Abstract
A hallmark of addiction is the loss of control over drug intake, which is seen only in a fraction of those exposed to stimulant drugs like cocaine. The cellular mechanisms underlying vulnerability or resistance to compulsive drug use are still unknown. Here we show that individual variability in the development of highly motivated and perseverative behavior toward cocaine is associated with synaptic plasticity in medium spiny neurons expressing dopamine D2 receptors (D2-MSNs) in the nucleus accumbens of mice. Potentiation of glutamatergic inputs onto indirect pathway D2-MSNs was associated with resilience towards compulsive cocaine seeking. Inhibition of D2-MSNs using a chemicogenetic approach enhanced the motivation to obtain cocaine while optogenetic activation of D2-MSNs suppressed cocaine self-administration. These results indicate that recruitment of D2-MSNs in nucleus accumbens functions to restrain cocaine self-administration and serves as a natural protective mechanism in drug-exposed individuals.
Individuals suffering from addiction endure large personal and financial losses in order to maintain drug use. Among other addictive behaviors, they show a strong perseverance and an extraordinary motivation to obtain the drug. These behaviors are expressed only by a fraction of those exposed to the drug, revealing a significant degree of individual variability and the existence of predisposing traits and conditions that may serve as risk or protective factors in the development of addiction. In humans, the vulnerability to develop compulsive behaviors towards stimulant drugs has been linked to deficits in cortico-striatal processing and low levels of dopamine D2 receptors in the striatum1-3. Moreover, impulsivity traits and low levels of dopamine D2 receptors have been associated with compulsive cocaine use in both rodents and non-human primates4, 5. Furthermore, rodents also show natural individual variability in the motivational properties of cocaine and the development of compulsive behaviors6-8.
Dopamine D2 receptors are expressed in the subpopulation of medium spiny neurons (D2-MSNs) in the striatum that form indirect projections to midbrain regions via pallidum and subthalamic nuclei (indirect pathway). The other subpopulation of MSNs expresses dopamine D1 receptors (D1-MSNs) and forms direct projections to midbrain neurons (direct pathway). Activation of dopamine receptors on each subpopulation of MSNs triggers different intracellular signaling cascades. While activation of D2 receptors inhibits PKA activity via Gi signaling in D2-MSNs, activation of D1 receptors stimulates PKA activity via Gs/olf signaling in D1-MSNs9. It is thought that these two MSN subtypes and their parallel pathways exert complementary, and sometimes opposing, actions on behaviors that are controlled by the cortico-striatal system10. Use of pharmacological tools that target D1 and D2 receptors have helped understand the relative contribution of the direct and indirect pathways in behavior. However, the complex expression pattern of dopamine D2 receptors present in both pre- and post-synaptic compartments in different neuronal types in the mesolimbic circuit has complicated the interpretation of these experimental results.
Cell-type specific approaches have been recently used to aid in this quest. In the dorsal striatum, optogentic activation of direct pathway D1-MSNs increases locomotion and serves as a reinforcer, while activation of indirect pathway D2-MSN increases freezing behaviors but is not a reinforcer11, 12. In the nucleus accumbens (NAc), a region involved in cue-induced reward learning, D1-MSNs and D2-MSNs have opposite effects on cocaine related behaviors9. Activation of D2-MSNs reduces conditioned place preference for cocaine while activation of D1-MSN increases it13. In addition, the ablation or inhibition of D2-MSNs in the NAc induces an increase in amphetamine conditioned place preference and facilitates locomotor sensitization to cocaine, uncovering a tonic role of D2-MSNs on limiting the actions of stimulant drugs 14, 15.
However, despite these findings, the role of the indirect pathway and D2-MSNs in voluntary cocaine self-administration and compulsive drug seeking remains unclear. We predicted that indirect pathway D2-MSNs would exert an inhibitory influence on behavioral output of this circuitry and limit drug seeking and that weakening this pathway would remove the inhibitory control and render individuals more susceptible to develop compulsive drug seeking. In this study, we found individual variability in the vulnerability to compulsive cocaine that was correlated to the synaptic strength of inputs to D2-MSNs. Moreover, inhibition or activation of the accumbal indirect pathway enhanced or suppressed cocaine self-administration behavior, respectively.
RESULTS
Individual variability in behaviors towards cocaine
Intravenous cocaine self-administration was established in an out-bred strain of mice using a cued-operant task that required naïve mice to nosepoke in an active hole to earn an intravenous infusion of cocaine (Suppl. Fig. 1). Approximately 55% of mice acquired the behavior within 5-10 days and the rest were removed from the study. Mice were then given access to cocaine during daily 2 hour sessions for 6-7 weeks. Two behaviors were measured to determine the degree of compulsive drug use: 1) the difficulty stopping or limiting drug use measured as perseverative drug seeking, and 2) high motivation to obtain the drug measured as the effort exerted to obtain it. These behaviors were adopted from the diagnostic criteria for drug dependence in humans described in the DSM-IV and have been successfully applied in a model developed in rats4, 7, 16.
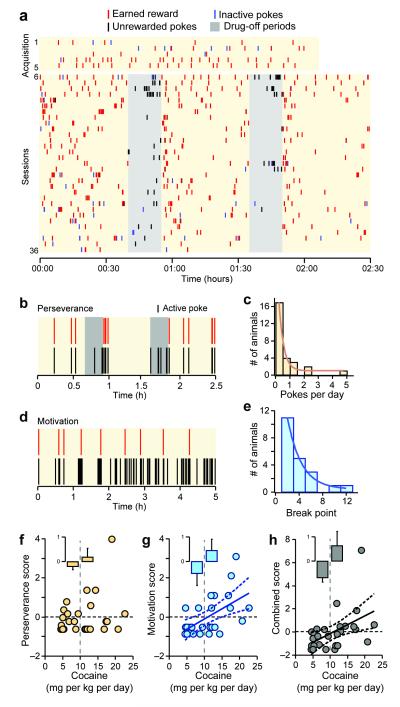
In order to measure perseverative responding, sessions were interrupted by two drug-off periods (15 min long) during which cocaine was not available (Fig. 1b). Initially, mice showed a typical extinction response during the drug-off periods that was characterized by a spike in the responding on days 1 and 2, followed by a decrease in responding selectively in the drug-off periods during subsequent days. While most animals maintained minimal responding for the rest of the sessions, a few developed perseverative responding after weeks of self-administration (Suppl. Fig. 2). The perseverance value was calculated as mean active pokes during the drug-off periods over the last 10 sessions (Fig. 1c). During this time, perseverative responding during the drug-off period was selective to the active nosepokes holeand the number of inactive pokes was similar between mice with high and low perseverance values (0.13 ± 0.07 inactive/day for mice with perseverance value > 2, n = 3 mice; 0.27 ± 0.08 inactive/day for mice with values < 2, n = 24 mice, unpaired t-test, t(24) = 0.5, p = 0.62).
Figure 1. Individual variability in the behaviors towards cocaine after extended self-administration.
(a) Raster plot showing the daily pattern of cocaine self-administration of a mouse during acquisition (day 1-5) and sessions (day 6-36). Red ticks = earned cocaine infusions, blue ticks = inactive pokes, black ticks = unrewarded active pokes, and shaded area= drug-off period. (b) Example of perseverative responding during the drug-off periods in session 35; red tick = cocaine infusion, black tick = active poke. (c) Distribution of the perseverance value (mean active pokes/day during drug-off) within the population. Line is log normal fit to the data (with yo=16.5, A=−14, xo=1.5, width=4; x2=22.9; n=10 mice). (d) Example of responding during progressive ratio session used to measure motivation. The number of active pokes (black ticks) required to earn a cocaine infusion (red tick) increases with each subsequent infusion. (e) Distribution of the breakpoint values within the population. Line is log normal fit to the data (with yo=15.5, A=−9, xo=10.8, width=2.2; x2=1.7; n=6 mice). (f, g, h) The scores of individual mice for perseverance (f), motivation (g) and combined bahevioral scores versus cocaine intake. Vertical dash line marks 10 mg/kg/day cocaine dose and horizontal dash line marks the mean of score (zero). Insets, mean ± SEM perseverance score for mice with cocaine intake (left) lower and (right) higher than 10 mg/kg/day.Solid and dash lines represent linear regression fit and 95% confidence interval.
Motivation to obtain cocaine was assessed in two progressive ratio sessions in which the number of pokes required to earn each consecutive cocaine reward was increased exponentially (see Methods; Fig. 1d). The breakpointbreakpoint, the number of pokes performed to earn the last reward, was defined as the motivation value. Similar to the measure of perseverative behavior, most animals showed low motivation values, but a few animals had high values (Suppl. Fig. 2). Importantly, the ratio of inactive to active pokes did not change during these progressive ratio sessions (0.27 ± 0.04 for session and 0.48 ± 0.17 for progressive ratio , paired t-test, t(26) = 1.2, p = 0.23, n = 27 mice).
A combined behavior score was calculated for each mouse by adding the z-score of perseverance and motivation values. By definition, z-scores have a mean of zero and a standard deviation of 1which ensures equal contribution of each behavior and eliminates inherent differences in the variance of each measurement. Mice varied on the degree of perseverance, motivation and combined behavior score as well as on their average cocaine intake through the study (5-24 mg/kg/day). However, there was no correlation between the perseverance scores and cocaine intake (rP = 0.27, r2 =0.08, p = 0.16, n = 27 mice; Fig. 1f). While the highest perseverance score was observed in a mouse with daily intake of 20 mg/kg cocaine, high intake was not sufficient to predict positive perseverance scores. As expected, there was a positive correlation between the motivation and the combined behavior scores and daily cocaine intake (motivation score: rP = 0.57, r2 =0.31, p <0.01, n = 27 mice, Fig. 1g; combined score: rP = 0.49, r2 = 0.24, p <0.01, n = 27 mice, Fig. 1h). A mean daily intake of 10 mg/kg/day, a dose that increases locomotion in this mouse strain17, was used as the threshold to identify mice with high and low cocaine intake. Mice with low daily cocaine intake displayed mean negative motivation scores (−0.5 ± 0.1) and behavior scores (−0.7 ± 0.2, n = 13 mice), while most animals with high cocaine intake showed mean positive motivation (0.5 ± 0.3) and behavior scores (0.6 ± 0.6, n = 14 mice) (Fig. 1g, h, insets).
Despite the correlation between intake and the behavior scores, there is a considerable degree of variability in the scores among animals with high intake. High intake did not always result in the development of compulsive cocaine use and some maintained negative behavior scores. We asked whether specific synaptic mechanisms are associated with and responsible for the vulnerability or resistance to develop compulsive behaviors among cocaine users.
Potentiation of excitatory synapses onto D1-MSNs
Glutamatergic excitatory transmission was examined in direct-pathway D1-MSNs and indirect-pathway D2-MSNs in the NAc core region of ex vivo brain slices. These neurons receive dense innervation from cortical regions that were activated by electrical stimulation. The expression of green fluorescent protein was used to discriminate between fluorescently labeled D1-MSNs and unlabeled putative D2-MSNs (Fig, 2a, b). Recordings were made from 30 mice that underwent at least 17 sessions of cocaine self-administration (22 of these with behavioral scores) and 20 sham surgery mice. In general, recordings were collected from D1-MSNs in some animals and D2-MSNs in other animals, although recordings were obtained from both D1-MSNs and D2-MSNs in a small subset of cocaine-exposed mice (Suppl. Table 1). In cocaine-exposed animals, the ratio of AMPA/NMDA excitatory postsynaptic currents (EPSCs) was increased selectively in D1-MSNs but not in D2-MSNs compared to sham controls (D1-MSNs: 2.77 ± 0.28 for cocaine, n = 46 cells/23 mice, and 1.93 ± 0.26 for sham, n= 27 cells /15 mice, Mann-Whitney test U = 415, p = 0.018; D2-MSNs: 2.74 ± 0.31 for cocaine, n = 29 cells/18 mice; and 2.25 ± 0.27 for sham, n= 23 cells/15 mice, Mann-Whitney test U = 312, p = 0.20; Fig. 2c-e).
Figure 2. Potentiation of glutamatergic inputs on D1-MSNs after cocaine self-administration.
(a) Sagittal section of Drd1-GFP mouse brain showing green fluorescent in dorsal striatum and nucleus accumbens core (NAc) and shell subregions with projections to subtantia nigra reticulate (SNr) overlaid on outline from mouse brain atlas. LV, lateral ventricle. Scale bar = 1 mm. (b) Fluorescently labeled soma of D1-MSN in NAc. Scale bar = 20 μm. (c,d) Traces of AMPAR and NMDAR mediate EPSCs from (c) D1-MSN in sham (black) and cocaine (green) and (d) D2-MSN in sham and cocaine (red) animals. Scale bars: 25 pA and 20 ms. (e) AMPA/NMDA ratios obtained in D1-MSNs (sham = 27 cells/15 mice, cocaine = 46 cells/23 mice) and D2-MSN (sham= 23 cells/15 mice, cocaine= 29 cells/18 mice). (f) Dendrite segment and dendritic spine from a D1-MSN acquired using 2-photon laser-scanning microscope. Star indicates site of uncaging laser pulse. Scale bar = 0.5 μm (g, h) Representative traces of AMPA mediated uEPSCs (g) and NMDAR mediated uEPSCs (h) recorded from D1-MSNs in NAc core from sham (black) and cocaine (green) mice. (i) Peak amplitude of AMPA and NMDAR mediated uEPSC in D1-MSNs of sham (black, AMPA= 22 spines/9 cells, NMDA= 14 spines/6 cells) and cocaine (green, AMPA= 23 spines/13 cells, NMDA=20 spines/5 cells) animals. (j) Amplitude distribution of all AMPA uEPSCs recorded in D1-MSNs from sham (black) and cocaine (green) mice. y-axis represents number of spines. * p < 0.05, Mann Whitney (e, i), unpaired t-test (i). Data are mean ± SEM.
This increase in the ratio in D1-MSNs was further investigated using two-photon laser uncaging of glutamate at single spines to measure the AMPA and the NMDA receptor mediated currents at individual spines (Fig. 2f-h). The amplitudes of AMPA receptor uncaging evoked excitatory postsynaptic currents (uEPSCs) in cocaine-exposed mice showed a tendency to be larger than in sham mice (sham= 23.3 ± 4.3 pA, n=24 spines/9 cells; cocaine= 32.9 ± 4.6 pA, n=23 spines/13 cells, Mann-Whitney test U= 195, p = 0.09 for two-tail and p = 0.04 for one-tail; Fig. 2i), while the amplitude of NMDA receptor uEPSC was similar in sham and cocaine-exposed mice (sham= 6.4 ± 0.7 pA, n=22 spines/6 cells; cocaine= 5.9 ± 0.8 pA, n=20 spines/5 cells, unpaired t-test t(40)= 0.26, p = 0.79; Fig. 2i). A rightward shift in the amplitude distribution of AMPA receptor uEPSC was seen in cocaine-exposed mice meaning there was a smaller percentage of spines with currents undistinguishable from noise (< 5 pA uEPSC, 4% spines for cocaine vs. 12.5% for sham; Fig. 2j), and a greater percentage of spines with large AMPA receptor uEPSCs (65% for cocaine vs. 33% for sham with uEPSC > 15 pA; Suppl. Fig. 3c). Furthermore, an increase in spine head width but not length was seen in D1-MSNs after cocaine (thin: sham= 0.81 ± 0.03 μm, n=23 spines, cocaine= 0.89 ± 0.03 μm, n=22 spines; wide: sham=1.17 ± 0.04 μm, n=23 spines, cocaine= 1.32 ± 0.06 μm, 22 spines; two-way ANOVA no interaction F(1,68)=0.6, p=0.4, effect of width F(1,68)=99.6, p < 0.01, effect of treatment F(1,68)=8.4, p <0.05; Bonferroni post-hoc wide spines sham vs. cocaine p < 0.05, thin spines sham vs. cocaine p > 0.05; Suppl. Fig. 3a-c). These results suggest that the enhancement of AMPA/NMDA ratio observed in D1-MSNs from cocaine-exposed animals is due to a potentiation of AMPA receptor mediated currents at excitatory synapses.
Potentiation at D2-MSNs only inthose with negative scores
While inputs to D2-MSNs did not show potentiation in the majority of animals, analysis of individual animals revealed that a fraction of cocaine-exposed mice showed an increase in the AMPA/NMDA ratio in D2-MSNs (Fig. 3a). Among mice with high daily cocaine intake, we found a strong negative correlation between the behavior scores and the ratio at D2-MSN inputs (rP = −0.81, r2 =0.66, p=0.01, n=8 mice; Fig. 3b). Mice with negative behavior scores displayed potentiated inputs while animals with positive behavior scores had ratios comparable to sham controls (positive: ratio= 2.6 ± 0.3, behavior score= 0.33 ± 0.14, n = 4 mice; negative: ratio=5 ± 1, behavior score = −0.64 ± 0.22, n= 4 mice; sham: ratio = 2.1 ± 0.9, n = 15 mice; one-way ANOVA F(2,20)=9.7, p<0.01; Bonferroni post-hoc negative vs. sham t=4.4, p <0.05; positive vs. sham t=0.6, p>0.05, Fig. 3c). Interestingly, mice with positive and negative behavior scores had similar cocaine intake (14.2 ± 1.3 for positive and 14.5 ± 2.1 mg/kg/day for negative), indicating that the dose of cocaine does not account for this difference.
Figure 3. Synaptic potentiation at D2-MSNs correlates negatively with negative behavior scores.
(a, d) AMPA/NMDA ratio as a function of cocaine intake in (a) D2-MSNs and (d) D1-MSNs for sham mice (grey) and all cocaine mice that underwent 17 or more self-administration sessions (red, n=18 mice/green, n=22 mice) . Shaded area represents sham mean ± SD. (b, e) Behavior scores as a function of AMPA/NMDA ratio in (b) D2-MSNs (n = 8 mice) and (e) D1-MSNs (n=15 mice) for mice with high intake and behavioral analysis completed. Color lines are linear regression fit and 95% confidence interval for D2-MSNs and D1-MSNs. (c, f) AMPA/NMDA ratios recorded in the (c) D2-MSNs and (f) D1-MSNs from sham (black, n=15 mice) and cocaine self-administration mice with positive (+) and negative (−) behavior scores (n = 4 and 4 mice for D2-MSNs and n= 8 and 7 mice for D1-MSNs). Dotted line marks the mean AMPA/NMDA ratio in sham animals. Data are mean ± SEM and sample size cells/animals. Refer to suppl. Table 1 for additional sample size information.* p < 0.05, one way ANOVA followed by Bonferroni.
No correlation was observed between D1-MSNs and the behavior scores (rP = −0.02, r2 <0.01, p=0.95, n=15 mice; Fig. 3d-e). Mice with positive and negative behavior scores had similar AMPA/NMDA ratios and thus synaptic potentiation in D1-MSNs was not correlated with the development of compulsive cocaine intake (positive: ratio= 2.9 ± 0.7, behavior score= 1.4 ± 0.8, n = 8 mice; negative: ratio= 3.2 ± 0.5, behavior score= −0.87 ± 0.2, respectively, n= 7 mice; sham: ratio=1.9 ± 1.1, n= 15 mice; one-way ANOVA F(2,20)=2.8, p>0.05, Fig. 3f).
D2-MSN inhibition enhances motivation for cocaine
The findings indicate that excitatory inputs with higher AMPA/NMDA ratios are present in D2-MSNs of individuals that did not develop compulsive behaviors towards cocaine suggesting that potentiation of excitatory inputs onto D2-MSNs might confer protection against cocaine-related behaviors. We reasoned that weakening of the indirect pathway may render individuals more vulnerable to the expression of compulsive behavior toward cocaine. To test this hypothesis, the activity of D2-MSNs was manipulated using a chemical-genetic approach with Designer Receptor Exclusively Activated by a Designer Drug (DREADD)18, 19. hM4Di is a Gi-coupled DREADD that has been previously used to inhibit MSN activity in the striatum15, 20. A conditional viral vector expressing hM4Di was injected bilaterally in the NAc core of mice expressing Cre recombinase selectively in D2-MSNs (Adora2a-Cre+/− mice). Fluorescently-tagged DREADDs were expressed at the soma of MSNs in the NAc core region around the anterior commissure and on axonal projections to the ventral pallidum (Fig. 4a, b). No labeling was seen in other brain regions.
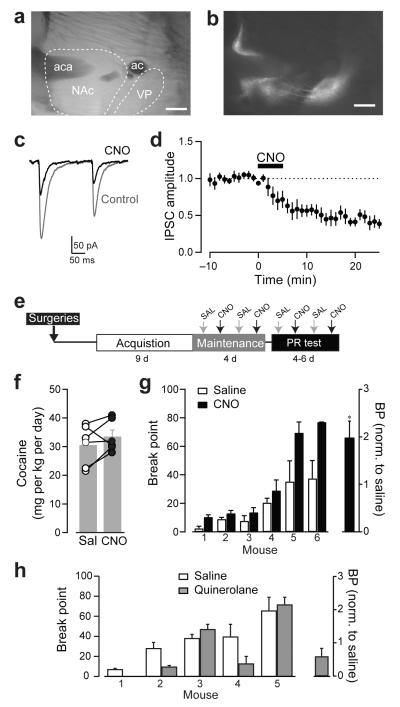
Figure 4. Inhibition of indirect-pathway D2-MSNs increases motivation for cocaine.
(a) Bright field image of sagittal section and (b) fluorescence image showing DREADD expression in NAc neurons and projections to ventral pallidum. aca, anterior commisure ant; ac, anterior commisure. Scale bars = 500 μm. (c) Representative traces of ChR2-evoked IPSC recorded in ventral pallidum neurons before (grey) and after CNO (10 μM) application. (d) Time course of the CNO inhibition of IPSC amplitude in ventral pallidum neurons (n = 7). (e) A diagram describing the experimental timeline. (f) Cocaine intake for animals receiving saline (open) or CNO (solid). (g) Left, Breakpoint values achieved by individual mouse during 4-6 consecutive progressive ratio sessions (PR test) in which mice randomly received saline (open) or CNO (solid, 1 mg/kg). Inset, BP values after CNO normalized to individual saline values (h) BP values achieved by individual mouse during 4 consecutive progressive ratio sessions (PR test) in which mice randomly received saline (open) or D2 agonist quinelorane (gray, 0.03 mg/kg). Inset, BP after quinelorane normalized to individual saline values. *p < 0.05, paired t-test (f) and one sample t-test (g, h). Data are mean ± SEM.
The inhibitory action of hM4Di on the indirect pathway was confirmed with recordings from ventral pallidum neurons. Synaptic responses were triggered by selective activation of fibers from D2-MSNs in the NAc that co-expressed channelrhodopsin-2 (ChR2) and hM4Di (injected at a 1:1 ratio). A laser pulse evoked GABAergic IPSCs that were completely blocked by the GABAA receptor antagonist gabazine (5 μM, Fig. 5d). IPSCs were depressed by 52.5 ± 3.7 % when the synthetic agonist for hM4Di clozapine N-oxide (CNO, 10 μM) was applied (paired t-test, t(6)= 4.0, p <0.01 n = 7 cells, Fig. 4c, d). Application of CNO had no effect on the amplitude of IPSC in slices expressing only ChR-2 in D2-MSN (data not shown). The experiments demonstrated that activation of hM4Di expressed in accumbal D2-MSNs inhibits the output of the accumbal-tegmental indirect pathway.
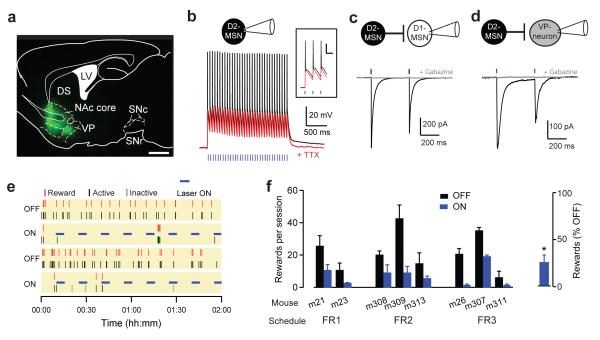
Figure 5. Activation of indirect-pathway D2-MSNs inhibits cocaine self-administration.
(a) Fluorescent image showing expression pattern of ChR2-EYFP vector in nucleus accumbens (NAc) core and projections to the ventral pallidum of Adora2a-Cre+/− mice overlayed with brain atlas outline. DS, dorsal striatum; LV, lateral ventricle; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata. Scale bar = 1 mm. (b) Representative traces of action potentials recorded from ChR2-expressing D2-MSNs in NAc core evoked by 0.5 ms-long laser stimulation (33 pulses at 16.6 Hz) before (black) and after TTX (0.5 μM) application (red). Inset, expanded traces showing ChR2-evoked action potentials before (black) and after TTX (red). Scale bar: 50 ms; 20 mV. (c, d) Representative traces of IPSCs evoked by laser stimulation recorded from (c) a ChR2 negative neurons (putative D1-MSN) in NAc core and (d) a neuron in the ventral pallidum region before (black) and after gabazine (gray). (e) Raster plot showing representative example of responding during four consecutive self-administration sessions with laser stimulation OFF and ON (5 min at 16 Hz every 10 min) for a mouse trained on a FR2 schedule. (f) Left, Rewards earned (mean ± SEM) by eight individual Adora2a-Cre+/− mice expressing ChR2 in the D2-MSNs and trained on a FR1, FR2 or FR3 schedule during interspersed cocaine self-administration sessions with laser OFF (black) or ON (blue). Right, Earned rewards during laser stimulation sessions normalized to the rewards earned during laser OFF sessions for every individual mouse. *p < 0.05, one sample t-test. Data are mean ± SEM
Mice expressing hM4Di in D2-MSNs in the NAc were allowed to acquire cocaine self-administration behavior for 9 days. The effect of D2-MSNs inhibition was first examined on sessions where the drug was easily accessible under a fixed poke/reward ratio of 1 (FR1). Mice alternately received CNO (1 mg/kg) and saline intravenously immediately before the session. The two groups showed similar daily cocaine intake (saline = 31 ± 2.9 mg/kg/day; CNO = 34 ± 2.3 mg/kg/day, paired t-test, t(13)= 0.86, p=0.24, n=6 mice; Fig. 4f), suggesting inhibition of the D2-MSNs output did not alter cocaine intake when the drug was available with minimum effort. The motivation to obtain cocaine was tested in 4-6 consecutive progressive ratio sessions in which the pokes/reward ratio was increased exponentially with each subsequent reward. Importantly, during these sessions, drug intake drops to 10% - 40% of the normal intake during sessions with low-effort access to cocaine (FR1). Following a latin-square design, CNO or saline was administered intravenously immediately before the session. Mice had different breakpointbreakpoint values on control saline days, (Fig. 4g left), a reflection of the individual differences in the motivation for cocaine also shown previously in Fig. 1. However, independently of their baseline breakpointbreakpoint, mice had more active pokes and achieved higher breakpoint on CNO days (breakpoint = 18.7 ± 6.1 for saline and 35.4 ± 12.3 for CNO, n= 6, paired t-test, t(5) = 2.6, p < 0.05). When normalized to saline, breakpoint doubled on CNO days indicating higher motivation to obtain cocaine upon inhibition of D2-MSNs (normalized breakpoint in CNO = 2 ± 0.2, one sample t-test, t(5) = 2.7, p < 0.02; Fig. 4g right). Treatment with the synthetic agonist CNO had no effect on breakpoint values in control mice that received intracranial injection but did not express hM4Di (Adora2a-CRE−/− mice or Adora2a-CRE+/− with unsuccessful injections) ruling out a non-specific effect of CNO on performance (breakpoint: saline= 41.5 ± 18.2, CNO = 33.9 ± 16.9, paired t-test, t(3) = 1.3, p > 0.2, n= 4 mice; breakpoint in CNO normalized to saline = 0.78 ± 0.16, one sample t-test t(3) = 1.35, p = 0.27; Suppl. Fig. 4a). When hM4Di was expressed in D2-MSNs of the dorsal striatum, CNO treatment did not increase breakpoint values, suggesting a selective regulation by NAc neurons of cocaine seeking during the first weeks following acquisition of the behavior (breakpoint: saline= 59.2 ± 24.1, CNO = 66.8 ± 23.6, paired t-test, t(5) = 0.5, p > 0.6, n= 6; breakpoint in CNO normalized to saline= 1.2 ± 0.2, one sample t-test, t(5)=1.3, p > 0.2; Suppl. Fig. 4b-c).
Activation of Gi signaling in D2-MSNs can also be achieved by administration of D2 receptor agonists. However, D2 receptors are also expressed in other cell types that include striatal cholinergic interneurons and midbrain dopaminergic neurons, where activation of these receptors inhibits dopamine release21. Systemic administration of the D2-like agonist quinelorane failed to increase breakpoint values for cocaine during progressive responding sessions (saline = 35 ± 9.5 and quinelorane = 28 ± 13.4, n= 5 mice, paired t-test, t(4) = 1.1, p > 0.3; Fig. 4h left). To the contrary, a trend to decreased responding for cocaine was observed in these experiments when a low dose of quinelorane (30 μg/kg) was administered i.v. before the start of the session (normalized breakpoint in quinelorane = 0.59 ± 0.24 %, one-sample t-test t(4)= 1.67, p = 0.17, n=5 mice; Fig. 4h right). This dose was chosen because higher doses of D2 receptor agonist depress locomotor activity in mice22, which would interfere with the task. This result is in agreement with previous reports that D2-like agonists produce a leftward shift in the dose-response curve for cocaine self-administration and decrease overall cocaine intake in rats and non-human primates23-25.
This same activity manipulation had no effect on responding when animals were trained on a cued-instrumental task to earn a food reward (Suppl. Fig. 5). Adora2a-Cre+/− mice expressing hM4Di in NAc D2-MSNs (n=3 mice) and sham surgery control mice Adora2a-Cre−/− (n=4 mice) were trained on a fixed ratio schedule and the effect of CNO on responding for food rewards was assessed under fixed ratio and progressive ratio schedules. Food rewards earned under fixed ratio were similar on days mice received saline and CNO in both genotypes (rewards / hour in Adora2a-Cre+/− mice: saline= 54 ± 10 and CNO=53 ± 11; in Adora2a-Cre+/− mice: saline= 57 ± 6 and CNO= 54 ± 10; no interaction or effect in repeated measure two-way ANOVA all F’s < 0.1, p’s > 0.1; Suppl. Fig. 5b). When normalized to rewards earned on saline days, food rewards earned on CNO days were comparable between genotypes (Adora2a-Cre+/− mice= 1.0 ± 0.1 and Adora2a-Cre−/− mice= 1.06 ± 0.1, unpaired t-test: t(12) = 0.34, p = 0.7; Suppl. Fig 5c). CNO had no effect during progressive ratio sessions and mice expressing hM4Di achieved similar breakpoints for food in saline and CNO days as did sham mice (Adora2a-Cre+/− mice saline= 983 ±362, CNO= 829 ± 277; Adora2a-Cre−/− mice saline= 950 ± 153, CNO= 1341 ± 388, no interaction or effect in repeated measure two-way ANOVA, all F(1, 5)’s <0.8, p’s > 0.4; Suppl. Fig. 5d). Normalized CNO breakpoint were similar between genotypes (Adora2a-Cre+/− mice= 0.98 ± 0.3, Adora2a-Cre−/− mice= 1.7 ± 0.8; unpaired t-test, t(5)< 0.98, p > 0.4; Suppl. Fig. 5e), supporting the findings that inhibition of accumbal D2-MSNs did not affect motivation for food reinforcement. Furthermore, striatal D1 receptor expression and function appeared unaltered in mmice that expressed hM4Di in the striatum and received repeated CNO treatment (1mg/kg for 2-4 days; Suppl. Fig. 6).
D2-MSN activation suppresses cocaine self-administration
In vivo optogenetic stimulation was used to selectively activate D2-MSNs in the NAc core and test the effect of accumbal indirect pathway stimulation on cocaine self-administration. A conditional viral vector expressing ChR2 (EYFP tagged) was injected bilaterally in the NAc core region of Adora2a-Cre+/− mice and fiber optics implanted in the injection area. Pathway-specific expression of ChR2 was confirmed using fluorescent microscopy that showed labeled cell bodies in the NAc core and labeled projections in the ventral pallidum but no other brain region (Fig. 5a). Electrophysiological recordings in brain slices containing NAc core showed that brief laser stimulation (0.5 ms duration pulse at 16.6 Hz) reliably triggered the firing of action potentials in D2-MSNs expressing ChR2 and, as expected, the firing was sensitive to TTX (n=2 cells, Fig.5b). The same brief laser stimulation elicited an inhibitory postsynaptic current (IPSC) in two types of neurons that are known to receive inputs from indirect pathway D2-MSNs: neighboring striatal MSNs (ChR2 negative, putative D1-MSNs, n= 3 cells) and ventral pallidum neurons (n= 9 cells, Fig. 5c,d). Application of the GABAA receptor antagonist gabazine (5 μM) abolished the laser evoked IPSCs in both cell types (n=2 for both cell types). These results confirm that optogenetic stimulation activates accumbal indirect pathway neurons and in turns inhibits the postsynaptic targets of D2-MSNs.
Mice expressing ChR2 in accumbal D2-MSNs were allowed to acquire cocaine self-administration behavior (5-9 days) and then habituated to perform with the optic fibers until responding was stable. Testing took place over 4 consecutive daily sessions in which the laser power was OFF or ON following the same pattern for all mice (OFF-ON-OFF-ON) (Fig. 5e). In the first set of experiments, optogenetic stimulation (10-ms pulses at 16.6 Hz, 10 min OFF - 5 min ON throughout the session) was delivered in the NAc core. Under these conditions, the mean number of earned rewards dropped significantly during ON sessions for all mice (OFF rewards/session = 21.8 ± 4.3, ON rewards/session = 7.3 ± 2.1, paired t-test, t(7) = 4.5, p < 0.01, n=8 mice; Fig. 5f left). When normalized to the OFF sessions, optogenetic stimulation suppressed cocaine self-administration by 69.1 ± 5.3 % (one-sample t-test, t(7) = 12.6, p < 0.001, n= 8 mice; Fig. 5f right). Furthermore, stimulation caused a similar decrease in mice trained on a FR1, FR2 and FR3 schedule (FR1 off=18 ± 7.5, on= 6.5 ± 4, n=2 mice; FR2 off= 25.7 ± 8.5, on= 7.8 ± 1.2, n=3 mice; FR3 off= 20.5 ± 8.3, on= 7.3 ± 5.8, n=3 mice; two way repeated measure ANOVA main effect of stimulation F(1,5) = 15.25, p < 0.02; no effect of fixed ratio schedule or interaction, both F’s < 0.3, p’s > 0.7). These results indicate that recruitment of the indirect pathway reduces cocaine self-administration independent of the ratio requirement.
DISCUSSION
Our findings demonstrated that resilience to compulsive cocaine use was accompanied by potentiation of glutamatergic inputs to D2-MSNs in the NAc, suggesting that this synaptic potentiation serves a protective mechanism. We challenged this conclusion by manipulating the activity of indirect pathway D2-MSNs. Inhibition of D2-MSNs during self-administration enhanced responding and increased the motivation to obtain cocaine, rendering mice more vulnerable to the expression of compulsive behaviors. In contrast, activation of D2-MSNs decreased cocaine self-administration. These experiments provide conclusive evidence of a causal link between the synaptic strength at D2-MSN in the NAc core and behavioral control over reward-motivated actions.
The study also found synaptic potentiation of glutamatergic inputs onto direct pathway D1-MSNs after cocaine self-administration. Enhanced AMPA/NMDA ratios and other evidence of synaptic potentiation were seen previously in D1-MSNs of the NAc after passive cocaine administration (Dobi at el. 2011, Pascoli et al., 2012). The results here add to previous observation by demonstrating that potentiation of D1-MSNs inputs is a generalized response to cocaine exposure and that it develops in the majority of the mice, independently of whether or not they show compulsive behaviors towards cocaine. In contrast, potentiation at D2-MSN inputs was observed only in the resilient individuals that did not develop compulsive behaviors. It is remains unclear whether resilient individuals have stronger inputs to accumbal D2-MSNs that preceeded the cocaine exposure or whether it developed as a consequence of repeated cocaine self-administration. Judging by the range of AMPA/NMDA ratios recorded in sham animals, it is unlikely that the potentiation of D2-MSNs inputs preceded drug exposure but further studies are required to determine the predisposing factors to this resilience.Manipulations of the activity of indirect pathway neurons in the NAc, even when introduced after several weeks of drug taking, significantly increased or decreased cocaine seeking and self-administration. Systemic administration of a D2-like agonist did not enhance seeking and failed to reproduce the effects of inhibiting D2-MSNs using cell-specific expression of hM4Di. This is possibly due to the different effects of activation of dopamine D2 receptors expressed on other cell types, such as dopaminergic neurons and cholinergic interneurons. These results highlight the importance of using cell-specific approaches and demonstrate that selective activation Gi signaling in D2-MSNs can increase drug seeking behavior. The same manipulation did not alter responding for food reward, suggesting specificity for cocaine-seeking. Dorsal regions of the striatum are more likely involved as they regulate cued-instrumental responding for food26. As expected for a strong reinforcer, breakpoint values achieved for food were 10-30 times higher than those achieved for cocaine. Therefore, it is possible that activity of indirect pathway neurons is already depressed when responding for such a potent reinforcer and the effect of hM4Di activation might be occluded. When D2-MSNs in the dorsal striatum were inhibited after 3 weeks of cocaine self-administration, responding for cocaine did not increase significantly, suggesting that indirect pathway neurons in the dorsal striatum are not involved in cued drug seeking at this early stage and under these experimental conditions.
D2-MSNs in the NAc core send GABAergic projections to the dorsolateral subregion of the ventral pallidum where neurons display sustained changes in firing rate that are time locked to responding during a cocaine self-administration task27. Reinstatement of cocaine seeking is associated with decreased levels of extracellular GABA in the ventral pallidum and blockers of glutamateric transmission in the NAc core prevent the change28, 29. The evidence implicates indirect D2-MSNs in NAc and its projections to the ventral pallidum in the processing of drug seeking behavior and inhibitory control of the output. It is tempting to speculate that selective silencing of ventral pallidum neurons would also decrease the motivation to seek cocaine. Using therapeutic brain stimulation to enhance indirect pathway or silence the output of ventral pallidum in patients could enhance self-control in those fighting dependence on stimulant drugs.
In conclusion, this study establishes synaptic potentiation in D2-MSN inputs as a critical mechanism for controlling the expression of compulsive behaviors towards cocaine. We propose that this cell-specific synaptic potentiation facilitates the recruitment of indirect pathway neurons and protects against the development of addictive behaviors.
Methods
Animals
Experiments were performed in accordance with guidelines from the NIAAA Animal Care and Use Committee. Drd1-EGFP BAC transgenic mice (GENSAT, Swiss Webster background) and Adora2a-Cre BAC transgenic mice (GENSAT, KG139Gsat/Mmcd, SW/B6 background) were used for this study. Mice were housed in groups until surgery and individually afterwards on reversed light cycle (lights on 18:30 – 06:30) with food and water ad libitum.
Intravenous cocaine self-administration
Mice (~ 40 days old) were implanted with a chronically indwelling catheter (CamCath) in the right jugular vein as described previously30. Mice received oral antibiotics and recovered for 5-7 days before behavioral testing started. Catheters were maintained by daily flushes with heparinized saline (100-275 U/ml). Sham surgeries permanently occluded blood flow through the jugular vein. Sham control mice (n = 20) were left undisturbed in their home cage for at least 3 weeks in the reversed light cycle before experiments. Modified operant boxes (Med-Associates) (size = 11×18×13cm WxLxH) were used and behavioral testing was performed during the dark phase of the light cycle. Pokes in active hole delivered intravenous cocaine infusions (1-2 mg/kg) paired with the cue light ON for 4 seconds and a 10 sec time out. The house light signaled the drug-off periods and the end of the sessions. Naïve mice were trained in 2-6 hr sessions for 5-13 days on a fixed poke/reward ratio of 1. The criteria for acquisition of self-administration behavior was a ratio of active to inactive pokes of at least 2.5 and cocaine intake higher than 5 mg/kg/day for the last two training session. An estimated 55% of Swiss Webster Drd1-EGFP mice (27 males, 9 females) reached criteria within 8.5 ± 0.5 days of training were moved to sessions (2:30 h long, cocaine 2 mg/kg/infusion) and self-administered cocaine for an average of 31 more days (min 17 – max 41) (Suppl. Table 1). The other 45% of the mice (31 mice) did not acquire The behavioral analysis was completed in 28 out of the 36 mice while 8 mice lost patency right before the last breakpoint session and were used for physiological recordings but lack behavioral measurements (Suppl. Table 1). Sessions consisted of three drug-on periods (40 min each) interspersed by two drug-off periods of 15 min. Perseverance was measured as the active pokes during the drug-off periods over the last 5 sessions. Motivation was measured in two exponentially progressing responding sessions (after days 15 and 30) and the breakpoint was assigned as the motivation value31. The motivation and perseverance scores for individual mice were calculated using the z-score, , where Xi is the behavior value for the individual animal, is the mean behavior value for all the mice included in the study, and SD is the standard deviation of the population. The combined behavior score was equal to the sum of the motivation and perseverance scores for each individual mouse.
In experiments using Adora2a-Cre+/− mice, sessions were 3:40 h long, the single infusion dose was 1 mg/kg cocaine, and mice were trained on a fixed ratio 1, 2 or 3 as mentioned and depending on the experiment purpose. CNO and quinelorane were dissolved in saline and administered i.v. (1 ml/kg) at 1mg/kg and 0.03 mg/kg, respectively in through the catheter right before the start of the session. Catheter patency was determined every 10 days by anesthetic cocktail (1.5 mg/kg ketamine, 0.75 mg/ml midazepan). Mice that failed patency before reaching session 25 were removed from the study. Behavioral data was analyzed and plotted in Igor. The log normal equation was used to fit the behavioral values.
Intracranial viral gene transfer and in vivo optogenetic stimulation
Cre-inducible AAV-hSyn-DIO-hM4Di-mCherry (serotype 1 or 2, 6×1012 VM/ml, OTTC at NIDA and UNC Vector Core) and AAV5-EF1a-DIO-ChR2(H134R)-EYFP (4×1012 VM/ml, Vector Core at UNC) were used. Stereotaxic bilateral injections (250-400 nl at 100 nl/min) were performed into the NAc core (AP, +1.4 mm; ML, ±0.1 mm; DV, −4.8 mm) or the dorsal striatum (AP, +0.8 mm; ML, ±1.75 mm; DV, −3.2 mm) of Adora2a-Cre+/− and control Adora2a-Cre−/− mice at 5-6 weeks of age. For self-administration experiments, mice recovered for 3-5 days before indwelling catheters were implanted in the jugular vein. To confirm hM4Di activity using electrophysiological recordings, Cre-inducible hM4Di-mCherry and ChR2-EYFP vectors were co-injected (1:1) into NAc core of Adora2a-Cre+/−. For in vivo optogenetic stimulation of indirect pathway, a two-ferrule cannula with fiber optics (200 μm/0.22 NA, 4.0 mm, Doric Lenses, Inc) was implanted immediately after the viral injection right above the injection area and secured to the skull with metabond and dental cement. Diode-pumped blue lasers (473 nm, 25 mW, CrystaLaser) was used and connected via optic fiber (200 μm/0.22 NA, M25L02, ThorLabs) to a 1in-2out fiberoptic rotary joint (Doric Lenses, Inc) to split laser power and facilitate movement during the task. Stimulation consisted of 10 ms-duration pulses delivered at 16.6 Hz for 5 min (2 min for one animal), mean power output of 5 mW (3.5-6 mW) at each fiber tip of the two-ferrule cannula implanted in the NAc core every 10 min throughout the session. The control animals for these experiments were Adora2a-Cre+/− mice injected with AAV-hSyn-DIO-mCherry (serotype 5, titer = 6×1012 VM/ml, Vector Core, UNC).
Electrophysiology
Sagittal slices (250 μm) were prepared from 103 ± 12 days old mice ( n = 35) 4-17 days after last self-administration session and from sham-surgery mice (97 ± 16 days old, n = 20) in cutting solution containing (in mM) 225 sucrose, 119 NaCl, 2.5 KCl, 0.1 CaCl2, 4.9 MgCl2, 26.2 NaHCO3, 1 NaH2PO4, 1.25 glucose, and 3 kynurenic acid32. Slices were incubated at 33 °C for 30 min and at room temperature until used. Recording were made at 30 °C using artificial cerebrospinal fluid (ACSF) containing (in mM): 124 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 26.2 NaHCO3, 1 NaH2PO4, and 20 glucose. Gabazine (5 μM) and d-serine (10 μM) were added to the ACSF in order to isolate glutamatergic EPSCs. Whole-cell voltage-clamp recordings were obtained from GFP positive and negative MSNs in the NAc core using electrodes (2.5-3.5 MΩ) filled with solution (in mM): 130 CsMeSO4, 10 CsCl, 10 HEPES, 0.2 EGTA, 4 Na-ATP, 0.4 Na-GTP, and 0.1 spermine (pH = 7.25, ~310 mOsm). Recordings were excluded if access resistance was greater than 25 MΩ or if input resistance was greater than the range expected for MSNs (>350 MΩ). eEPSCs were elicited by a pair of current pulses (0.2 ms width, 50 ms interval, every 20 s) using an ACSF–filled glass pipette placed ~250 μm rostro-dorsally. AMPAR mediated eEPSCs were recorded when holding cells at −70 mV. eEPSCs were then recorded at +40 mV in the presence or absence of NMDAR antagonist (R)-CPP (10 μM) and the NMDA receptor response was calculated by subtraction off-line. For current clamp recording from D2-MSNs, K-based internal solution was used. Measurements of D2-MSNs output in either core or the ventral pallidum were carried out by whole-cell voltage-clamp recordings using CsCl based internal solution (all other components kept the same as above) while holding the cells at −20 mV in the presence of AMPA receptor antagonist NBQX (5 μM) in order to isolate GABAergic IPSCs. IPSCs were evoked by activation of ChR2 and triggered by a brief light pulse (0.2 ms width, 20 sec interval) produced by a diode-pumped blue laser (473 nm, 25 mW, CrystaLaser) and delivered via a fiber optic (200 μm/0.22 NA, ThorLabs). All data was analyzed and plotted in Igor. Stock of CNO was prepared in water and tested at final concentration of 10 μM in ACSF. Data was acquired using Multiclamp 700B (Molecular Devices), filtered at 1 kHz and digitized at 5 kHz. Electrophysiological recordings were made blind to the behavior score of the mice, except for sham surgery mice that did not carry anintravenous port and were easily distinguishable to the experimenter. Data was collected from 16 sham surgery mice and 27 mice after cocaine self-administration within 1-38 days of the last cocaine exposure. Behavioral data was available for 22 mice (5 mice had no behavior; Suppl. Table 1). Recordings from D1-MSNs were collected in 9 mice, from D2-MSNs in 3 mice and from D1-MSNs and D2-MSNs in 14 mice. Note that there is no behavioral data for all of them and refer to Suppl. Table 1 for detail.
Two photon glutamate uncaging
A custom-built 2-photon laser scanning microscope equipped with two Ti-Sapphire lasers was used to simultaneously image dendritic spines using a 840 nm laser and photo-release caged glutamate next to the spines using a 720 nm laser. Slices and solutions were prepared as described above. Whole-cell recordings were obtained from GFP positive MSNs in the NAc core region at 25 °C. Alexa Fluor® 594 (10 uM) was added to the internal solution to visualize second and third order dendrites and spines within 200 μm from the soma were randomly selected for imaging. For all experiments, MNI-glutamate (5 mM), GABAzine (5 μM), TTX (1 μM), and the Ca2+ channel blockers mibefradil (20 μM) and conotoxin GVIA (1 μM) were added to ACSF. For recordings of AMPAR uEPSC, cells were held at −80 mV and AP5 (5 μM) was added to the external solution. For recordings of NMDA-R uEPSC, cells were held at −50 mV and low Mg2+ (50 μM) ACSF containing NBQX (5 μM) was used. Glutamate uncaging was achieved with 1-2-ms pulse of 720-nm light delivered next to the spine head every 30 sec and the laser power was standardized in advance by measuring the fluorescence recovery from photobleaching (FRAP) of the red dye in the spine head33. Data were acquired using National Instruments data acquisition boards and custom software written in MATLAB34. Ten to fifteen uEPSCs were average per spine and the peak amplitude calculated in 1-ms window around the peak. Recordings were collected from D1-MSNs in 3 cocaine mice and 4 sham surgery mice (Suppl. Table 1).
Operant food self-administration
Adora2a-Cre+/− and sham Adora2a-Cre−/− littermate mice were stereotaxically injected into NAc core with Cre-inducible AAV vectors expressing hM4Di-mCherry and allowed to recover for 9 days. Mice were placed in operant chambers in sound attenuating boxes (Med-Associates) in which they pressed a cued-active lever (left or right; counterbalanced across mice) for an outcome of regular “chow” pellets (20 mg pellet). Presses on an inactive lever did not result in reinforcement. Two days before training commenced, mice were food restricted to 90% of their baseline weight at which they were maintained for the duration of experimental procedures. On the first day, mice were trained to approach the food magazine (no levers or cue light present) on a random time schedule, with a reinforcer delivered on average every 60 sec for a total of 30 min. All other training sessions commenced with illumination of the house light, active and inactive lever extension, and illumination of a cue light located directly above the active lever, and ended after 60 min with the levers retracting and the cue and house-light turning off. Mice were trained in on a FR1 schedule for 3-5 days and, once lever-pressing behavior was acquired, the fixed ratio requirement was increased to FR10. One hour before each session, mice received an injection of saline (10 ml/kg, i.p.) during training for habituation and they received either saline or CNO (1 mg/kg dose, 10 ml/kg, i.p.) during testing. Testing of FR10 responding began after 3 days of baseline FR10 responding and consisted of two days of CNO and two days of saline injections. The order of drug test (Sal first vs. CNO first) was counterbalanced across mice and kept constant across CNO testing. Progressive ratio testing followed in which mice were given CNO or saline on alternating days (counterbalanced across mice) and with a FR10 training session in between progressive ratio test days. The same progressive ratio schedule used for cocaine self-administration was used here31 with an initial breakpoint of 10, and maximum breakpoint to be achieved of 2012.
Locomotor assessment
Locomotor activity was recorded in cages (10.25 × 6 inches) constructed out of clear polycarbonate walls and floors under dim illumination (150 lux). Horizontal activity was detected as infrared beam crosses (1 inch spacing, 10 beams per cage) made on consecutive beams (ambulatory counts) using Opto M3 activity monitors (Columbus Instruments). AThe response to the D1 agonist SKF81297 was assessing using a previous protocol 22. Briefly, Adult mice (8–16 weeks old) were allowed to run freely for 60 minutes, during which time baseline locomotor activity was determined. They received an i.p. injection of either saline or 2.5 mg/kg SKF81297, a dose that increases locomotor activity in wild-type C57BL/6J mice35, and were immediately returned to the cage and horizontal locomotion was measured for the next 150 min. In all cases, locomotor activity is expressed as the number of ambulatory counts per ten minutes, one hour, or normalized to the baseline value obtained for each mouse prior to drug administration.
Quantitative polymerase chain reaction
Striatal tissue was microdissected from two adult mice (9-16 weeks old) of each genotype (Adora2a-Cre+/− and control Adora2a-Cre−/−). All mice received an intra-striatal injection of a viral vector that conditionally expresses hM4Di and 2-4 days of CNO (1 mg/kg) treatment. Total RNA was purified using RNeasy Micro (Qiagen), and cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad). mRNA expression of dopamine D1 receptor Drd1a and the endogenous control gene β-actin Actb were determined using TaqMan Gene Expression Assays (Applied Biosystems, catalog No. Mm01353211_m1 and Mm01205647_g1, respectively). Quantitative PCR (qPCR) runs were performed using TaqMan Fast Polymerase (Applied Biosystems) in a StepOnePlus Real-Time PCR system. Cycling conditions were as follows: initial hold at 95°C for 20 s; 40 cycles of step 1 (95°C for 1 s); and set 2 (60°C for 20 s). Samples were run in quadruplicates, and negative controls were run in parallel. cDNA synthesis and qPCR experiments were repeated three times. Relative quantification was calculated using the ΔΔCt method (StepOne System Software, Applied Biosystems).
Statistics
All statistical comparisons were two-sided and were performed with IGOR or Prism. Paired and unpaired Student’s t tests, one-way and repeated measures two-way ANOVA were used when appropriate, and n and p values are included in the results section. Bonferroni tests were used for multiple comparisons post-hoc analysis. One sample t-test was used to compare normalized breakpoint values all rewards earned when mentioned. F test was used to compare the variance among groups and test the assumption of normality for each statistical test. In the case of AMPA/NMDA ratios and uncaged evoked responses at single spines (uEPSC), the normality assumption was not met and the non-parametric test Mann-Whitney was used instead. . All replicates are biological, and the errors are calculated as SEM. Sample size was not justified with statistical power.
Drugs
Cocaine-HCL was obtained from NIDA, NIH. Gabazine (SR-95531), (R)-CPP, NBQX, AP5, kynurenic acid and conotoxin GVIA were purchased from Ascent Scientific; MNI-glutamate, mibefradil and quinelorane from Tocris; Alexa Fluor® 594 from Molecular Probes and clozapine N-oxide (CNO) and all other chemicals from Sigma.
Supplementary Material
Acknowledgements
The authors are grateful to the staff of FLAC and to Dr. Chedester for the technical support provided and to the members of the Alvarez laboratory and Drs. J.T. Williams, D.M. Lovinger and B.L. Sabatini for the helpful comments on the manuscript. The authors also thank Drs. B. Roth and K. Deisseroth for their generosity in providing the constructs for hM4Di and ChR2 , respectively, and Dr. B. Harvey at OTTC at NIDA for the viral vector preparation. This study was funded by the Intramural Programs of NIAAA and NINDS (ZIA-AA000421).
Footnotes
The authors have no competing financial interests to declare.
Author Contributions: R.B. carried out behavioral studies with the help from C.H.C, E.M., P.F.K and C.M.G.; J.H.S carried out all electrophysiological experiments with the help from M.F.A. and A.D. performed all two photon glutamate uncaging experiments; A.R.K., C.M.G., R.B. and M.F.A. performed the behavioral experiments with hM4Di and ChR2; R.B., J.H.S. and V.A.A. designed the study and wrote the manuscript.
References
- 1.Ersche KD, et al. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology. 2009;56(Suppl 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkow ND, et al. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 4.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nader MA, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 6.Beckmann JS, Marusich JA, Gipson CD, Bardo MT. Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat. Behav Brain Res. 2011;216:159–165. doi: 10.1016/j.bbr.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 8.Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012 doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobo MK, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durieux PF, et al. D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci. 2009;12:393–395. doi: 10.1038/nn.2286. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson SM, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasanetz F, et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 17.Kramer PF, et al. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J Neurosci. 2011;31:126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong S, Rogan SC, Roth BL. Directed molecular evolution of DREADDs: a generic approach to creating next-generation RASSLs. Nat Protoc. 2010;5:561–573. doi: 10.1038/nprot.2009.239. [DOI] [PubMed] [Google Scholar]
- 19.Alexander GM, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozorovitskiy Y, Saunders A, Johnson CA, Lowell BB, Sabatini BL. Recurrent network activity drives striatal synaptogenesis. Nature. 2012;485:646–650. doi: 10.1038/nature11052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bello EP, et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. J Pharmacol Exp Ther. 2005;312:733–741. doi: 10.1124/jpet.104.074468. [DOI] [PubMed] [Google Scholar]
- 23.Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Caine SB, Negus SS, Mello NK. Effects of dopamine D(1-like) and D(2-like) agonists on cocaine self-administration in rhesus monkeys: rapid assessment of cocaine dose-effect functions. Psychopharmacology (Berl) 2000;148:41–51. doi: 10.1007/s002130050023. [DOI] [PubMed] [Google Scholar]
- 25.Caine SB, Negus SS, Mello NK, Bergman J. Effects of dopamine D(1-like) and D(2-like) agonists in rats that self-administer cocaine. J Pharmacol Exp Ther. 1999;291:353–360. [PubMed] [Google Scholar]
- 26.Corbit LH, Janak PH. Posterior dorsomedial striatum is critical for both selective instrumental and Pavlovian reward learning. Eur J Neurosci. 2010;31:1312–1321. doi: 10.1111/j.1460-9568.2010.07153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Root DH, et al. Differential roles of ventral pallidum subregions during cocaine self-administration behaviors. J Comp Neurol. 2012 doi: 10.1002/cne.23191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang XC, McFarland K, Cagle S, Kalivas PW. Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. J Neurosci. 2005;25:4512–4520. doi: 10.1523/JNEUROSCI.0685-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torregrossa MM, Tang XC, Kalivas PW. The glutamatergic projection from the prefrontal cortex to the nucleus accumbens core is required for cocaine-induced decreases in ventral pallidal GABA. Neurosci Lett. 2008;438:142–145. doi: 10.1016/j.neulet.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomsen M, Caine SB. Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci. 2005 doi: 10.1002/0471142301.ns0920s32. Chapter 9, Unit 9 20. [DOI] [PubMed] [Google Scholar]
- 31.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 32.Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31:1895–1904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giessel AJ, Sabatini BL. M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron. 2010;68:936–947. doi: 10.1016/j.neuron.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pologruto TA, Sabatini BL, Svoboda K. ScanImage: flexible software for operating laser scanning microscopes. Biomed Eng Online. 2003;2:13. doi: 10.1186/1475-925X-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Napolitano F, et al. Role of aberrant striatal dopamine D1 receptor/cAMP/protein kinase A/DARPP32 signaling in the paradoxical calming effect of amphetamine. J Neurosci. 2010;30:11043–11056. doi: 10.1523/JNEUROSCI.1682-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.