Abstract
Exposure to the pesticide paraquat (PQ) increases the risk of Parkinson’s disease (PD), and its effect may be modulated by genetic or other environmental factors. The neuropeptide PACAP (pituitary adenylyl cyclase activating polypeptide, Adcyap1) has been shown to enhance tyrosine hydroxylase (TH) and VMAT2 expression, protect dopaminergic (DA) neurons against the neurotoxin 6-hydroxydopamine, regulate neuronal mitochondria, and inhibit inflammation. Decreased expression of PACAP may thus interact with environmental factors such as PQ to increase the risk of PD. To mimic a low level environmental exposure to PQ, wild type (WT) and PACAP knockout (KO) mice were given a single [10 mg/kg] dose of PQ, a regimen that did not induce loss of TH expression or DA neurons in WT mice. This treatment reduced the number of TH-positive cell bodies in the substantia nigra pars compacta (SNpc) of PACAP KO. Because inflammation is also a risk factor for PD, we performed a quantitative analysis of SNpc Iba+ microglia. As expected, PQ increased the number of larger microglial profiles, indicative of activation, in WT mice. Strikingly, microglial activation was already evident in PACAP KO mice in the basal state. PQ caused no further activation in these mice, although TNF-α gene expression was enhanced. In the periphery, PQ had no effects on the abundance of proinflammatory Th1 or Th17 cells in WT mice, but increased the numbers of anti-inflammatory regulator T cells (Tregs). PACAP KO mice, in contrast, had elevated numbers of Th17 cells after PQ, and the induction of Tregs was impaired. The results indicate that endogenous PACAP acts to maintain the integrity of dopaminergic neurons during exposure to PQ, an action that may be linked to its ability to regulate microglia and/or other immune cells.
Keywords: Parkinson’s disease, pesticide, paraquat, PACAP, dopamine, tyrosine hydroxylase, microglia, mice
Introduction
Parkinson’s disease (PD) is thought to develop as a result of an interplay between genetic and environmental factors, and the identification of genetic factors that modulate an individual’s sensitivity to environmental factors may lead to both a better risk assessment and a better understanding of disease mechanisms (Hamza et al., 2012; Fitzmaurice et al. 2012; Ritz et al., 2012; Ritz et al., 2009) Epidemiological studies have shown that long-term exposure to pesticides is associated with an increased risk of developing PD (Baldi et al., 2003; Costello et al., 2009; Engel et al., 2001; Gorell et al., 2004; Tanner et al., 2011; Wang et al., 2011). For example, exposure to paraquat, (PQ) a widely used pesticide, results in up to 2.5 times the risk of developing PD in humans (Costello et al., 2009; Tanner et al., 2011) and has been shown at some doses to induce selective dopaminergic (DA) neurodegeneration in mice (Brooks et al., 1999; Fernagut et al., 2007; Peng et al., 2004). Recently, inflammatory changes in the brain have also emerged as a possible risk factor in sporadic PD. Activated microglia and/or proinflammatory cytokines interleukin (IL)-1, IL-6, IL-8 and tumor necrosis factor (TNF)- have been identified in post-mortem PD brains (McGeer et al., 1988; Mogi et al., 1994; Mogi and Nagatsu, 1999; Nagatsu et al., 2000a, b) and in living PD patients (Gerhard et al., 2006; Ouchi et al., 2009), although consequences (harmful or protective) of such inflammatory events on dopaminergic neurons remain controversial. Moreover, the use of non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen is negatively correlated with disease risk in some studies suggesting that suppression of the inflammatory response may be beneficial in preventing PD (Bower et al., 2006; Chen et al., 2005; Ton et al., 2006), but only if started at least 5 years before the clinical onset of manifest PD (Manthripragada et al., 2011). While not directly proven, inflammation caused during pesticide exposure may be a contributing factor in the development of PD later in life. In mice, it has been reported that a single exposure to PQ induces activation of microglia even in the absence of observable DA neuron damage (Purisai et al., 2007). Therefore, these studies suggest that a combination of exposure to pesticides such as PQ and ensuing inflammation may be involved in the development and pathogenesis of PD.
Pituitary adenylyl cyclase activating polypeptide (PACAP) is a neuropeptide expressed in the rat brain at particularly high concentrations in the substantia nigra and nucleus accumbens (Ghatel et al, 1993), and has been shown in numerous investigations to protect dopaminergic and other neurons and non-neuronal cell types in vitro and in vivo (reviewed in (Reglodi et al., 2011) and (Reglodi et. al., 2012)). For example, PACAP reversed DA neurodegeneration caused by 6-hydroxydopamine (6-OHDA) in vitro and protected PC12 cell lines against 1-methyl-4-phenylpyridinium (MPP+) toxicity (Chung et al., 2005; Takei et al., 1998). Furthermore, local pretreatment with PACAP in a unilateral 6-OHDA-induced nigral degeneration model significantly improved behavioral alterations and rescued DA neurons (Reglodi et al., 2004a; Reglodi et al., 2004b). Similarly, PACAP has been shown to provide neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced toxicity in mice (Wang et al., 2008). PACAP was injected intravenously for 7 days following 5 days MPTP treatment and ameliorated the effect of MPTP on tyrosine hydroxylase (TH) positive neurons and TH protein expression in the substantia nigra (Wang et al., 2008). In addition to effects of PACAP on neuron survival, PACAP treatment of cultured cells has been shown to promote several aspects of dopamine metabolism, stimulating TH mRNA and protein expression in rat PC12 pheochromocytoma cells (Corbitt et al., 1998), enhancing TH activity and dopamine synthesis (Houchi et al., 1995; Moser et al., 1999), in part by increasing TH serine 40 phosphorylation (Bobrovskaya et al., 2007), and by increasing dopamine uptake (Takei et al., 1998) and the expression of the vesicular monoamine transporter (VMAT2) (Guillot et al., 2008;). Moreover, it was recently reported that PACAP inhibited oxidative DNA stress in vivo after transient global ischemia (Stetler et al., 2010), and enhanced mitochondrial membrane potential in neuronal cultures along with the expression of the master transcriptional co-regulator peroxisome proliferator-activated receptor γ co-activator 1α (PGC1α) (Kambe and Miyata, 2012). Finally, an additional set of studies has suggested that PACAP exerts some of its neuroprotective effects indirectly by regulating inflammatory responses (Armstrong et al., 2008; Ohtaki et al., 2006; Tan et al., 2009). In this regard, in addition to its aforementioned expression in the SN and striatum which could regulate local inflammation, PACAP is also present in autonomic circuits presumed to regulate inflammatory cells in lymph nodes and other peripheral immune sites (Beaudet et al., 1993). Taken together, these properties of PACAP suggest that it may modulate the effects of PQ on DA neurons. The demonstration of important modulatory effects of PACAP would encourage assessing a role for PACAP and PACAP receptor polymorphisms in modulating PD risk, especially in patients exposed to pesticides. In this study we exposed PACAP knockout (KO) mice to a single dose of PQ to determine potential protective actions of endogenous PACAP after exposure to this pesticide.
Materials and Methods
Animals
Male 2–3 month old PACAP KO (null at both alleles) and WT mice from the same colony, both on a C57BL/6 background (Colwell et al., 2004) (backcrossed for at least 12 generations) were used in our experiments. The genotypes of all mice were determined by polymerase chain reaction (PCR) amplification analysis of tail DNA at one month of age and verified at the end of the experiment. Animals were housed and fed ad libitum at the University of California at Los Angeles (UCLA) vivarium. All procedures were approved by UCLA Animal Care and Use Committee (protocol 93–302) and conducted in accordance with the guidelines in National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Treatment and tissue preparation
Two cohorts of mice received a single intraperitoneal injection of either saline or PQ dichloride hydrate (10 mg/kg; Sigma-Aldrich, St. Louis, MO) and were sacrificed 7 days later. The first cohort (n=6/group) were deeply anesthetized with sodium pentobarbital [100 mg/kg, intraperitoneal (ip)] and transcardially perfused with 0.1 M phosphate buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2P04, pH 7.4), followed by 4% paraformaldehyde (PFA). Brains were quickly removed, post-fixed with PFA for 2 hours, cryoprotected in 30% sucrose in 0.1 M PBS, frozen on powdered dry ice and stored at −80 C. Free-floating coronal sections (40 m) were cut on a cryostat and collected for immunohistochemical analysis. The second cohort (n=6–9/group), used for real-time RT-PCR assay, were deeply anesthetized and transcardially perfused with 0.1 M PBS. Brains were quickly removed and placed on an ice-cold plate. Coronal blocks of the substantia nigra (SN) were micro-dissected using an acrylic mouse brain slicer matrix with 1.0 mm coronal intervals (Mouse Brain Matrix, AL-1175; Roboz Surgical Instrument Co., USA). Tissue was frozen on powdered dry ice and stored at −80 C for mRNA analysis.
Immunohistochemistry and stereological analysis of dopaminergic neurons in substantia nigra
Every fourth section of the SN was processed for TH-immunoreactivity (TH-IR) as described previously (Fernagut et al., 2007). Sections were washed with 0.1 M PBS, incubated in 0.5% H2O2 in methanol for 30 min (to inhibit endogenous peroxidase activity), washed in PBS and blocked for 1 hour with 10% normal goat serum (NGS) and 0.5% Triton-X in PBS. Sections were incubated with a polyclonal anti-TH (1:600; Pel Freez Biologicals, Rogers, AR) diluted in 5% NGS and 0.5% Triton-X in PBS overnight at 4°C. Sections were washed with PBS and incubated in biotinylated secondary goat anti-rabbit IgG (1:1000 dilution; Vector Laboratories, Inc., Burlingame, CA) in 5% NGS in PBS. Sections were washed in 0.1 M PBS and subsequently incubated in avidin-biotin complex (ABC; Vector Laboratories, Burlingame, CA) for 45 minutes and washed again in 0.1 M PBS followed by an incubation in 0.05 M Tris buffered saline (TBS) containing 3-3'diamino benzidine (DAB; Sigma-Aldrich, St. Louis, MO) and 0.3% H2O2 (Sigma-Aldrich, St. Louis, MO) to reveal staining. Following the DAB reaction, all sections were mounted on charged glass slides, counterstained in 0.5% Cresyl violet, dehydrated, cleared with xylene and coverslipped with Eukit mounting medium (Calibrated Instruments, Hawthorne, NY).
TH positive neurons and Nissl stained neurons were counted as follows in the SN pars compacta (SNc) using the optical fractionator method, an unbiased quantitative technique independent of neuronal size and shape or any conformational changes in the tissue, by an investigator blind to genotype and treatment. Sampling was performed using the Stereo Investigator software (MicroBrightField, Colchester, VT) coupled to a Leica DM-LB microscope with a Ludl XYZ motorized stage and z-axis microcator (MT12, Heidenheim, Traunreut, Germany). The SNc was delineated under a 63× objective by outlining the region of dense cellularity as illustrated in (McCormack et al., 2002) while counting was performed using a 100× objective. To ensure the exclusion of lost profiles, guard zones of 1.5 µm were used on the top and bottom of the section. The estimated total number of TH-IR neurons in the SNc was calculated based on the formula: N = Q−1 × 1/ssf × 1/asf × t/h (West et al., 1991), where N is the estimate of the total number of cells, Q−1 is the number of objects counted, ssf is the section sampling fraction, asf is the area sampling fraction, and t/h is the section thickness divided by the height of the dissector. Subregions of the substantia nigra were delineated as previously described (Fernagut et al. 2007). Briefly, after delineating the SNc, three regions corresponding to one third of the substantia nigra each were outlined; the medial part abutted the ventral tegmental area and the lateral part abutted the external tip of the cerebral peduncle. The central portion was then further divided by outlining a ventral region corresponding to one-third of the size of this central part Approximately 300 objects were counted per animal to generate the stereological estimates. Gundersen coefficients of error were less than 0.1.
Quantification of TH immunoreactivity in striatum
Rostral, medial, and caudal striatal sections were stained with primary antibody anti-TH, followed by secondary antibody anti-rabbit Cy3. Sections were scanned using a microarray scanner and scanned images were converted to grey scale and mean pixel intensity was measured using ImageJ (Meurers et al., 2009). Analysis was performed by an investigator blind to genotype and treatment condition. Images of TH immunofluorescence-labeled sections from striatum were taken using an Agilent microarray scanner equipped with a krypton/argon laser at 10 µm resolution with the photomultiplier tube set at 5%. Immunofluorescence intensity of TH staining was quantified by an investigator blind to genotype using ImageJ (NIH). Values are expressed as the mean pixel intensity for each region.
Immunohistochemistry of IBA-1
Sections of substantia nigra were washed in 0.1 M PBS, incubated in 0.5% H2O2 in methanol for 30 min, washed in PBS and incubated in 10% NGS and 0.5% Triton-X in PBS. Sections were then incubated overnight with a primary antibody against ionized calcium binding adapter molecule-1 (IBA-1) (polyclonal rabbit anti-IBA-1; 1:500 dilution; Wako Pure Chemical Industries Ltd., Japan) at 4° C in 5% NGS and 0.5% Triton-X in PBS. Sections were washed in PBS followed by a 2-hour incubation at room temperature with a biotinylated secondary antibody (goat anti-rabbit; 1:200 dilution; Vector Laboratories, Inc., Burlingame, CA) in 5% NGS in PBS. Sections were washed in PBS, incubated in avidin-biotin complex (ABC; Vector Laboratories, Burlingame, CA) for 45 minutes and washed again in PBS followed by an incubation in 0.05 M Tris buffered saline (TBS) containing 3-3'diamino benzidine (DAB; Sigma-Aldrich, St. Louis, MO) and 0.3% H2O2 (Sigma-Aldrich, St. Louis, MO) to reveal staining. Sections were washed with PBS, mounted on charged glass slides, dehydrated in ethanol, cleared with xylene and mounted with Eukit mounting medium (Calibrated Instruments, Hawthorne, NY).
Quantification of microglial activation
Microglial size was measured by an investigator blind to genotype in sections of SN from all groups. IBA-1 is expressed in microglia in both resting and activated state (Rappold et al., 2006) and different classes of IBA-1-positive cells can be distinguished based on cell body diameter (Streit et al., 1999). Microglia with 1–4 µm cell body are characterized by multiple ramified processes (resting microglia). Microglia with diameters of 5–6 µm exhibit hyper-ramification, whereas microglia with diameters of 7–14 µm are characterized by an amoeboid morphology with few, short processes (activated microglia). Accordingly, microglial activation was quantified by measuring diameters of IBA-1-positive cells. Measurements was made on a Leica DM-LB microscope with a Ludl XYZ motorized stage and z-axis microcator (MT12, Heidenheim, Traunreut, Germany) and StereoInvestigator software (MicroBrightField, Colchester, VT). Under the 5× objective lens, a contour was drawn to delineate the SN boundary, then diameters of microglial cell bodies were measured at 40× magnification in the first counting frame (100µm) and then in every fifth counting frame. This sampling frequency was selected in order to count approximately 30–50 IBA-1+ cells in each side of the SN. The first frame was positioned in the upper left corner of the contour and moved systematically from left to right and from top to bottom until the entire delineated contour region was sampled. The abundance of IBA-1+ microglia cells with cell diameters ranging from 1µm to 14µm was determined and then expressed as a percentage of total microglial cells counted in each section (Watson et al., 2012).
Assessment of TNF- by quantitative real-time PCR
RNA was isolated from microdissected fresh frozen SN using Nucleospin RNAII isolation kit (Machery-Nagel, Inc., Germany) according to the manufacturer’s instructions. RNA concentration was equalized across genotypes before cDNA synthesis using a High Capacity cDNA RT Kit (Applied Biosystems, USA). Real-time PCR primers were obtained as “Taqman® Gene Expression Assays” containing forward and reverse primers, and a FAM-labeled MGB Taqman probe for each gene (Applied Biosystems, US). Primers were purchased from Applied Biosystems and were the following accession numbers: TNF-α (Mm0043258_m1) and β-actin (Mm00607939_s1). A 1:4 dilution of cDNA was prepared and real-time PCR performed using Applied Biosystems 7900 Real-time PCR System. cDNA was mixed with qPCR™ Mastermix Plus (Applied Biosystems, US) and the respective gene assay. Mouse β-actin, was used as an endogenous controls and expression was measured using a gene expression assay containing forward and reverse primers. For quantification, real-time quantitative PCR was performed using the Applied Biosystems 7900 real-time PCR system. Forty to sixty cycles were run as follows: 10 min at 95 °C and for each cycle, 15 s at 95 °C and 1 min at 60 °C. Expression of TNF-α was calculated using the 2−ΔΔCT method and normalized to the housekeeping gene -actin.
Quantification of Th1, Th17, and regulatory T (Treg) cells
Immune cells were isolated from cervical lymph nodes and spleen of WT and PACAP KO mice 7 days following treatment with PQ [10 mg/kg] by tapping the organs through a 40µm nylon mesh. Whole blood was collected at this time point via retro-orbital bleeding and cardiac puncture into tubes containing heparin from mice deeply anesthetized with sodium pentobarbital (100 mg/kg, ip) just before perfusion. Erythrocyte lysis was performed by incubating blood and spleen cell suspensions for 10 minutes at 4 C with red blood cell lysis buffer (Sigma Aldrich, USA). Single cell suspensions from blood, spleens and lymph nodes were prepared equalized to 1 × 106 cells/ml. Cells (1 × 106 cells/sample) were washed and resuspended in 1% BSA/PBS. For quantification of Th1 and Th17 cells, cytokine intracellular staining of IFN-γ, and IL-17 were performed, respectively. Cells were incubated in RPMI with 2% fetal bovine serum (FBS) and 1% penicillin/streptomycin and with 50ng/ml PMA (Sigma-Aldrich), 1 mg/ml of ionomycin (Sigma-Aldrich) and 1X brefeldin and 1X monensin (eBioscience, San Diego, CA) for 4 hours at 37°C. After surface staining with FITC-anti-CD4 antibody (0.5 g/ l; eBioscience), cells were fixed for 15 min with 2% PFA and then permeabilized with PBS/0.2% Tween 20. Then, cells were incubated with PE-anti-IFNγ (500ug/ml; BD Bioscience) and Pe-anti-IL-17 (200ug/ml BD Bioscience) antibodies in PBS/0.2% Tween 20/5% FBS/2% BSA for 1h at 4°C at the dilutions recommended by the manufacturer (eBioscience). For Treg quantification, cells were stained with FITC-anti-CD4 (0.5 g/ l; eBioscience) and PE-anti-CD25 (0.2 g/ l; eBioscience). These cells were then fixed and permeabilized with fix/perm solution. Cells were washed in 1% BSA/PBS and stained intracellularly with APC-anti-FOXP3 (0.2 g/ l; eBioscience). Cells were centrifuged at 1800rpm for 10 min and the pellets were re-suspended in 300 µl of 1% BSA/PBS. Flow cytometric analysis was performed using a BD FACS Calibur with FloJo software. Weasel program was used for flow cytometric analysis. For Th1/Th17 profile, the T lymphocyte population was gated on the unstained sample in the FS and SS dotplot identified as it is low in this dotplot. In the case of Th1 and Th17 cells, the quadrants were set up using the single positive stains for CD4, IFN and IL-17. The quadrants were fixed and samples were run. For T reg cells, the T lymphocyte population was gated on the unstained sample in the FS and SS dotplot. The quadrants were set up using the single positive stains for CD4, CD25 and Foxp3. The quadrants were fixed and samples were run.
Statistics
Two-way repeated measures (RM) ANOVAs were performed on all data. Post hoc tests performed were Fisher’s least significant difference or planned comparison Student’s t test. To determine the degree of microglial activation in PACAP KO and WT mice treated with saline and PQ, we used the bootstrapping method (Efron and Tibshirani, 1991) using the MATLAB program, which requires no special probability assumptions regarding the shape of the populations being sampled from, and thus is fitting to use for populations that are not normally distributed, as described in (Watson et al., 2012). The frequency of distribution of the WT means was our test statistic, M. We re-sampled this test statistic by using a box model for simulation, and generated psuedo M values. This resampling process was repeated 1000 times, and the pseudo M values of the WT microglial diameters were plotted onto a histogram. The 2.5% and 97.5% cut-off values of the pseudo M values were calculated to find the 95% confidence interval (CI). These 95% CI bands were utilized to assess whether the actual mean microglial diameters of the PACAP KO mice were statistically significant at the p<0.05 level, independent of assumptions about probability distributions. Statistical analyses were conducted using MATLAB (MathWorks, Natick, MA, USA) and GB-STAT software (Dynamic Microsystems, Inc. Silver Spring, MD). The level of significance was set at p<0.05.
Results
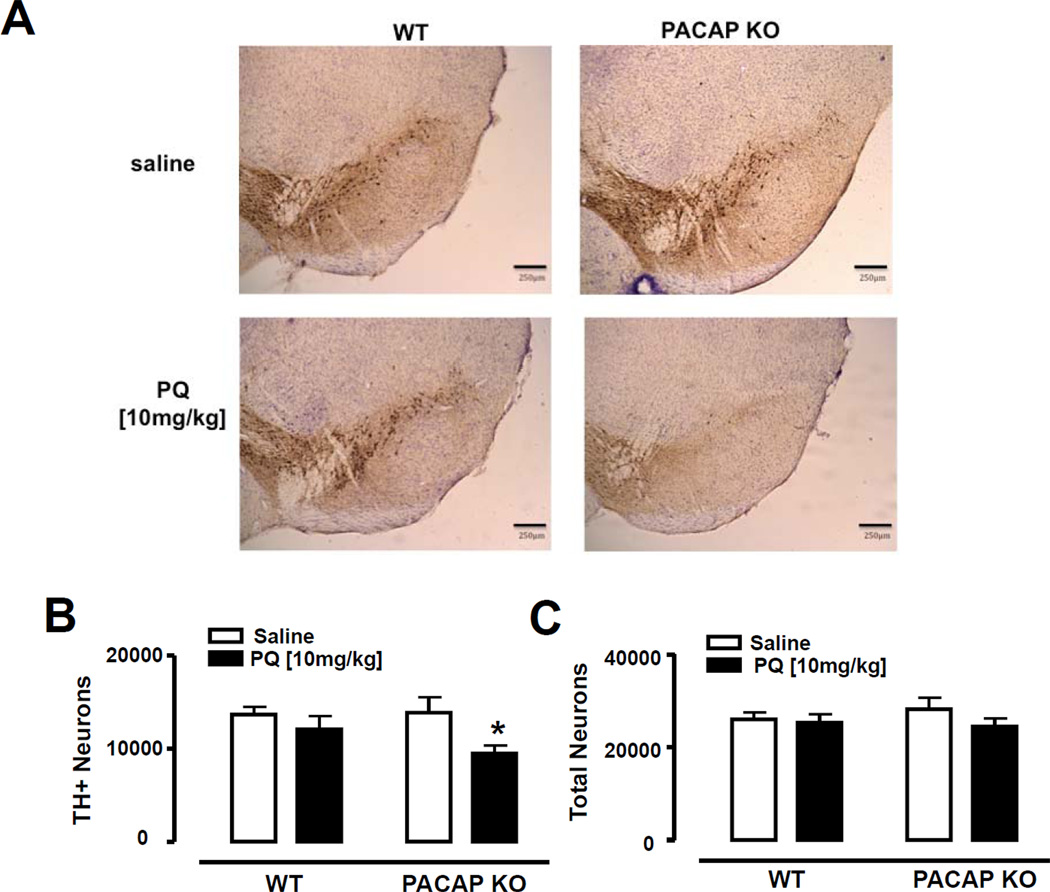
PQ treatment did not cause mortality in either WT or PACAP KO mice. Consistent with previous data (McCormack et al., 2002), a single dose of PQ [10 mg/kg] in WT mice did not affect the number of THpositive cells in the SNc (Fig. 1B). In contrast, in PACAP KO mice, this subthreshold dose of PQ was sufficient to cause a conspicuous loss of TH immunoreactivity in the SNc in several animals (Fig. 1A), with a 30% reduction in the number of TH-positive neurons in PACAP KO mice compared with salinetreated PACAP KO mice (p<0.05; Fisher’s LSD) (Fig. 1 B). Subregion analysis indicated a trend for a reduction in all SNc subdivisions, with a statistically-significant decrease in the medial and lateral subdivisions (Table 1). To determine if treatment with PQ also resulted in an overall loss of neurons, we counted cresyl violet counterstained cells that exhibited a neuronal morphology. We found that there was no significant differences between any of the groups in the numbers of cresyl violet stained neurons in the SNc (Fig. 1C), or in SNc subdivisions as delineated at each level as illustrated in McCormack et al., 2002 (data not shown), suggesting that the loss of TH in PQ-treated PACAP KO mice might be due to a failure to maintain expression of the TH protein rather than overall loss of DA neurons.
Figure 1. Loss of TH in SNpc of paraquat-treated PACAP KO mice.
Stereological counting of THpositive (TH+) and Total neurons (TH+Nissl+) in the substantia nigra pars compacta (SNpc) after administration of saline (white bars) or paraquat [10 mg/kg] (black bars) in WT and PACAP KO mice. (A) Representative images of SNpc in saline and paraquat treated WT and PACAP KO mice (blue:Nissl, brown:TH). Stereological estimates of TH+ (B) and Nissl+ (C) in the whole SNpc. *p<0.05 compared with corresponding saline group; using two-way ANOVA followed by Fisher’s LSD test, n=5–6 mice per group. Scale bar 250 µm.
Table 1.
Regional analysis of TH-IR neurons in SNc
| Mean | SEM | ||
|---|---|---|---|
| Lateral SNc | WT + Saline | 1048.56 | 156.53 |
| WT + PQ | 1139.31 | 152.26 | |
| PACAP + Saline | 1222.71 | 259.20 | |
| PACAP + PQ | 798.98 * | 162.98 | |
| Ventral SNc | WT + Saline | 2434.88 | 251.30 |
| WT + PQ | 2286.63 | 326.04 | |
| PACAP + Saline | 2582.70 | 633.32 | |
| PACAP + PQ | 1672.41 | 276.66 | |
| Dorsal SNc | WT + Saline | 5988.51 | 557.73 |
| WT + PQ | 4793.12 | 809.65 | |
| PACAP + Saline | 5021.06 | 395.34 | |
| PACAP + PQ | 4059.72 | 492.42 | |
| Medial SNc | WT + Saline | 4201.96 | 308.30 |
| WT + PQ | 3886.81 | 488.20 | |
| PACAP + Saline | 4909.54 | 753.48 | |
| PACAP + PQ | 2948.21* | 378.54 |
p < 0.05 compared with PQ + Saline. Two way ANOVA followed by Fisher‘s LSD
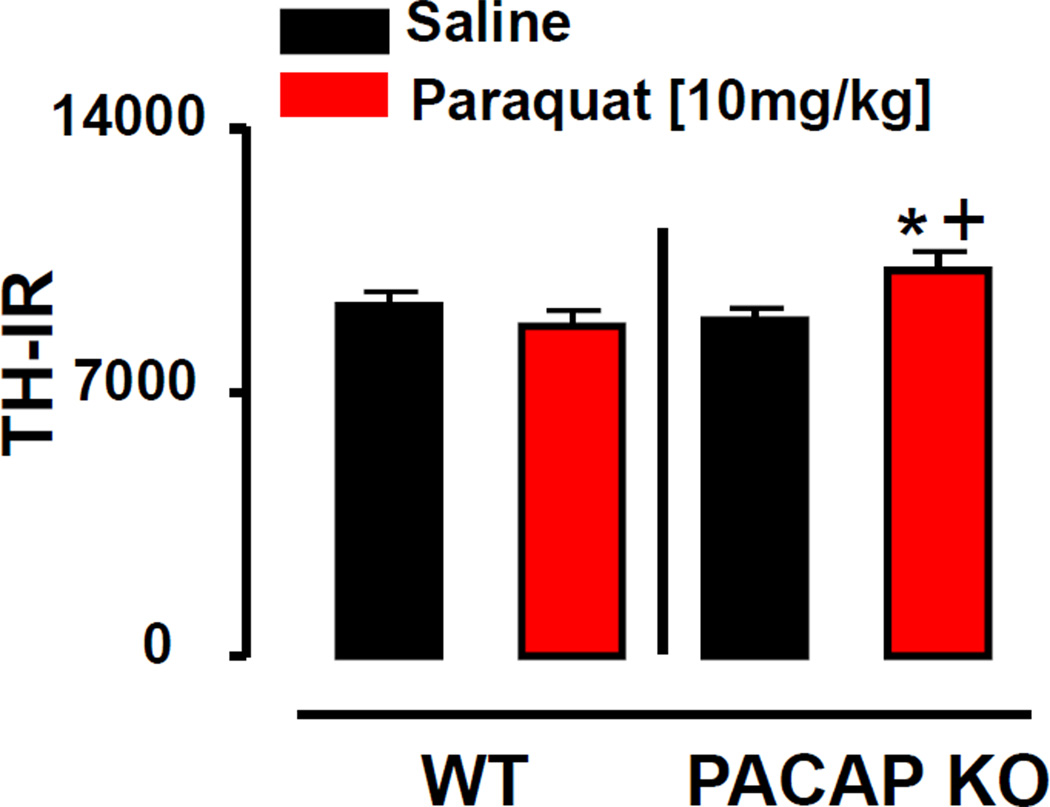
In addition to the changes in the SN, we also sought to determine whether treatment with PQ imparted an effect on the terminals of the DA system in PACAP KO mice. Similar to the effect in the SNc, we found no effect of PQ on TH immunoreactivity in the striatum in WT mice. In contrast, PQ induced significantly higher levels of TH immunoreactivity in the striatum of PACAP KO mice compared to either saline-treated PACAP KO mice or saline- or PQ-treated WT mice. (p<0.05; Fisher’s LSD; Fig. 2).
Figure 2. Increased TH immunoreactivity in striatum of paraquat-treated PACAP KO mice.
TH Immunoreactivity (TH-IR) in the striatum after administration of saline (white bars) or paraquat [10 mg/kg] (black bars) in WT and PACAP KO mice. *p<0.05 compared with corresponding saline group; +p<0.05 compared with WT mice; using two-way ANOVA followed by Fisher’s LSD test, n=6 mice per group.
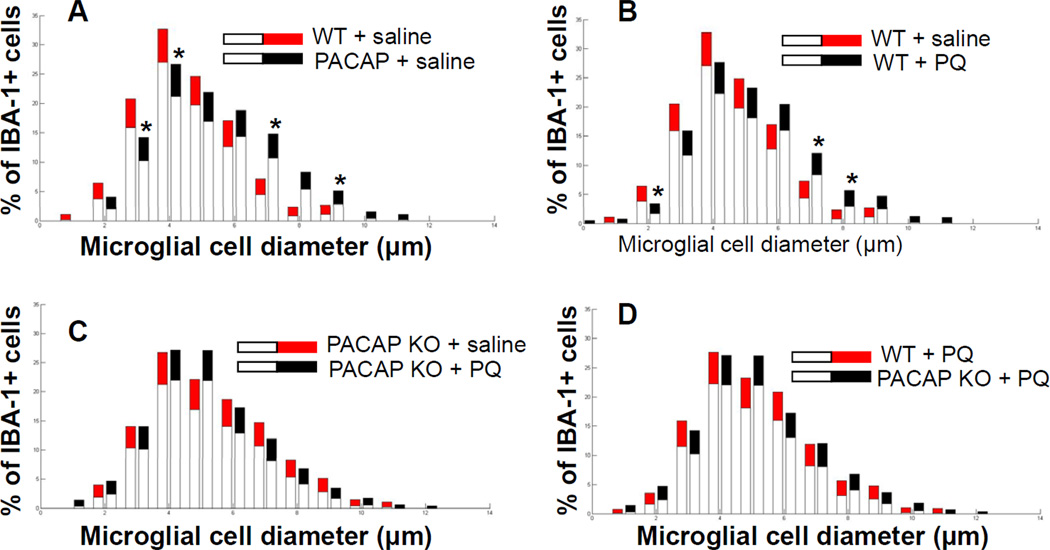
Recent studies have suggested that the effects of PQ on the DA system are mediated through microglial activation (Purisai et al., 2007). Therefore, we assessed the morphology of microglial in the substantia nigra in WT and PACAP KO mice after saline or PQ administration. We identified microglia by immunostaining with an antibody against IBA-1, an antigen that is expressed by both resting and activated microglia (Rappold et al., 2006). Microglial activation was assessed based upon cell morphology with resting microglia exhibiting small cell bodies (mean diameters of 1–4 µm) and activated microglia exhibiting mean large cell bodies (mean diameters of 7–14 µm) (Kreutzberg, 1996). We thus measured the diameters of microglial cell bodies to determine microglial activation in substantia nigra of WT and PACAP KO mice in response to PQ, quantified the cell numbers for each size, and expressed the numbers as a percentage of the total microglia counted in each section. Quantitative analysis revealed a marked difference in microglia morphology in the substantia nigra of saline-treated PACAP KO mice compared with that observed in saline-treated WT mice, exemplified by an increase in large diameter microglia (activated) with a parallel decrease in the number of microglia with smaller diameters (resting) (p<0.05; Bootstrapping; Fig. 3A). PQ-treated WT mice exhibited a microglial phenotype that was qualitatively similar to, but less pronounced than that observed in salinetreated PACAP KO mice (Fig. 3B), but had no effect on the microglia activation in PACAP KO animals beyond the observed elevated basal activity (Fig. 3C). As a result of the latter, the genotype difference seen in vehicle-treated WT and PACAP KO was no longer apparent in mice treated with PQ (Fig. 3D).
Figure 3. Microglial activation in saline-treated PACAP KO mice.
Morphological analysis of microglial activation in substantia nigra in saline-treated and paraquat-treated WT and PACAP KO mice. Diameter of IBA-1+ microglial cells measured in SN of 2–3 month old WT and PACAP KO mice treated with saline or paraquat [10 mg/kg]. Significance is represented if black confidence intervals fall outside of 95% confidence interval (grey segment of bar means), calculated by bootstrapping (*p<0.05). (A) Saline-treated PACAP KO mice exhibited increased numbers of activated IBA-1+ microglial cells in substantia nigra compared with saline-treated WT mice. (B) PQ increased the numbers of activated microglia in WT mice, but (C) produced no significant alterations in PACAP KO animals. (D) No difference between genotypes in microglial profiles were observed after PQ treatment (Values are mean ± 95% CI, n=6).
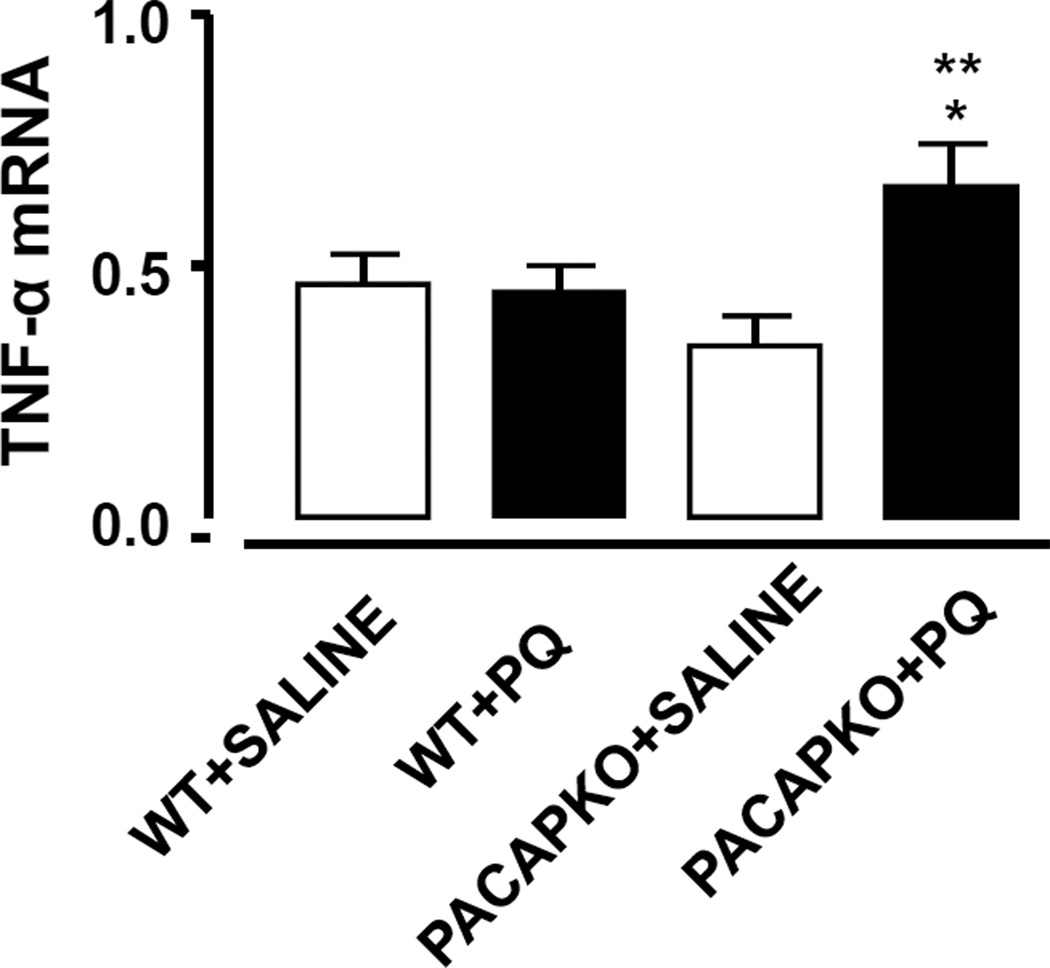
Pro-inflammatory cytokines may contribute to the neurodegenerative response induced by PQ by inducing the release of oxidative species or other mechanisms. In the present investigation, PQ induced a significant increase in TNF-α mRNA expression in the SN of PACAP KO mice compared with salinetreated PACAP KO mice, and no such induction was observed in WT mice (p<0.01; Fisher’s LSD; Fig. 4).
Figure 4. Increased TNF-α mRNA expression in SN of paraquat-treated PACAP KO mice.
TNF-α mRNA expression as measured by QPCR was significantly increased in SN of 2–3 month old paraquattreated PACAP KO mice compared with saline-treated PACAP KO mice (**p<0.01, ANOVA followed by Fisher’s LSD). This effect was also significantly different than paraquat-treated WT mice (+p<0.05, ANOVA followed by Fisher’s LSD). Values are mean ± SEM, n= 6–9).
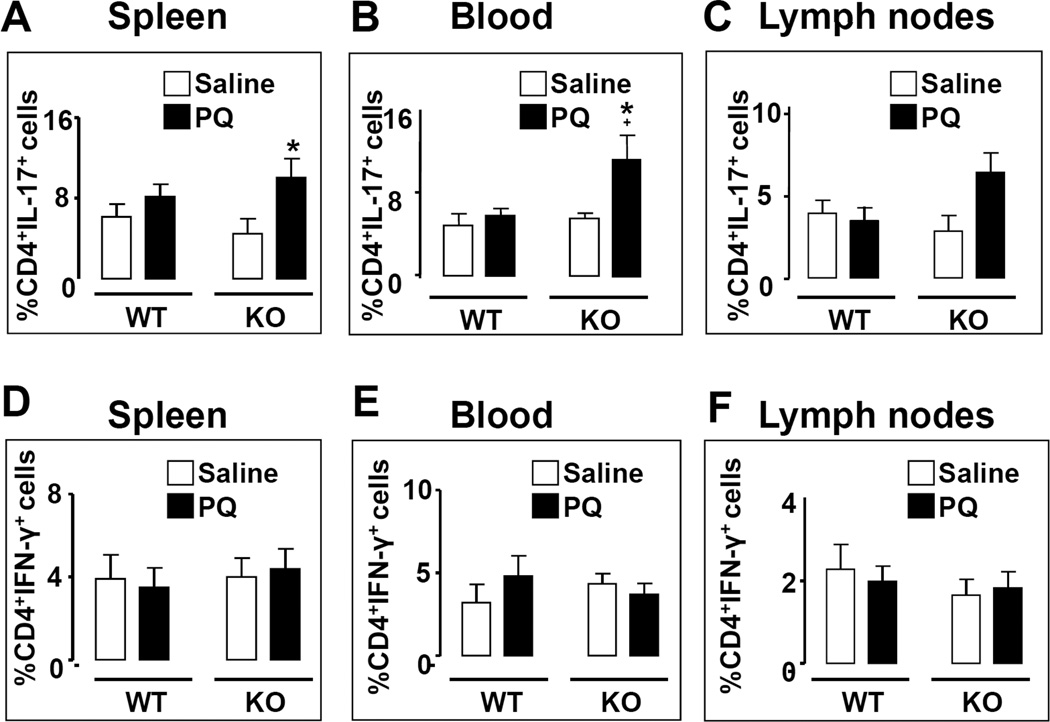
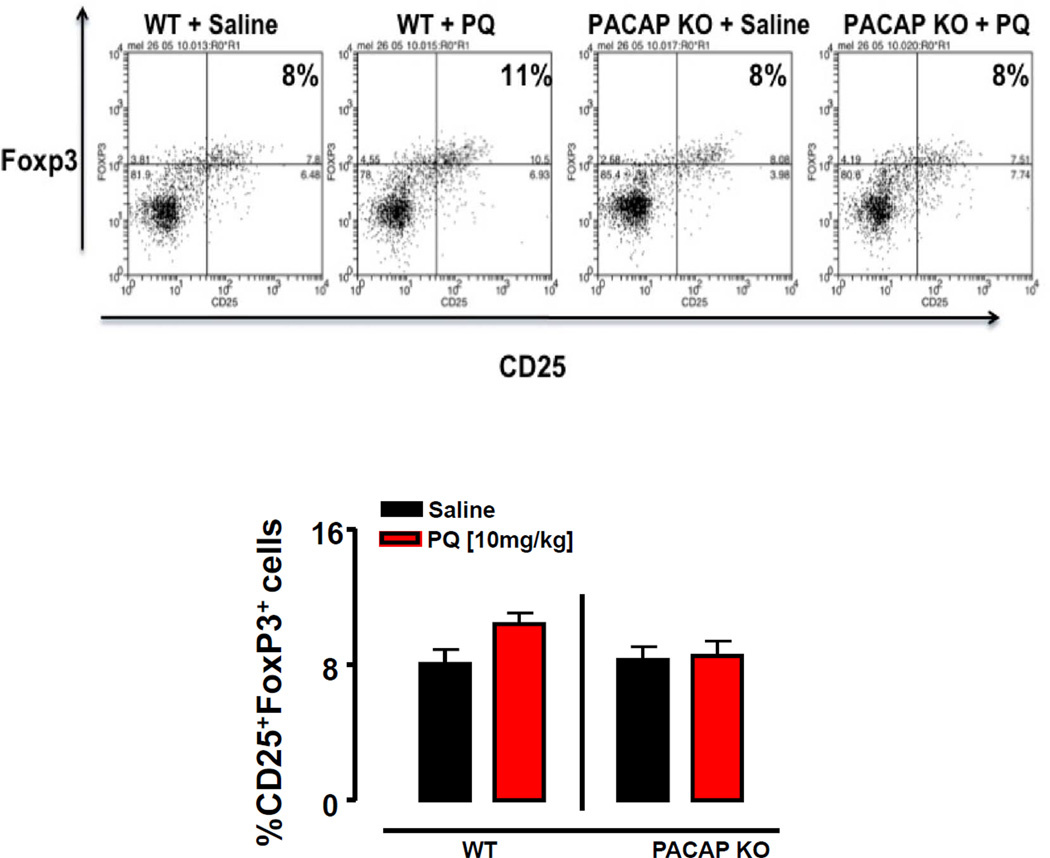
Recent studies have shown that T lymphocytes may also be involved in PD pathogenesis. For example, the presence of CD8+ and CD4+ T cells has been demonstrated in post mortem PD brain samples specifically in the SNc (Brochard et al., 2009). Moreover, PD patients were found to exhibit and higher ratios of Th1 to Th2 cells in the blood, as well as reduced numbers and percentages of Tregs (Baba et al., 2005). We thus determined the abundances of the proinflammatory subsets (Th1 and Th17 cells) and Tregs at peripheral sites of saline- and PQ-treated WT and PACAP KO mice. PQ was found to have no effect on the abundances of Th1 and Th17 cells in the spleen, blood, and lymph nodes of WT mice (Fig. 5A–F). In contrast, PQ selectively increased the numbers of Th17 lymphocytes in both the spleen and blood of PACAP KO mice, but not significantly in their lymph nodes (Fig 5B and C). Finally, Tregs were found to be induced in WT mice by PQ specifically in the lymph nodes, an effect which was abolished in PACAP KO mice (Fig. 6A, B; p<0.05; Fisher’s LSD). Thus, the peripheral inflammatory response to PQ was shifted towards a more pro-inflammatory phenotype in PACAP-deficient mice, with increased Th17 and reduced Treg abundances, in line with that observed with these mice in the experimental autoimmune encephalomyelitis model (Tan et al., 2009).
Figure 5. FACS analysis of peripheral Th1 and Th17 lymphocytes in WT and PACAP KO mice 7 days after a single dose of paraquat.
Lymphocytes were isolated from spleen (A, D), blood (B, E), and lymph nodes (C, F) of WT and PACAP KO mice 7 days following treatment with PQ [10 mg/kg] and stained with antibodies to CD4, IFN-γ, and IL-17 as described in the methods. Flow cytometric analysis was performed using a BD FACS Calibur with Weasel software. % Th1 and % Th17 represent the % of the CD4+ population that are IFN-γ+ and IL-17+, respectively. Panels A–C: Th17 cells; Panels D–F: Th1 cells. * p<0.05 compared with saline-treated;+ p<0.05 compared with WT PQ treated, planned comparison Student’s t test, n=5–6 mice per group.
Figure 6. Paraquat increases T regulatory cells in the lymph nodes of WT mice.
Measurement of T regulatory cells (Tregs) in the lymph nodes after administration of saline (black bars) or paraquat [10 mg/kg] (red bars) in WT and PACAP KO mice. (A) Representative dot plots of CD4+CD25+Foxp3+ (Tregs). (B) Mean percentages of Tregs (represented as the % of the CD4+ cell population that are CD25+Foxp3+) in the lymph nodes of saline and paraquat treated WT and PACAP KO mice. +p<0.05 compared with saline-treated WT mice, planned comparison Student’s t test, n=5–6 mice per group.
Discussion
In the present study we showed that dopaminergic neurons of the substantia nigra are more susceptible to a subthreshold dose of PQ in PACAP KO mice compared to their WT littermates. Indeed, a single dose of PQ that did not affect TH expression in nigrostriatal DA neurons in WT mice resulted in a 30% decrease in the number of TH-positive neurons in PACAP KO mice. We detected only a trend for a reduction in the number of neurons in the SNc, implying that the decreased TH immunoreactivity observed in PQ-treated PACAP KO mice is due to a loss of TH expression rather than actual neuronal loss. One interpretation of this is that PACAP normally functions to protect the phenotypic integrity of DA neurons after exposure to subtoxic doses of PQ. The maintenance of normal TH immunoreactivity after single dose PQ in WT but not PACAP KO mice is compatible with previous observations that exogenous PACAP promotes TH expression in PC12 pheochromocytoma cells at the level of both mRNA and protein (Corbitt et al., 1998). Whether endogenous PACAP can significantly protect against the loss of DA neurons induced by more aggressive PQ administration protocols or by other neurotoxins, as suggested by studies in which exogenous PACAP was administered (Reglodi et al., 2011), remains uncertain. However, our model of subtoxic PQ administration is more likely to reflect environmental exposure in agricultural or residential settings that have been shown to increase PD risk (Costello et al., 2009; Tanner et al., 2011) compared to the acute toxicity of higher doses of neurotoxic agents.
PQ also induced selectively in PACAP KO mice a change in TH expression in the striatum. However, in contrast to the observed reduction of TH+ cells in the SN in these mice, TH immunoreactivity in the striatum was slightly, but significantly enhanced. An increase in TH immunoreactivity at the level of terminals has been suggested to correspond to a compensatory mechanism occurring in early injured or degenerating DA neuron, and might function to maintain adequate dopamine levels in the target sites (Ossowska et al., 2005). For example it was reported that three doses of PQ 10 mg/kg to mice separated by one week resulted in a significant reduction of THpositive neurons in the SNpc, but a significant increase in TH activity in the striatum (McCormack et al., 2002). Potential mechanisms for such a compensatory increase would be decreased TH degradation in axon terminals and/or sprouting of remaining dopaminergic terminals.
The mechanism by which PQ induces selective DA cell damage in rodents is not fully known, however, microglial activation is suggested to play a role (Purisai et al., 2007). Microglial activation is a pathological feature associated with neurodegeneration in the PD brain (Forno et al., 1992; Hirsch, 2000). Interestingly, PACAP appeared to protect DA neurons from endotoxin exposure in rat mesencephalic neuron-glia cultures via inhibition of microglial activity (Yang et al., 2006), and PACAP has been shown to inhibit the endotoxin-induced release of proinflammatory mediators from microglia (Delgado et al., 2003). Here, using rigorous quantitative assessment of microglial diameters (Watson et al., 2012), we observed a higher mean number of activated microglia in WT mice treated with low dose PQ. PACAP KO mice, however, already exhibit signs of increased microglial activation in the basal (untreated) state. The altered morphology of microglia in untreated PACAP KO mice suggests these cells may exist in a ‘primed’ state in these mice. Consistent with this possibility, an increase in gene expression of the pro-inflammatory cytokine TNF-α was induced by PQ in the SN of PACAP KO mice, whereas, no induction was observed in WT animals. Polymorphisms in inflammatory genes, including TNF-α, are reported to be risk factors for PD (Wahner et al., 2007).
Patients with PD are reported to have alterations in immune cells in the periphery (Baba et al., 2005). Thus, in addition to the observed inflammatory changes in the brain, we measured the effects of single dose PQ on immune cells in the periphery. We focused on proinflammatory Th subsets and on Tregs because 1) numbers of these cells are reported to be altered in PD patients (Baba et al., 2005), 2) adoptive transfer of Tregs was reported to protect mice from MPTP-induced DA neuron loss in the SN, an effect potentiated by pretreatment of donor mice with the closely-related peptide VIP (Reynolds et al., 2007), and 3) PACAP loss has been shown to effect Th responses and Treg abundance in at least one other inflammatory setting (Tan et al., 2009). We found that PQ treatment induced a significant increase in the percentages of Tregs in the lymph nodes of WT mice, an effect that was abolished in PACAP KO mice. This suggests that WT mice respond to pesticide exposure with a PACAP-dependent upregulation of Tregs. In parallel, the abundance of Th17 cells, a highly inflammatory Th subset, was elevated specifically in PACAP KO mice. Although the link between these peripheral changes in lymphocytes and the observed loss of TH expressing neurons in PQ-treated PACAP KO mice remains to be determined, taken with the observation of enhanced microglia activation, the results suggest that heightened inflammatory responses may underlie the toxic effect of PQ in these mice and that the anti-inflammatory effects of PACAP may counteract this mechanism.
The present results clearly demonstrate an interaction between a gene (encoding PACAP), and an environmental exposure (PQ) implicated in PD. The interplay between environmental and genetic factors is emerging as a major modulator of PD risk (Hamza et al., 2012; Fitzmaurice et al. 2012; Ritz et al., 2012; Ritz et al., 2009), and our results extend the range of potential genes that may interact with environmental exposures to modulate the risk of PD in exposed individuals. In addition, the present data further support a role for modulators of inflammation in PD risk, and suggest that genetic factors leading to differences in PACAP levels or function may contribute to the inflammatory burden, pointing to the importance of examining such factors in epidemiological and genetic studies of PD.
An interplay between environmental factor and a gene is demonstrated in a mouse model
PACAP loss increases sensitivity to pesticide implicated in Parkinson's Disease
Alterations in microglia, Th17 and regulatory T cell activities are demonstrated
Acknowledgements
Grant support for this project was received from PHS grants P01ES016732 (the UCLA Center for Gene Environment in Parkinson's Disease), NS-P50 NS38367 (UCLA Morris K. Udall Parkinson Disease Research Center of Excellence), PHS NS070580, the American Parkinson’s Disease Association UCLA Advanced Center for Parkinson’s Disease Research and gifts to the UCLA Center for the Study of Parkinson’s Disease, and the National Multiple Sclerosis Society RG4859.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong BD, Abad C, Chhith S, Cheung-Lau G, Hajji OE, Nobuta H, Waschek JA. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience. 2008;151:63–73. doi: 10.1016/j.neuroscience.2007.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord. 2005;11:493–498. doi: 10.1016/j.parkreldis.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Baldi I, Cantagrel A, Lebailly P, Tison F, Dubroca B, Chrysostome V, Dartigues JF, Beaudet MM, Braas KM, May V. Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol. 1998;36:325–336. [PubMed] [Google Scholar]
- Brochard P. Association between Parkinson's disease and exposure to pesticides in southwestern France. Neuroepidemiology. 2003;22:305–310. doi: 10.1159/000071194. [DOI] [PubMed] [Google Scholar]
- Bobrovskaya L, Gelain DP, Gilligan C, Dickson PW, Dunkley PR. PACAP stimulates the sustained phosphorylation of tyrosine hydroxylase at serine 40. Cell Signal. 2007;19:1141–1149. doi: 10.1016/j.cellsig.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Bower JH, Maraganore DM, Peterson BJ, Ahlskog JE, Rocca WA. Immunologic diseases, anti-inflammatory drugs, and Parkinson disease: a case-control study. Neurology. 2006;67:494–496. doi: 10.1212/01.wnl.0000227906.99570.cc. [DOI] [PubMed] [Google Scholar]
- Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest. 2009;119:182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Res. 1999;823:1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14:1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Waschek JA. Selective deficits in the circadian light response in mice lacking PACAP. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1194–R1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- Corbitt J, Vivekananda J, Wang SS, Strong R. Transcriptional and posttranscriptional control of tyrosine hydroxylase gene expression during persistent stimulation of pituitary adenylate cyclase-activating polypeptide receptors on PC12 cells: regulation by protein kinase A-dependent and protein kinase A-independent pathways. J Neurochem. 1998;71:478–486. doi: 10.1046/j.1471-4159.1998.71020478.x. [DOI] [PubMed] [Google Scholar]
- Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol. 2009;169:919–926. doi: 10.1093/aje/kwp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M, Leceta J, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit the production of inflammatory mediators by activated microglia. J Leukoc Biol. 2003;73:155–164. doi: 10.1189/jlb.0702372. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Statistical data analysis in the computer age. Science. 1991;253:390–395. doi: 10.1126/science.253.5018.390. [DOI] [PubMed] [Google Scholar]
- Engel LS, Checkoway H, Keifer MC, Seixas NS, Longstreth WT, Jr, Scott KC, Hudnell K, Anger WK, Camicioli R. Parkinsonism and occupational exposure to pesticides. Occup Environ Med. 2001;58:582–589. doi: 10.1136/oem.58.9.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut NA, Salcedo J, Masliah E, Chesselet MF. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein over-expression. Synapse. 2007;61:991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzmaurice AG, Rhodes SL, Lulla A, Murphy NP, Lam HA, O'Donnell KC, Barnhill L, Casida JE, Cockburn M, Sagasti A, Stahl MC, Maidment NT, Ritz B, Bronstein JM. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110:636–641. doi: 10.1073/pnas.1220399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno LS, DeLanney LE, Irwin I, Di Monte D, Langston JW. Astrocytes and Parkinson's disease. Prog Brain Res. 1992;94:429–436. doi: 10.1016/s0079-6123(08)61770-7. [DOI] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ghatei MA, Takahashi K, Suzuki Y, Gardiner J, Jones PM, Bloom SR. Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues. J Endocrinol. 1993;136:159–166. doi: 10.1677/joe.0.1360159. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Peterson EL, Rybicki BA, Johnson CC. Multiple risk factors for Parkinson's disease. J Neurol Sci. 2004;217:169–174. doi: 10.1016/j.jns.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Guillot TS, Richardson JR, Wang MZ, Li YJ, Taylor TN, Ciliax BJ, Zachrisson O, Mercer A, Miller GW. PACAP38 increases vesicular monoamine transporter 2 (VMAT2) expression and attenuates methamphetamine toxicity. Neuropeptides. 2008;42:423–434. doi: 10.1016/j.npep.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza TH, Chen H, Hill-Burns EM, Rhodes SL, Montimurro J, Kay DM, Tenesa A, Kusel VI, Sheehan P, Eaaswarkhanth M, et al. Genome-wide gene-environment study identifies glutamate receptor gene GRIN2A as a Parkinson's disease modifier gene via interaction with coffee. PLoS Genet. 2012;7:e1002237. doi: 10.1371/journal.pgen.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch EC. Glial cells and Parkinson's disease. J Neurol. 2000;247(Suppl 2):II58–II62. doi: 10.1007/pl00007762. [DOI] [PubMed] [Google Scholar]
- Houchi H, Azuma M, Kitamura K, Okuno M, Oka M. Pituitary adenylate cyclase-activating polypeptide stimulates the synthesis of dopamine in cultured bovine adrenal chromaffin cells. Hypertens Res. 1995;18(Suppl 1):S169–S171. doi: 10.1291/hypres.18.supplementi_s169. [DOI] [PubMed] [Google Scholar]
- Kambe Y, Miyata A. Role of Mitochondrial Activation in PACAP Dependent Neurite Outgrowth. J Mol Neurosci. 2012;48:550–557. doi: 10.1007/s12031-012-9754-0. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Manthripragada AD, Schernhammer ES, Qiu J, Friis S, Wermuth L, Olsen JH, Ritz B. Non-steroidal anti-inflammatory drug use and the risk of Parkinson's disease. Neuroepidemiology. 2011;36:155–161. doi: 10.1159/000325653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Thiruchelvam M, Manning-Bog AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson's disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- Meurers BH, Zhu C, Fernagut PO, Richter F, Hsia YC, Fleming SM, Oh M, Elashoff D, Dicarlo CD, Seaman RL, Chesselet MF. Low dose rotenone treatment causes selective transcriptional activation of cell death related pathways in dopaminergic neurons in vivo. Neurobiol Dis. 2009;33:182–192. doi: 10.1016/j.nbd.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Mogi M, Nagatsu T. Neurotrophins and cytokines in Parkinson's disease. Adv Neurol. 1999;80:135–139. [PubMed] [Google Scholar]
- Moser A, Scholz J, Gansle A. Pituitary adenylate cyclase-activating polypeptide (PACAP-27) enhances tyrosine hydroxylase activity in the nucleus accumbens of the rat. Neuropeptides. 1999;33:492–497. doi: 10.1054/npep.1999.0768. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl. 2000a;60:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson's disease. J Neural Transm Suppl. 2000b;58:143–151. [PubMed] [Google Scholar]
- Ohtaki H, Nakamachi T, Dohi K, Aizawa Y, Takaki A, Hodoyama K, Yofu S, Hashimoto H, Shintani N, Baba A, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc Natl Acad Sci U S A. 2006;103:7488–7493. doi: 10.1073/pnas.0600375103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowska K, Wardas J, Smialowska M, Kuter K, Lenda T, Wieronska JM, Zieba B, Nowak P, Dabrowska J, Bortel A, et al. A slowly developing dysfunction of dopaminergic nigrostriatal neurons induced by long-term paraquat administration in rats: an animal model of preclinical stages of Parkinson's disease? Eur J Neurosci. 2005;22:1294–1304. doi: 10.1111/j.1460-9568.2005.04301.x. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yagi S, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson's disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S200–S204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- Peng J, Mao XO, Stevenson FF, Hsu M, Andersen JK. The herbicide paraquat induces dopaminergic nigral apoptosis through sustained activation of the JNK pathway. J Biol Chem. 2004;279:32626–32632. doi: 10.1074/jbc.M404596200. [DOI] [PubMed] [Google Scholar]
- Purisai MG, McCormack AL, Cumine S, Li J, Isla MZ, Di Monte DA. Microglial activation as a priming event leading to paraquat-induced dopaminergic cell degeneration. Neurobiol Dis. 2007;25:392–400. doi: 10.1016/j.nbd.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold PM, Lynd-Balta E, Joseph SA. P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Res. 2006;1089:171–178. doi: 10.1016/j.brainres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Kiss P, Lubics A, Tamas A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des. 2011;17:962–972. doi: 10.2174/138161211795589355. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Kiss P, Szabadfi K, Atlasz T, Gabriel R, Horvath G, Szakaly P, Sandor B, Lubics A, Laszlo E, Farkas J, Matkovits A, Brubel R, Hashimoto H, Ferencz A, Vincze A, Helyes Z, Welke L, Lakatos A, Tamas A. PACAP is an endogenous protective factor-insights from PACAP-deficient mice. J Mol Neurosci. 2012;48:482–492. doi: 10.1007/s12031-012-9762-0. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Lubics A, Tamas A, Szalontay L, Lengvari I. Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson's disease. Behav Brain Res. 2004a;151:303–312. doi: 10.1016/j.bbr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Reglodi D, Tamas A, Lubics A, Szalontay L, Lengvari I. Morphological and functional effects of PACAP in 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Regul Pept. 2004b;123:85–94. doi: 10.1016/j.regpep.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson's disease. J Leukoc Biol. 2007;82:1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- Ritz B, Rhodes SL, Bordelon Y, Bronstein J. alpha-Synuclein genetic variants predict faster motor symptom progression in idiopathic Parkinson disease. PLoS One. 2012;7:e36199. doi: 10.1371/journal.pone.0036199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz BR, Manthripragada AD, Costello S, Lincoln SJ, Farrer MJ, Cockburn M, Bronstein J. Dopamine transporter genetic variants and pesticides in Parkinson's disease. Environ Health Perspect. 2009;117:964–969. doi: 10.1289/ehp.0800277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler RA, Gao Y, Zukin RS, Vosler PS, Zhang L, Zhang F, Cao G, Bennett MV, Chen J. Apurinic/apyrimidinic endonuclease APE1 is required for PACAP-induced neuroprotection against global cerebral ischemia. Proc Natl Acad Sci U S A. 2010;107:3204–3209. doi: 10.1073/pnas.1000030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Takei N, Skoglosa Y, Lindholm D. Neurotrophic and neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on mesencephalic dopaminergic neurons. J Neurosci Res. 1998;54:698–706. doi: 10.1002/(SICI)1097-4547(19981201)54:5<698::AID-JNR15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tan YV, Abad C, Lopez R, Dong H, Liu S, Lee A, Gomariz RP, Leceta J, Waschek JA. Pituitary adenylyl cyclase-activating polypeptide is an intrinsic regulator of Treg abundance and protects against experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2009;106:2012–2017. doi: 10.1073/pnas.0812257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, et al. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton TG, Heckbert SR, Longstreth WT, Jr, Rossing MA, Kukull WA, Franklin GM, Swanson PD, Smith-Weller T, Checkoway H. Nonsteroidal anti-inflammatory drugs and risk of Parkinson's disease. Mov Disord. 2006;21:964–969. doi: 10.1002/mds.20856. [DOI] [PubMed] [Google Scholar]
- Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch Neurol. 2007;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- Wang A, Costello S, Cockburn M, Zhang X, Bronstein J, Ritz B. Parkinson's disease risk from ambient exposure to pesticides. Eur J Epidemiol. 2011;26:547–555. doi: 10.1007/s10654-011-9574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Pan J, Tan YY, Sun XK, Zhang YF, Zhou HY, Ren RJ, Wang XJ, Chen SD. Neuroprotective effects of PACAP27 in mice model of Parkinson's disease involved in the modulation of K(ATP) subunits and D2 receptors in the striatum. Neuropeptides. 2008;42:267–276. doi: 10.1016/j.npep.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, Effros RB, Chesselet MF. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp Neurol. 2012;237:318–334. doi: 10.1016/j.expneurol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Yang S, Yang J, Yang Z, Chen P, Fraser A, Zhang W, Pang H, Gao X, Wilson B, Hong JS, Block ML. Pituitary adenylate cyclase-activating polypeptide (PACAP) 38 and PACAP4-6 are neuroprotective through inhibition of NADPH oxidase: potent regulators of microglia-mediated oxidative stress. J Pharmacol Exp Ther. 2006;319:595–603. doi: 10.1124/jpet.106.102236. [DOI] [PubMed] [Google Scholar]