Abstract
Bisphenol A (BPA) is an environmental estrogenic endocrine disruptor that may have adverse health impacts on a range of tissue/systems. In previous studies, we reported that BPA rapidly promoted arrhythmias in female rodent hearts through alteration of myocyte calcium handling. In the present study we investigated the acute effects of BPA on ventricular arrhythmias and infarction following ischemia-reperfusion in rat hearts. Rat hearts were subjected to 20 minutes of global ischemia followed by reperfusion. In female, but not male hearts, acute exposure to 1 nM BPA, either alone or combined with 1 nM 17β-estradiol (E2), during reperfusion resulted in a marked increase in the duration of sustained ventricular arrhythmias. BPA plus E2 increased the duration ventricular fibrillation, and the duration of VF as a fraction of total duration of sustained ventricular arrhythmia. The pro-arrhythmic effects of estrogens were abolished by MPP combined with PHTPP, suggesting the involvements of both ERα and ERβ signaling. In contrast to their pro-arrhythmic effects, BPA and E2 reduced infarction size, agreeing with previously described protective effect of estrogen against cardiac infarction. In conclusion, rapid exposure to low dose BPA, particularly when combined with E2, exacerbates ventricular arrhythmia following IR injury in female rat hearts.
Keywords: Bisphenol A, heart, cardiac arrhythmias, ischemic-reperfusion injury
2. INTRODUCTION
Bisphenol A (BPA) is a common environmental endocrine disrupting chemical. It is used in the manufacturing of polycarbonate plastic and epoxy resins, and is found in a wide range of consumer products such as water bottles, food containers, baby bottles, water pipes, metal food and beverage can lining and paper products (Vandenberg et al., 2007). With annual production exceeding 2 million metric tons, BPA is one of the highest production volume chemicals worldwide. BPA leaches from the plastics, particularly when heated or exposed to acidic or basic solutions (Le et al., 2008; Vandenberg et al., 2007). There is near ubiquitous human exposure to BPA, from diet, inhalation and other exposure routes (Geens et al., 2012; Vandenberg et al., 2007). In various human exposure assessments, BPA was detected in urine, serum or blood in most individuals at low µg/L, or low nanomolar, concentrations (Vandenberg et al., 2010).
BPA is increasingly becoming a health concern due to the wide human exposure and its ability to act as an estrogenic endocrine disruptor. Extensive experimental and epidemiological evidence has linked BPA to a number of adverse health effects such as cancer, obesity, diabetes, and disorders of the reproductive and immune systems (Diamanti-Kandarakis et al., 2009). Emerging evidence also points to adverse impact of BPA on the cardiovascular system. Both cross section and longitudinal epidemiological studies in the US and UK populations have demonstrated an association between higher human BPA exposure levels and cardiovascular disease including coronary artery disease and peripheral arterial disease (Lang et al., 2008; Melzer et al., 2012; Melzer et al., 2010; Shankar et al., 2012). We have recently shown that rapid exposure to low doses of BPA promotes the development of arrhythmias in the hearts and ventricular myocytes from female, but not male, rodents (Yan et al., 2011). We showed that the pro-arrhythmic effect of BPA is more pronounced in the presence of physiological level of 17β-estradiol (E2) or under stress conditions; the cardiac actions of BPA is mediated by alteration of myocyte calcium handling, particularly increased in calcium “leak” from the sarcoplasmic reticulum, and by estrogen receptor (ER) β signaling (Belcher et al., 2012; Yan et al., 2011).
Here, we examined the influence of rapid BPA exposure on ischemic-reperfusion (IR) injury, particularly reperfusion arrhythmias, in rat hearts. Acute myocardial infarction (AMI, or myocardial infarction, i.e., heart attack) is estimated to impact over 900,000 individuals annually, with 610,000 new attacks and 325,000 recurrent attacks (Roger et al., 2012). During myocardial infarction, IR injury occurs as a result of severe reduction or arrest of coronary blood flow and subsequent re-establishment of blood flow to the ischemic myocardium (Moens et al., 2005). IR injury can lead to irreversible cell damage, infarction, ventricular dysfunction, and ultimately heart failure. It also results in electrophysiological perturbation of the heart and acute reperfusion arrhythmias, which can lead to sudden cardiac death (Balke et al., 1981; Bernier et al., 1989; Scherlag et al., 1974; Yamazaki et al., 1986). It is well recognized that estrogen has a strong influence on the response of myocardium to IR injury, conferring a generally cardio-protective effect in female animal models (Babiker et al., 2002; Gabel et al., 2005; Kuhar et al., 2007; Murphy and Steenbergen, 2007). However, it should be noted that the cardio-protective effects of estrogen has not been observed in women receiving menopausal hormone therapy (LaCroix et al., 2011; Nelson et al., 2012). Importantly, the potential impact of estrogenic endocrine disruptors such as BPA on IR injury of the heart is not known. Such impact has potential relevance to the outcome of AMI, particularly in women, and is the focus of the present study.
3. MATERIALS AND METHODS
3.1 Reagents
All reagents and solvents used were of the highest purity available. Aqueous solutions were prepared using BPA-free water (18 MΩ; <6 ppb total oxidizable organics; Millipore A10 system). Bisphenol A (BPA), CAS 80-05-7 was from TCI America, lot 111909 (ground by Battelle), and was provided by the Division of the National Toxicology Program (DNTP) at the National Institute of Health/National Institute of Environmental Health Sciences. Dimethyl sulfoxide (Chromasolv Plus, HPLC ≤ 99.7%; batch no. 00451HE) was from Sigma-Aldrich (St. Louis, MO). 1,3,5 (10)-estratriene-3,17β-diol (17β-estradiol, 17β-E2) cat E0959, batch B0356 was from Steraloids (Newport RI). Methyl-piperidino-pyrazole, 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP), CAS 289726-02-9 and 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP), CAS 805239-56-9 were from Tocris Cookson (Ellisville, MO). Other chemicals were from Sigma-Aldrich unless otherwise stated.
3.2 Animals
Animal procedures were done as previously described (Yan et al., 2011), and in accordance with protocols approved by the University of Cincinnati Institutional Animal Care and Use Committee and followed recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Adult Sprague-Dawley rats (200–250 grams; Harlan; Indianapolis, IN) were maintained on a 14 h light, 10 h dark light cycle in standard polycarbonate caging with Sani-chip bedding (Irradiated Aspen Sani-chip; P.J. Murphy Forest Products Corp. Montville, NJ) to eliminate possible corn-based mycoestrogen exposure. All animals are fed ad libitum Teklad diet 2020 (Harlan Laboratories Inc.) which lacks soybean meal, alfalfa or animal products that may introduce uncontrolled levels of estrogenic compounds. Sterile drinking water was generated by a dedicated water purification system (Millipore Rios 16 with ELIX UV/Progard 2) that reduces oxidizable organics to less than 1% of source levels. Drinking water was dispensed from glass water bottles.
3.3 Ex vivo ischemia-reperfusion experiments and cardiac electrogram recordings
Rats were anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and heart quickly dissected. Following dissection, hearts were quickly cannulated via the aorta, and retrograde perfused on a Langendorff apparatus with 37°C Krebs-Henseleit solution containing (mM) NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, EDTA 0.5, CaCl2 2.5, NaHCO3 25, and glucose 11, pH = 7.4, bubbled with 95% O2 and 5% CO2, at a pressure of 80 mmHg and perfusion rate of ~15 ml/min.
Bipolar cardiac electrogram was measured from the surface of the heart, with electrodes positioned at the base and apex of the heart. Hearts were perfused under control conditions for at least 1 hour to allow stabilization and equilibration before ischemia-reperfusion experiments. Hearts that showed any baseline arrhythmias under control conditions after equilibration were rejected. Hearts were subjected to 20 minutes no-flow global ischemia, followed by reperfusion for 60 minutes (for TTC staining experiments, after 60 minutes of reperfusion and cardiac electrogram recording, the hearts were reperfused for another 60 minutes, for a total of 120 minutes). Reperfusion solution contained either vehicle or the treatment chemicals for the duration of the reperfusion. Cardiac electrogram data collection and analysis were performed using the Powlab 4/30 data acquisition system and LabChart 7 software (AD Instruments, Colorado Springs, CO).
3.4 Infarct size analysis using TTC staining
After 120 min of reperfusion, infarct size was determined by the histochemical stain with 2,3,5-triphenyltetrazolium chloride (TTC), which differentiates viable versus necrotic tissue (Klein et al., 1981). Hearts were perfused with 37°C 1% TTC solution in phosphate buffer via the aortic cannula, transferred to 10% formalin solution, frozen at −20°C, and sectioned into 5 to 6 transverse slices. The slices were digitally photographed and weighed. Viable tissue is stained as brick-red whereas necrotic tissue remained pale-white. Infarct area of each section was determined by computer morphometry (NIH imaging software, 1.61 version), and was calculated as a percentage of the entire area of the section. Infarct area of the whole heart was calculated based on infarct area and weight of each section.
3.5 Myocyte simulated ischemia-reperfusion and after-contraction analysis
Ventricular myocytes were enzymatically dissociated using Langendorff perfusion with a Tyrode solution composed of (mM) NaCl 118, KCl 5.4, HEPES 10, NaH2PO4 0.33, MgCl2 2, glucose 10 (pH = 7.4) and containing 0.7 mg/ml type II collagenase (Worthington Biochem, Lakewood, NJ), 1 mg/ml BSA, 0.2 mg/ml hyaluronidase, and 0.025 mM CaCl2. Isolated ventricular myocytes were suspended in 1.0 mM Ca2+-Tyrode solution and allowed to sediment by gravity. Supernatant was then removed and myocytes were resuspended and studied in the same day.
Simulated myocyte ischemia-reperfusion experiments followed previously described protocol (Ren et al., 2009). Myocytes were cultured in either 1.1 mM CaCl2-Tyrode control solution or ischemia buffer composed of (mM) NaCl 128, KCl 5, CaCl2 1.1, KH2PO4 0.3, MgCl2 0.5, MgSO4 0.4, NaHCO3 4, HEPES 10, pH 6.8, and placed in a hypoxic incubator (100% N2, 37°C) for 1 hour, followed by 2 hours of “reperfusion” under control culture conditions (control Tyrode solution, 95% O2 and 5% CO2, 37°C). The reperfusion solution also contained either vehicle or various treatment drugs. Myocytes were then placed in a plexiglass cell chamber filled with 1.1 mM CaCl2-Tyrode test solution at room temperature (24°C). The test solution did not contain any estrogen for all groups. Myocytes were excited with field stimulation (Grass S48 stimulator, Grass Instruments, Quincy, MA) with 2 ms 1.5× threshold pulses at a rate of 0.5 Hz. Steady state myocyte shortening was imaged with a CCD camera and examined using a video-edge detector (Crescent Electronics, Sandy, UT). Data were sampled through an Axon Digidata 1322A board using the PCLAMP 9 software (both from Molecular Devices, Sunnyvale, CA).
3.6 Statistical analysis
Statistical analysis was conducted using unpaired t-test, or one-way analysis of variance (ANOVA) with differences between treatment groups assessed using a multiple comparison post-test. Frequency of events (e.g., percentage of myocyte with after-contractions) was analyzed using a chi-square (χ2) test. Minimal level of statistical significance for differences in values is considered to be P < 0.05. Data was analyzed with SigmaPlot and Excel.
4. RESULTS
4.1 BPA and E2 exacerbate reperfusion arrhythmias in female rat hearts
The effects of BPA and E2 on reperfusion arrhythmia were examined using cardiac electrogram in ex vivo rat hearts. Hearts were subjected to 20 minutes no-flow global ischemia followed by reperfusion. Prior to ischemia-reperfusion (IR) all hearts showed normal sinus rhythm, which quickly converted to asystole upon global ischemia. In a typical heart, junctional and ventricular ectopic beats were first observed 10 – 20 seconds after start of reperfusion, and the ectopic beats were interrupted by short runs of sinus rhythm (not shown). This activity deteriorated into prolonged episodes of ventricular tachycardia (VT; likely originating from a mid to apical ventricular site) that subsequently deteriorated into ventricular fibrillation (VF). Hearts typically spontaneously reverted to sinus rhythm following continued reperfusion. Examples of recorded traces at various time points are shown for the control heart in figure 1, left panel.
Figure 1.
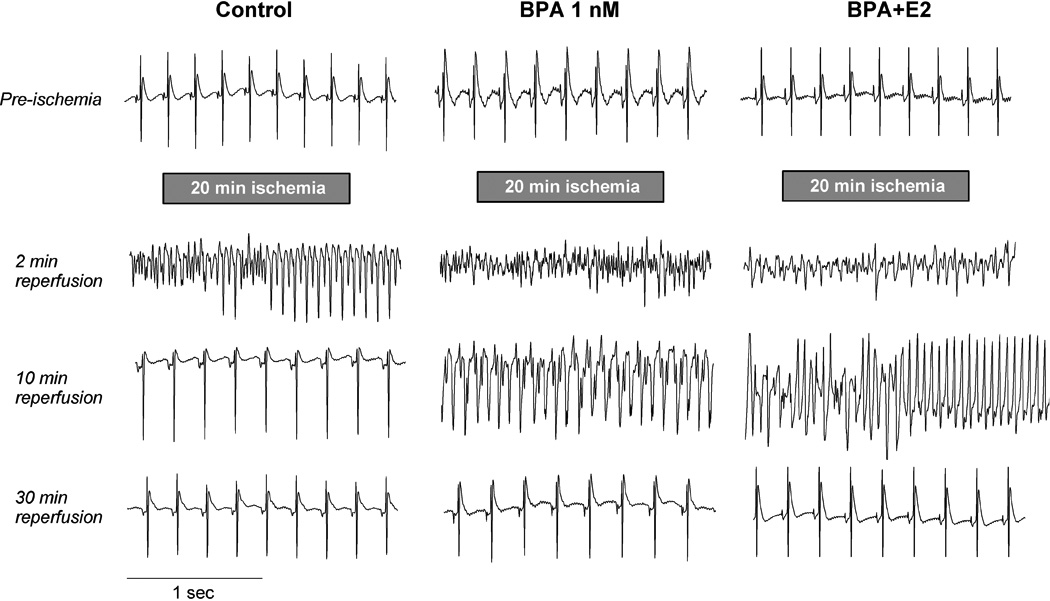
Exposure to low-dose BPA exacerbates ventricular arrhythmia following ischemia/reperfusion in female rat hearts. Shown are typical cardiac electrogram traces recorded from the surface of female rat hearts prior to ischemia, and at various time points (around 2, 10 and 30 minutes) during reperfusion following 20 minutes of global no-flow ischemia. Hearts were exposure to control perfusate, 1 nM BPA or BPA combined with E2 (both at 1 nM) during reperfusion as indicated. All three traces show normal sinus rhythm pre-ischemia. For the control heart, traces show transition from ventricular fibrillation (VF) to ventricular tachycardia (VT) at 2 minutes of reperfusion, and sinus rhythm at 10 and 30 minutes of reperfusion. For the heart exposed to BPA, traces show VF (2 minutes), VT (10 minutes) and sinus rhythm (30 minutes). For the heart exposed to BPA plus E2, traces show VF (2 minutes) followed by transition from VF to VT (10 minutes) and sinus rhythm (30 minutes). It should be noted that a fair amount of variation was observed in terms of the duration of arrhythmias and the nature of arrhythmia at any particular time point; for average data see Figure 2.
In female hearts, exposure to 1 nM BPA during reperfusion significantly increased the duration of reperfusion arrhythmia (Fig. 1, center panel). On average, duration of sustained reperfusion ventricular arrhythmia was increased from 394 sec in control (n = 20) to 744 sec in the BPA exposed group (n = 14; P < 0.05; Fig. 2A). There was a trend of increase in the duration of VF upon BPA exposure, although this increase did not reach statistical significance (Fig. 2B).
Figure 2.
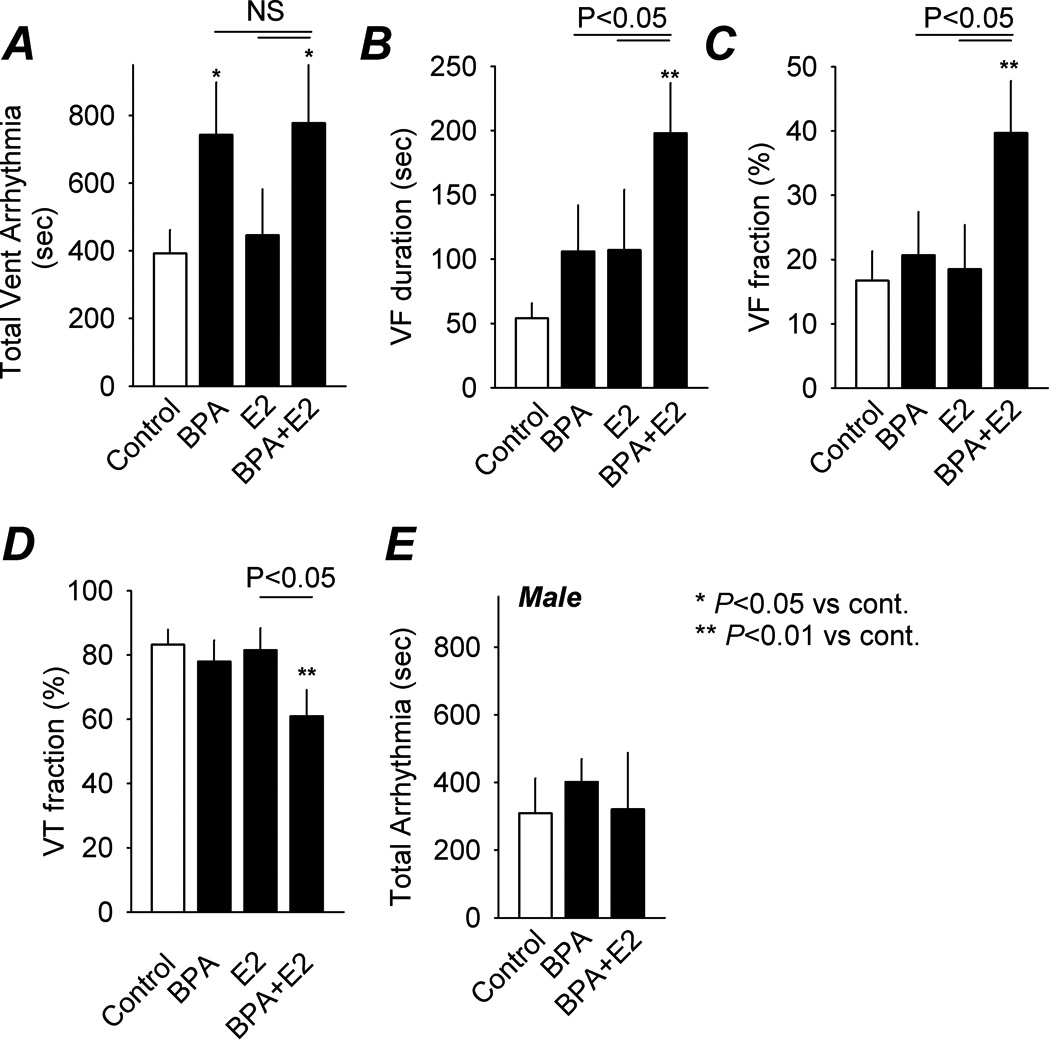
Effects of BPA and estrogen on ischemia/reperfusion-induced arrhythmia. A, total duration of sustained IR-induced ventricular arrhythmia in female rat hearts under control (n = 20 hearts) and when exposed to BPA (n = 14), E2 (n = 13) and BPA plus E2 (n = 16; all at 1 nM) during reperfusion. Duration of ventricular fibrillation (VF) and VF expressed as fraction of total duration ventricular arrhythmia are shown in B and C, respectively. Duration of ventricular tachycardia (VT) expressed as fraction of total duration ventricular arrhythmia is shown in D. E, total duration of IR-induced ventricular arrhythmia under various reperfusion conditions recorded from male rat hearts (n = 6 – 8). Error bars and SEM. *: P < 0.05 vs control; **: P < 0.01 vs control; NS (not significant): P > 0.1.
The effects of endocrine disruptors are most physiologically relevant when evaluated in the context of endogenous hormones. The combined effect of BPA and physiological levels of E2 was examined in female hearts. Exposure to BPA plus E2 during reperfusion increased total duration of sustained ventricular arrhythmia from 394 sec in control to 778 sec (P < 0.05; Fig. 1 and 2A), similar to the effect observed under BPA alone. Of importance, combined exposure markedly increased the duration of VF, a malignant and life threatening form of ventricular arrhythmia, from 54 in control to 198 sec (P < 0.01), and the duration of VF as a fraction of total duration of ventricular arrhythmia from 16.8% in control to 38.7% (P < 0.01; Fig. 2B and 2C). These effects of BPA plus E2 on VF durations were statistically significant when compared with BPA or E2 alone (P < 0.05). In parallel with the increase in VF fraction, BPA plus E2 decreased the duration of VT as a fraction of total duration of ventricular arrhythmia from 83% in control to 61.3% (P < 0.01; Fig. 2D).
Unlike the sensitivity of female hearts to estrogens, reperfusion arrhythmias were not affected by either BPA alone or BPA combined with E2 in male rat hearts (P > 0.1 vs control for both BPA and BPA+E2; Fig. 2E).
4.2 Role of ER mediated signaling
In previous studies, we showed that BPA rapidly increased arrhythmogenic events and modulated mechanical properties in female cardiac myocytes, and that these rapid actions of BPA were mediated by estrogen receptor (ER) β signaling (Belcher et al., 2012; Yan et al., 2011). Using both total duration of ventricular arrhythmia and percentage of VF as indices, the role of ERs in mediating estrogens’ action on reperfusion arrhythmias were examined. Neither PHTPP, an ERβ selective blocker (5 µM) nor MPP, an ERα selective blocker (1 µM) significantly affected the pro-arrhythmic actions of BPA plus E2 in female hearts (Fig. 3; P > 0.2 vs BPA+E2). Interestingly, PHTPP combined with MPP abolished the pro-arrhythmic effects of BPA plus E2 on IR-induced arrhythmias and VF duration (P < 0.05 vs BPA+E2; P > 0.1 vs control), suggesting the involvement of both ERα and ERβ-mediated signaling.
Figure 3.
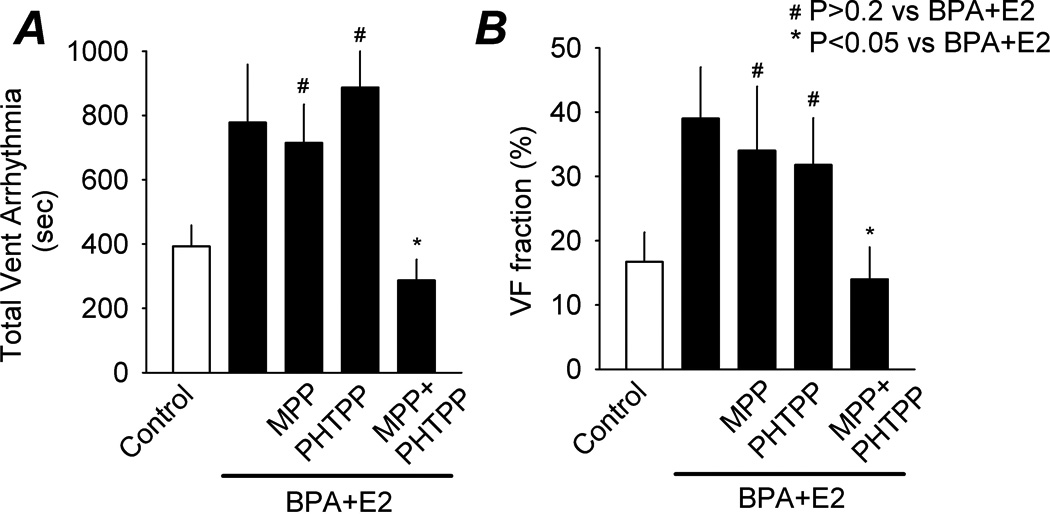
Roles of ERs in mediating the pro-arrhythmic effect of estrogens on ischemia/reperfusion-induced arrhythmia. Shown are total duration of sustained ventricular arrhythmia (A) and VF as fraction of total ventricular arrhythmia (B) in female rat hearts following IR injury, under control, BPA plus E2 (both at 1 nM), and BPA plus E2 in the presence of MPP (1 µM), PHTPP (5 µM), or MPP plus PHTPP (1 and 5 µM, respectively). N was 8 hearts for all ER blocker groups. Error bars and SEM. *: P < 0.05 vs BPA plus E2; #: P > 0.2 vs BPA plus E2.
4.3 BPA increases triggered activities in myocytes following simulated IR
The mechanisms of reperfusion arrhythmia and the actions of estrogen on the heart are complex and involve both vascular and myocardial effects. Whether BPA has direct effect on IR-induced arrhythmia at the myocyte level was examined. Myocytes from female hearts were subjected to simulated IR treatment (see Methods) and incidence of spontaneous excitation following repeated pacing was examined (Fig. 4). Such spontaneous excitation is indicative of delayed after-depolarization, a well recognized cellular mechanism of arrhythmogenesis in the heart (Pogwizd and Bers, 2004). Six percent of non-IR treated myocytes (control) showed after-contractions (n = 29). Simulated IR injury increased the percentage of myocytes with after-contractions to 33% (n = 34). Exposure to 1 nM BPA or BPA plus E2 (both at 1 nM) during reperfusion further increased the percentage to 60% and 67%, respectively (n = 32 and 37, respectively; P < 0.05 vs simulated IR alone). These results suggest that the pro-arrhythmic effects of estrogens post IR injury in female hearts likely involve direct actions on cardiac myocytes.
Figure 4.
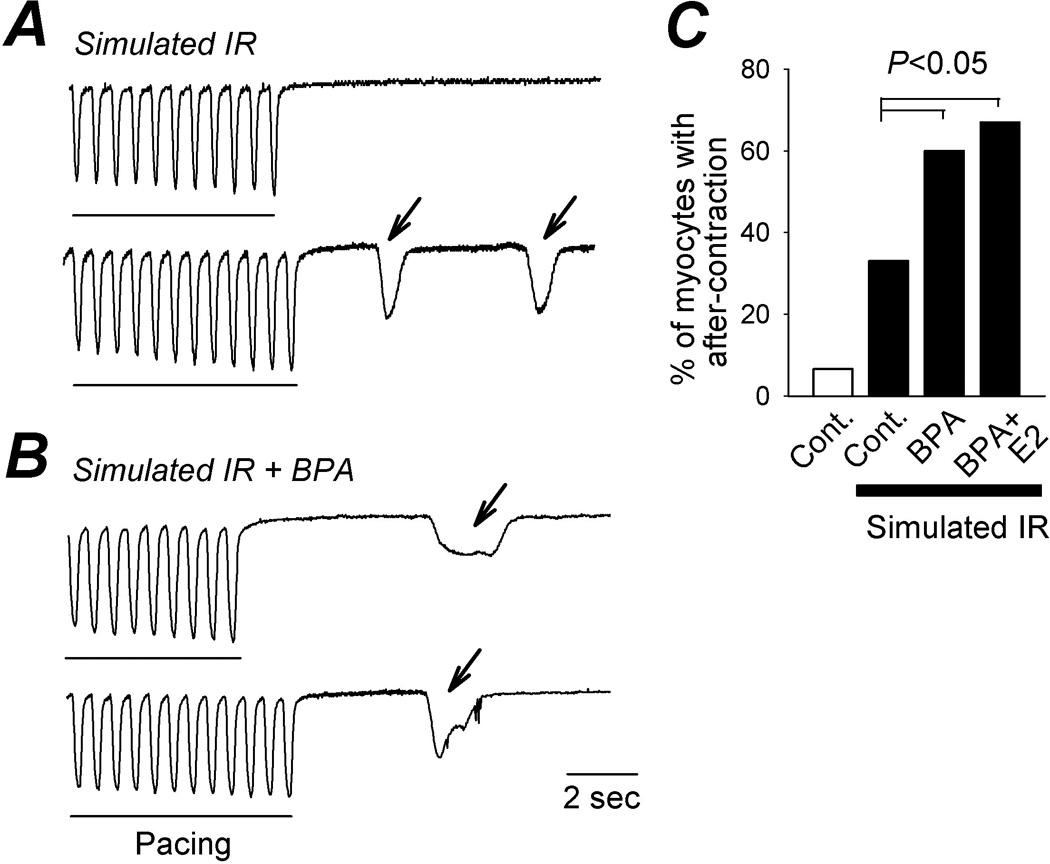
Effect of BPA on triggered activities induced by simulated ischemia-reperfusion in female ventricular myocytes. A and B, representative contraction traces of female rat myocytes elicited by pacing, following simulated IR treatment (A) or simulated IR treatment (B) with the presence of 1 nM BPA during reperfusion. Arrows indicate spontaneous after-contraction (i.e., triggered activity). Rising rates and duration of the spontaneous contractions varied among the myocytes, and were not influenced by BPA treatment. C, percentages of myocytes with after-contractions under various conditions. N = 29 – 37 myocytes.
4.4 Protective effect of estrogens on IR-induced infarction
Estrogen has been shown to be cardio-protective and reduces IR-induced infarction (Gabel et al., 2005; Murphy and Steenbergen, 2007). The effect of BPA exposure on infarction size in female rat hearts following IR was assessed using TTC staining, which differentiates viable versus necrotic tissue (Klein et al., 1981). Consistent with previous findings, treatment of the hearts with 1 nM E2 during reperfusion reduced the infarctions size from 13.2% of the whole heart to 6.6% (Fig. 5B; P < 0.05). Intriguingly, exposure to 1 nM BPA or BPA combined with E2 during reperfusion had similar effects, attenuating the infarction size to 5.1% and 4.7% of the whole heart, respectively (Fig. 5; P < 0.05 vs control). These results suggest that the detrimental effect of estrogens on reperfusion arrhythmia is mediated by a mechanism that is separate from estrogens’ influence on cell death and myocardial infarction.
Figure 5.
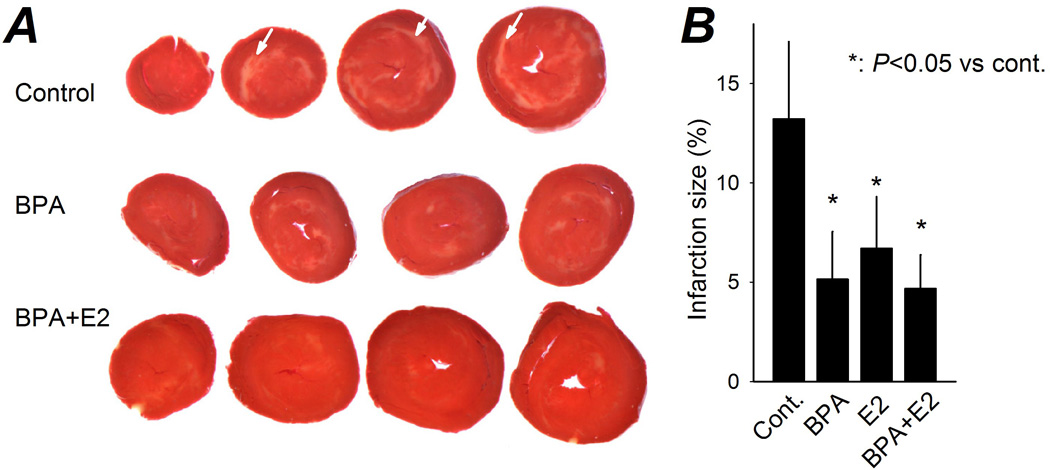
Effect of estrogens on size of IR-induced cardiac infarction. A, representative female rat heart sections following IR injury, with reperfusion under control or in the presence of BPA or BPA plus E2 (all at 1 nM). TTC stained red area indicates viable tissue, pale-white regions (arrows) indicate infarction. B, infarction size (as a percentage of the whole heart) when exposed to various treatments during reperfusion. N = 7 – 11 hearts. Error bars are SEM. *: P < 0.05 vs control.
5. DISCUSSION
The endogenous physiological and pathophysiological factors that determine the responses of the heart to IR injury have been extensively investigated due to their significant clinical relevance; however, the potential impact of environmental chemicals on such responses is not known. In the present study, we show that in female rat hearts, rapid exposure to environmentally-relevant low dose BPA during reperfusion increased the duration of ventricular arrhythmia following IR injury. The pro-arrhythmic effect of BPA was particular significant in the presence of physiological level of E2, but is independent of the effect of estrogens on infarction size. Our results suggest that estrogens have a double-edged sword effect on cardiac response to IR injury; the cardiac toxicity of BPA may be a factor that influences the development of arrhythmias following AMI, particularly in female hearts.
Women, especially prior to menopause, have significantly lower risk of coronary heart disease, including AMI (Lloyd-Jones et al., 1999), and have later onset of the first AMI (Vaccarino et al., 1999; Wolff et al., 2009). Such gender difference is at least in part due to the more favorable blood lipid profile in women (Roeters van Lennep et al., 2002), and contributes to the prevailing view that female sex hormones, particularly estrogens, are cardio-protective. However, it is well documented that women have a worse prognosis following AMI. The female gender has been shown to be an independent predictor of inhospital mortality in the total population (Andrikopoulos et al., 2006). The high mortality rate post AMI is particularly pronounced in younger age women (< 55yr). The short term mortality rate for younger women is more than twice that of same aged men (Andrikopoulos et al., 2006; Vaccarino et al., 1999; Zhang et al., 2012). While the reasons for the worse prognosis following AMI for women are not fully understood, for the short term deaths (ie, in-hospital deaths), lethal ventricular arrhythmia appears to be a contributing factor. Women have more ventricular fibrillation-complications following AMI, and a higher arrhythmic death rate than men in the first 6 months post-AMI (Barakat et al., 2000; Yap et al., 2005). Our results from animal experiments suggest that exposure to BPA, particularly when combined with E2, exacerbates IR-induced ventricular arrhythmia in female rat hearts by increasing the total duration of arrhythmia and the duration of ventricular fibrillation. While we fully recognize the marked species difference in cardiac physiology and pathophysiology, the sensitivity of the female rat heart to estrogens and the pro-arrhythmic cardiac toxicity of BPA observed in rats may have implication for risk factors for malignant arrhythmias in females following AMI.
It has been widely demonstrated that estrogen is protective against IR injuries and reduces IR-induced infarction (Booth and Lucchesi, 2008; Gabel et al., 2005; Murphy and Steenbergen, 2007). Interestingly, acute treatment with BPA mimicked the protective effect of estrogen against infarction. The contrasting protective effect of BPA against infarction and its deleterious effect on reperfusion arrhythmias suggest that BPA (and estrogen) has a double-edged sword impact on the response of the heart to IR injury. As such, that the influence of estrogens on IR injury of the heart is more complex than currently recognized; while the protective effect of estrogens against infarction should reduce the risk of myocardial dysfunction and heart failure, their pro-arrhythmic effect may contribute to malignant arrhythmias and short-term mortality. The diverging effect of BPA on infarction and arrhythmias also suggest that the pro-arrhythmic effect of BPA is not secondary to BPA’s influence on infarction, but is likely mediated by a separate mechanism.
The mechanism mediating BPA’s influence on IR injury is likely to be complex, and remains to be fully elucidated. Vascular and microvascular injury contributes to IR injury, resulting in reduced coronary flow, or even “no-reflow” conditions following initial reactive hyperaemia (Maxwell and Lip, 1997; Moens et al., 2005). We show that, in isolated ventricular myocytes, treatment with BPA significantly increased the incidence of arrhythmogenic triggered activities (i.e., spontaneous excitation following pacing) following simulated IR. Such myocyte level effects are independent from any impact that estrogens have on the vasculature, and suggest that the pro-arrhythmic effect of BPA involves direction actions on the myocytes. Disregulation of calcium homeostasis has long been known to play an important role in IR injury, including IR-induced abnormality of the electrophysiology of the heart (Carmeliet, 1999; Maxwell and Lip, 1997; Moens et al., 2005). IR injury results in elevated cytosolic calcium concentration, which leads to the development of triggered activities and arrhythmias both directly and indirectly through its activation of other cardiac ionic channels, carriers and exchangers. Previously, we showed that exposure to BPA rapidly alters myocyte calcium handling, and in particular, resulting in increased calcium leakage from the sarcoplasmic reticulum (Yan et al., 2011). Such increased calcium leak is expected to exacerbate IR-induced cytosolic calcium overload, and likely plays a role in the potentiation of reperfusion arrhythmias by BPA. It remains to be determined the effect of BPA on other IR injury mechanisms, such as free radical generation, inflammation, and mitochondrial electron transport disregulation.
Previously we showed that low dose BPA activates both ERα- and ERβ-mediated rapid signaling in rodent cardiac myocytes, and that the pro-arrhythmic effect of BPA on female rodent myocytes are mediated by ERβ signaling (Belcher et al., 2012; Yan et al., 2011). Here, we show that blockade of either ERα or ERβ is not sufficient to abolish the effect of estrogens on IR-induced arrhythmias, and that blockade of both ERα and ERβ is required. This observation is consistent with the notion that the mechanism of estrogen’s action on IR-induced arrhythmia is not limited to direct influence on cardiac myocytes. The female-specificity of the pro-arrhythmic effects of estrogens is notable, and is consistent with the previously described female-specific sensitivity of cardiac myocytes and hearts to the rapid actions of BPA and E2 (Belcher et al., 2012; Yan et al., 2011). In those previous studies, we showed that the balance of ERα to ERβ signaling is the prime regulator of sex-specific estrogen sensitivity of rodent cardiac myocytes (Belcher et al., 2012). Estrogen receptors, due to their role in estrogen-mediated cardioprotection, have clear potential as drug targets. However, their influence on the heart may be a double-edged sword. Clear understanding of the molecular mechanisms underlying their cardiac actions is necessary for the development of therapeutical strategies that preserving their desired effects while avoiding their harmful impact, as well as for protection against the cardiac toxicity of environmental chemicals such as BPA.
An isolated rat heart preparation was used as the model system in our study. The isolated heart model is a well-accepted and widely used model for examining IR injuries, including IR-induced ventricular arrhythmias (Curtis, 1998; Skrzypiec-Spring et al., 2007). For our study, the model offers the important advantage of allowing the assessment of the cardiac actions of BPA independent of neuro-humoral influences and systemic estrogenic effects. Limitations of the model include significant variations in duration of ventricular arrhythmias post IR, possibly due to the many factors that influence reperfusion arrhythmias (Curtis, 1998). Such variation was observed in our experiments, and accounts for the large standard errors in our data. Other limitations of our study include the lack of full elucidation of the cellular and molecular mechanisms underlying the pro-arrhythmic actions of BPA. IR injury and reperfusion arrhythmias are complex events (Maxwell and Lip, 1997), and the impact of BPA on these events likely involves multiple mechanisms. At the whole animal level, cardiac electrical properties and function are well-known to be regulated by the autonomic nervous system, and such regulation likely also influences the response of the heart to IR injury and the impact of BPA exposure. Further, BPA metabolism also likely influences its toxicity in vivo. Elucidation of such mechanisms and further investigation at the in vivo level are important future steps for understanding the cardiac toxicity of BPA.
In conclusion, we show that rapid exposure to low dose of BPA and estrogen has a double-edged effect on the response of female hearts to IR injury; while conferring a protective effect against infarction, it exacerbates ventricular arrhythmia following IR injury in female rat hearts. This pro-arrhythmic action may contribute to the higher susceptibility to sudden death following AMI reported in women. Growing evidence suggests that it is important to consider not only endogenous physiological and pathophysiological factors, but also exposure to environmental chemicals when evaluating the risk of, and prognosis following cardiac diseases.
HIGHLIGHTS.
We studied the rapid effects of BPA on ischemia-reperfusion injury of rat hearts
In female hearts, exposure to BPA increased duration of reperfusion arrhythmias
BPA combined with E2 increased the duration ventricular fibrillation
BPA and/or E2 reduced ischemia-reperfusion induced cardiac infarction
Our result may have relevance to response of female hearts to myocardial infarction
Acknowledgements
This work was supported by National Institute of Health grant RO1 ES017262 (HSW).
Abbreviations
- BPA
bisphenol A
- IR
ischemia-reperfusion
- E2
17β-estradiol
- VF
ventricular fibrillation
- VT
ventricular tachycardia
- ER
estrogen receptor
- AMI
acute myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andrikopoulos GK, Tzeis SE, Pipilis AG, Richter DJ, Kappos KG, Stefanadis CI, Toutouzas PK, Chimonas ET. Younger age potentiates post myocardial infarction survival disadvantage of women. Int J Cardiol. 2006;108:320–325. doi: 10.1016/j.ijcard.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Babiker FA, De Windt LJ, van Eickels M, Grohe C, Meyer R, Doevendans PA. Estrogenic hormone action in the heart: regulatory network and function. Cardiovasc Res. 2002;53:709–719. doi: 10.1016/s0008-6363(01)00526-0. [DOI] [PubMed] [Google Scholar]
- Balke CW, Kaplinsky E, Michelson EL, Naito M, Dreifus LS. Reperfusion ventricular tachyarrhythmias: correlation with antecedent coronary artery occlusion tachyarrhythmias and duration of myocardial ischemia. Am Heart J. 1981;101:449–456. doi: 10.1016/0002-8703(81)90135-6. [DOI] [PubMed] [Google Scholar]
- Barakat K, Wilkinson P, Suliman A, Ranjadayalan K, Timmis A. Acute myocardial infarction in women: contribution of treatment variables to adverse outcome. Am Heart J. 2000;140:740–746. doi: 10.1067/mhj.2000.110089. [DOI] [PubMed] [Google Scholar]
- Belcher SM, Chen Y, Yan S, Wang HS. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17beta-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology. 2012;153:712–720. doi: 10.1210/en.2011-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier M, Manning AS, Hearse DJ. Reperfusion arrhythmias: dose-related protection by anti-free radical interventions. Am J Physiol. 1989;256:H1344–H1352. doi: 10.1152/ajpheart.1989.256.5.H1344. [DOI] [PubMed] [Google Scholar]
- Booth EA, Lucchesi BR. Estrogen-mediated protection in myocardial ischemia-reperfusion injury. Cardiovasc Toxicol. 2008;8:101–113. doi: 10.1007/s12012-008-9022-2. [DOI] [PubMed] [Google Scholar]
- Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev. 1999;79:917–1017. doi: 10.1152/physrev.1999.79.3.917. [DOI] [PubMed] [Google Scholar]
- Curtis MJ. Characterisation, utilisation and clinical relevance of isolated perfused heart models of ischaemia-induced ventricular fibrillation. Cardiovasc Res. 1998;39:194–215. doi: 10.1016/s0008-6363(98)00083-2. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel SA, Walker VR, London RE, Steenbergen C, Korach KS, Murphy E. Estrogen receptor beta mediates gender differences in ischemia/reperfusion injury. J Mol Cell Cardiol. 2005;38:289–297. doi: 10.1016/j.yjmcc.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, Maghuin-Rogister G, Pironnet AM, Pussemier L, Scippo ML, Van Loco J, Covaci A. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. 2012;50:3725–3740. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Klein H, Puschmann S, Schaper J, Schaper W. The mechanism of the tetrazolium reaction in identifying experimental myocardial infarction. Virchows Arch. 1981;393:287–297. [Google Scholar]
- Kuhar P, Lunder M, Drevensek G. The role of gender and sex hormones in ischemic-reperfusion injury in isolated rat hearts. Eur J Pharmacol. 2007;561:151–159. doi: 10.1016/j.ejphar.2007.01.043. [DOI] [PubMed] [Google Scholar]
- LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- Maxwell SR, Lip GY. Reperfusion injury: a review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol. 1997;58:95–117. doi: 10.1016/s0167-5273(96)02854-9. [DOI] [PubMed] [Google Scholar]
- Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, McCormack P, Luben R, Khaw KT, Wareham NJ, Galloway TS. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2012;125:1482–1490. doi: 10.1161/CIRCULATIONAHA.111.069153. [DOI] [PubMed] [Google Scholar]
- Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One. 2010;5:e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens AL, Claeys MJ, Timmermans JP, Vrints CJ. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179–190. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res. 2007;75:478–486. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal Hormone Therapy for the Primary Prevention of Chronic Conditions: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendations. Ann Intern Med. 2012;157:104–113. doi: 10.7326/0003-4819-157-2-201207170-00466. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan GC. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeters van Lennep JE, Westerveld HT, Erkelens DW, van der Wall EE. Risk factors for coronary heart disease: implications of gender. Cardiovasc Res. 2002;53:538–549. doi: 10.1016/s0008-6363(01)00388-1. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherlag BJ, el-Sherif N, Hope R, Lazzara R. Characterization and localization of ventricular arrhythmias resulting from myocardial ischemia and infarction. Circ Res. 1974;35:372–383. doi: 10.1161/01.res.35.3.372. [DOI] [PubMed] [Google Scholar]
- Shankar A, Teppala S, Sabanayagam C. Bisphenol A and Peripheral Arterial Disease: Results from the NHANES. Environ Health Perspect. 2012;120:1297–1300. doi: 10.1289/ehp.1104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrzypiec-Spring M, Grotthus B, Szelag A, Schulz R. Isolated heart perfusion according to Langendorff---still viable in the new millennium. J Pharmacol Toxicol Methods. 2007;55:113–126. doi: 10.1016/j.vascn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Wolff T, Miller T, Ko S. Aspirin for the primary prevention of cardiovascular events: an update of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;150:405–410. doi: 10.7326/0003-4819-150-6-200903170-00009. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Fujibayashi Y, Rajagopalan RE, Meerbaum S, Corday E. Effects of staged versus sudden reperfusion after acute coronary occlusion in the dog. J Am Coll Cardiol. 1986;7:564–572. doi: 10.1016/s0735-1097(86)80466-1. [DOI] [PubMed] [Google Scholar]
- Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang H-S. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One. 2011;6:e25455. doi: 10.1371/journal.pone.0025455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap YG, Duong T, Bland M, Malik M, Torp-Pedersen C, Kober L, Connolly SJ, Marchant B, Camm J. Temporal trends on the risk of arrhythmic vs. non-arrhythmic deaths in high-risk patients after myocardial infarction: a combined analysis from multicentre trials. Eur Heart J. 2005;26:1385–1393. doi: 10.1093/eurheartj/ehi268. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Fang J, Gillespie C, Wang G, Hong Y, Yoon PW. Age-specific gender differences in in-hospital mortality by type of acute myocardial infarction. Am J Cardiol. 2012;109:1097–1103. doi: 10.1016/j.amjcard.2011.12.001. [DOI] [PubMed] [Google Scholar]


