Abstract
Neurotrophic factors may play a role in exercise-induced neuroprotective effects, however it is not known if exercise mediates changes in glial cell line-derived neurotrophic factor (GDNF) protein levels in the spinal cord. The aim of the current study was to determine if 2 weeks of exercise alters GDNF protein content in the lumbar spinal cord of young and old rats. GDNF protein was quantified via an enzyme-linked immunosorbent assay and Western blot. Immunohistochemical analysis localized GDNF in choline acetyltransferase (ChAT)-positive motor neurons and cell body areas were measured. Involuntary running in the young animals appeared to elicit the greatest increase in GDNF protein content (6-fold increase), followed by swimming (3-fold increase) and voluntary running (2-fold increase); however there was no significant difference between the modalities of exercise. Low-intensity running of the old animals significantly increased GDNF protein content in the spinal cord. Both young and old exercised animals showed a doubling in ChAT-positive motor neuron cell body areas. These results suggest that GDNF protein content in spinal cord is modulated by exercise.
Keywords: Spinal Cord, Exercise, Aging, Neural Plasticity, GDNF, Motor Neuron
1. Introduction
A significant loss of skeletal muscle mass and strength are commonly observed in aging individuals (Kallman et al., 1990; Frontera et al., 2000) and contribute to an increased incidence of falls and disability (Fries et al., 1994; Toulotte et al., 2003). Changes with age are observed both in skeletal muscle and motor neurons innervating skeletal muscles. Alterations in motor neurons with increased age include loss of somatic motor neurons (Jacob, 1998) and loss of inputs to motor nerve cell bodies (Kullberg et al., 1998). In the aging rat there is a decrease in muscle innervation, loss of myelinated nerve fibers and changes in expression of neuropeptides and growth factors, similar to what is observed following axon lesion (Johnson et al., 1999). One possible contributing factor for the loss of motor neurons with age could be diminished neurotrophic factor signaling (Bergman et al., 1999).
Glial cell line-derived neurotrophic factor (GDNF) was first discovered in glial cells (Lin et al., 1993), and its expression has been found in a variety of tissues both in the central and peripheral nervous systems (Henderson et al., 1994; Suter-Crazzolara and Unsicker, 1994; Springer et al., 1995; Suzuki et al., 1998). To date, GDNF is the most potent survival factor identified for motor neurons (Henderson et al., 1994), where heterozygous GDNF knockout mice lack 22% of their lumbar motor neurons (Moore et al., 1996), and GDNF receptor alpha-1 (GFRα-1) knockout mice lack 24% of their lumbar motor neurons (Cacalano et al., 1998). One possible source of GDNF for somatic motor neurons is skeletal muscle, where GDNF is transported in a retrograde fashion (Yan et al., 1995; Trupp et al., 1997; Wang et al., 2002).
Increased expression of GDNF in developing skeletal muscle leads to increased axonal branching and increased motor unit size (Nguyen et al., 1998; Zwick et al., 2001), while treatment with exogenous GDNF causes continuous synaptic remodeling at the neuromuscular junction (Keller-Peck et al., 2001) and prevents motor neuron degeneration following axotomy (Oppenheim et al., 1995). GDNF increases choline acetyltransferase (ChAT) activity of embryonic motor neurons (Zurn et al., 1994), rescues somatic motor neurons from natural occurring cell death (Oppenheim et al., 2000) and from axotomy-induced cell death (Oppenheim et al., 1995), and protects motor neurons from chronic degeneration (Corse et al., 1999). Neurotrophic factors, such as brain derived neurotrophic factor (BDNF), insulin-like growth factor 1, and vascular endothelial growth factor (Wu et al., 2008; Trejo et al., 2001; Fabel et al., 2003) have been suggested to play a role in exercise-mediated neuroprotective effects, however it is not known if GDNF plays a similar role. While independent studies have found similar beneficial effects following exercise to those observed with exogenous treatment with GDNF, no one has been able to link the two together. One of the goals of our studies is to determine if the beneficial effects of exercise for the motor nervous system may, in part, be driven by changes in GDNF levels. Here, we report that short-term exercise increases GDNF protein content in the lumbar spinal cord of young (6-month-old) and old (24-month-old) rats, at the same time we observed morphological changes of motor neuron cell bodies.
2. Experimental Procedures
2.1 Subjects
All experiments were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council) and protocols were approved by the Institutional Animal Care and Use Committee at Western Michigan University. Male Sprague-Dawley rats (Charles River, Kalamazoo, MI) were given access to food and water ad libitum and were maintained on a 12h light/dark cycle. Rats were euthanized via CO2 asphyxiation followed by thoracotomy.
2.2 Training Protocol
We tested the effects of 2 weeks of exercise on GDNF protein content in the lumbar spinal cord of young (6-month-old) and old (24-month-old) animals. Two weeks of exercise was chosen as we have previously shown that this duration alters GDNF protein content in rat skeletal muscle (McCullough et al., 2011). The 6-month-old rats were randomly divided into four groups. One group was kept as sedentary controls (n=12). The remaining groups underwent different exercise protocols (swimming, voluntary running and involuntary running). The voluntary running group (n=6) had continuous access to individually housed running wheels, where activity was recorded with an activity wheel monitoring system (Lafayette Instruments, Lafayette, IN). The involuntary running group (n=5) were placed in individual forced running wheels (Lafayette Instruments). These animals underwent 5 bouts of 24 min of running plus 10 min of rest, at a pace of 10m/min (McCullough et al., 2011). Two hours of involuntary exercise was chosen to match the distance run by the voluntary running group. The swimming group (n=6) had 3 rats/barrel placed in water (35°C) and these animals swam for a total of 2 hours, with bouts of rest, to match the animals of the running groups. The 24-month-old rats were randomly divided into two groups, a voluntary running group (n=6), as this was the least stressful of our exercise protocols, and an age-matched sedentary control group (n=5). Aged animals reached a peak running speed of only 2m/min.
2.3 Tissue Processing
In order to minimize the number of animals used for this study, we selected different regions of the spinal cord from each animal to quantify and visualize GDNF protein. The L1 – L3 lumbar spinal cord region was chosen for quantification of GDNF protein content, as these motor neurons innervate the quadriceps, gluteus, adductor muscles, flexor muscles and extensor muscles, including the extensor hallucis longus and extensor digitorum longus, and the soleus (Nicolopoulous-Stournaras and Iles, 1983). Others have published GDNF protein content data from this region of the spinal cord (Tokumine et al., 2003). The lumbar spinal cord region of L4 – L5 was chosen to examine localization of GDNF protein, as these motor neurons innervate the muscles of the hamstrings, adductor muscles, flexor muscles, extensor muscles including the extensor hallucis longus and extensor digitorum longus, gastrocnemius and the soleus (Nicolopoulous-Stournaras and Iles, 1983). Others have utilized immunohistochemical techniques to localize GDNF in this lumbar spinal cord region (Tokumine et al., 2003). To determine GDNF protein content, lumbar spinal cord sections (L1–L3) were removed and frozen on dry ice and samples were subsequently dipped in liquid nitrogen and smashed into a fine powder. Sample processing buffer (0.55 M NaCl, 0.02 M NaH2PO4, 0.08 M Na2HPO4, 2 mM EDTA, 0.1 mM benzethonium chloride, 2 mM benzamidine, 20 KIU/ml aprotinin, 0.5% bovine serum albumin, and 0.05% Tween-20) was added and was homogenized on ice. Samples were centrifuged for 30 min at 4°C and supernatant was collected and stored at −80°C.
2.4 GDNF protein quantification
GDNF protein content was measured using an enzyme-linked immunosorbent assay (ELISA) as previously described (McCullough et al., 2011). Briefly, 96-well plates were incubated overnight at room temperature in a humidified chamber with a monoclonal antibody raised against GDNF (R&D Systems, Minneapolis, MN). The following day, plates were rinsed with wash buffer and blocked with phosphate buffered saline containing 1% bovine serum albumin and 5% sucrose for 1 hour at room temperature. Plates were rinsed with wash buffer and the GDNF standard (R&D Systems) or tissue supernatants were added to the wells. For each assay, a standard curve was calculated from the known GDNF standard concentration, ranging from 1000 – 2pg/ml. Following a 2 hour incubation at room temperature, the plates washed then incubated with biotinylated anti-GDNF secondary antibody (R&D Systems) for 2 hours at room temperature. The plates were then washed and coated with β-galactosidase conjugated to streptavidin (Molecular Probes, Eugene, OR) for 20 minutes at room temperature. The plates had a final wash and chlorophenol red-β-D-galactopyranoside (CPRG) substrate was added (in phosphate buffered saline + bovine serum albumin) and incubated until the color had developed.
2.5 Western Blot
Total protein content of the spinal cord samples were measured by a Pierce® BCA protein assay (Thermo Scientific, Rockford, IL) according to manufacturers specifications. Tissue samples were prepared for Western blot analysis of GDNF protein as previously described (Vianney and Spitsbergen, 2011). Briefly, protein extracts (20μg), a protein ladder (New England BioLabs, Ipswich, MA) and a loading control of α-Tubulin (Developmental Studies Hybridoma Bank, Iowa City, IA) were prepared with Laemmli 2X loading buffer and loaded into a 15% polyacrylamide gel. The gel was submerged and was run in separating buffer at different voltages followed by transfer to a polyvinylidene difluoride (PVDF; Invitrogen) membrane in tris-glycine buffer. The PVDF membrane was blocked with I-Block (Applied Biosystems, Foster City, CA) followed by overnight incubation with a primary antibody against GDNF (Santa Cruz Biotechnologies). The following day, the membrane was washed in buffer followed by incubation with a HRP-conjugated secondary antibody (ECL; GE Healthcare) in I-Blocking buffer. The ECL detection kit was used to detect the proteins and was visualized on BioMax XAR film (Kodak). ImageJ software was used to measure the relative density of GDNF bands and values were expressed as ratios of controls.
2.6 Immunohistochemistry
Lumbar spinal cord sections (L4–L5) were fixed in 4% paraformaldehyde overnight at 4°C and then washed in fresh phosphate buffered saline. Tissues were embedded in O.C.T. compound mounting medium, cut into 40μm transverse sections on a cryotome, and thaw mounted onto Histobond® slides (VWR International, Bridgeport, NJ). Slides were incubated overnight at 4°C with primary antibodies (1:200) of rabbit anti-GDNF (Santa Cruz Biotechnology, Santa Cruz, CA), (1:200) mouse anti-ChAT (Millipore, Temecula, CA), and (1:50) goat-anti GM130 (Santa Cruz Biotechnology) in phosphate buffered saline containing 1% bovine serum albumin and 0.1% triton X-100. Slides were then incubated with secondary antibodies (1:500) of donkey anti-mouse conjugated to Alexafluor 568, donkey anti-rabbit conjugated to Alexafluor 488, and donkey anti-goat conjugated to Alexafluor 568, for 2 hours at room temperature. Negative control slides had primary antibodies omitted. Slides were viewed with a Zeiss LSM 510 laser scanning confocal microscope and images were examined with the Zeiss LSM 5 Image Examiner program.
2.7 Measurement of motor neuron cell body size
Since somatic motor neurons stain positively for ChAT immunoreactivity (Wetts and Vaughn, 1996), ChAT-positive cells were measured in Lamina IX of the spinal cord from all animals. Twenty randomly selected motor neurons from the L4–L5 spinal cord levels were counted from each animal to determine cell body area. The cells that had a mid-section through the nucleus were examined. Cell body areas were determined with the Zeiss LSM 5 Image Examiner program.
2.8 Statistical Analysis
All data values are reported as mean ± the standard error of the mean (SEM). GDNF protein values are expressed as pg GDNF/mg of wet tissue weight. Data were analyzed using a one-way ANOVA and Tukey’s post-hoc comparison to test for differences between groups. p values ≤ 0.05 were considered as statistically significant.
Results
3.1 Short-term exercise increases GDNF protein in the lumbar spinal cord of 6-month-old rats
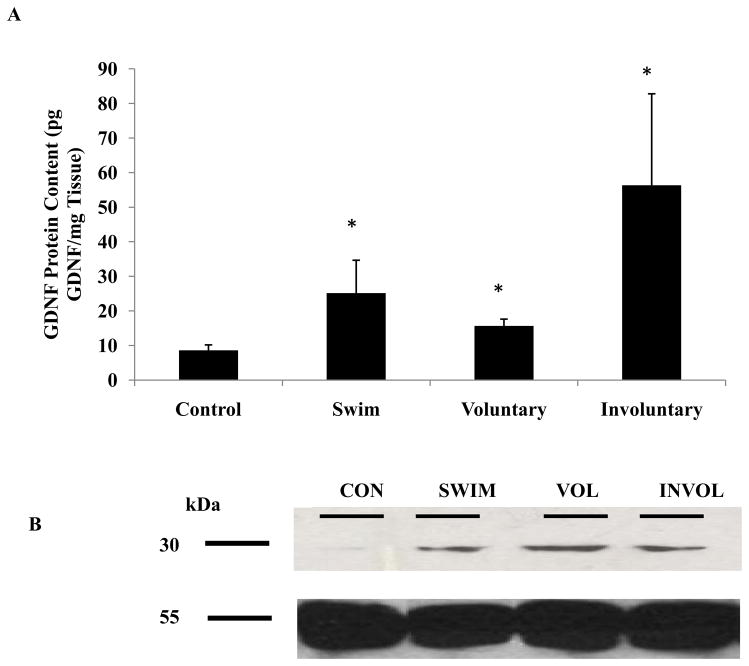
Six-month-old animals underwent voluntary running, involuntary running, or swimming for 2 weeks. Animal weights were lower in the pooled exercised animals (388.8g ±16.7g) compared to sedentary controls (401.9g ± 24.8g). The maximum running speed of the voluntary running group was 28m/min, whereas the involuntary running group was maintained at 10m/min. While the intensities were quite different between the two running groups, the average distance run per day was similar (Table 1). Two weeks of all modes of exercise significantly increased GDNF protein content in the lumbar spinal cord as compared to sedentary controls (8.6 ± 1.5pg GDNF/mg tissue weight). Involuntary running resulted in the greatest change in GDNF protein content in the lumbar spinal cord (56.3 ± 26.4pg GDNF/mg tissue weight), followed by swimming (25.1 ± 9.5pg GDNF/mg tissue weight) and voluntary running (15.7 ± 1.9pg GDNF/mg tissue weight) (Figure 1), however there were no significant differences between the exercise groups. The involuntary running group had a 6.5-fold increase of GDNF protein content as compared to controls, followed by a 2.9-fold increase from the swimming group and a 1.8-fold increase from the voluntary running group (Table 1).
Table 1.
Low-intensity, forced running (10m/min) elicited the greatest increase in GDNF protein content in spinal cord compared to other modalities of exercise. Changes in GDNF protein content in spinal cord, as detected by Western blot, show similar trends as those measured via ELISA. Old rats (24-month-old) ran a shorter distance and at a lower intensity than young (6-month-old) rats.
| Age of animals (Months) | Duration and type of exercise | Fold change of GDNF protein content (via ELISA) from controls | Fold change of GDNF protein content (via Western blot) from controls | Maximum Intensity of exercise (m/min) | Distance Run/day (m) |
|---|---|---|---|---|---|
| 6 | 2 Weeks Voluntary Running | 2 ↑ | 1.5 ↑ | 28 | 1434.9 ± 77.4 |
| 6 | 2 Weeks Involuntary Running | 6.5 ↑ | 3.5 ↑ | 10 | 1200 |
| 6 | 2 Weeks Swimming | 3 ↑ | 1.7 ↑ | N/A | N/A |
| 24 | 2 weeks Voluntary Running | 2 ↑ | 2 ↑ | 2 | 132.3 ± 86.6 |
Involuntary running elicits the largest increase of GDNF protein content in the rat spinal cord as measured via ELISA.
Figure 1.
Note: Staining for α-tubulin as a loading control was added to Panel B GDNF protein content was increased in the spinal cord after 2 weeks of exercise in 6-month-old rats. The lumbar spinal cord (L1–L3) was removed from control and exercised 6-month-old animals. (A) Tissues were processed for GDNF protein content using an ELISA. A significant increase in GDNF protein content was detected in the spinal cord of animals that had undergone 2 weeks of voluntary running, involuntary running and swimming as compared to sedentary control animals. Values are displayed as mean ± SEM. Asterisk (*) indicates significance (p≤0.05) from controls. (B) Tissues were processed for Western blot to determine GDNF protein content (top) and a loading control of α-tubulin (bottom). An increase in GDNF protein content was detected in the spinal cord of animals that had undergone 2 weeks of involuntary running, followed by swimming and then voluntary running as compared to controls.
Using Western blot analysis we found that GDNF had a molecular weight of 30kDa (Figure 1B), which is close to the 34kDa previously reported in human fetal spinal cord (Koo and Choi, 2001). Densitometry analysis of GDNF bands showed a 3.5-fold increase in GDNF expression for the involuntary runners, followed by a 1.7-fold increase for the swimmers and a 1.5-fold increase for the voluntary runners, which follows the same trend as our ELISA results (Table 1).
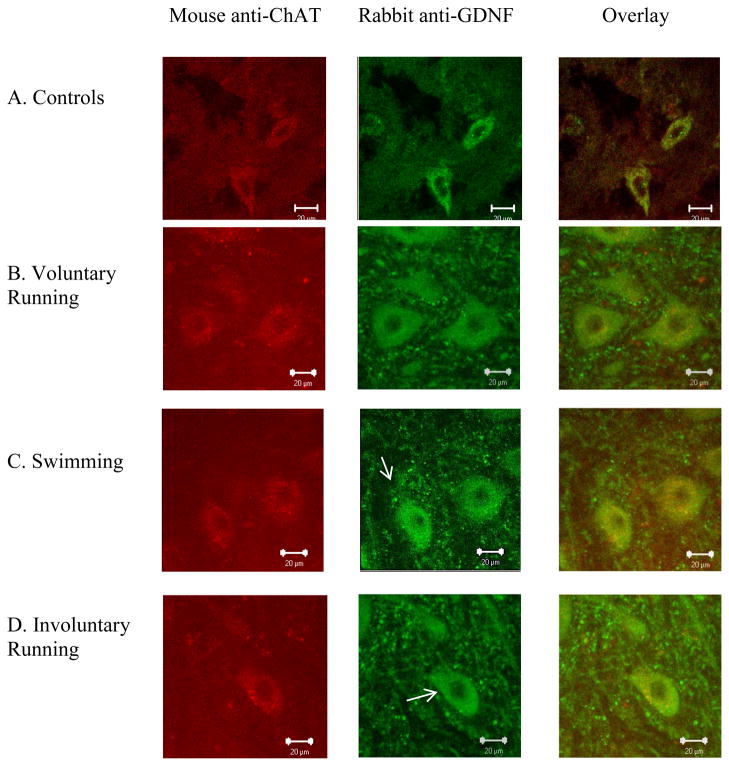
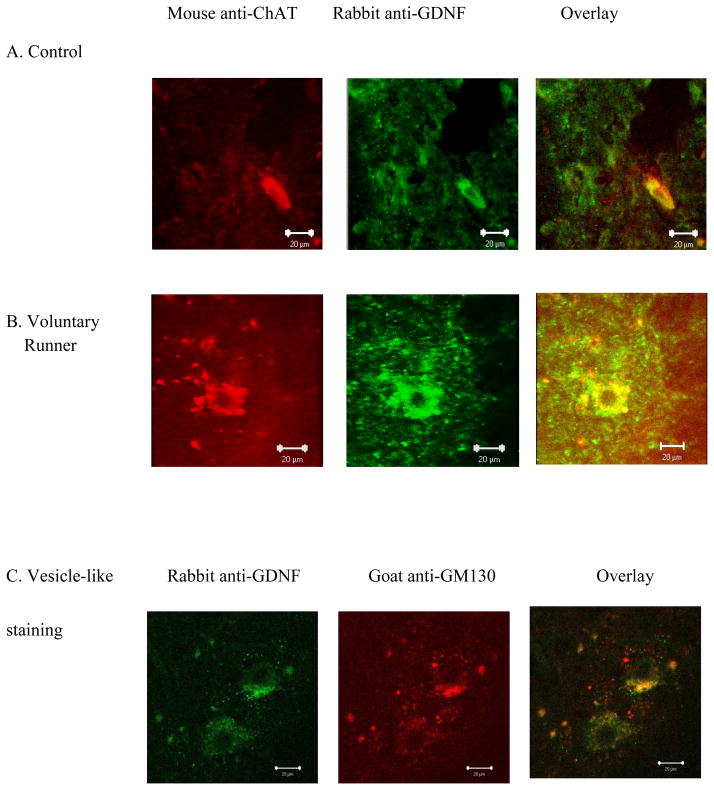
Positive immunoreactivity for GDNF was found in ChAT-positive cells, which are presumed to be motor neurons (Wetts and Vaughn, 1996), in the lumbar spinal cord from control and exercised animals (Figure 2). We observed more vesicle-like structures containing GDNF surrounding motor neurons in exercised rats (arrows in Figure 2B–D) compared to those from sedentary controls (Figure 2A).
Figure 2.
Exercise increased motor neuron size and vesicle-like structures of GDNF in 6-month-old rat spinal cord. Representative lumbar spinal cord sections from a 6-month-old sedentary control animal (A), voluntary exercised animal (B), swimming exercised animal (C) and an involuntary exercised animal (D). Spinal cord sections were immunolabeled with primary antibodies against ChAT (red) and GDNF (green). ChAT immunoreactivity is co-localized with GDNF immunoreactivity in the lumbar spinal cord. Exercised animals appeared to have more vesicle-like structures containing GDNF that surround the motor neurons (indicated by arrows), as compared to controls. The scale bar represents 20μm.
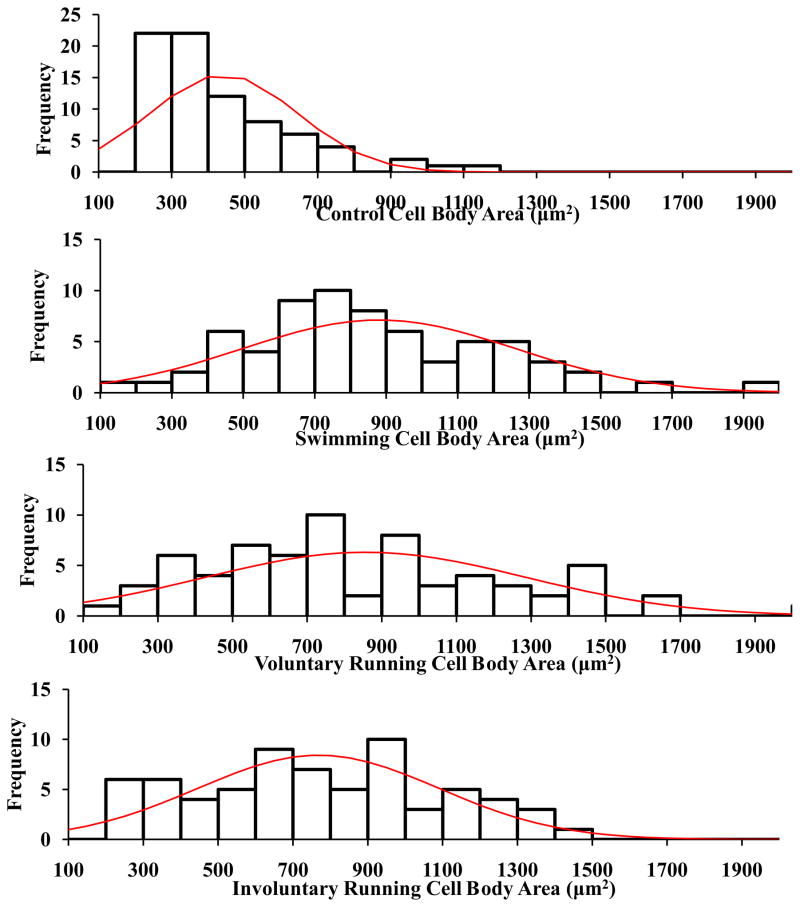
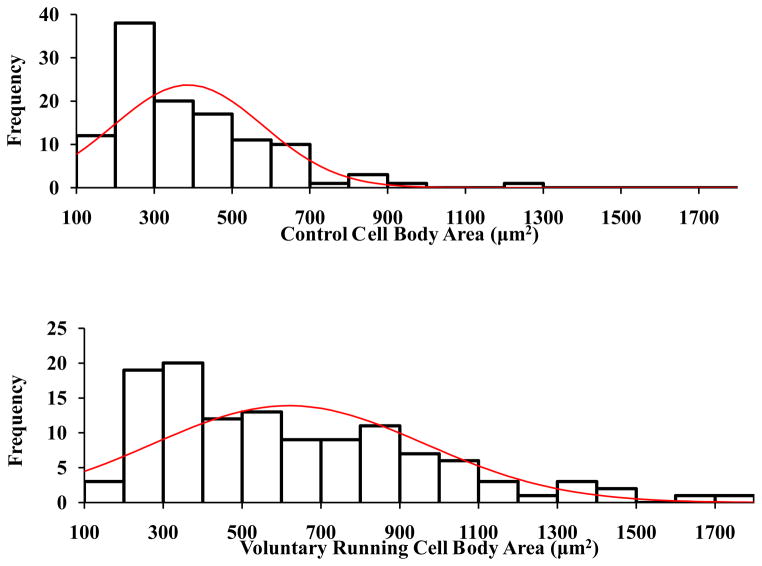
Analysis of motor neuron cell body areas revealed a significant increase following voluntary running (841.5 ± 49.6μm2), involuntary running (749.8 ±29.5μm2), and swimming (879.9 ± 46.2μm2), compared to that in controls (454.8 ± 25.6μm2). No significant differences were observed between exercise groups. Histogram analysis of motor neuron cell body areas displayed more occurrences of the large-sized motor neurons (>1500 μm2) belonging to the fast motor units (Deforges et al., 2009) among the voluntary and swimming groups than the involuntary running group and controls (Figure 3).
Figure 3.
Histogram analysis of ChAT-positive motor neuron cell body area of 6-month-old rats. Following 2 weeks of exercise, the voluntary running group and swimming group displayed a higher frequency of cells >1500μm2 as compared to the involuntary running group and controls.
3.2 Short-term exercise increases GDNF protein in the spinal cord of 24-month-old rats
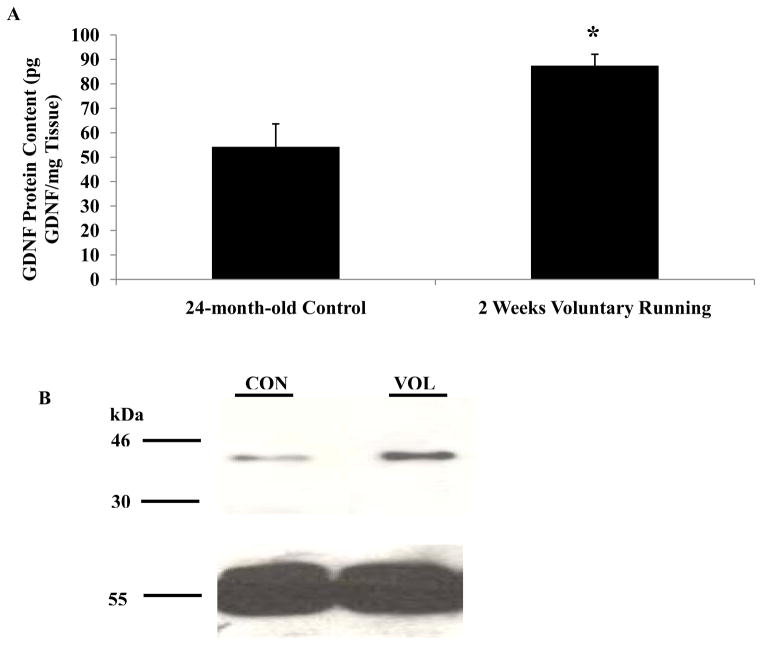
To determine if short-term exercise alters GDNF protein content in the spinal cord of old animals, 24-month-old rats underwent voluntary running for 2 weeks. These animals ran at a peak speed of only 2m/min (Table 1), which is considered to be low-intensity running. Average animal weights were significantly lower in the exercised animals (355.0g ± 34.9g) as compared to age-matched sedentary controls (415.4g ± 56.0g). The average distance run per day by these old animals was around 1km less than the 6-month-old runners (Table 1). Two weeks of voluntary running significantly increased GDNF protein content 2-fold in the lumbar spinal cord of the 24-month-old rats (87.4 ± 4.6pg GDNF/mg Tissues) as compared to age-matched sedentary controls (54.4 ± 9.3pg GDNF/mg Tissue) (Figure 4a). GDNF protein levels in the spinal cord were significantly higher among the 24-month-old controls than the 6-month-old controls.
Figure 4.
Note: Staining for α-tubulin as a loading control was added to Panel B GDNF protein content was increased in the spinal cord after 2 weeks of exercise in 24-month-old rats. The lumbar spinal cord (L1–L3) was removed from control and exercised 24-month-old animals. (A) Tissues were processed for determination of GDNF protein content using an ELISA. A significant increase in GDNF protein content was detected in the lumbar spinal cord of animals that had undergone 2 weeks of voluntary running as compared to age-matched sedentary controls. Values are displayed as mean ± SEM. Asterisk (*) indicates significance (p≤0.05) from controls. (B) Tissues were processed for Western blot to determine GDNF protein content (top) and a loading control of α-tubulin (bottom). An increase in GDNF protein content was detected in the spinal cord of animals that had undergone 2 weeks of voluntary running as compared to controls.
Western blot analysis also confirmed that 2 weeks of exercise increased GDNF protein expression 2-fold in the lumbar spinal cord of old rats as compared to age-matched sedentary controls (Figure 4b). Interestingly, the molecular weight of GDNF appeared to be around 41kDa in old rats compared to 30kDa in young adult rats.
Immunoreactivity for GDNF followed a similar pattern to that observed in 6-month-old rats, where we found more vesicle-like structures containing GDNF outside of ChAT-positive motor neurons in exercised animals compared to sedentary controls (Figure 5A–B). Antibody staining for colocalization of GDNF to vesicle-like structures was confirmed with the GM130 antibody (Figure 5C).
Figure 5.
Note: Panel C was added displaying staining for vesicle-like structures using anti-GM130 GDNF immunoreactivity and motor neuron cell body size increased with exercise in 24-month-old rats. Representative lumbar spinal cord sections from a 24-month-old control animal (A) and a 2 week voluntary exercised animal (B). Spinal cord sections were immunolabeled with primary antibodies against ChAT (red) and GDNF (green). Exercised animals appeared to have more vesicle-like structures containing GDNF that surrounded the motor neurons (arrows in B) as compared to controls (arrows in A). GDNF immunoreactivity was also colocalized to the GM130 antibody for vesicle-like structures (C). The scale bar represents 20μm.
ChAT-positive motor neuron cell body area in 24-month-old animals was significantly greater following 2 weeks of exercise (618.8 ± 31.5 μm2) compared to that in age-matched sedentary controls (387.1 ± 18.0 μm2). The ChAT-positive motor neuron cell body area from the 24-month-old sedentary controls were significantly smaller than the 6-month-old sedentary controls (p=0.02). Furthermore, the ChAT-positive motor neuron cell body area from the 24-month-old voluntary runners were significantly smaller than the 6-month-old voluntary runners (p=0.003). Again, histogram analysis of ChAT-positive motor neuron cell body areas displayed a higher frequency of the large-sized motor neurons (>1500μm2) following 2 weeks of voluntary running as compared to controls (Figure 6).
Figure 6.
Exercise increased ChAT-positive motor neuron cell body area of 24-month-old rats. Histogram analysis of ChAT-positive motor neuron cell body area. Following 2 weeks of exercise, voluntary runners displayed a higher frequency of cells >1500μm2 as compared to 24-month-old sedentary controls.
4. Discussion
While other investigators have found that exercise increases neurotrophin levels in the spinal cord (Gomez-Pinilla et al., 2001, 2002; Dupont-Versteegden et al., 2004), few studies have examined the effects of exercise on spinal cord GDNF levels. The present study was designed to determine if short-term exercise (2 weeks) would alter GDNF protein content in the spinal cord of young and old animals. In young rats, involuntary running resulted in the greatest fold-change in GDNF protein content in the spinal cord, followed by swimming and voluntary running, however these levels were not statistically different. Interestingly, both young and old voluntary runners displayed the same fold-change in GDNF protein content as compared to their age-matched controls. In the exercise groups, ChAT-positive motor neuron cell body area doubled in size compared to that from age-matched sedentary controls.
4.1 Motor neuron size increases at the same time as GDNF levels following short term exercise
In animal models of aging, there is selective atrophy of large-sized motor neurons and a decrease in the total number of motor neurons that innervate hindlimb muscles (Hirofuji et al., 2000; Hashizume and Kanda, 1990). Our results confirm that motor neuron cell body size decreases with advancing age in sedentary animals. Mature motor neurons obtain trophic support from various types of cells, including Schwann cells, skeletal muscle cells and other neurons (Nishi, 1994; Oppenheim, 1996), and may resist death by increasing production of neurotrophic factors in these tissues. While there may not be a direct correlation to the increase in neurotrophic factor content with the decrease in motor neuron size observed with aging, our observations of increased neurotrophic levels with advancing age may suggest a steady increase is in response to motor neuron loss with senescence. Acute effects of exercise were also found to increase production of neurotrophic factors. Recent studies have demonstrated links between beneficial effects of exercise, changes in neurotrophic factor levels and neuronal plasticity. Our observations of increased neurotrophic factor expression in the lumbar spinal cord following short-term exercise coupled with the increase in motor neuron size suggest acute bouts of exercise as a possible mechanism to protect motor neurons from undergoing atrophy with senescence.
4.2 Low-intensity exercise is a potent stimulus for enhancing neurotrophic factor levels
While some studies report that voluntary exercise increases mRNA and protein levels for neurotrophic factors in the spinal cord (Ferraiuolo et al., 2009; Macias et al., 2002; Skup et al., 2002; Ying et al., 2003), others show decreasing levels of neurotrophic factors in the spinal cord following exercise (Siamilis et al., 2009; Engesser-Casar et al., 2007). One possible contributing factor to these discrepancies may be due to variations in intensity and duration of exercise. BDNF and neurotrophin-3 mRNA and protein levels in the lumbar spinal cord are known to increase following short-term exercise (Gomez-Pinilla et al., 2001; Molteni et al., 2002; Neeper et al., 1996). Moderate-intensity exercise (13m/min) increases BDNF, but high intensity exercise decreases BDNF levels in the brain (Aguiar et al., 2007), suggesting that low to moderate levels of exercise may be a more potent stimulus for increasing neurotrophic factor levels. Our results lend support to this idea, where we find that our moderate-intensity involuntary running protocol yielded the greatest change in GDNF protein content of all exercise regimens examined. Together, these results may suggest that short-term, moderate-intensity exercise programs may be a better stimulus for enhancing neurotrophic factor content in the spinal cord.
4.3 Punctate immunoreactivity for GDNF is altered with exercise and age
A punctate staining pattern for GDNF has been found in neuronal cell bodies, dendrites and axons (Kawamoto et al., 2000). In cultured neuroendocrine cells the staining pattern for GDNF appears to be localized to vesicle-like structures (Lonka-Nevalaita et al., 2010). Moreover, in axons of dorsal root ganglion neurons GDNF is present in dense-core vesicles (Ohta et al., 2001) and in rat primary cortical and hippocampal neurons, the immunostaining pattern of GDNF appears in vesicle-like structures at the tips of neurites, where the authors suggest transportation of GDNF to the cell periphery (Lonka-Nevalaita et al., 2010). Within the spinal cord, we observed vesicle-like staining for GDNF that was confirmed with the GM130 antibody, where our results suggest that exercise increases the incidence of GDNF positive immunoreactivity. These observations are in accordance with our ELISA values, where GDNF protein content is increased following exercise in both young and old animals as well as increased with advancing age in control animals.
4.4 Molecular weight of GDNF changes with age
The reported molecular weight of GDNF varies in the literature depending on the cells/tissues examined. Lin et al. (1994) describe GDNF from cultured dopaminergic cells as having a molecular weight of 33–45kDa in non-reduced gels and 15–21kDa from reduced gels. GDNF protein size was reported to be 24kDa in cultured primary fibroblasts (Blesch and Tuszynkski, 2001), 30kDa in cultured neuroblastoma cells (Larsen et al., 2006) and 34kDa in 18-week-old human fetal spinal cord (Koo and Choi, 2001). We found the molecular weight of GDNF in the rat lumbar spinal cord to be 30kDa in young animals and 41kDa in old animals. Similarly, several NGF isoforms have been reported in various whole tissues of both humans and animals (reviewed by Al-Shawi et al., 2007). One explanation for the variations in molecular weights may be due to modifications of the prodomain regions of the protein. Pro-neurotrophins, which are the precursor forms of neurotrophins, are synthesized and then cleaved by furin and other proteases to produce mature neurotrophins (Lee et al., 2001). Mature NGF and BDNF induce neuronal survival, differentiation and synaptic modulation (Huang and Reichardt, 2001). Results of other studies suggest that the precursor of NGF may be either neurotoxic (Ibanez, 2002) or significantly less neurotrophic than the mature form of NGF (Fahnestock et al., 2004). Pro-neurotrophins, such as pro-BDNF and pro-NGF, induce cell death by activating an apoptotic cascade via binding to cell death complexes involving sortilin and p75 receptors (Lee et al., 2001; Nykjaer et al., 2004). Moreover, pro-NGF has been found to be upregulated with aging and disease. Pro-NGF is increased in the superior cervical ganglia of old rats (Bierl and Isaacson, 2007), the parietal cortex of patients with Alzheimer’s disease (Peng et al., 2004) and in spinal cord oligodendrocytes from a murine model of spinal cord injury (Beattie et al., 2002). Our observation of increasing molecular weight for GDNF with advancing age may suggest a similar phenomenon is occurring in spinal cord of rat, possibly demonstrating increased expression of a higher molecular weight pro-form of GDNF in the older animals. It has been shown that pro-NGF is secreted by reactive astrocytes, and may affect motor neuron survival (Domeniconi et al., 2007). The spinal cord samples processed in the current study would contain motor neurons, interneurons and glial cells, which could account for the pro-GDNF, if it is associated with glial cells. While it is still unknown if the pro form of GDNF activates similar apoptotic pathways as NGF, it is known that post-translational modifications of GDNF are due to prohormone convertase that cleaves five consensus sites giving rise to four different peptide forms of processed GDNF (Oh-hashi et al., 2009; Immonen et al., 2008). Future studies are warranted to determine if proGDNF is less neuroprotective or neurotoxic and how aging affects its expression.
4.5 GDNF transport following exercise
GDNF is produced by motor neurons, oligodendrocytes and Schwann cells in the spinal cord (Henderson et al., 1994; Rind and von Bartheld, 2002; Russell et al., 2000; Yamamoto et al., 1996) as well as by skeletal muscles (Yamamoto et al., 1996). Both anterograde and retrograde transport between neurons and target tissues have been demonstrated for GDNF (Rind and von Bartheld, 2002; Russell et al., 2000). While our results did not determine which cells are producing the GDNF protein observed in the spinal cord, we have previously shown that GDNF protein is increased in skeletal muscle following short term exercise (McCullough et al., 2011; Wehrwein et al., 2002), which could be transported back to the spinal cord, resulting in the elevated levels observed in the current study. It has been shown that skeletal muscle derived GDNF has more potent effects for the neuromuscular system than that supplied by anterograde transport (Li et al., 2007), thus elevated retrograde transport of GDNF from the muscle following exercise may represent an important stimulus for enhanced plasticity.
4.6 Exercise stressors
There is evidence that stress is unlikely to be the critical factor underlying the differential effects of voluntary and forced running (Leasure and Jones, 2008). Forced running acutely elevates corticosterone levels, the rodent stress hormone, more than voluntary running (Ploughman et al., 2005, 2007), however these levels return to baseline within a few hours after exercise (Stranahan et al., 2006; Ploughman et al., 2007) and after several weeks of exercise these levels are no longer elevated (Fediuc et al., 2006). Stress is also known to activate microglia (Nair and Bonneau, 2006; Sugama et al., 2007), however neither forced nor voluntary running enhances microglial activity in the brain (Leasure and Jones, 2008). These observations may suggest that the exercise-induced changes in neurotrophic factor expression observed in the current study are not likely to be dependent on a stress response.
Conclusion
In conclusion, the results demonstrate that short-term exercise increases GDNF protein content, GDNF immuno-labeling and motor neuron size in the spinal cord of young and old animals. These results are consistent with our hypothesis that the neural protection/neural plasticity caused by exercise may be driven, in part, by enhanced GDNF production. In addition, there may be a relationship between the intensity of exercise and the amount of GDNF protein produced, where a low-intensity exercise protocol yields the greatest increase in GDNF protein content. We believe that exercise has the advantage of enhancing neurotrophic factor levels by physiological means using intrinsic mechanisms in the spinal cord rather than attempting to increase neurotrophic factor levels via exogenous administration where all the physiological implications are not well understood.
Highlights.
Exercise increases GDNF protein content in spinal cord.
Low-intensity, forced running elicits the greatest fold-change in GDNF content.
Motor neuron cell body size increases over the same time course as GDNF protein.
Molecular weight of GDNF protein in spinal cord changes with advancing age.
Exercise increases GDNF staining in and around motor neurons in spinal cord.
Acknowledgments
This work was supported by NIH grant 1 R15 AG022908-01A2 and Western Michigan University. The monoclonal α-Tubulin antibody developed by Charles Walsh was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Abbreviations
- BDNF
Brain-derived neurotrophic factor
- ChAT
Choline acetyltransferase
- GDNF
Glial cell line-derived neurotrophic factor
- NGF
Nerve growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Monica J. McCullough, Email: monicajmccullough@gmail.com.
Amy M. Gyorkos, Email: amy.gyorkos@wmich.edu.
M. Spitsbergen John, Email: john.spitsbergen@wmich.edu.
References
- Aguiar AS, Tuon T, Pinho CA, Silva LA, Andreazza AC, Kapczinski F, Quevedo J, Streck EL, Pinho RA. Mitochondrial IV complex and brain neurotrophic derived factor response of mice brain cortex after downhill training. Neurosci Lett. 2007;426:171–174. doi: 10.1016/j.neulet.2007.08.058. [DOI] [PubMed] [Google Scholar]
- Al-Shawi R, Hafner A, Chun S, Raza S, Crutcher K, Thrasivoulou C, Simons P, Cowen T. ProNGF, sortilin, and age-related neurodegeneration. Ann NY Acad Sci. 2007;1119:208–215. doi: 10.1196/annals.1404.024. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury. Neuron. 2002;36:375–386. doi: 10.1016/s0896-6273(02)01005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman E, Kullberg S, Ming Y, Ulfhake B. Upregulation of GFRalpha-1 and c-ret in primary sensory neurons and spinal motoneurons of aged rats. J Neurosci Res. 1999;57:153–165. doi: 10.1002/(SICI)1097-4547(19990715)57:2<153::AID-JNR1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bierl MA, Isaacson LG. Increased NGF proforms in aged sympathetic neurons and their targets. Neurobiol Aging. 2007;28:122–134. doi: 10.1016/j.neurobiolaging.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. GDNF gene delivery to injured adult CNS motor neurons promotes axonal growth, expression of the trophic neuropeptide CGRP, and cellular protection. J Comp Neurol. 2001;436:399–410. doi: 10.1002/cne.1076. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corse AM, Bilak MM, Bilak SR, Lehar M, Rothstein JD, Kuncl RW. Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiol Dis. 1999;6:335–346. doi: 10.1006/nbdi.1999.0253. [DOI] [PubMed] [Google Scholar]
- Deforges S, Branchu J, Biondi O, Grondard C, Pariset C, Lecolle S, Lopes P, Vidal P-P, Chanoine C, Charbonnier F. Motoneuron survival is promoted by specific exercise in a mouse model of amyotrophic lateral sclerosis. J Physiol. 2009;587:3561–3571. doi: 10.1113/jphysiol.2009.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Hempstead BL, Chao MV. Pro-NGF secreted by astrocytes promotes motor neuron cell death. Mol Cell Neurosci. 2007;34:271–279. doi: 10.1016/j.mcn.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Houle JD, Dennis RA, Zhang J, Knox M, Wagoner G, Peterson CA. Exercise-induced gene expression in soleus muscle is dependent on time after spinal cord injury in rats. Muscle Nerve. 2004;29:73–81. doi: 10.1002/mus.10511. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Cotman CJ. Wheel running and fluoxetine antidepressant treatment have differential effects in the hippocampus and the spinal cord. Neurosci. 2007;144:1033–1044. doi: 10.1016/j.neuroscience.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Yu G, Michalski B, Mathew S, Colquhoun A, Ross GM, Coughlin MD. The nerve growth factor precursor proNGF exhibits neurotrophic activity but is less active than mature nerve growth factor. J Neurochem. 2004;89:581–592. doi: 10.1111/j.1471-4159.2004.02360.x. [DOI] [PubMed] [Google Scholar]
- Fediuc S, Campbell JE, Riddell MC. Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. J Appl Physiol. 2006;100:1867–1875. doi: 10.1152/japplphysiol.01416.2005. [DOI] [PubMed] [Google Scholar]
- Ferraiuolo L, De Bono JP, Heath PR, Holden H, Kasher P, Channon KM, Kirby J, Shaw PJ. Transcriptional response of the neuromuscular system to exercise training and potential implications for ALS. J Neurochem. 2009;109:1714–1724. doi: 10.1111/j.1471-4159.2009.06080.x. [DOI] [PubMed] [Google Scholar]
- Fries JF, Singh G, Morfeld D, Hubert HB, Lane NE, Brown BW., Jr Running and the development of disability with age. Annal Intern Med. 1994;121:502–509. doi: 10.7326/0003-4819-121-7-199410010-00005. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 2000;88:133–137. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Hashizume K, Kanda K. Neuronal dropout is greater in hindlimb motor nuclei than in forelimb motor nuclei in aged rats. Neurosci Lett. 1990;113:267–269. doi: 10.1016/0304-3940(90)90595-z. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simpson LC, Moffet B, Vandlen RA, Koliatsos VE, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Hirofuji C, Ishihara A, Roy RR, Itoh K, Itoh M, Edgerton VR, Katsuta S. SDH activity and cell size of tibialis anterior motoneurons and muscle fibers in SAMP6. NeuroRep. 2000;11:823–828. doi: 10.1097/00001756-200003200-00033. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez CF. Jekyll-hyde neurotrophins: the story of proNGF. Trends Neurosci. 2002;25:284–286. doi: 10.1016/s0166-2236(02)02169-0. [DOI] [PubMed] [Google Scholar]
- Immonen T, Alakuijala A, Hytonen M, Sainio K, Poteryaev D, Saarma M, Pasternack M, Sariola H. A proGDNF-related peptide BEP increases synaptic excitation in rat hippocampus. Exp Neurol. 2008;210:793–796. doi: 10.1016/j.expneurol.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Jacob JM. Lumbar motor neuron size and number is affected by age in male F344 rats. Mech Ageing Dev. 1998;106:205–216. doi: 10.1016/s0047-6374(98)00117-1. [DOI] [PubMed] [Google Scholar]
- Johnson H, Hokfelt T, Ulfhake B. Expression of p75NTR, trkB and trkC in nonmanipulated and axotomized motoneurons of aged rats. Mol Brain Res. 1999;69:21–34. doi: 10.1016/s0169-328x(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45:M82–M88. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- Kawamoto Y, Nakamura S, Matsuo A, Akiguchi I, Shibasaki H. Immunohistochemical localization of glial cell line-derived neurotrophic factor in the human central nervous system. Neurosci. 2000;100:701–712. doi: 10.1016/s0306-4522(00)00326-2. [DOI] [PubMed] [Google Scholar]
- Keller-Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD. Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci. 2001;21:6136–6146. doi: 10.1523/JNEUROSCI.21-16-06136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Choi BH. Expression of glial cell line-derived neurotrophic factor (GDNF) in the developing human fetal brain. Int J Devl Neurosci. 2001;19:549–558. doi: 10.1016/s0736-5748(01)00042-9. [DOI] [PubMed] [Google Scholar]
- Kullberg S, Ramirez-Leon V, Johnson H, Ulfhake B. Decreased axosomatic input to motoneurons and astrogliosis in the spinal cord of aged rats. J Gerontol A Biol Sci Med Sci. 1998;53:B369–B379. doi: 10.1093/gerona/53a.5.b369. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Benn SC, Ay I, Chian R-J, Celia SA, Remington MP, Bejarano M, Liu M, Ross J, Carmillo P, Sah D, Phillips KA, Sulzer D, Pepinsky RB, Fishman PS, Brown RH, Jr, Francis JW. A glial cell line-derived neurotrophic factor (GDNF):tetanus toxin fragment C protein conjugate improves delivery of GDNF to spinal cord motor neurons in mice. Brain Res. 2006;1120:1–12. doi: 10.1016/j.brainres.2006.08.079. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neurosci. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Li W, Brakefiled D, Pan Y, Hunter D, Myckatyn TM, Parsadanian A. Muscle-derived but not centrally derived transgene GDNF is neuroprotective in G93A-SOD1 mouse model of ALS. Exp Neurol. 2007;203:457–471. doi: 10.1016/j.expneurol.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Lin L-FH, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lin L-FH, Zhang TJ, Collins F, Armes LG. Purification and Initial characterization of rat B49 glial cell line-derived neurotrophic factor. J Neurochem. 1994;63:758–768. doi: 10.1046/j.1471-4159.1994.63020758.x. [DOI] [PubMed] [Google Scholar]
- Lonka-Nevalaita L, Lume M, Leppanen S, Jokitalo E, Peranen J, Saarma M. Characterization of the intracellular localization, processing, and secretion of two glial cell line-derived neurotrophic factor splice isoforms. J Neurosci. 2010;30:11403–11413. doi: 10.1523/JNEUROSCI.5888-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias M, Fehr S, Dwornik A, Sulejczak D, Wiater M, Czarkowska-Bauch J, Skup M, Schachner M. Exercise increases mRNA levels for adhesion molecules N-CAM and L1 correlating with BDNF response. Neurorep. 2002;13:2527–2530. doi: 10.1097/00001756-200212200-00029. [DOI] [PubMed] [Google Scholar]
- McCullough MJ, Peplinski NG, Kinnell KR, Spitsbergen JM. Glial cell line-derived neurotrophic factor protein content in rat skeletal muscle is altered by increased physical activity In vivo and In vitro. Neurosci. 2011;174:234–244. doi: 10.1016/j.neuroscience.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1117. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF over-expression in muscle. Science. 1998;279:1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- Nishi R. Neurotrophic factors: two are better than one. Science. 1994;265:1052–1053. doi: 10.1126/science.8066443. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Oh-hashi K, Ito M, Tanaka T, Hirata Y, Kiuchi T. Biosynthesis, processing, and secretion of glial cell line-derived neurotrophic factor in astroglial cells. Mol Cell Biochem. 2009;323:1–7. doi: 10.1007/s11010-008-9958-3. [DOI] [PubMed] [Google Scholar]
- Ohta K, Inokuchi T, Gen E, Change J. Ultrastructural study of anterograde transport of glial cell line-derived neurotrophic factor from dorsal root ganglion neurons of rats towards the nerve terminal. Cells Tissues Organs. 2001;169:410–421. doi: 10.1159/000047909. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Neurotrophic survival molecules for motoneurons: an embarrassment of riches. Neuron. 1996;17:195–197. doi: 10.1016/s0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S. Developing motor neurons rescued from programmed and axotomy- induced cell death by GDNF. Nature. 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Houenou LJ, Parsadanian AS, Prevette D, Snider WD, Shen L. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: regulation of programmed cell death among motoneuron subtypes. J Neurosci. 2000;20:5001–5011. doi: 10.1523/JNEUROSCI.20-13-05001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63:641–649. doi: 10.1093/jnen/63.6.641. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Granter-Button S, Chernenko G, Attwood Z, Tucker BA, Mearow KM, Corbett D. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res. 2007;1150:207–216. doi: 10.1016/j.brainres.2007.02.065. [DOI] [PubMed] [Google Scholar]
- Ploughman N, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neurosci. 2005;136:991–1001. doi: 10.1016/j.neuroscience.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Rind HB, von Bartheld CS. Anterograde axonal transport of internalized GDNF in sensory and motor neurons. Neuroreport. 2002;13:659–664. doi: 10.1097/00001756-200204160-00025. [DOI] [PubMed] [Google Scholar]
- Russell FD, Koishi K, Jiang Y, McLennan IS. Anterograde axonal transport of glial cell line-derived neurotrophic factor and its receptors in rat hypoglossal nerve. Neurosci. 2000;97:575–580. doi: 10.1016/s0306-4522(00)00079-8. [DOI] [PubMed] [Google Scholar]
- Siamilis S, Jakus J, Nyakas C, Costa A, Mihalik B, Falus A, Radak Z. The effect of exercise and oxidant-antioxidant intervention on the level of neurotrophins and free radicals in spinal cord of rats. Spinal Cord. 2009;47:453–457. doi: 10.1038/sc.2008.125. [DOI] [PubMed] [Google Scholar]
- Skup M, Dwornik A, Macias M, Sulejczak D, Wiater M, Czarkowska-Bauch J. Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin-4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp Neurol. 2002;176:289–307. doi: 10.1006/exnr.2002.7943. [DOI] [PubMed] [Google Scholar]
- Springer JE, Seeburger JL, He J, Gabrea A, Blankenhorn EP, Bergman LW. cDNA sequence and differential mRNA regulation of two forms of glial cell line-derived neurotrophic factor in Schwann cells and rat skeletal muscle. Exp Neurol. 1995;131:47–52. doi: 10.1016/0014-4886(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neurosci. 2007;146:1388–1399. doi: 10.1016/j.neuroscience.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Suter-Crazzolara C, Unsicker K. GDNF is expressed in two forms in many tissues outside the CNS. Neurorep. 1994;5:2486–2488. doi: 10.1097/00001756-199412000-00020. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hase A, Miyata Y, Arahata K, Akazawa C. Prominent expression of glial cell line-derived neurotrophic factor in human skeletal muscle. J Comp Neurol. 1998;402:303–312. [PubMed] [Google Scholar]
- Toulotte C, Fabre C, Dangremont B, Lensel G, Thevenon A. Effects of physical training on the physical capacity of frail, demented patients with a history of falling: a randomised controlled trial. Age Aging. 2003;32:67–73. doi: 10.1093/ageing/32.1.67. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor 1 mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF). c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianney J-M, Spitsbergen JM. Cholinergic neurons regulate secretion of glial cell line-derived neurotrophic factor by skeletal muscle cells in culture. Brain Res. 2011;1390:1–9. doi: 10.1016/j.brainres.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto K, Okada T, Mizukami H, Matsushita T, Hanazono Y, Kime A, Nagatsu T, Ozawa K, Nakano I. Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002;22:6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrwein EA, Roskelley EM, Spitsbergen JM. GDNF is regulated in an activity-dependent manner in rat skeletal muscle. Muscle Nerve. 2002;26:206–211. doi: 10.1002/mus.10179. [DOI] [PubMed] [Google Scholar]
- Wetts R, Vaughn JE. Differential vulnerability to two subsets of spinal motor neurons in Amyotrophic Lateral Sclerosis. Exp Neurol. 1996;141:248–255. doi: 10.1006/exnr.1996.0159. [DOI] [PubMed] [Google Scholar]
- Wu CW, Chang YT, Yu L, Chen HI, Jen CJ, Wu SY, Lo CP, Kuo YM. Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J Appl Physiol. 2008;105:1585–1594. doi: 10.1152/japplphysiol.90775.2008. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sobue G, Yamamoto K, Terao S, Mitsuma T. Expression of glial cell-line derived growth factor mRNA in the spinal cord and muscle in amyotrophic lateral sclerosis. Neurosci Lett. 1996;204:117–120. doi: 10.1016/0304-3940(96)12342-9. [DOI] [PubMed] [Google Scholar]
- Yan Q, Matheson C, Lopez OT. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton R, Gomez-Pinilla F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003;987:93–99. doi: 10.1016/s0006-8993(03)03258-x. [DOI] [PubMed] [Google Scholar]
- Zurn AD, Baetge EE, Hammang JP, Tan SA, Aebischer P. Glial cell line-derived neurotrophic factor (GDNF), a new neurotrophic factor for motoneurones. Neurorep. 1994;6:113–118. doi: 10.1097/00001756-199412300-00030. [DOI] [PubMed] [Google Scholar]
- Zwick M, Teng L, Mu X, Springer JE, Davis BM. Overexpression of GDNF induces and maintains hyperinnervation of muscle fibers and multiple end-plate formations. Exp Neurol. 2001;171:342–350. doi: 10.1006/exnr.2001.7753. [DOI] [PubMed] [Google Scholar]