Abstract
Lipopolysaccharide (LPS)-induced white matter injury in the neonatal rat brain is associated with inflammatory processes. Cyclooxygenase-2 (COX-2) can be induced by inflammatory stimuli, such as cytokines and pro-inflammatory molecules, suggesting that COX-2 may be considered as the target for anti-inflammation. The objective of the present study was to examine whether celecoxib, a selective COX-2 inhibitor, can reduce systemic LPS-induced brain inflammation and brain damage. Intraperitoneal (i.p.) injection of LPS (2 mg/kg) was performed in postnatal day 5 (P5) of Sprague-Dawley rat pups and celecoxib (20 mg/kg) or vehicle was administered i.p. 5 min after LPS injection. The body weight and wire hanging maneuver test were performed 24 hr after the LPS exposure, and brain injury was examined after these tests. Systemic LPS exposure resulted in an impairment of behavioral performance and acute brain injury, as indicated by apoptotic death of oligodendrocytes (OLs) and loss of OL immunoreactivity in the neonatal rat brain. Treatments with celecoxib significantly reduced systemic LPS-induced neurobehavioral disturbance and brain damage. Celecoxib administration significantly attenuated systemic LPS-induced increments in the number of activated microglia and astrocytes, concentrations of IL-1β and TNFα, and protein levels of phosphorylated-p38 MAPK in the neonatal rat brain. The protection of celecoxib was also associated with a reduction of systemic LPS-induced COX-2+ cells which were double labeled with GFAP+ (astrocyte) cells. The overall results suggest that celecoxib was capable of attenuating the brain injury and neurobehavioral disturbance induced by systemic LPS exposure, and the protective effects are associated with its anti-inflammatory properties.
Keywords: lipopolysaccharide, cyclooxygenase-2, celecoxib, oligodendrocyte, microglia, astrocyte
INTRODUCTION
Increasing evidence has indicated that perinatal infection or inflammation and hypoxia-ischemia are major contributors to perinatal brain injury such as periventricular leukomalacia (PVL), a predominant form of injury in the premature infant brain (Dammann and Leviton, 1997, 2004: Goldenberg et al., 2008; Hagberg et al., 2002; Volpe, 2003). The intrinsic vulnerability of late oligodendrocyte (OL) progenitors (O4+/O1-), which are the predominant OL lineage during the peak period of PVL (i.e. 24-32 gestation weeks), is considered central to pathogenesis of PVL in the infant brain (Back et al., 2001, 2002; Rezaie and Dean, 2002). Elevated concentrations of inflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNFα) are frequently observed in the brain (Kadhim et al., 2001, 2003; Yoon et al., 1997b), cord blood (Yoon et al., 1996) and amniotic fluid (Yoon et al., 1997a) in infants with PVL. Therefore, treatments aiming at anti-inflammation might provide protection to OLs.
In our previous study, we found that intracerebral (i.c.) injection of LPS resulted in acute white matter and neuronal injury in the neonatal rat (Cai et al., 2003; Fan et al., 2005a, 2008c; Pang et al., 2003). Activation of microglia plays a critical role in neonatal i.c. LPS-induced brain injury in the rat brain (Fan et al., 2005a, 2008c). Interaction of microglial cells with apoptotic neurons has been reported to selectively promote COX-2 expression, and COX-2 may mediate microglial activation and may play a key role in amplifying the inflammatory response with toxic effects (Bartels and Leenders, 2010; De Simone et al., 2004). Two COX isoforms have been characterized: COX-1, which is constitutively expressed in most tissues and is thought to mediate physiological responses, and COX-2, which is rapidly expressed in several cell types in response to cytokines, growth factors and pro-inflammatory molecules (Bartels and Leenders, 2010; Choi et al., 2009a; Minghetti, 2004). In the central nervous system (CNS), the expression of COX-2 may contribute to fundamental brain functions; however, COX-2 is induced in inflammatory cells in response to cytokines and pro-inflammatory molecules, suggesting that COX-2 has a role in the inflammatory processes (Bartels and Leenders, 2010; Minghetti, 2004).
Celecoxib is a selective COX-2 inhibitor, currently being used in the treatment of various painful and inflammatory conditions, and is the safest COX-2 inhibitor relating to the cardiovascular safety data (Jones, 2005). Celecoxib may decrease the phosphorylation state of p38 and p44/42 of mitogen-activated protein kinase (MAPK) in human osteoarthritic chondrocytes (Takahashi et al., 2005) and celecoxib also can enhance heme oxygenase-1 (HO-1) mediated anti-inflammatory activity in vascular endothelium (Hamdulay et al., 2010). The neuroprotective action of celecoxib has been observed in the LPS-induced nigrostriatal neurodegeneration (Hunter et al., 2007) and 6-hydroxydopamine (6-OHDA)-induced progressive dopamine neuron degeneration in a rat model of Parkinson's disease (Sanchez-Pernaute et al., 2004). The present study has a two-fold objective: to investigate whether the systemic LPS exposure through an i.p. injection, an exposure route more likely to be encountered in newborn infants with infections, also induces the brain inflammation and brain injury in our neonatal rat model (P5) and whether celecoxib offers protection against LPS-induced brain inflammation and brain damage in the neonatal rat brain.
EXPERIMENTAL PROCEDURES
Chemicals
Unless otherwise stated, all chemicals used in this study were purchased from Sigma (St. Louis, MO, USA). Monoclonal mouse antibodies against late oligodendrocyte (OL) progenitor cell marker O4 (O4) and glial fibrillary acidic protein (GFAP) were purchased from Millipore (Billerica, MA, USA). Polyclonal rabbit antibodies against caspase-3 (active form); ionized calcium binding adapter molecule 1 (Iba1); and p38 mitogen-activated protein kinase (p38 MAPK) or phospho-p38 mitogen-activated protein kinase (p-p38 MAPK) were obtained from Millipore (Billerica, MA, USA), Wako Chemicals USA (Irvine, CA, USA), and Cell Signaling (Danvers, MA, USA), respectively. Polyclonal goat antibody against cyclooxygenase-2 (COX-2) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The TUNEL staining kit was purchased from Millipore (Billerica, MA, USA). ELISA kits for immunoassay of rat interleukin-1β (IL-1β) and tumor necrosis factor-α (TNFα) were purchased from R&D Systems (Minneapolis, MN, USA).
Animals
Timed pregnant Sprague-Dawley rats arrived in the laboratory on day 19 of gestation. Animals were maintained in a room with a 12 h light/dark cycle and at constant temperature (22 ± 2°C). The day of birth was defined as postnatal day 0 (P0). After birth, the litter size was adjusted to twelve pups per litter to minimize the effect of litter size on body weight and brain size. All procedures for animal care were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center. Every effort was made to minimize the number of animals used and their suffering.
Animal treatment
Intraperitoneal (i.p.) injection of LPS (2 mg/kg, from Escherichia coli, serotype 055: B5) was performed in 5 day old (P5) Sprague-Dawley rat pups of both sexes. The control rats were injected with the same volume of sterile saline (0.1 ml). All animals survived the injection. Both LPS- and saline-injected animals were further divided into two groups: one received i.p. injections of celecoxib (20 mg/kg) and the other vehicle. Celecoxib (20 mg/kg) was dissolved in 20% DMSO in normal saline (Cunha et al., 2010; Sato et al., 2007) and administered immediately after the LPS injection. Core temperature was acquired using a rectal probe and digital thermometer (Fisher Scientific, Suwanee, GA, USA) every one hour, beginning from prior to LPS injection until 9 hours and 24 hours after the LPS injection, and hypothermia was defined as rectal temperature less than 32°C (Garrett-Cox et al., 2003). Room temperature was maintained at 24 ± 2°C throughout. A total of ninety-six rats from eight litters were used in the present study. One pup from each litter was assigned to each group, then the n number was equal to eight for each group. Behavioral tests were conducted in eight rats from each group from P5 to P6. Rats were sacrificed on P6. Eighteen rats from each group were sacrificed by decapitation to collect fresh brain tissue for Western blot analysis (6 rats for each group), and ELISA assay (12 rats for each group). Six additional rats from each group were sacrificed by transcardiac perfusion with normal saline followed by 4% paraformaldehyde for brain section preparation. Free-floating coronal brain sections at 40 μm of thickness were prepared in a freezing microtome (Leica, SM 2000R, Wetzlar, Germany) for immunohistochemistry staining.
Behavioral testing: wire hanging maneuver
The body weight of rat pups was recorded daily. A behavioral test, wire hanging maneuver, was performed for all rat pups from P5 to P6 as described by Fan et al. (2005b, 2008a) with modification. This maneuver tests neuromuscular and locomotor development (Altman and Sudarshan, 1975; Hermans et al., 1992). Pups suspended by their forelimbs from a horizontal rod (5 × 5 mm2 area, 35 cm long, between two poles 50 cm high) tend to support themselves with their hind limbs, preventing them from falling and aiding in progression along the rod. A sawdust-filled box at the base served as protection for the falling pups. Suspension latencies were recorded and the cut-off time was 120 sec.
Immunohistochemistry
Brain injury was estimated based on the results of immunohistochemistry in consecutive brain sections prepared from rats sacrificed 1 day (P6) after LPS injection. For immunohistochemistry staining, primary antibodies were used in the following dilution: O4, 1 μg/ml; Iba1, 1:500; GFAP, 1:200; and caspase-3, or COX-2, 1:100. O4 was used to detect late OL progenitor cells in white matter. Caspase-3 (active form) was used to confirm apoptotic cell death. Microglia were detected using Iba1 immunostaining, which recognizes both the resting and the activated microglia. GFAP was used to detect astrocytes. COX-2 provides selective staining of inducible cyclooxygenase. Sections were incubated with primary antibodies at 4 °C overnight and further incubated with secondary antibodies conjugated with fluorescent (rhodamine, 1:200; Alexa Fluor 555, 1:500 or Alexa Fluor 488, 1:200; Jackson Immunoresearch, West Grove, PA, USA) for 1 h in the dark at room temperature. 4′, 6-Diamidino-2-phenylindole (DAPI) (100 ng/ml) was used simultaneously to identify nuclei in the final visualization. Sections incubated in the absence of primary antibody were used as negative controls. When double-labeling was required, primary antibodies from different hosts were used in combination with appropriate secondary antibodies, which were against the immunoglobulin from the corresponding hosts. The resulting sections were examined under a fluorescent microscope (Olympus, BX60) at appropriate wavelengths.
Detection of cell death
Cell death was detected using TUNEL kits. When double-labeling of O4 cells was required, O4 immunostaining was first performed as described above, with rhodamine as the fluorophore. TUNEL staining was then performed in these sections following the manufacturer's instructions. The TUNEL positive cells show a green color and the O4 positive cells show a red color.
Since both necrosis and apoptosis may result in TUNEL-positive staining (Stadelmann and Lassmann, 2000), we further performed caspase-3 and O4 double-labeling to confirm apoptotic cell death of OLs following LPS exposure. Caspase-3 is a key mediator of apoptotic cell death (Stadelmann and Lassmann, 2000). Free-floating brain sections were simultaneously incubated with rabbit antibody against caspase-3 and mouse antibody against O4. Alexa Fluor 488-conjugated secondary antibody against rabbit IgG and rhodamine-conjugated secondary antibody against mouse IgM were used to visualize the result.
Immunoblotting analysis
Protein expression of p38 MAPK and p-p38 MAPK was determined in the P6 rat brain by western blotting according to the methods of Fan et al. (2011a, 2011b) with modifications. One day after LPS injection (P6), brains were quickly removed and tissues were frozen in liquid nitrogen and stored at -80°C. Tissues were homogenized in an extraction buffer (Biosource, Camarillo, CA, USA) added with a mixture of protease inhibitors (Calbiochem, La Jolla, CA, USA) and 1 mM PMSF by application of a Sonic Dismembrator (Fisher Scientific, Suwanee, GA, USA) 3 times for 10 seconds each. Protein levels of homogenates were determined by the Bradford method. The homogenates were diluted with 1:2 (v/v) Laemmli sample buffer (including 5% (w/v) β-mercaptoethanol) and boiled for 5 minutes. Equal quantities of protein (10 μg/10 μl) were loaded into each well of a 4 to 20% SDS-polyacrylamide gradient gel (MINI-PROTEAN TGX, 4-20%, Bio-Rad Laboratories, Hercules, CA, USA). The separated proteins were transferred electrophoretically to PVDF membranes (Bio-Rad Laboratories, Hercules, CA, USA) at 100V for one hour. The blots were incubated with a blocking solution containing 5% non-fat milk and 0.1% Tween-20 in Tris-buffered saline (TBS) for one hour before incubation with the primary antibody (1:1000) in the blocking solution overnight at 4°C. The blots were then incubated with peroxidase-conjugated antibodies in the blocking solution (1:4,000) for one hour at room temperature. The immunoreactivity was detected by the Enhanced Chemiluminescence Plus or advanced ECL system (GE Healthcare, Piscataway, NJ, USA) and then determined with the Chemidoc MP Imaging System followed by quantification using Image Lab software (both from Bio-Rad Laboratories, Hercules, CA, USA). To ensure that equal amounts of protein were applied to the immunoblot, the membranes were stripped with a stripping buffer (Thermo Scientific, Rockford, IL, USA) and re-probed for β-actin (1:4,000, Sigma) to normalize the results.
Determination of IL-1β and TNFα protein by ELISA
Two major proinflammatory cytokines, IL-1β and TNFα, were determined by ELISA as previously described (Fan et al., 2005a, 2008c). Briefly, brain tissues from each pup were collected 6 or 24 h after LPS injection, when the LPS-stimulated increase in inflammatory cytokines in the rat brain reaches a peak value (Pang et al., 2003). Brain tissues were homogenized by sonication in 1 ml ice-cold PBS (pH 7.2) and centrifuged at 12,000g for 20 min at 4°C. The supernatant was collected and the protein concentration was determined by the Bradford method. ELISA was performed following manufacturer's instructions and data were acquired using a 96-well plate reader (Bio-Tek instruments, Inc., VT, USA). The cytokine contents were expressed as pg cytokines/mg protein.
Quantification of data and statistics
Our previous studies indicate that neonatal i.c. LPS injection produces white matter injury primarily in the cigulum white matter area of the forebrain (Cai et al., 2003; Fan et al., 2005a; Pang et al., 2003). To compare the i.c. LPS injection-induced effects, therefore, brain sections at the bregma level and the middle dorsal hippocampus level were used for determination of all pathological changes caused by systemic LPS injection. Most immunostaining data were quantified by counting of positively stained cells. When the cellular boundary was not clearly separated, numbers of DAPI-stained nuclei from the superimposed images were counted as the cell number. Three digital microscopic images were randomly captured in each of the three sections and the number of positively stained cells in the three images was counted and averaged (cells/mm2). The mean value of cell counting from the three brain sections was used to represent one single brain. For convenience of comparison among the treatment groups, results were standardized as the average number of cells/mm2. COX-2 staining was quantified by using NIH image software to determine the percentage area that contains COX-2 positive staining in the entire area of the captured image (Fan et al., 2011a, 2011b). In response to LPS challenge, the number of Iba1 positive microglia and GFAP positive astrocytes increases and the soma of these cells become larger. In addition to cell density, the Iba1 or GFAP immunoreactivity was also quantified by calculating the percentage area of the whole image that contains Iba1 or GFAP immunostaining (Fan et al., 2011a, 2011b).
The body temperature data were presented as the mean ± SEM and analyzed by two-way repeated measures ANOVA (for tests conducted continuously at different hours), followed by Student-Newman-Keuls test. The behavioral data were presented as the mean ± SEM and analyzed by one-way ANOVA followed by the Student-Newman-Keuls test. Data from immunostaining, immunoblotting analysis, and ELISA assay were presented as the mean ± SEM and analyzed by one-way ANOVA followed by the Student-Newman-Keuls test. Results with a p < 0.05 were considered statistically significant.
RESULTS
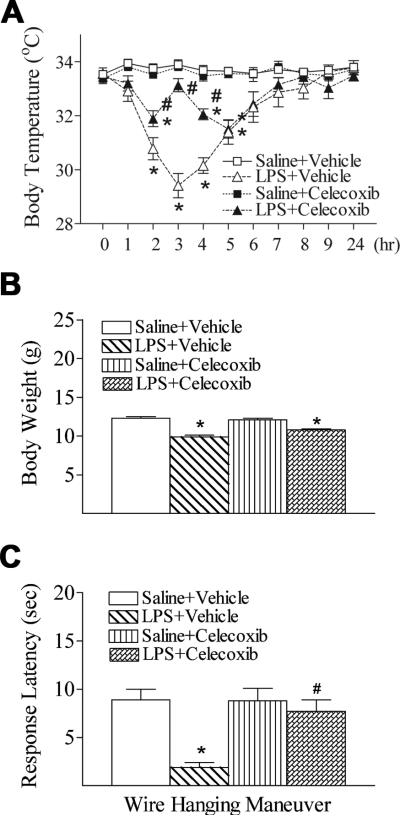
Celecoxib attenuated LPS-induced hypothermia
The rectal temperature dropped in LPS treated group from 2 to 5 hours after LPS injection (29.4 ± 0.4°C to 31.4 ± 0.4°C) as compare to control group (33.7 ± 0.2°C to 33.9 ± 0.2°C) (p < 0.05) (Fig. 1A). No gender differences in LPS-induced hypothermia were observed in the rats. Celecoxib treatment significantly protected against LPS-induced hypothermia (< 32.0°C) from 2 to 4 hours after LPS injection (31.5 ± 0.3°C to 33.1 ± 0.2°C) (p < 0.05) (Fig. 1A).
Fig. 1.
Celecoxib attenuated systemic LPS-induced hypothermia (A), body weight loss (B), and reduction of mean latency times in the wire hanging maneuver (C) in the rat. The results are expressed as the mean ± SEM of eight animals in each group, and analyzed by two-way repeated measures ANOVA for data from tests conducted continuously at different hours after LPS injection (A) or one-way ANOVA (B and C). * P < 0.05 represents a significant difference for the LPS + Vehicle group or LPS + Celecoxib group as compared with the Saline + Vehicle group. # P < 0.05 represents a significant difference for the LPS + Celecoxib group as compared with the LPS + Vehicle group.
Celecoxib improved neurobehavioral deficits induced by LPS exposure
Compared with the control group, systemic LPS-injection on P5 rats resulted in a lower body weight at P6 (Fig. 1B). No gender differences in LPS-induced lower body weigh were observed in the rats. Celecoxib treatment did not reduce the LPS-induced weight reduction in rats, but significantly improved neuromuscular deficits following systemic LPS exposure (Fig. 1C). In the wire hanging maneuver test, the mean latency time of the LPS-injected group was significantly less than that of the control group at P6 (p < 0.05) (Fig. 1C). The reduction in wire hanging latency in the LPS + celecoxib group was much less prominent than in the LPS group (p < 0.05), and there was no difference in wire hanging maneuver between the control and the LPS + celecoxib groups (Fig. 1C).
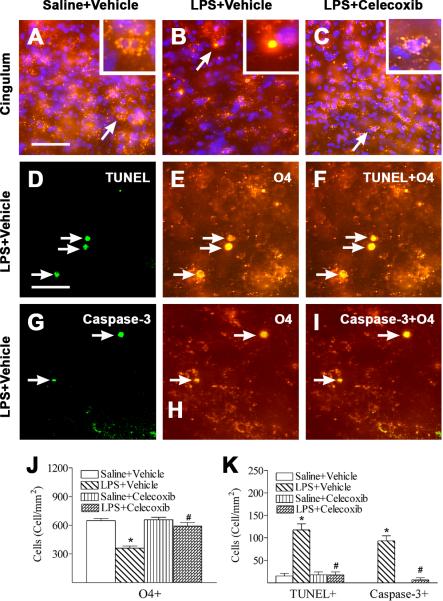
Celecoxib attenuated LPS-induced apoptotic cell death of OLs
In the present study, systemic LPS exposure (i.p.) resulted in similar brain injury induced by intracerebral (i.c.) LPS injection as we reported previously (Fan et al., 2008b, 2008c). No gender differences in LPS-induced brain injury were observed in the P6 rat brain. Late OL progenitor cells (O4+/O1-) are the main OL population in the P6 rat brain (Fan et al., 2005a; Follett et al., 2000). Abundant O4 positive cells, which had positive staining primarily localized at the cell membrane and processes, were observed in the P6 control rat brain (Fig. 2A, red), mostly in the subcortical white matter. DAPI was used simultaneously to identify nuclei in the final visualization (blue) (A, B, and C). LPS-induced injury to developing OLs was primarily found in the cingulum area (Fig. 2B, red). LPS treatment resulted in pyknotic O4 positive cells in this area (Fig. 2B, red, arrows indicated). These pyknotic cells displayed acute degeneration features that included apparent condensation of the cell body and the appearance of O4 immunoreactivity at the whole shrunken cell body (see insert in Fig. 2B, red). Celecoxib treatment reduced the number of pyknotic OLs (Fig. 2C). As compared to the control group, LPS significantly reduced the number of O4 positive cells in the cingulum area of P6 rat brain (P < 0.05) (Fig. 2J). Celecoxib treatment significantly attenuated the LPS-induced loss of O4 positive cells in the P6 rat brain (P < 0.05) (Fig. 2J).
Fig. 2.
Representative photomicrographs of O4 immunostaining (A to C, E and H) and cell death (D and G) in rat brain 24 hours (P6) after LPS injection. Abundant O4 positive cells (red), which had positive staining at the cell membrane and processes, were found in the cingulum white matter of the brain sections at the bregma level in P6 control rat brains (A). DAPI (blue) was used simultaneously to identify nuclei in the final visualization (A, B, and C). LPS injection resulted in pyknotic O4 cells with condensed cell body and immunoreactivity in the cytoplasm (arrow indicated in B, red) and reduced the number of normal O4 positive cells. Inserts in A, B and C are magnified parts indicated by the white boxes. Celecoxib attenuated LPS-induced loss of O4 positive cells (C). Many TUNEL positive cells (green) (D, arrows indicated) were observed in the LPS-injected rat brain (D), and significantly fewer TUNEL positive cells were observed in the control (K) or the LPS + celecoxib rat brain (K). Double-labeling (yellow) showed that many TUNEL positive cells (F) were O4 positive cells (E). F is a merged image of D and E. Double-labeling also showed that many caspase-3 positive cells (green) (G, arrows indicated) in the LPS-injected rat brain were O4 positive cells (red) (H, arrows indicated). I (yellow) is a merged image of G and H. The scale bar shown in A represents 50 μm for A to C, or shown in D represents 25 μm for D to I. Quantitation of the O4 positive cells (J), TUNEL positive cells and caspase-3 positive cells (K) was performed as described in Methods. The results are expressed as the mean ± SEM of six animals in each group, and analyzed by one-way ANOVA. * P < 0.05 represents a significant difference for the LPS + Vehicle group or LPS + Celecoxib group as compared with the Saline + Vehicle group. # P < 0.05 represents a significant difference for the LPS + Celecoxib group as compared with the LPS + Vehicle group.
LPS-induced apoptotic OL cell death was demonstrated by TUNEL and caspase-3 staining. Double-labeling showed that many TUNEL positive (Fig. 2D) or caspase-3 positive cells (Fig. 2G) were also O4 positive (Figs. 2E and 2H), indicating that apoptosis was involved in LPS-induced OL cell death. Few TUNEL positive cells were found in the control rat brain (Fig. 2K). An increased number of TUNEL positive cells was observed primarily at the periventricular areas 24 h after the LPS injection (P < 0.05) (Fig. 2K). Celecoxib treatment effectively prevented the LPS-induced increase in the number of TUNEL positive (P < 0.05) (Fig. 2K) or caspase-3 positive O4 cells (P < 0.05) (Fig. 2K).
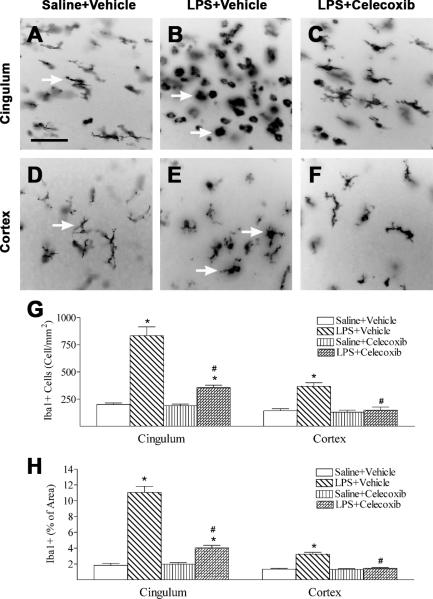
Celecoxib decreased the LPS-induced increase in microglial activation and inflammatory responses
LPS treatment caused activation of microglia in the rat brain as indicated by Iba1 immunostaining (Figs. 3B, 3E, 3G and 3H). In the control rat brain, a few Iba1 positive cells were detected and most of those cells were in resting status with a ramified shape (arrows indicated in Figs. 3A and 3D). Significantly increased numbers of activated microglia showing bright staining of an elongated or a round shaped cell body with blunt or no processes were found in the white matter in cingulum area and cortex (arrows indicated in Figs 3B and 3E) of the rat brain 24 hours after LPS injection (p < 0.05) (Fig. 3G). Iba1 staining was also quantified by measuring the percentage area that contains Iba1 immunostaining in the captured images. A higher percentage of Iba1 immunostaining area was observed in the cingulum area and cortex of the neonatal LPS-exposed rat brain (Fig. 3H). Celecoxib treatment reduced the number of activated microglia and percentage of Iba1 immunostaining area following LPS injection (p < 0.05) (Figs. 3C, 3F, 3G and 3H).
Fig. 3.
Representative photomicrographs of microglia (A to C, cingulum area; D to F, cortex) in the rat brain 24 hours (P6) after LPS injection. As shown by Iba1 immunostaining in the cingulum white matter (A) and cortex (D), a few microglia at the resting status with a small rod shaped soma and ramified processes (arrows indicated in A and D) were found in the control rat brain. Numerous activated microglia showing bright staining of an elongated or a round shaped cell body with blunt or no processes (arrows indicated in B and E) were observed in the cingulum white matter (B) and cortex (E) of the rat brain with neonatal LPS exposure. Celecoxib treatment reduced the number of activated microglia stimulated by LPS in the above areas (C, F and G). The scale bar shown in A represents 50 μm for A to F. Quantitation of the number of Iba1 positive cells (G) and the percentage area of image that contained Iba1 staining (H) in the cingulum white matter and cortex was performed as described in Methods. The results are expressed as the mean ± SEM of six animals in each group, and analyzed by one-way ANOVA. * P < 0.05 represents a significant difference for the LPS + Vehicle group or LPS + Celecoxib group as compared with the Saline + Vehicle group. # P < 0.05 represents a significant difference for the LPS + Celecoxib group as compared with the LPS + Vehicle group.
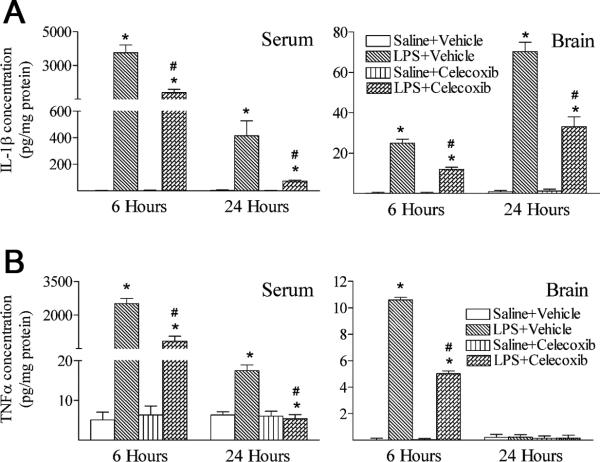
Systemic exposure to LPS resulted in inflammatory responses in the rat brain, as evidenced by the immediate elevation of two major proinflammatory cytokine levels (Fig. 4A, IL-1β and Fig. 4B, TNFα). Six hours following LPS injection, IL-1β and TNFα concentrations in the serum of LPS-exposed rats were dramatically increased as compared to those in the saline injected rats (p < 0.05) (Figs. 4A and 4B). IL-1β and TNFα concentrations in the LPS-exposed rat brain were also significantly increased as compared to those in the control rat brain (p < 0.05) (Figs. 4A and 4B). As shown in Figs. 4A and 4B, concentrations of IL-1β and TNFα in the serum of LPS-exposed rats were still significantly higher than those in the control rats 24 hours following LPS injection (p < 0.05) (Figs. 4A and 4B). The concentration of TNFα in the rat brain returned to the control level 24 hours after the LPS exposure (Fig. 4B). However, IL-1β concentration in the LPS-exposed rat brain remained elevated at 24 hours (p < 0.05) (Fig. 4A). Treatment with celecoxib attenuated induction of IL-1β and TNFα contents by LPS (p < 0.05) (Figs. 4A and 4B).
Fig. 4.
Celecoxib attenuated systemic LPS-stimulated increases in inflammatory cytokines (A, IL-1β and B, TNFα) in the rat brain 6 hours or 24 hours after LPS injection. A, IL-1β and B, TNFα concentrations were determined by ELISA kit and presented in the unit of pg/mg protein, as described in Methods. Six hours following LPS injection, serum and brain levels of IL-1β (A) and TNFα (B) were elevated as compared with the Saline + Vehicle group. Twenty-four hours following LPS injection, serum levels of IL-1β (A) and TNFα (B) were still elevated as compared with the Saline + Vehicle group. The concentration of TNFα in the rat brain returned to the control level 24 hours after the LPS exposure (right panel of B), but IL-1β concentration in the LPS-exposed rat brain remained increased as compare with that in the control rat brain (right panel of A). Treatment with celecoxib attenuated induction of IL-1β and TNFα contents by LPS. The results are expressed as the mean ± SEM of six animals in each group, and analyzed by oneway ANOVA. * P < 0.05 represents a significant difference for the LPS + Vehicle group or LPS + Celecoxib group as compared with the Saline+Vehicle group. # P < 0.05 represents a significant difference for the LPS + Celecoxib group as compared with the LPS + Vehicle group.
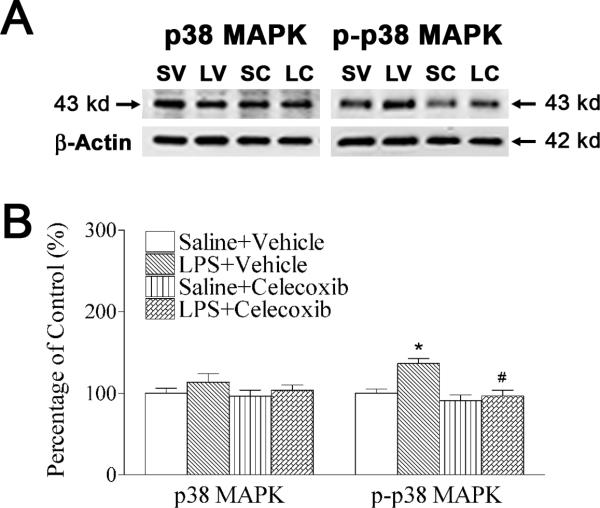
As a downstream event of IL-1 signaling, p38 MAPK plays important roles in dopaminergic neuron death in animal models of Parkinson's disease (Ruano et al., 2006). In the current study, p-P38 MAPK, but not p38 MAPK expression was significantly increased in P6 LPS-exposed rat brain (Figs. 5A and 5B), suggesting that p38 MAPK in the brain might be functioning abnormally following neonatal LPS exposure. Celecoxib reduced LPS-induced increments in the phosphorylation of p38 MAPK (p-p38 MAPK expression) (p < 0.05) (Figs. 5A and 5B).
Fig. 5.
Celecoxib attenuated systemic LPS-stimulated increases in phosphorylation of p38 MAPK in the rat brain 24 hours after LPS injection. A, western blotting of protein expression of p38 MAPK and p-p38 MAPK in P6 rat brain. B, expression of p38 MAPK (left panel of B) and p-p38 MAPK (right panel of B) is presented as the percentage of expression in the control group (Saline + Vehicle). LPS exposure increased phosphorylation of p38 MAPK (p-p38 MAPK expression), but not p38 MAPK expression in P6 rat brain. Celecoxib treatment attenuated the LPS-induced increase in expression of p-p38 MAPK expression in the P6 rats. The results are expressed as the mean ± SEM of six animals in each group, and analyzed by one-way ANOVA. * P < 0.05 represents a significant difference for the LPS + Vehicle group as compared with the Saline + Vehicle group. # P < 0.05 represents a significant difference for the LPS + Celecoxib group as compared with the LPS + Vehicle group.
Celecoxib decreased the LPS-induced increase in astrocyte activation and COX-2 expression
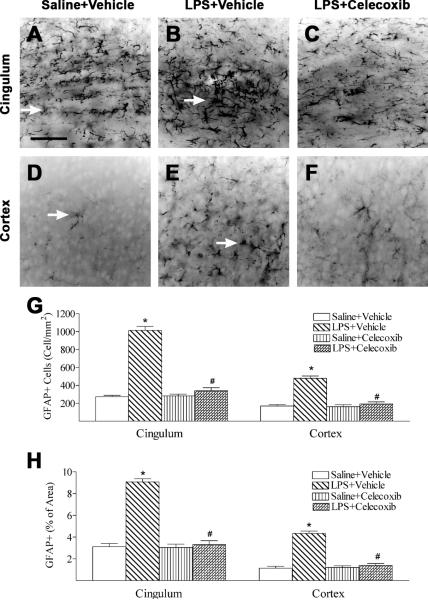
Development of the hypertrophic morphology and upregulation of intermediate filament proteins, in particular GFAP by reactive astrocytes are perhaps the best known hallmarks of reactive astrocytes and reactive gliosis. (McGeer and McGeer, 2008; Pekny and Nilsson, 2005). Increased expression of GFAP, an indication of astrogliosis, was observed at the cingulum area (Fig. 6B) and cortex (Fig. 6E) in the systemic LPS-exposed group 24 h after the injection (Fig. 6G). In the control rat brain, some GFAP positive cells were detected and most of those cells were in resting status with fine processes extending from the main cellular processes (arrows indicated in Figs. 6A and 6D). Significantly increased numbers of reactive astrocytes showing hypertrophy of cellular processes of astrocytes (GFAP+ cells) were found in the cingulum area (arrows indicated in Fig 6B) and cortex (arrows indicated in Fig. 6E) of the rat brain 24 h after LPS injection (p < 0.05) (Fig. 6G). GFAP staining was also quantified by measuring the percentage area that contains GFAP immunostaining in the captured images. A higher percentage of GFAP immunostaining area was observed in the cingulum area and cortex of the neonatal LPS-exposed rat brain (Fig. 6H). Celecoxib treatment reduced the number of activated astrocytes and percentage of GFAP immunostaining area following LPS injection (p < 0.05) (Figs. 6C, 6F, 6G and 6H).
Fig. 6.
Representative photomicrographs of astrocytes (A to C, cingulum area; D to F, cortex) in the rat brain 24 hours (P6) after LPS injection. As shown by GFAP immunostaining in the cingulum white matter (A) and cortex (D), some GFAP positive cells were detected and most of those cells were in resting status with fine processes extending from the main cellular processes (arrows indicated A and D) in the control rat brain. Significantly increased numbers of reactive astrocytes showing hypertrophy of cellular processes of astrocytes (arrows indicated in B and E) were observed in the cingulum white matter (B) and cortex (E) of the rat brain following neonatal LPS exposure. Celecoxib treatment reduced the number of reactive astrocytes stimulated by LPS in the above areas (C, F and G). The scale bar shown in A represents 50 μm for A to F. Quantitation of the number of GFAP positive cells (G) and the percentage area of image that contained GFAP staining (H) in the cingulum white matter and cortex was performed as described in Methods. The results are expressed as the mean ± SEM of six animals in each group, and analyzed by one-way ANOVA. * P < 0.05 represents a significant difference for the LPS + Vehicle group as compared with the Saline + Vehicle group. # P < 0.05 represents a significant difference for the LPS + Celecoxib group as compared with the LPS + Vehicle group.
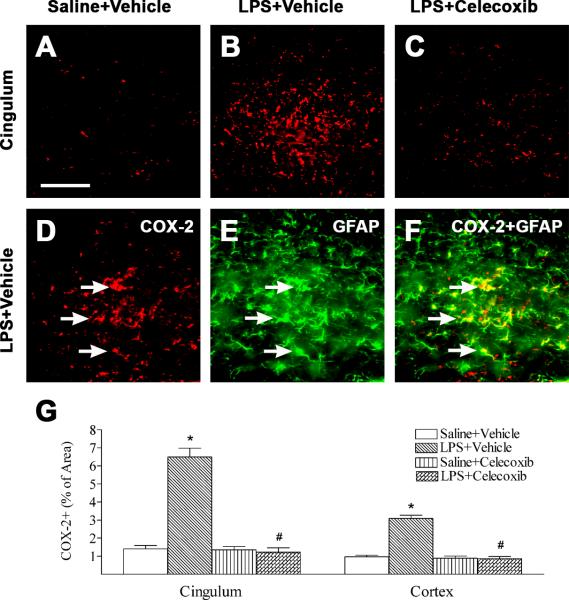
Neonatal systemic LPS-induced inflammatory responses also can be indicated by the increase in the number of COX-2 positive cells in the rat brain (Figs. 7B, 7D and 7G) as compared to that in the control rat brain (Figs. 7A and 7G). Double staining showed that most COX-2 positive cells in the cingulum were GFAP positive cells (Figs. 7E and 7F). There were few COX-2 positive cells that were Iba1 expressing microglia (data not shown). Treatment with celecoxib reduced the percentage of COX-2 immunostaining area following LPS injection (p < 0.05) (Figs. 7C and 7G).
Fig. 7.
Representative photomicrographs of COX-2 immunostaining (A to C, D, cingulum) in rat brain 24 hours (P6) after LPS injection. Weak COX-2 positive staining was detectable in the brain sections of control brain (A). LPS injection increased the expression of inducible cyclooxygenase, as indicated by COX-2 positive staining, in cingulum (B, D and G, red) and cortex (G) of P6 rat brain. Celecoxib treatment attenuated the LPS-increased COX-2 positive staining in the above areas (C and G). Double-labeling (yellow) showed that many COX-2 positive cells (F) were GFAP+ cells (astrocytes) (E, green). F is a merged image of D and E. The scale bar in A represents 50 μm for A to F. Quantitation of the percentage area of image that contained COX-2 positive staining in the cingulum white matter and cortex was performed as described in Methods. The results are expressed as the mean ± SEM of six animals in each group, and analyzed by one-way ANOVA. * P < 0.05 represents a significant difference for the LPS + Vehicle group as compared with the Saline + Vehicle group. # P < 0.05 represents a significant difference for the LPS + Celecoxib group as compared with the LPS + Vehicle group.
DISCUSSION
The present results indicate that, similar to i.c. LPS injection, systemic exposure to LPS through i.p. injection in the neonatal rats may cause brain inflammatory responses and brain injury, such as increments in the number of activated microglia and the elevated concentrations of IL-1β and TNFα, and apoptotic death of OLs (Cai et al., 2003; Fan et al., 2005a, 2005b, 2008a, 2008b, 2008c). LPS has been detected in the amniotic fluid (Romero et al., 1987). It is possible that LPS may reach the fetal brain during maternal infection. Our preliminary study has found that endogenous LPS concentration in the rat brain was very low, but it quickly increased to the range of 50-90 pg/mg protein in the rat brain 2-24 hr after i.p. injection of LPS (2 mg/kg), suggesting that peripherally administered LPS can reach the neonatal rat brain (Cai et al., 2012). Therefore, the systemically administered LPS can penetrate into the neonatal rat brain and cause inflammation and brain injury.
The present data show that neonatal systemic LPS treatment resulted in general physical effects, as indicated by the loss of body weight, hypothermia, and a deficit in wire hanging maneuver in the neonatal rat (Fig. 1). Neonatal systemic LPS (2 mg/kg) exposure caused hypothermia (< 32.0°C) from 2 to 5 hours after LPS injection in the neonatal rat (Fig. 1A). It has been reported that a low dose of systemic LPS induces fever in rats, but hypothermia may precede the polyphasic fever when the dose of LPS is high (> 1 mg/kg) (Romanovsky et al., 1996; Steiner et al., 2009). LPS-induced hypothermia may result from LPS-promoted vasodilation, decreased huddling behavior, increased heat losses, and reduced thermogenesis (Garrett-Cox et al., 2003). Celecoxib treatment significantly protects against LPS-induced hypothermia from 2 to 4 hours after LPS injection in rats (Fig. 1A). The inhibition of hypothermic response to systemic LPS exposure may be associated with the inhibition of COX-induced hypotension (Steiner et al., 2009). Hypothermia is neuroprotective after hypoxia-ischemia in the neonatal rats (Xiong et al., 2012) and therapeutic hypothermia has been used to treat newborn with hypoxic ischemic encephalopathy (Gancia and Pomero, 2012). Other studies have indicated that low dose LPS preconditioning may provide potential therapies against hypoxic ischemic brain injury in neonatal rats (Lin et al., 2009, 2010). Taken together, systemic LPS preconditioning-induced hypothermia may associate with the possible neuroprotective mechanism after hypoxic ischemic brain injury in the neonates.
Systemic LPS exposure resulted in acute brain injury, as indicated by increases in caspase-3 activity and apoptotic cell death in the neonatal rat cingulum white matter and the decreased immunoreactivity of O4 OLs, some of which are TUNEL positive apoptotic cells (Fig. 2). Treatments with celecoxib significantly reduced systemic LPS-induced cingulum white matter damage (Fig. 2). The LPS-induced increments of TUNEL positive apoptotic cells were also found in the cortex and striatum of neonatal brain 24 hr after LPS exposure. In our preliminary study, we also found that neonatal systemic LPS exposure resulted in dopaminergic neuronal damage, as indicated by a decrease in the number of tyrosine hydroxylase positive cells in substantia nigra of neonatal rats (A. Kaizaki, unpublished data). Therefore, systemic LPS exposure-induced central brain injury is not limited to brain white matter, but also involves other brain areas, suggesting further studies are needed.
Neonatal systemic LPS exposure resulted in brain inflammatory responses in the rat, as indicated by an increased number of activated microglia (Fig. 3), and elevated IL-1β and TNFα concentrations in the LPS-treated rat brain (Fig. 4). Microglia, the major resident immune cells in the brain, have been identified as the major LPS-responsive cells in the CNS (Lehnardt et al., 2002). The largest population of newborn microglia emerges in late gestation and early postnatal period in both humans and rats (Pont-Lezica et al., 2011; Harry and Kraft, 2012). Therefore, the LPS exposure in the neonatal rat brain (P5, relevant to human intrauterine infection in late gestation) can dramatically induce brain inflammatory responses. In the present study, systemic exposure to LPS resulted in immediate elevation of two major proinflammatory cytokine levels, IL-1β and TNFα (Figs. 4A and 4B), and increments in the phosphorylation of p38 MAPK (p-p38 MAPK expression), a downstream event of IL-1 signaling (Fig. 5). It suggested that p38 MAPK in the brain might be functioning abnormally following neonatal LPS exposure. The present study shows that treatment with a selective COX-2 inhibitor, celecoxib, provides anti-inflammatory effects as evidenced by the attenuation of LPS-induced increments in the number of activated microglia, the concentrations of IL-1β and TNFα, and protein levels of phosphorylated-p38 MAPK in the neonatal rat brain.
In the brain cells, COX-1 is detected in microglial cells, while COX-2 is found in neuronal and glial cells and dramatically up-regulated during inflammatory processes (Esposito et al., 2007). Systemic neonatal LPS exposure induced the expression of COX-2 in cells which were double labeled with GFAP positive (astrocyte) cells in the neonatal rat brain (Fig. 7). Reactive astrocytes usually appear not to attack a pathological target, as do microglia, but to wall it off they form a syncytium of interconnected cells both in health and in diseases (McGeer and McGeer, 2008; Pekny and Nilsson, 2005). Astrocytes produce both proinflammatory and anti-inflammatory responses, such as: they might be stimulating the microglia but at the same time secreting protective factors in the peripheral area (McGeer and McGeer, 2008). Interaction of microglia with apoptotic cells has been reported to selectively promote COX-2 expression, and COX-2 may mediate microglial activation and may play a key role in amplifying the inflammatory response with toxic effects (Bartels and Leenders, 2010; De Simone et al., 2004). Treatments of celecoxib affected LPS-induced astrogliosis (Fig. 6) and reduced the number of GFAP positive and COX-2 positive double labeled cells in the LPS-exposured rat brain (Fig. 7).
It has been suggested that COX-2 is associated with various inflammatory settings and involved in neurodegenerative processes, such as multiple sclerosis, amyotrophic lateral sclerosis, Parkinson's disease, Creutzfeldt-Jakob disease, and Alzheimer's disease (Bartels and Leenders, 2010; Minghetti, 2004). COX-2 is mainly induced in response to inflammatory stimuli and selective inhibition of COX-2 can reduce inflammation without affecting the physiological functions of COX-1-derived prostaglandins (Choi et al., 2009a). Celecoxib is one of the selective COX-2 inhibitors currently being used in the treatment of various pains and inflammatory conditions and is the safest COX-2 inhibitor relating to the cardiovascular safety data (Jones, 2005). Celecoxib can decrease the phosphorylation state of p38 and p44/42 of mitogen-activated protein kinase (MAPK) in human osteoarthritic chondrocytes (Takahashi et al., 2005). The present results also indicated that celecoxib attenuated of LPS-induced increments in the protein levels of phosphorylated-p38 MAPK in the neonatal rat brain. The neuroprotective action of celecoxib has been observed in the LPS-induced nigrostriatal neurodegeneration (Hunter et al., 2007) and 6-hydroxydopamine (6-OHDA)-induced progressive dopamine neuron degeneration in a rat model of Parkinson's disease (Sanchez-Pernaute et al., 2004). The neuroprotective effect of celecoxib may result from its direct COX-2 inhibition properties (Bartels and Leenders, 2010; Minghetti, 2004). Besides celecoxib, the protecting effect of other COX-2 inhibitors also needs to be further investigated. COX-2 is primarily responsible for inflammatory stimuli, however, the expression of COX-2 may contribute to fundamental brain functions in the CNS (Bartels and Leenders, 2010; Minghetti, 2004). In addition, COX-2 activity modulates matrix metalloproteinase-9 (MMP-9) and matrix metalloproteinase-3 (MMP-3) activities and is necessary to maintain blood–brain barrier (BBB) integrity during LPS exposure in mice (Choi et al., 2009b). Therefore, the possible side effects of COX-2 inhibitors should be also considered in the further study.
In summary, celecoxib attenuates systemic LPS-induced injury to developing OLs in the neonatal rat brain. The protection of celecoxib can be attributed to its ability to reduce systemic LPS-induced inflammatory responses, as indicated by inhibition of LPS induced increments in the number of activated microglia and astrocytes, and elevated concentrations of IL-1β and TNFα, increased protein levels of phosphorylated-p38 MAPK, and expression of COX-2 in the neonatal rat brain.
Systemic LPS resulted in an impairment of behavioral performance in neonatal rats
Systemic LPS caused brain inflammation and brain damage in neonatal rats
Celecoxib attenuated systemic LPS-induced behavioral impairments in neonatal rats
Celecoxib reduced systemic LPS-induced brain inflammation in neonatal rats
Celecoxib reduced systemic LPS-induced brain damage in neonatal rats
Acknowledgements
This work was supported by a NIH grant NS 54278, Newborn Medicine Funds from the Department of Pediatrics, University of Mississippi Medical Center, Jackson, Mississippi.
Abbreviations
- CNS
central nervous system
- COX-2
cyclooxygenase-2
- DAPI
4′,6-Diamidino-2-phenylindole
- ELISA
enzyme-linked immunosorbent assay
- GFAP
glial fibrillary acidic protein
- Iba1
ionized calcium binding adapter molecule 1
- IL1-β
interleukin 1-β
- i.c.
intracerebral
- i.p.
intraperitoneal
- LPS
lipopolysaccharide
- O4
late oligodendrocyte progenitor cell marker O4
- OL
oligodendrocyte
- PVL
periventricular leukomalacia
- P5
postnatal day 5
- p38 MAPK
p38 mitogen-activated protein kinase
- p-p38 MAPK
phospho-p38 mitogen-activated protein kinase
- TNFα
tumor necrosis factor-α
- TUNEL
terminal deoxynucleotidyl transferase (TdT)-mediated uridine 5′-triphosphate-biotin nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975;23:896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels AL, Leenders KL. Cyclooxygenase and neuroinflammation in Parkinson's disease neurodegeneration. Curr Neuropharmacol. 2010;8:62–68. doi: 10.2174/157015910790909485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Fan LW, Kaizaki A, Tien LT, Ma T, Pang Y, Lin S, Lin RCS, Simpson KL. Neonatal systemic exposure to lipopolysaccharide enhances vulnerability of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Dev Neurosci. 2012 doi: 10.1159/000346156. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- Choi SH, Aid S, Bosetti F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol Sci. 2009a;30:174–181. doi: 10.1016/j.tips.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Aid S, Choi U, Bosetti F. Cyclooxygenases-1 and -2 differentially modulate leukocyte recruitment into the inflamed brain. Pharmacogenomics J. 2009b;10:448–457. doi: 10.1038/tpj.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha NV, de Abreu SB, Panis C, Grassiolli S, Guarnier FA, Cecchini R, Mazzuco TL, Pinge-Filho P, Martins-Pinge MC. COX-2 inhibition attenuates cardiovascular and inflammatory aspects in monosodium glutamate-induced obese rats. Life Sci. 2010;87:375–381. doi: 10.1016/j.lfs.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Inflammatory brain damage in preterm newborns--dry numbers, wet lab, and causal inferences. Early Hum Dev. 2004;79:1–15. doi: 10.1016/j.earlhumdev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- De Simone R, Ajmone-Cat MA, Minghetti L. Atypical antiinflammatory activation of microglia induced by apoptotic neurons: possible role of phosphatidylserinephosphatidylserine receptor interaction. Mol Neurobiol. 2004;29:197–212. doi: 10.1385/MN:29:2:197. [DOI] [PubMed] [Google Scholar]
- Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson's disease. Exp Neurol. 2007;205:295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Fan LW, Chen RF, Mitchell HJ, Lin RCS, Simpson KL, Rhodes PG, Cai Z. α-Phenyl-n-tert-butyl-nitrone attenuates lipopolysaccharide-induced brain injury and improves neurological reflexes and early sensorimotor behavioral performance in juvenile rats. J Neurosci Res. 2008a;86:3536–3547. doi: 10.1002/jnr.21812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Rhodes PG, Cai Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience. 2005a;133:1359–1368. doi: 10.1016/j.neuroscience.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Fan LW, Michell HJ, Rhodes PG, Cai Z. Alpha-phenyl-n-tert-butyl-nitrone attenuates lipopolysaccharide-induced neuronal injury in the neonatal rat brain. Neuroscience. 2008b;151:159–168. doi: 10.1016/j.neuroscience.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Mirchell HJ, Tien LT, Zheng B, Pang Y, Rhodes PG, Cai Z. Alpha-phenyl-n-tert-butyl-nitrone reduces lipopolysaccharide-induced white matter injury in the neonatal rat brain. Dev Neurol. 2008c;68:365–378. doi: 10.1002/dneu.20591. [DOI] [PubMed] [Google Scholar]
- Fan LW, Pang Y, Lin S, Tien LT, Ma T, Rhodes PG, Cai Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J Neurosci Res. 2005b;82:71–82. doi: 10.1002/jnr.20623. [DOI] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Lin RCS, Simpson KL, Rhodes PG, Cai Z. Neonatal exposure to lipopolysaccharide enhances vulnerability of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Neurobiol Dis. 2011a;44:304–316. doi: 10.1016/j.nbd.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LW, Tien LT, Zheng B, Pang Y, Lin RCS, Simpson KL, Ma T, Rhodes PG, Cai Z. Dopaminergic neuronal injury in the adult rat brain following neonatal exposure to lipopolysaccharide and the silent neurotoxicity. Brain Behev Immun. 2011b;25:286–297. doi: 10.1016/j.bbi.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett P, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancia P, Pomero G. Therapeutic hypothermia in the prevention of hypoxic-ischaemic encephalopathy: new categories to be enrolled. J Matern Fetal Neonatal Med Suppl. 2012;4:94–96. doi: 10.3109/14767058.2012.715023. [DOI] [PubMed] [Google Scholar]
- Garrett-Cox RG, Pierro A, Spitz L, Eaton S. Body temperature and heat production in suckling rat endotoxaemia: beneficial effects of glutamine. J Pediatr Surg. 2003;38:37–44. doi: 10.1053/jpsu.2003.50006. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- Hamdulay SS, Wang B, Birdsey GM, Ali F, Dumont O, Evans PC, Haskard DO, Wheeler-Jones CP, Mason JC. Celecoxib activates PI-3K/Akt and mitochondrial redox signaling to enhance heme oxygenase-1-mediated anti-inflammatory activity in vascular endothelium. Free Radic Biol Med. 2010;48:1013–1023. doi: 10.1016/j.freeradbiomed.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Harry GJ, Kraft AD. Microglia in the developing brain: a potential target with lifetime effects. Neurotoxicology. 2012;33:191–206. doi: 10.1016/j.neuro.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans RH, Hunter DE, McGivern RF, Cain CD, Longo LD. Behavioral sequelae in young rats of acute intermittent antenatal hypoxia. Neurotoxicol Teratol. 1992;14:119–129. doi: 10.1016/0892-0362(92)90060-n. [DOI] [PubMed] [Google Scholar]
- Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- Jones SC. Relative thromboembolic risks associated with COX-2 inhibitors. Ann Pharmacother. 2005;39:1249–1259. doi: 10.1345/aph.1E654. [DOI] [PubMed] [Google Scholar]
- Kadhim H, Tabarki B, De Prez C, Sebire G. Cytokine immunoreactivity in cortical and subcortical neurons in periventricular leukomalacia: are cytokines implicated in neuronal dysfunction in cerebral palsy? Acta Neuropathol (Berl) 2003;105:209–216. doi: 10.1007/s00401-002-0633-6. [DOI] [PubMed] [Google Scholar]
- Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sebire G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56:1278–1284. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett P, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The Toll-like receptor TLP4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Huang CC, Chang KF. Lipopolysaccharide preconditioning reduces neuroinflammation against hypoxic ischemia and provides long-term outcome of neuroprotection in neonatal rat. Pediatr Res. 2009;66:254–259. doi: 10.1203/PDR.0b013e3181b0d336. [DOI] [PubMed] [Google Scholar]
- Lin HY, Wu CL, Huang CC. The Akt-endothelial nitric oxide synthase pathway in lipopolysaccharide preconditioning-induced hypoxic-ischemic tolerance in the neonatal rat brain. Stroke. 2010;41:1543–1551. doi: 10.1161/STROKEAHA.109.574004. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–910. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Pont-Lezica L, Bechade C, Belarif-Cantaut Y, Pascual O, Bessis A. Physiological roles of microglia during development. J Neurochem. 2011;119:901–908. doi: 10.1111/j.1471-4159.2011.07504.x. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Dean A. Periventricular leukomalacia, inflammation and white matter lesions within the developing nervous system. Neuropathology. 2002;22:106–132. doi: 10.1046/j.1440-1789.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Kulchitsky VA, Akulich NV, Koulchitsky SV, Simons CT, Sessler DI, Gourine VN. First and second phases of biphasic fever: two sequential stages of the sickness syndrome? Am J Physiol. 1996;271:R244–R253. doi: 10.1152/ajpregu.1996.271.1.R244. [DOI] [PubMed] [Google Scholar]
- Romero R, Kadar N, Hobbins JC, Duff GW. Infection and labor: the detection of endotoxin in amniotic fluid. Am J Obstet Gynecol. 1987;157:815–819. doi: 10.1016/s0002-9378(87)80061-3. [DOI] [PubMed] [Google Scholar]
- Ruano D, Revilla E, Paz Gavilan M, Vizuete ML, Pintado C, Vitorica J, Castano A. Role of p38 and inducible nitric oxide synthase in the in vivo dopaminergic cells’ degeneration induced by inflammatory processes after lipopolysaccharide injection. Neuroscience. 2006;140:1157–1168. doi: 10.1016/j.neuroscience.2006.02.073. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, Isacson O. Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease. J Neuroinflammation. 2004;1:6. doi: 10.1186/1742-2094-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Bolli R, Rokosh GD, Bi Q, Dai S, Shirk G, Tang XL. The cardioprotection of the late phase of ischemic preconditioning is enhanced by postconditioning via a COX-2-mediated mechanism in conscious rats. Am J Physiol Heart Circ Physiol. 2007;293:H2557–H2564. doi: 10.1152/ajpheart.00858.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann C, Lassmann H. Detection of apoptosis in tissue sections. Cell Tissue Res. 2000;301:19–31. doi: 10.1007/s004410000203. [DOI] [PubMed] [Google Scholar]
- Steiner AA, Hunter JC, Phipps SM, Nucci TB, Oliveira DL, Roberts JL, Scheck AC, Simmons DL, Romanovsky AA. Cyclooxygenase-1 or -2--which one mediates lipopolysaccharide-induced hypothermia? Am J Physiol Regul Integr Comp Physiol. 2009;297:R485–R494. doi: 10.1152/ajpregu.91026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Ogawa Y, Kitaoka K, Tani T, Uemura Y, Taguchi H, Kobayashi T, Seguchi H, Yamamoto H, Yoshida S. Selective COX-2 inhibitor regulates the MAP kinase signaling pathway in human osteoarthritic chondrocytes after induction of nitric oxide. Int J Mol Med. 2005;15:213–219. [PubMed] [Google Scholar]
- Volpe JJ. Cerebral white matter injury of the premature infant--more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- Xiong M, Ma SM, Shao XM, Yang Y, Zhou WH. Hypoxic ischaemic hypothermia promotes neuronal differentiation and inhibits glial differentiation from newly generated cells in the SGZ of the neonatal rat brain. Neurosci Lett. 523:87–92. doi: 10.1016/j.neulet.2012.06.054. 1212. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997a;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Kim CJ, Koo JN, Choe G, Syn HC, Chi JG. High expression of tumor necrosis factor-α and interleukin-6 in periventricular leukomalacia. Am J Obstet Gynecol. 1997b;177:406–411. doi: 10.1016/s0002-9378(97)70206-0. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]