finally, the intervention was tested in just 4 NHs. Outcomes may vary in other facilities based on their organizational and resident characteristics. That noted, the intervention’s monitoring-and feedback component is intended to help NHs tailor the intervention to achieve optimal results.
Conclusion
In summary, this controlled trial, the first of its kind, found that a staff training and management intervention significantly increased resident-directed care during morning care activities. The intervention is intentionally designed to be replicated as a QAPI project in NHs.
Acknowledgments
This research was supported by NIA grant #RO1 AGO 32446 awarded to John Schnelle, Principal Investigator, with support to Dr. Rahman provided by NIA T32AGOOOO37.
Footnotes
None of the authors have any conflicts of interest to report related to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Doty MM, Koren MJ, Sturla EL. [Accessed on July 17, 2012.];Culture Change in Nursing Homes: How Far Have We Come? Findings from The Commonwealth Fund 2007 National Survey of Nursing Homes. http://www.commonwealthfund.org/Content/Publications/Fund-Reports/2008/May/Culture-Change-in-Nursing-Homes--How-Far-Have-We-Come--Findings-From-The-Commonwealth-Fund-2007-Nati.aspx.
- 2.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 3.Centers for Medicare and Medicaid Services. Nursing Homes - Issuance of Revisions to Interpretive Guidance at Several Tags, as Part of Appendix PP, State Operations Manual (SOM), and Training Materials. [Accessed on July 17, 2012.];Letter to State Survey Agency Directors. 2009 http://www.cms.gov/SurveyCertificationGenInfo/downloads/SCletter09_31.pdf.
- 4.Lynn J, West J, Hausmann S, et al. Collaborative clinical quality improvement for pressure ulcers in nursing homes. J Am Geriatr Soc. 2007;55:1663–69. doi: 10.1111/j.1532-5415.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- 5.Rahman AN, Simmons SF, Applebaum R, et al. The coach is in: Improving nutritional care in nursing homes. Gerontologist. 2012;52:571–80. doi: 10.1093/geront/gnr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rantz MJ, Zwygart-Stauffacher M, Hicks L, et al. Randomized multilevel intervention to improve outcomes of residents in nursing homes in need of improvement. J AmMed Dir Assoc. 2012;13:60–8. doi: 10.1016/j.jamda.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnelle JF, McNees P, Crooks V, et al. The use of a computer-based model to implement an incontinence management program. Gerontologist. 1995;35:656–65. doi: 10.1093/geront/35.5.656. [DOI] [PubMed] [Google Scholar]
- 8.Schnelle JF, Bertrand R, Hurd D, et al. Resident choice and the survey process: The need for standardized observation and transparency. Gerontologist. 2009;49:517–24. doi: 10.1093/geront/gnp050. [DOI] [PubMed] [Google Scholar]
- 9.Simmons SF, Rahman A, Beuscher L, et al. Resident-directed long term care: Staff provision of choice during morning care. Gerontologist. 2011;51:867–75. doi: 10.1093/geront/gnr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boscart VM. A communication intervention for nursing staff in chronic care. J Adv Nurs. 2009;65:823–32. doi: 10.1111/j.1365-2648.2009.05035.x. [DOI] [PubMed] [Google Scholar]
- 11.Christenson AM, Buchanan JA, Houlihan D. Command use and compliance in staff communication with elderly residents of long-term care facilities. Behav Ther. 2011;42:47–58. doi: 10.1016/j.beth.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services. [Accessed on July 17, 2012.];Nursing Home Quality Assurance & Program Improvement: Background. http://www.cms.gov/Medicare/Provider-Enrollment-andCertification/SurveyCertificationGenInfo/QAPI.html.
- 13.Deming WE. Out of the Crisis. Cambridge, Massachusetts: Massachusetts Institute of Technology, Center for Advanced Engineering Study; 1986. [Google Scholar]
- 14.Senge P, Kleiner A, Roberts C, et al. The Dance of Change: The Challenges to Sustaining Momentum in Learning Organizations. New York: Doubleday; 1999. [Google Scholar]
- 15.Pronovost P, Vohr E. Safe Patients, Smart Hospitals: How One Doctor’s Checklist Can Help Us Change Health Care from the Inside Out. New York: Hudson Street Press; 2010. [Google Scholar]
- 16.Neily J, Mills PD, Young-Xu Y, et al. Association between implementation of a medical team training program and surgical mortality. JAMA. 2010;304:1693–1700. doi: 10.1001/jama.2010.1506. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Medicare and Medicaid Services. [Accessed on July 17, 2012,];Nursing Home Quality Assurance & Performance Improvement: National Rollout of Nursing Home Quality Assurance & Performance Improvement - Set for Summer 2012. http://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/QAPI.html.
- 18.Sloane PD, Miller LL, Mitchell CM, et al. Provision of morning care to nursing home residents with dementia: Opportunity for improvement? Am J Alzheimers Dis other Demen. 2007;22:369–77. doi: 10.1177/1533317507305593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Medicare and Medicaid Services. [Accessed on July 17, 2012];Nursing Home Quality Assurance & Program Improvement: The 5 Elements of QAPI. http://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/QAPI.html.
- 20.Michael J. Where’s the evidence that active learning works? Adv Physiol Educ. 2006;30:159–67. doi: 10.1152/advan.00053.2006. [DOI] [PubMed] [Google Scholar]
- 21.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci. 1999;54:M546–53. doi: 10.1093/gerona/54.11.m546. [DOI] [PubMed] [Google Scholar]
- 22.Folstein M, Folstein S, McHugh P. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Simmons SF, Durkin DW, Rahman AN, et al. The value of resident choice during daily care: Do staff and families differ? J Appl Gerontol. doi: 10.1177/0733464812454010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi L, Dominici F, Zeger SL, et al. Estimating treatment efficacy over time: A logistic regression model for binary longitudinal outcomes. Stat Med. 2005;24:2789–805. doi: 10.1002/sim.2147. [DOI] [PubMed] [Google Scholar]
- 25.Sowell VA, Schnelle JF, Hu TW, et al. A cost comparison of five methods of managing urinary incontinence. Qual Rev Bull. 1987;13:411–14. doi: 10.1016/s0097-5990(16)30175-0. [DOI] [PubMed] [Google Scholar]
- 26.Schnelle JF, Simmons SF, Cretin S. Report to Congress: Appropriateness of minimum nurse staffing ratios in nursing homes, Phase II final. Vol. 3. Washington, DC: Department of Health and Human Services; 2001. Minimum nurse aide staffing required to implement best practice care in nursing homes. [Google Scholar]
- 27.Persson T, Wästerfors D. “Such Trivial Matters:” How staff account for restrictions of residents’ influence in nursing homes. J Aging Stud. 2009;23:1–11. [Google Scholar]
- 28.Simmons SF, Durkin DW, Rahman AN, et al. Resident characteristics related to the lack of morning care provision in long-term care. Advance online publication. Gerontologist. doi: 10.1093/geront/GNS065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King J, Yourman L, Ahalt C, et al. Quality of life in late-life disability: “I don’t feel bitter because I am in a wheelchair”. J Am Geriatr Soc. 2012;60:569–76. doi: 10.1111/j.1532-5415.2011.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neupert SD, Allaire JC. I think I can, I think I can: Examining the within-person coupling of control beliefs and cognition in older adults. Psychol Aging. 2012 doi: 10.1037/a0026447. Advance online publication. [DOI] [PubMed] [Google Scholar]