Abstract
Dry eye is a common ocular surface disease of multifactorial etiology characterized by elevated tear osmolality and inflammation leading to a disrupted ocular surface. The latter is a risk factor for ocular surface infection, yet overt infection is not commonly seen clinically in the typical dry eye patient. This suggests that important innate mechanisms operate to protect the dry eye from invading pathogens. This article reviews the current literature on epidemiology of ocular surface infection in dry eye patients and laboratory-based studies on innate immune mechanisms operating at the ocular surface and their alterations in human dry eye and animal models. The review highlights current understanding of innate immunity in dry eye and identifies gaps in our knowledge to help direct future studies to further unravel the complexities of dry eye disease and its sequelae.
Keywords: antimicrobial peptides, contact lens, cornea, conjunctiva, cyclosporine, dry eye, infection, innate immunity, ocular surface inflammation, tears
I. Introduction
Dry eye is defined as a multifactorial disease that is caused by a decrease in tear production or an increase in tear evaporation and is associated with elevated tear osmolarity and symptoms of ocular irritation.1 Patients often report a variety of symptoms, including ocular burning, itching, foreign body sensation, photophobia, redness, and reduced visual acuity.2 Dry eye is thought to be one of the most common causes for a patient to consult an eye care professional.3 Prevalence rates are greatest among women and the elderly population,4 with various epidemiological studies estimating a 5–30% prevalence of dry eye in people who are 50 years and older.2 Dry eye often has a significant impact on the patient’s visual function and overall quality of life,5–7 and the use of various therapeutic and palliative treatment options imposes a major economic burden on the patient.4, 8, 9 Treatment can be challenging, as patients may present with ocular surface pathology but have no or few symptoms.10 Numerous factors have been associated with dry eye disease, such as poor systemic health, concurrent ocular disease, use of drying systemic medications, and the external environment.2
In dry eye, a chronic inflammatory reaction, possibly subclinical, is generated at the ocular surface, which can result in vital dye staining of the cornea and conjunctiva.11 The accumulation of inflammatory molecules at the ocular surface of dry eye patients,12–15 accompanied by a stagnant tear film15 and decreased level of mucins,16 can lead to destruction of epithelial tight junctions, and result in sloughing of the ocular surface epithelia.17 Epithelial anatomy (tight junctions, in particular) is one of several so-called innate immune response mechanisms that play a crucial role in preventing invasion of microorganisms into the ocular surface.18–20 Innate responses are the first line of defense against infection, responding immediately but in a nonspecific manner to invading pathogens.21, 22 Mucous membranes (eg, conjunctiva), secreted proteins (eg, lysozyme), mechanical barriers (eg, closure of eyelids), and the aforementioned physical barriers of the ocular surface epithelia, are just some features of the innate immune system at the ocular surface.23 These mechanisms work together to prevent the negative outcomes of organism colonization in an effort to keep the ocular surface free from infection. However, if these mechanisms are overwhelmed and/or can be circumvented, then an organism can take hold, requiring activation and participation of the adaptive immune system for effective elimination of the pathogen.
Adaptive immunity results from an encounter with a specific antigen (eg, microbial proteins, allergens) and is therefore acquired through experience.24 The adaptive system, which demonstrates memory for future encounters as well as specificity to antigens, is triggered by innate antigen-presenting cells, such as dendritic cells, and involves activity of various subsets of T lymphocytes and antibody-producing B lymphocytes, which facilitate pathogen removal and disease resolution. It should be noted that uncontrolled activity of the innate immune system can lead to a damaging inflammatory response, while erroneous activation of the adaptive system can result in autoimmune diseases. Indeed, a significant role for T lymphocytes in perpetuating dry eye inflammation itself is now recognized, and it appears that ocular surface epithelial cells secrete inflammatory molecules17, 25 in response to cytokines produced by activated T lymphocytes.26
Alterations in the ocular surface armor (ie, disrupted surface and altered tear film) in dry eye would be expected to give pathogens a “foot in the door.” However, as discussed below, the concept that dry eye patients have an increased risk for corneal infection is not well supported in the literature. This suggests that dry eye patients may have an enhanced innate immune response, preventing an organism from taking advantage of the disrupted ocular surface and reducing the risk for infection. The objective of this article is to review and discuss the evidence for innate immune mechanisms that may play a significant role in protecting the ocular surface in dry eye patients. While the 2007 Dry Eye WorkShop (DEWS) report formulated a definition for dry eye,1 there is still inconsistency in the literature as to how dry eye is defined in both population-based epidemiological studies (largely symptom-defined) and clinical trials (symptom and sign-defined). For the purposes of this review article, the terms “dry eye” and related “ocular surface disease (indicating dry eye)” were used, with the original author’s interpretation of appropriateness in using the term.
II. Dry Eye, Ocular Surface Inflammation and Corneal Infection
This section addresses evidence from published studies for an association between dry eye disease and ocular surface infection. The influence of contact lens wear is also discussed, as it is well recognized that this modality of refractive error correction is linked to both corneal infection and dry eye. Finally, reported changes in the ocular surface microbiome in dry eye are described, as they are pertinent to understanding a possible association between dry eye and infection.
A. Infection Risk in Dry Eye Patient Populations
1. Dry Eye Population in General
An association between dry eye disease and microbial keratitis is often cited in publications, including the DEWS report,27 textbooks,28 and the popular optometric/ophthalmic press. Also, in informal discussions among eye care practitioners, it is often concluded that dry eye patients are likely to have an increased risk for infection. The rationale for the association between dry eye and microbial keratitis is largely two-fold. First, dry eye disease can be associated with a quantitative reduction or qualitative alteration in the tear film, thereby causing a possible decrease in protective tear proteins.29 Changes can occur in various protective molecules (see section II. B); while some are indeed decreased, others are actually increased at the ocular surface. Secondly, dry eye is often associated with a disruption of the corneal epithelium, thus creating a possible opening for microbial invasion.30, 31
Clinically, varying degrees of fluorescein staining of the cornea and conjunctiva are seen in dry eye patients.32 While it is accepted that fluorescein staining indicates some level of compromise to the ocular surface epithelia, there continues to be much debate regarding the underlying etiology for the staining. Studies have suggested that loss of tight junction integrity allows deeper penetration and pooling of fluorescein between cells or that the dye is staining dead or damaged cells.33–35 A recent study by Mokhtarzadeh et al suggests that the punctate epithelial staining characteristic of ocular surface disease such as dry eye results from enhanced fluorescence by some epithelial cells in the superficial layers.36 The authors further speculate that these cells are likely interacting differently with fluorescein, perhaps because they are apoptotic or have lost the protective mucin barrier, a component of innate immunity.36 Fluorescein staining does not necessarily correlate with susceptibility to infection. This has been shown in studies on contact-lens related microbial keratitis37 and in mice deficient in MyD88, an adaptor molecule involved in the innate immune response.38 However, irrespective of fluorescein staining and its interpretation, studies in murine experimental dry eye and human patients indicate a disrupted ocular surface and, in severe cases, epithelial sloughing, ie, a compromise to the normal protective epithelial barrier.
Despite the controversy regarding the nature of fluorescein staining, the procedure continues to be a mainstay in the diagnosis of dry eye and is often considered one of the key features in ocular surface and lid disease.39,40 However, it is not clear whether the changes seen in staining patterns are part of the dry eye ocular surface disease process, or are a part of the inflammatory response, or a combination of both. Overall, very little literature exists to support the hypothesis that dry eye patients demonstrate an increased risk for microbial keratitis; however, it should be acknowledged that the diagnosis of microbial keratitis in patients with dry eye may well be under-reported. The few peer-reviewed published studies reporting an association between dry eye and microbial keratitis are described below.
A 2009 study of patients residing in nursing homes found that 26% of microbial keratitis cases, with Staphylococcus being the most prevalent isolate, were associated with the presence of dry eyes.41 However, most patients in this study suffered from rheumatoid arthritis (RA) and were concurrently using topical and/or systemic steroid therapy, which could increase the risk for infection, as well as alter the dry eye status.41 A case of mycobacterium keratitis was reported in a patient with Sjögren syndrome (SS)-related dry eye, but, notably, this patient also suffered from RA.42 It has been shown that RA is associated with dry eye disease, yet the link between RA and increased risk of infection in dry eye disease has not been established.
Boiko et al found an increased prevalence of dry eye in patients testing positive for Chlamydia conjunctivitis.43 However, Krasny et al noted that patients who were successfully treated for chronic follicular conjunctivitis due to Chlamydia infection demonstrated improvement in their dry eye condition,44 suggesting that, in some instances, ocular surface infection can predispose to dry eye rather than the dry eye predisposing a patient to infections. Several studies indicate that patients with dry eye-associated systemic autoimmune diseases, such as SS, RA, and ocular cicatricial pemphigoid, have an increased risk for sterile, but not infectious, corneal ulceration.45–49
Other studies also allude to a link between dry eye and microbial keratitis, but use the catch-all term “ocular surface disease,” making it difficult to determine true prevalence of dry eye in contrast to other “ocular surface diseases,” including infection and allergy. In one such study, ocular surface diseases such as herpetic corneal infection, bullous keratopathy, dry eye, blepharitis, and other eyelid disorders were shown to increase the risk for bacterial keratitis in 64 of 300 (21.3%) eyes (291 patients).50 Indeed, a history of ocular surface disease was the second most common factor associated with bacterial keratitis in this study, with contact lens wear being the primary association and acute corneal trauma the third. Of the 64 patients in this “ocular surface diseases” subset,50 28 had pre-existing keratopathies (herpetic/bullous/exposure), while the remaining 36 patients had “other disorders,” including dry eye and eyelid diseases. If all of these 36 patients originally suffered from dry eye, that would mean a 12% (36/300) risk for dry eye to predispose for bacterial corneal infections.
Keay et al found in a retrospective review of medical records that 5.8% of patients presenting with microbial keratitis had ocular surface disease as a predisposing factor.51 In a similar retrospective study for treatment of “keratitis,” Green et al found that a pre-existing history of ocular surface disease was present in 45 of 177 patients (18%) who had microbial keratitis.52 In many of these studies, ocular surface disease was not specifically defined, but it was noted that these patients tended to have more severe keratitis and took longer to recover.53 However, other factors may have contributed to the association between ocular surface disease and keratitis. In this same patient population,52 the authors reported that contact lens wear (22% of the patients) was the most common risk factor for keratitis.
Overall, from the evidence in existing literature, it is apparent that in certain situations (coexisting systemic autoimmune disease/patients on corticosteroid therapy), dry eye patients may have a slightly increased risk for bacterial infections. Surprisingly, however, there is insufficient evidence to strongly suggest that a typical dry eye patient will have an increased risk for microbial keratitis. Indeed, in a recent preliminary study where Pseudomonas aeruginosa (P. aeruginosa) was topically inoculated in a murine model of dry eye, there was no visible pathology in either dry eye mice or controls.54 The authors concluded that a dry eye state does not increase susceptibility to infection. It is possible that the animal model used was not ideal (no corneal staining was demonstrated), but the findings warrant further investigation.
2. Contact Lens Vs Non-Contact Lens Wearers
Although no firm evidence exists for an association between dry eye in general and an increased risk of microbial keratitis, can the same be said about contact lens wearers with dry eye? The risk of microbial infections in the soft contact lens-wearing population is well known and has been extensively cited in the literature.55–58 The reported incidence of microbial keratitis is equivalent regardless of soft contact lens material, varying from 5.2/10,000 people using daily wear soft contact lenses to 18.2/10,000 in those wearing reusable extended wear soft contact lenses.59,60 Contrary to expectations, silicone hydrogel contact lenses with an increased level of oxygen transmissibility are not associated with a reduced rate of keratitis compared to traditional hydrogel contact lenses, with both materials having a similar risk.61,62 As lack of oxygen does not appear to be the prime associated factor for eliciting microbial keratitis, other factors have been suggested, including impaired tear exchange, bacterial binding to contact lens material, decreased epithelial cell desquamation, and increased corneal permeability.37,63–66 Although highly oxygen-permeable silicone hydrogel lens materials are considered a risk factor, especially in a closed-eye modality, the cases of microbial keratitis occurring with these lenses have been less severe than those reported in hydrogel lens wearing populations.67
The biggest risk factor, whether with silicone hydrogel or hydrogel lenses, continues to be the modality of continuous or extended wear, fundamentally a closed-lid scenario. This may relate to factors mentioned above or could result from the combination of tear film alteration by the contact lens and tear film changes that take place in a closed-eye situation.68–71 Both appear to be independent factors that occur during lid closure; however, the relationship between the two is not as well defined. A contact lens, regardless of material, will alter the environment and therefore the ocular surface when worn on a regular basis. The cornea during contact lens wear will have a decrease in epithelial cell sloughing with a concomitant increase in epithelial cell size, as well as a decrease in epithelial thickness.72–77 The alteration of the epithelial surface may alter normal apoptosis and allow for bacteria to enter the epithelium via lipid rafts.78,79 Additionally, the post-lens tear film dynamics are altered in contact lens wear, which may affect receptor sites and allow for a potentially greater adherence of bacteria such as P. aeruginosa.79,80 Lin et al have shown that in addition to hypoxia, tear stagnation in the post-lens tear film increases corneal epithelial permeability, which may put the cornea at a greater risk for infection.81 To date, it has also been shown that sIgA, an important antimicrobial protein, decreases with closed-eye contact lens wear, and interleukin (IL)-8 increases with a lessened effect noted for both in silicone hydrogel lens wear.82
It is not known if the risk of microbial keratitis in contact lens wearers with dry eye is greater than in contact lens wearers without dry eye symptoms. Contact lens-related dry eye is a common complaint with manifest signs in a large proportion of these patients. Depending on the studied population, it has been reported that between 50% and 94% of contact lens wearers complain of dry eye symptoms,83–88 often leading to decreased wearing time or discontinuation of contact lens wear.89–92 Some studies have suggested that high-water-content soft contact lenses are associated with more pronounced symptoms of dry eye.93 The reason for this has not been clearly elucidated, although it has been suspected that it is due to increased evaporation from this type of lens. However, data to support this claim is lacking.89,94 Interestingly, while contact lens material can play a role in exacerbating the dry eye, it can also serve to ameliorate it, with studies showing a decreased occurrence of dry eye symptoms with silicone hydrogel contact lenses.88,95–97 Recently, scleral gas-permeable contact lenses have been promoted as a therapeutic option for severe dry eye,98,99 and increased occurrence of bacterial keratitis in patients using this therapeutic modality of lens wear for dry eye has not been reported.
Contact lenses can affect aqueous tear production by acting on the neurosensory loop. They can also be an extrinsic factor interrupting the normal patency of the tear film, creating an evaporative dry eye. Recent work has indicated that even silicone hydrogel lenses worn on a daily wear basis alter tear film stability and tear film components such as MUC5AC and IL-6, which have antimicrobial actions.100,101 As noted above, tear stagnation under a contact lens may put the cornea at a greater risk for infection.81 It might be suspected that tear stagnation could occur to an even greater extent in the dry eye, hence presenting an increased risk, especially in the aqueous-deficient dry eye, ie, the available volume of tears is less, so the amount of fresh tear film exchanged under the contact lens with each blink is less.
In a recent study, Berry et al compared protein expression in contact lens wearers with and without dry eye.102 Using several proteomic approaches, they found a number of potential biomarker proteins associated with the dry eye disease state in contact lens wearers. In particular, beta-2 microglobulin, proline rich 4, lacritin, and secretoglobin 1D1 were downregulated, while secretoglobin 2A2, serum albumin, glycoprotein 340, and prolactin-inducible protein were upregulated. The authors concluded that the functions of several of these proteins suggest roles in altered tear secretion in addition to possible increased susceptibility to infection.
Other factors associated with microbial keratitis include male gender, age, smoking status, humidity, and lens care system compliance and modality.62,103–105 Most risk factor profiles appear to implicate young contact lens wearers and those who have just started wearing contact lenses, neither of which are patient groups that are particularly known to exhibit dry eye disease. As is evident from this discussion, despite the known risk of microbial keratitis with contact lens wear, a relative increased risk with contact lens-related dry eye is circumstantial at best and has not been specifically investigated in epidemiologic studies.
In summary, in peer-reviewed and non-peer-reviewed literature and presentations in public forums, dry eye is often cited as a predisposing factor for infectious keratitis. Although this is widely believed as logical and possibly credible, evidence linking dry eye and infectious keratitis is minimal for both the general dry eye population and those with contact lens-related dry eye. Specific epidemiological studies to determine the risk of infectious keratitis in dry eye would be informative but costly and time-consuming, and have not been completed to date. However, as population-based studies of keratitis continue to be reported, meta-analysis of published data or nested case control study designs may be feasible to better determine the risk for infection in dry eye patients.
B. Dry Eye and Ocular Surface Microbial Load
In a recent review, Miller and Iovieno noted that the normal ocular surface harbors a diverse group of microorganisms, with Gram-positive bacteria such as Staphylococci species being the primary commensals recovered from lids, conjunctiva, and tears.106 A recent DNA sequencing-based study showed that the healthy human conjunctiva can have a wide variety of microbes, such as Pseudomonas, Propionibacterium, Bradyrhizobium, Corynebacterium, Acinetobacter, Brevundimonas, Staphylococci, Aquabacterium, Sphingomonas, Streptococcus, Streptophyta and Methylobacterium.107 Further, the microbial community remains relatively stable unless interrupted by events such as contact lens wear, exposure to preservatives, and ocular surface disease.106
A number of studies have addressed the ocular surface microbial load and changes in dry eye patients. The earliest (1975) study estimating load did not reveal the presence of adenovirus (types 3, 7, 8 and 14) or herpes simplex in 50 patients.108 However, in a more recent study, Robert et al found human herpes virus-6 (HHV-6) in the tear fluid of 2 of 28 dry eye patients.109 Albeitz and Lenton reported that the ocular surface of dry eye patients had a greater bacterial load compared to healthy patients and that SS patients had a greater bacterial load (Cornyebacterium species and Propionibacterium species) than non-SS dry eye patients.110 Similarly, Graham et al reported greater loads of coagulase negative staphylococci (normal flora at the ocular surface) in dry eye patients. They found certain common ocular surface bacteria (Cornyebacterium and Propionibacterium) as well as uncommon ones (Rhodococcus erythropolis, Klebsiella oxytoca and Erwinia species) to be present in normal patients and dry eye patients.111 They concluded that the presence of these microbial pathogens at the ocular surface of normal and dry eye patients presented a “diagnostic dilemma”: since the pathogens were present in all patient groups, antimicrobial therapy could not be justified for one group (dry eye patients).
Previous studies have shown that conditions that cause dry eye (such as anterior blepharitis, meibomian gland dysfunction, and ocular rosacea) are associated with a variety of bacteria such as coagulase negative staphylococci,112–114 Staphylococcus aureus (S. aureus),115 Streptococcus species, Bacillus subtilis,116,117 Rhodococcus species,118 P. aeruginosa,119 and Hemophilus influenza.116,117 Hori et al found no significant difference in bacterial isolation rates from conjunctiva of normal and dry eye patients, although the dry eye patients (SS and non-SS) had an increased presence of fluoroquinolone-resistant bacterial strains.120
It is not clear that any changes in ocular surface flora in dry eye are the result of the altered environment or if an altered flora may contribute to the disease process. Bacterial lipases and bacteriocins121 produced by commensals at the ocular surface have been shown to damage cells of the surface and destabilize the lipid layer,111 induce desiccating stress,26 and cause loss of goblet cells,122,123 indicating that bacterial action may exacerbate dry eye disease. Topical (tobramycin, azithromycin) and oral (doxycycline, minocycline) antibiotics alone or in combination with steroids such as dexamethasone or loteprednol have been shown to be beneficial in the treatment of blepharitis, which causes a secondary dry eye.124–128 It has been hypothesized that these topical medications alter the normal eyelid and ocular surface flora, reduce inflammation, and possibly contribute to antibiotic resistance.120,129,130 Some data suggest that drugs such as oral minocycline aid the treatment of blepharitis by decreasing or eliminating ocular surface flora.128 Other data suggest that oral minocycline also inhibits lipases in blepharitis patients,127 while oral doxycycline inhibits matrix metalloproteinase (MMP)-9 and thus helps blepharitic conditions.125 A recently published case report of a methicillin-resistant S. aureus keratitis in a dry eye patient with a therapeutic contact lens highlights the complicated and multifactorial nature of ocular surface conditions and the difficulty in determining causative factors.131
Based on the limited evidence available, it appears that dry eye patients do tend to have an altered/greater microbial load at the ocular surface compared to healthy patients. However, greater prevalence of infections from these microbes does not seem to exist in dry eye patients. This suggests that dry eye patients may not be at a higher risk for infection, because innate immunity (and perhaps other mechanisms) adequately protects the ocular surface despite the presence of dry eye disease.
III. Dry Eye, Innate Immune System and Endogenous Antimicrobials
A. Ocular Surface Innate Defense Mechanisms
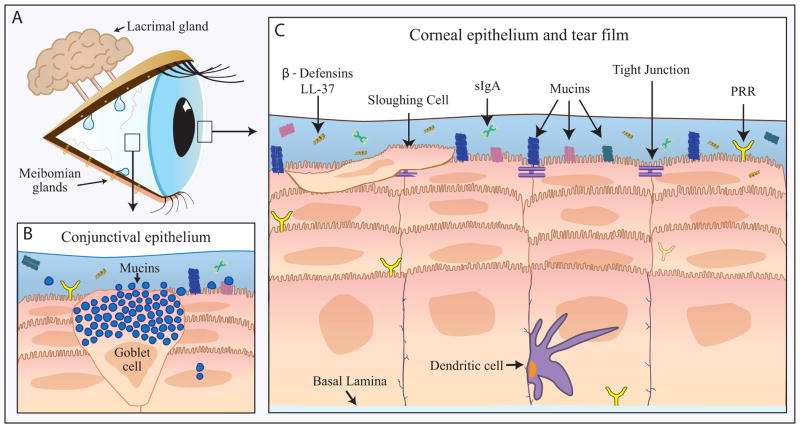
A comprehensive review of ocular surface innate defense mechanisms is beyond the scope of this article; suffice to say the ocular surface is well equipped for defense against pathogens through the physical barrier presented by the epithelia, the sloughing of epithelial cells, sensory nerves to trigger tearing/blinking, and a myriad of molecules, large and small, with direct or indirect antimicrobial properties. The latter arise from a number of cellular sources, including the ocular surface epithelial cells and glands involved in tear production. Some are derived from neutrophils that can be found in the tears on awakening. Figure 1 summarizes the major defense mechanisms. For more detail, the reader is referred to recent review articles by Gregory132 and McDermott.133 Current literature on changes in innate defense mechanisms reported to occur with dry eye are reviewed below. The major findings are also summarized in Table 1.
Figure 1.
Innate immune system of the ocular surface.
The innate immune system is the first line of defense against invasion for the ocular surface (A) and includes components of the conjunctival (B) and corneal epithelium and tear film (C). PRR=pattern recognition receptors, LL-37= cathelicidin, sIgA=secretory Immunoglobulin A.
Table 1.
Modulation of Innate Immune Molecules in Dry Eye
| Molecule | Classification of Dry Eye | Fluid/Tissue Tested | Change (References) |
|---|---|---|---|
|
| |||
| Lysozyme | Non-SS and SS | Tears | Decreased114, 45–149 |
| Non-SS and SS | Unchanged150,151 | ||
|
| |||
| Lactoferrin | Non-SS and SS | Tears | Decreased 114,145, 146,151 |
|
| |||
| Lipocalin | Non-SS and SS | Tears | Decreased 150,151 |
|
| |||
| sIgA | Non-SS and SS | Tears | Decreased145 |
| Mixed results++114 | |||
| SS | Unchanged155 | ||
|
| |||
| sPLA2 | Non-SS and SS | Tears | Increased163,164 |
| Non-SS* | Conjunctival epithelium* | Increased165* | |
|
| |||
| Mucins | |||
| MUC1 | Non-SS and SS | Tears, CIC | Increased184 |
| MUC5AC | SS | CIC, conjunctival biopsy, tears | Decreased167,185,179 |
| MUC16 | Non-SS | CIC | Altered distribution/glycosylation187 |
| MUC19 | SS | CIC, conjunctival biopsy | Decreased185 |
|
| |||
| AMPs | |||
| hBD-1 | Non-SS | CIC | Unchanged198 |
| hBD-2 | Non-SS and SS | CIC | Increased 137,198,208 |
| hBD-3 | Non-SS | CIC | Unchanged198 |
| hBD-9 | Unspecified | Corneal and conjunctival impression cytology | Decreased210 |
| LL-37 | Non-SS | CIC | Unchanged137 |
|
| |||
| TLRs | |||
| TLR2 | Non-SS | CIC | Increased (mRNA but not protein136) |
| TLR4 | Non-SS* | Corneal epithelium and stroma* | Increased137,138 |
| TLR9 | Non-SS | CIC | Decreased137 |
All data pertain to human studies, except where * denotes a murine model of dry eye. Tears were collected using Whatman filter paper,114 microcapillary tube,147,163 or micropipette,151 Schirmer strip,145,148,149 or surgical sponge extraction,155 or by a tear wash.150,179,184
SS = Sjogren’s syndrome. CIC = conjunctival impression cytology. MUC= Mucin. AMPs=Antimicrobial peptides.; TLRs=Toll-like receptors. ++ IgA was decreased in 3 of 12 eyes, and was in the low normal range for the others.
B. Pathogen Recognition in Dry Eye
Sensing of pathogens via pattern recognition receptors (PRRs) is the primary way host cells detect the presence of unwanted invaders. The major PRRs are toll-like receptors (TLRs) and NOD-like receptors (NLRs), and these generally recognize specific pathogen-derived ligands referred to as pathogen-associated molecular patterns. Ocular surface epithelial cells and corneal epithelial-associated Langerhans cells are known to express a range of both TLRs and NLRs and thus can readily detect and respond to invading organisms via production of chemokines, cytokines, and antimicrobial peptides.134,135 Enhanced expression of ocular surface PRRs may confer greater pathogen-sensing capabilities to the dry eye, contributing to a reduced risk of overt infection. However, it may also be a source of inflammation, as TLR activation stimulates the production of many proinflammatory cytokines and chemokines found in dry eye.
Some preliminary studies have addressed modulation of TLR expression in vitro and in vivo. Culture conditions mimicking a dry eye environment upregulated ocular surface epithelial cell expression of TLR4 and 5, which detect bacterial lipopolysaccharide and flagellin respectively, but downregulated TLR9, an endosomal receptor that detects unmethylated CpG motifs on bacterial and viral DNA (Redfern and McDermott [unpublished data]). In conjunctival epithelia of patients with dry eye disease, TLR2 (which detects bacterial lipopeptides) mRNA but not protein was upregulated,136 TLR4 was increased (although not significantly so) and TLR9 was downregulated.137
In a recent study using an experimental dry eye mouse model, Lee et al found that corneal epithelial and stromal TLR4 expression was increased, and that TLR4 inhibition decreased the severity of dry eye corneal staining and significantly reduced cytokine expression and infiltration of immune cells into the cornea and lymph node.138 In the same mouse model, we have observed upregulation of TLR2-4 and 9 in conjunctiva, cornea, and/or lacrimal gland.137 These investigations show that TLR expression is modulated at the ocular surface in dry eye conditions. They are corroborated by other studies showing enhanced TLR expression in labial salivary glands of human SS patients, a form of dry eye with an autoimmune basis, and SS mouse models.135 However, the significance of modulated TLR expression in dry eye and its influence on risk of ocular surface infection in dry eye has yet to be elucidated. Further NLRs and the complement system, which is constitutively active at low levels in the tears and may be viewed as a pathogen detection system, have not yet been investigated in dry eye.
C. Lactoferrin, Lysozyme, Lipocalin, sIgA, and Phospholipase A2 in Dry Eye
The levels of lactoferrin and lysozyme in human tear fluid have been of great interest to dry eye researchers for over three decades. Lactoferrin is secreted by acinar cells of the lacrimal gland.139 Lactoferrin binds iron in tear fluid, and, thus, bacteria are unable to colonize the ocular surface due to the lack of this nutrient.140,141 Lactoferrin is known to have anti-inflammatory, anticancer, and immune-modulating properties, in addition to possessing antimicrobial activity against a wide range of microbes (bacteria, fungi and viruses).142 Lysozyme, which attacks cell walls of bacteria,141 is secreted by the main lacrimal gland acinar cells143 and conjunctival accessory lacrimal glands.144 Several studies have demonstrated that the levels of lysozyme and lactoferrin are decreased in the tear fluid of SS and non-SS dry eye patients compared to healthy patients. 114,145–149
Two recent studies have reported the levels of lipocalin in the tear fluid of dry eye patients.150,151 Lipocalin, which is secreted by the main lacrimal gland acinar cells,143 produces bacteriostatic activity by binding to bacterial ferric siderophores and preventing siderophore-mediated iron uptake by bacteria. Caffery et al showed that lipocalin is reduced in SS patients compared to non-SS dry eye and healthy patients.150 They also demonstrated that lipocalin levels are not different between non-SS dry eye patients and healthy humans. Versura et al showed decreased levels of lipocalin and lactoferrin in the tear fluid of evaporative dry eye patients.151 However, both these studies demonstrated no difference in lysozyme levels in the tear fluid of SS, non-SS, or healthy patients.150,151
Secretory IgA (sIgA) plays a major role in antimicrobial protection of mucosal tissue.152 Plasma cells in the main lacrimal gland as well as in the conjunctival accessory lacrimal glands produce immunoglobulin-A (IgA).153,154 When excreted in to the tear film, a protein referred to as secretory component is associated with IgA dimers. SIgA may modulate the normal flora at the ocular surface and thus provide protection to the surface. In the intestine, sIgA uses “immune exclusion” to clear antigens and pathogens by either preventing access to epithelial receptors or by capturing them in mucus and promoting their removal by peristalsis.152 Seal et al showed that tear fluid sIgA levels were decreased in 3 of 12 eyes (6 dry eye patients), and sIgA levels were in the low normal ranges for the other eyes.114 Boukes et al found a decrease in sIgA in the tear fluid of SS patients compared to healthy controls,145 while Wehmeyer et al did not find any difference between these two groups of patients.155
sIgA might play an important role in preventing bacterial adhesion on contact lenses.156 Contact lens-wear decreases the levels of sIgA in tear fluid,157 and extended wear of contact lenses, which is associated with a greater risk of corneal infections, also decreases tear concentrations of sIgA epitopes.158 It can be hypothesized that decreased levels of sIgA inhibits proper modulation of ocular surface flora, whereby pathogenic/opportunistic organisms colonize the ocular surface. This situation can arguably increase the risk for microbial infections at the ocular surface.
Secreted Phospholipase A2 (sPLA2) is a pro-inflammatory enzyme that catalyzes the initial step of the arachidonic acid pathway.159 sPLA2 binds to the anionic bacterial surface due to its cationic nature and kills via its phospholipolytic enzymatic activity.160,161 Group II sPLA2 in the tear fluid plays a major role in killing a broad spectrum of Gram-positive bacteria at the ocular surface under physiologyical conditions.162 sPLA2 can also kill Gram-negative bacteria with the help of additional antibacterial compounds, such as the bactericidal/permeability-increasing protein.160,161 The ocular surface has increased levels of sPLA2 in tear fluid of dry eye patients,163 increased activity in tear fluid in dry eye patients,164 and increased expression of mRNA and protein in dry eye mouse conjunctival tissue.165 The mouse study did not detect sPLA2 in the normal or dry eye corneas and noted that a sPLA2 inhibitor reduced the level of sPLA-2-induced inflammation in this model.165
From the above discussion, it is very clear that sPLA2 plays a major role in the prevention of microbial infections at the ocular surface in dry eye conditions.
D. Mucins in Dry Eye
Mucins are high molecular-weight proteins, with tandem repetitions in the central portion of the molecule.166 The ocular surface expresses at least 9 of the 18 known human mucin genes: MUC1, 2, 4, 5AC, 7, 13, 15, 16, and 17.167–178 Mucins keep the ocular surface wet and protected from adverse environmental conditions. Based on their amino acid sequences, mucins are categorized in three distinct families: gel forming (MUC2, 5AC, 5B, 6, and 19), soluble (MUC7 and MUC9), and transmembrane (MUC1, 3A, 3B, 4, 12, 13, 15–17, 20, and 21); other mucins remain unclassified (MUC8 and MUC11). MUC1 and MUC16 exist as membrane-bound mucins on ocular surface epithelia as well as soluble mucins in the tear fluid.179 The membrane-bound form of MUC1 clears bacteria from the ocular surface by binding the bacteria and is later cleared out of the surface as a bacterial-mucin complex.179 MUC16 helps prevent bacterial adhesion.180 It has been shown previously that the frequency of non-SS aqueous-deficient dry eye patients expressing only the MUC1/A splice variant of the mucin MUC1 may be lower than that of a normal control group.181 Thus, a longer repeat sequence of amino acids on MUC1 variants may play role in susceptibility to dry eye syndrome, as they provide better quality of lubrication and protect the surface from inflammation.181
Differences in MUC1 genotype can explain the loss of ocular surface integrity that is often observed in dry eye patients.12,16 Blalock et al demonstrated that molecules such as neutrophil elastase and tumor necrosis factor-alpha (TNF-α) promote the release of MUC1, MUC4, and MUC 16 in human corneal-limbal epithelial cells.182 Similar release of membrane-associated mucins can be induced in dry eye patients who often demonstrate increased levels of inflammatory cytokines, such as TNF-α.183 The release of these mucins from the corneal epithelial surface can lead to loss of integrity of the ocular surface in dry eye patients (evidenced in clinical practice as vital dye positive staining), which can open pathways for pathogens to invade the cornea. Interestingly, Caffery et al found an increase of soluble MUC1 in SS patients and non-SS patients compared to healthy patients.184 They also found MUC1 mRNA expression to be similar in non-SS and healthy patients, while membrane-bound MUC1 expression was different only between the SS and healthy patients.184 The difference in results for MUC1 expression shown in this study could be attributed to factors such as pooling impression cytology samples, which can mask individual patient variability.184 The authors suggested that the differences in MUC1 expression between the three groups of patients indicates a preventative response from the ocular surface to avoid infections/inflammation.184
Other recent studies have demonstrated a decrease in MUC5AC (mRNA and protein), altered distribution of MUC16 epitopes, and decrease in MUC19 (mRNA and protein) levels in SS patients compared to healthy patients.167,179, 185–187 The chemical composition of gel-forming mucins such as MUC5AC can be altered by bacterial ligands, such as lipoteichoic acid (Gram-positive), flagellin A, and LPS (Gram-negative).188 Alteration of these gel-forming mucins can cause epithelial stress and thereby activate the adaptive immune system, resulting in T lymphocyte infiltration, cytokine secretion, and death of surface epithelial/goblet cells.188 Mucins can be thought of as providing a physical barrier to the entry of bacteria.189 As described earlier, the presence of MUC16 at the ocular surface provides a nonadhesive barrier to prevent bacterial entry.180
In summary, loss of ocular surface mucins may result in loss of a physical barrier against pathogens, loss of corneal integrity (vital dye staining), and epithelial stress that activates the adaptive immune system, causing T lymphocyte infiltration and cytokine secretion (leading to ocular surface epithelial cell death). Thus, it can be argued that the alteration of ocular surface mucins in dry eye states places the eye at risk for microbial infections.
E. Antimicrobial Peptides and Dry Eye
Antimicrobial peptides (AMPs) such as defensins and cathelicidins, are important innate defense molecules at the ocular surface.190–200 We have shown that the ocular surface epithelia constitutively express human β-defensin (hBD)-1 and hBD-3, while hBD-2 expression is observed only in response to inflammatory cytokines, infections, and injury.190,192–196,199–201 We have also shown that enhanced expression of the cathelicidin LL-37 is observed at the ocular surface in response to inflammatory cytokines and infection.190,191,197,202 Low levels of α-defensins (human neutrophil peptides [HNP])-1, -2 and -3 have been detected in the tear fluid, with the primary source being neutrophils that can be found in the tears upon eye opening.203 hBD-2 and -3 have also been detected in low levels in reflex tears and basal tears (hBD-2 only).204 The origin of these tear β-defensins is not known for certain, but it may be ocular surface epithelial cells and/or lacrimal gland.
In terms of antimicrobial effectiveness, hBD-1 is not significantly effective against the common ocular pathogens P. aeruginosa or S. aureus.191,205 However, hBD-2 has good activity against P. aeruginosa, while hBD-3 and LL-37 have good activity against both P. aeruginosa and S. aureus. Several studies using murine models have shown the importance of defensins and the murine cathelicidin cathelin-related antimicrobial peptide (CRAMP), the ortholog of human LL-37, in protection against P. aeruginosa keratitis. A recent study with stratified cultured human corneal epithelial cells showed that defensins were essential to prevent traversal of the bacteria across the epithelial barrier.202,206,207
We found that dry eye patients demonstrate an upregulated expression of hBD-2 compared to healthy patients, which may be mediated by enhanced pro-inflammatory cytokine expression.137,200,201 However, there was no difference in the expression of hBD-1 and -3 between the two groups of patients.201 Later, Huang et al, in another laboratory, confirmed increased conjunctival expression of hBD-2 in SS dry eye patients as well.208 It should be noted, however, that salivary gland expression of hBD-1 and -2 has been shown to be decreased in SS patients.209 We found that conjunctival expression of LL-37 does not change in dry eye patients compared to healthy patients,137 and Abedin et al reported decreased corneal and conjunctival expression of hBD-9 in dry eye.210 Thus, these data indicate that the dry eye retains significant protection through hBD-1, hBD-3 and LL-37, the levels of which do not appear to be decreased compared to normal, and that additional protection may arise from the enhanced expression of hBD-2. The decrease in hBD-9 is not expected to be of significance, as this particular defensin is not predicted to have potent antimicrobial activity.210
It should be noted that the antimicrobial action of some AMPs (eg, hBD-1 and -2) is salt- sensitive and is compromised by salt and mucins in the tear film,191,192,205 calling into question their role as antimicrobial agents at the ocular surface. However, at least for hBD-2, the elevated levels in dry eye may go some way to compensate for these detrimental effects.191 We have performed a limited investigation of AMP expression in a dry eye mouse model and found decreased corneal and/or conjunctival expression of mBD-1 and CRAMP but increased or no change in expression of mBD-3 and -4 (the latter are orthologs of human hBD-2)17,137.
Although more study is required, these early data mostly show similar trends to changes in AMP expression in human dry eye; thus, the murine dry eye model may be useful for investigating the functional consequences of modulated AMP expression in vivo.
While AMPs are known to have direct antimicrobial activity, they also exhibit a variety of immunomodulatory behaviors and modulate wound healing. Thus, their protective role in dry eye may be not only to effect direct pathogen killing, but they may also, for example, influence immune cell actions, thus indirectly maintaining and enhancing antimicrobial protection. AMP mechanisms of antimicrobial action and their plethora of other actions are the subject of several recent reviews.141,211–213
F. Modulation of Other Defense Mechanisms
Some patients with dry eye disease frequently experience significant epiphora214 as a feedback response to an irritated and dry ocular surface. This response serves to bathe the ocular surface with replenished tear film and help rid it of irritants or foreign objects. Might this same response also protect the patient with dry eye disease from infection by decreasing the microbial load on the ocular surface? No studies examining this have been performed. However, it has been shown that SS patients having decreased salivary flow rates exhibited a higher load of Candida albicans.215 Since the oral cavity does not benefit from the same compensatory mechanism as the eye, it might be inferred that an increased rate of tear fluid production would serve to decrease certain microbes. Interestingly, dry eye patients demonstrate decreased corneal sensitivity216 and reduced blink efficiency.217 Therefore, the mechanism for the increased reflex epiphora in dry eye patients seems unclear.
The basement membrane of the corneal epithelium is known to provide an important barrier to pathogen entry into the stroma, although this can be compromised, for example, by bacterial proteases.218 The dry eye environment enhances production of degradative MMPs, and an increased risk of corneal ulceration has been reported in severe cases of dry eye.34,219,220 These data suggest the possibility of basement membrane compromise in dry eye, although direct evidence for this in the literature is lacking.
In summary, there is evidence to support reduction in some ocular surface antimicrobial molecules and possible compromise of other defense mechanisms. However, to balance this, important chemical defense molecules, such as sPLA2 and AMPs, are either unchanged or enhanced. Further, in addition to the molecules discussed above, there are a number of other ocular surface molecules with known antimicrobial properties, such as surfactant protein D.221 How their expression is modulated, if at all, in dry eye is not yet known, but they may possibly also be increased, so contributing to enhanced ocular surface protection. Thus, we propose that in dry eye, it is possible that the enhancement of innate immune molecules as described above serves to effectively clear any pathogens attempting to invade, preventing them from reaching and causing damage to or taking advantage of a possibly compromised basement membrane to gain entry to the stroma. In contrast, contact lens wear appears to reduce the ability of the ocular surface to respond to pathogens. For example, a reduced ability of P. aeruginosa to induce hBD-2 expression was noted in an in vitro model of contact lens wear, leaving the cornea with a reduced capacity to clear pathogens.222 Hence, the disrupted epithelial surface of the contact lens wearer is likely at greater risk of infection than the dry eye-disrupted epithelium. An important comparison yet to be investigated is the state of innate defenses in contact-lens wearing patients who also have dry eye.
IV. Dry Eye Treatment and Modulation of Risk for Keratitis
Treatment of dry eye is often aimed at replenishing the tear film, increasing tear retention, or dampening inflammation on the ocular surface. The palliative and therapeutic agents used for these purposes may also contribute to limiting the microbial load on the ocular surface by simply washing out and/or diluting invading pathogens or by altering the ocular surface physiology to increase microbial killing or decrease their capacity to invade. Common dry eye treatment modalities and their potential impact in modulating the risk for microbial keratitis are discussed below.
A. Artificial Tears
Artificial tears are often one of the first treatment options given by eye care practitioners to dry eye patients,223 and they are sometimes recommended to patients with microbial keratitis to relieve ocular surface irritation. A variety of artificial tears are available over-the-counter with a plethora of formulations.224 Benzalkonium chloride (BAK) is the most commonly used preservative in ophthalmic products, largely due to its proven antimicrobial efficacy.225 BAK, at a low concentration (0.005%) is able to kill S. aureus and coagulase-negative Staphylococcus,226,227 but not P. aeruginosa.227 These data suggest that in addition to replenishing the tear film, artificial tears may also reduce the risk of staphylococcal infection in dry eye patients. However, preservative-free artificial tears are more commonly being recommended for frequent use to replenish the tear film, as BAK can be cytotoxic to human corneal epithelial cells,205 thus nullifying their potential antimicrobial effect. Further, although implicated in reducing microbial loads, topical artificial tears may increase corneal epithelial permeability,228 perhaps leading to increased corneal staining clinically. As previously mentioned, increased corneal permeability may allow microbial penetration into the cornea,19, 229 although it has been suggested that, for this to occur, additional risk factors need to be present.63
As mentioned in section III. E, the ocular surface epithelial cells produce antimicrobial peptides, including hBD-2 and LL-37, which are capable of killing a variety of pathogens.191,192,202 However, it has been observed that both hBD-2 and LL-37 lose their ability to kill P. aeruginosa in the presence of carboxymethylcellulose-containing artificial tears in vitro, possibly adding to apparent detrimental effects of artificial tear solutions on ocular surface immunity.230 Clinically, we often observe that dry eye patients use artificial tear solutions several times per day, which may itself contribute to the flushing out of pathogenic microbes.
B. Cyclosporine
It is well documented that inflammation is a key component in the pathogenesis of dry eye. This is borne out by the efficacy of anti-inflammatory agents, such as corticosteroids (discussed in section IV. C) and cyclosporine for treatment of dry eye disease.231 Topical cyclosporine significantly alleviates the signs and symptoms of dry eye232 and is often prescribed for long-term use by eye care practitioners. Topical steroids have also been used to treat more severe forms of dry eye disease, often in combination with cyclosporine at the initiation of dry eye therapy. Cyclosporine selectively inhibits T lymphocyte-dependent production of proinflammatory cytokines involved in a myriad of ocular surface inflammatory conditions. It exerts its effects by forming a complex with cyclophilin, which binds and inhibits calcineurin,233 preventing the translocation of nuclear factor of activated T cells, a family of transcription factors, from the cytoplasm to the nucleus. This interaction ultimately inhibits the production of proinflammatory cytokines, therefore dampening the immune response on the ocular surface.234
In addition to reducing the production of proinflammatory cytokines in dry eye disease,235 cyclosporine is also thought to dampen the expression of immune activation markers (HLA-DR and CD40) by conjunctival epithelial cells236 and decrease CD3, CD4, and CD8 positive-T lymphocytes in the conjunctiva of dry eye patients.237 Guzey et al showed that in patients with trachomatous dry eye, central corneal thickness increased with cyclosporine treatment.238 They attributed this to an improvement in the integrity of the ocular surface and resolution of the underlying inflammation. However, cyclosporine may have adverse side effects, especially when used chronically. With the reduction in lymphocytes and the compromised ocular surface, does the use of cyclosporine in dry eye disease increase the risk of microbial keratitis? Does cyclosporine’s effect on the inflammatory cascade coincide with an alteration of the localized immune response on the ocular surface?
In an in vitro study, Hara et al showed that cyclosporine may increase the susceptibility of human corneal epithelial cells to viral infection by reducing the production of IL-6, IL-8 and NF-kB activation in response to TLR3 activation by pathogen-associated molecular pattern sequences.239 As discussed below, several studies have examined the risk for infection with topical cyclosporine use in human dry eye patients and animals.
In patients with concurrent herpes simplex keratitis (HSK) and dry eye disease, a 1-year treatment with cyclosporine actually reduced the duration of HSK recurrences rather than increasing the risk of infection.240 In a multicenter, double-masked study, Stevenson et al investigated the safety of twice-daily dosing topical cyclosporine treatment at doses ranging from 0.05% up to 0.4% for 12 weeks in patients with moderate-to-severe dry eye.241 This study found no significant adverse effects, no microbial overgrowth on the ocular surface, and no increased risk of ocular infection in any of the cyclosporine-treatment groups. Similarly, in an in vivo study on the effect of topical cyclosporine on microbial colonization of the corneal surface of dogs with dry eye, Salisbury et al found that in dogs that responded to cyclosporine treatment, as indicated by a significant increase in tear production, the percentage of eyes from which bacteria were isolated after 3, 6, and 12 months of cyclosporine treatment was significantly less than it was prior to treatment.242 They also found that the percentage of eyes from which fungi were isolated decreased during the course of cyclosporine treatment. Consistent with this, cyclosporine treatment has been shown to have a significant suppressive effect on the growth of Fusarium oxysporum and Fusarium solani, compared to vehicle or methylprednisolone treatment243 and can also have a synergistic effect with the antifungal medication fluconazole in cases of fungal keratitis.244 Together, these studies suggest that use of cyclosporine is not associated with an increased risk of ocular surface infection.
In severe dry eye disease, the ocular surface may become compromised, possibly leading to ulceration, ultimately initiating a wound healing response. Cyclosporine has been shown to inhibit the production of cytokines and chemokines involved in wound healing,239,245 and studies have investigated the effect of cyclosporine on modulating the immune response when the ocular surface is compromised. Flueckiger et al used an ex vivo, whole-globe porcine model to investigate corneoepithelial wound healing in response to cyclosporine, and found that cyclosporine had no influence on corneoepithelial wound healing.246 However, Garweg et al, in a study of human conjunctival epithelial cells, found that cyclosporine inhibited cell proliferation, with a corresponding decline in cell viability, as detected by a decrease in calcein metabolism.247
Previous studies have shown that wound healing and dry eye increase the expression of antimicrobial peptide, hBD-2 in ocular surface cells,137,200,201 suggesting that hBD-2 may provide additional antimicrobial protection when the ocular surface is compromised. Interestingly, cyclosporine has been shown to downregulate hBD-2 expression in human corneal epithelial cells in vitro,248 and, therefore, the same scenario may exist when cyclosporine is used in patients to treat dry eye inflammation. A potential downregulation in hBD-2 could increase the risk for infection in dry eye patients. This change may not be physiologically relevant in dry eye, as hBD-2 may lose some of its antimicrobial effectiveness on ocular surface of dry eye patients due to hyperosmolar stress and potential interactions with mucins in the tear film.192,195
C. Steroids
In addition to cyclosporine, other immunomodulatory drugs such as steroids are often used to dampen inflammation on the ocular surface in dry eye. The effect of corticosteroids on the inflammatory cascade, specifically the blockade of cyclooxygenase and production of prostanoids from arachidonic acid, is well known and is likely the reason this form of therapy has been efficacious in practice. Corticosteroids also exert local immunomodulatory activity through the inhibition of certain transcription factor activity.223
An increased susceptibility to infection with the use of topical steroids has been cited in the literature.249 This has been documented with the case of susceptibility to fungal infections.249,250 However, Ilyas et al reported no significant corneal adverse events, including corneal infections, in a subject population using loteprednol etabonate 0.2% for allergic conjunctivitis for a minimum of 1 year.251 This could in part be due to the specific design of loteprednol, which replaces the typical ketone group in other steroids with a 17α-chloromethyl ester.252,253
Recently, Suto et al examined the bacteria isolated from the conjunctival sac in pre-cataract surgery patients with and without dry eye, who either were taking oral steroids or using artificial tears or other topical medications (steroid).254 The bacterial isolation rate was significantly lower in dry eye patients (using only artificial tears) than for those without dry eye disease, and the use of artificial tears reduced the bacterial isolation rate compared to patients not using topical medication. Patients taking oral steroids did not have a significant difference in bacterial isolation rates. Similar results were found in dry eye patients taking topical steroids. This study reported that topical steroid use by dry eye patients did not alter the bacterial profile on the ocular surface compared to dry eye patients treated with punctal plugs or artificial tears.
These studies suggest that topical steroids are effective at reducing ocular surface inflammation in dry eye while not increasing the risk for infection.
D. Antibiotics
Macrolide antibiotics (azithromycin) and tetracycline derivatives (tetracycline, doxycycline, and minocycline) have been commonly used in the management of ocular surface and dermatological conditions, including anterior and posterior blepharitis, meibomian gland dysfunction, and rosacea. As a class, tetracyclines and their synthetic counterparts have been a frequently used therapy for the underlying causes of dry eye disease, and are thought to decrease inflammation and normalize production by the meibomian glands, in addition to having an antibacterial effect.255,256 Their action in reducing MMPs257 has also been useful in ameliorating the effects of inflammation after corneal ulcer treatment.258
Tetracyclines also serve to decrease the production of inflammatory cytokines IL-1α and TNF-α.256,259,260 Although tetracyclines are broad spectrum antibiotics effective against Gram-positive and Gram-negative bacterial species, their effective prophylaxis against microbial infections in patients with dry eye has not been studied. Additionally, only minocycline and doxycycline reach levels that would be considered beneficial as an antimicrobial at the tear film ocular surface interface.261 This was shown most recently in a small clinical study that demonstrated a decrease in ocular surface bacterial flora in patients who were started on 50 mg minocycline daily for 2 weeks, then switched to 100 mg daily for a total of 3 months.127 At high concentrations, the tetracycline class of medications has been shown to have an inhibitory decrease in Staphylococcal exotoxin-elicited increases in cytokines and chemokines.262 Tetracyclines may also act upon bacterial lipases with a resultant decrease in free fatty acids in the tear film.263 However, to date, little is known about the relative proportion of the beneficial mechanisms involved in tetracycline therapy.
Topical azithromycin has been prescribed for use off-indication, alone or in combination with oral tetracycline derivatives, in the treatment of meibomian gland dysfunction and blepharitis, which often coexist with dry eye disease.264 Studies to date have focused on the effectivity in reducing signs and symptoms of the disease, and have not reported a negative impact or evaluated the effect of chronic use on the normal microbiome and defense mechanisms of the ocular surface.265–267 While no cases of ocular infection have been reported in these studies, further work is warranted to investigate the impact of preservative BAK as well as the chronic use of a topical antibiotic.
E. Future Studies
In the clinical management of dry eye patients, little thought is given to the potential negative effects of a systemic or topical therapy on the innate and adaptive immune systems of the ocular surface, although each therapy has the opportunity to directly or indirectly impact ocular surface immunity. Existing data do not seem to indicate that topical and oral therapies significantly reduce the ability of the ocular surface to respond to microbial threat, but further studies, including surveillance and natural history studies, may help to further elucidate the impact of chronic topical therapies on the ocular surface. In addition, techniques to accurately evaluate innate and acquired immunity in a clinical setting (or for clinical trials) are yet to be developed. The existing platforms of micro-tear collection used by the RPS viral detector as well as the TearLab osmolarity systems are technological advances that could be applied to further “biomarker” tear analysis. A detectable tear marker for increased bacterial load, for example, would be welcome in further understanding of the complex mechanisms at play on the ocular surface, with and without disease prior to and during treatment.
V. Summary and Conclusions
This article provides a comprehensive review of the current evidence, circumstantial and otherwise, for a link between microbial keratitis and dry eye. Although such a link is widely believed as logical and possibly credible, we conclude that convincing supportive evidence linking dry eye and infectious keratitis is minimal. For some situations, such as concomitant systemic autoimmune disease, the evidence appears a little stronger, but for the typical dry eye patient, contact lens-wearer or not, dry eye does not appear to be associated with overt microbial keratitis, even though there may be some changes in ocular flora. Further, commonly used dry eye treatments do not appear to contribute to modulating infection risk.
One problem with the existing literature is that none of the clinical studies were designed to specifically investigate a link between dry eye and infectious keratitis; rather, a link, when found, has been more an incidental finding. Epidemiological studies to specifically determine the risk of infectious keratitis in dry eye would help to provide conclusive findings. However, until such trials are forthcoming, available data indicate that dry eye (whether defined via symptom survey or a battery of clinical tests) in and of itself does not appear to be associated with an increased risk of microbial keratitis.
How then is an ocular surface that is compromised by dry eye able to protect itself? We believe that innate immune defenses are of the utmost importance in this respect. While evidence indicates that some innate defenses are breached (disrupted epithelium, reduction of some antimicrobial molecules), others appear unchanged or enhanced, including sPLA2 and AMP production. Thus, the redundancy in the innate immune system appears essential for protecting the ocular surface of dry eyes. While a number of innate mechanisms/molecules have been compared among the dry eye and the normal ocular surface, there are others (both innate and adaptive) to be investigated. Such investigations will shed further light on to how a compromised ocular surface resists infection.
Acknowledgments
This work was supported by EY13175 (AMM), EY07024 and EY18113 (RLR), EY015519 (KKN) and EY007551 (University of Houston, College of Optometry CORE Grant).
Footnotes
The authors have no commercial or proprietary interests in any concept or product discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. (No authors listed) [DOI] [PubMed] [Google Scholar]
- 2.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 3.Schaumberg DA, Sullivan DA, Dana MR. Epidemiology of dry eye syndrome. Adv Exp Med Biol. 2002;506:989–98. doi: 10.1007/978-1-4615-0717-8_140. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder SC. Prevalence, burden, and pharmacoeconomics of dry eye disease. Am J Manag Care. 2008;14:S102–6. [PubMed] [Google Scholar]
- 5.Abetz L, Rajagopalan K, Mertzanis P, et al. Development and validation of the impact of dry eye on everyday life (IDEEL) questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patients. Health Qual Life Outcomes. 2011;9:111. doi: 10.1186/1477-7525-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman NJ. Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol. 2010;21:310–6. doi: 10.1097/ICU.0b013e32833a8c15. [DOI] [PubMed] [Google Scholar]
- 7.Pouyeh B, Viteri E, Feuer W, et al. Impact of ocular surface symptoms on quality of life in a United States Veterans Affairs population. Am J Ophthalmol. 2012;153:1061–6. doi: 10.1016/j.ajo.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Clegg JP, Guest JF, Lehman A, Smith AF. The annual cost of dry eye syndrome in France, Germany, Italy, Spain, Sweden and the United Kingdom among patients managed by ophthalmologists. Ophthalmic Epidemiol. 2006;13:263–74. doi: 10.1080/09286580600801044. [DOI] [PubMed] [Google Scholar]
- 9.Reddy P, Grad O, Rajagopalan K. The economic burden of dry eye: a conceptual framework and preliminary assessment. Cornea. 2004;23:751–61. doi: 10.1097/01.ico.0000134183.47687.75. [DOI] [PubMed] [Google Scholar]
- 10.Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol. 2008;146:350–56. doi: 10.1016/j.ajo.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Perry HD, Donnenfeld ED. Dry eye diagnosis and management in 2004. Curr Opin Ophthalmol. 2004;15:299–304. doi: 10.1097/00055735-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Calonge M, Enriquez-de-Salamanca A, Diebold Y, et al. Dry eye disease as an inflammatory disorder. Ocul Immunol Inflamm. 2010;18:244–53. doi: 10.3109/09273941003721926. [DOI] [PubMed] [Google Scholar]
- 13.Paiva CS, Pflugfelder SC. Rationale for anti-inflammatory therapy in dry eye syndrome. Arq Bras Oftalmol. 2008;71:89–95. doi: 10.1590/s0004-27492008000700017. [DOI] [PubMed] [Google Scholar]
- 14.Stern ME, Siemasko KF, Gao J, et al. Evaluation of ocular surface inflammation in the presence of dry eye and allergic conjunctival disease. Ocul Surf. 2005;3:S161–4. doi: 10.1016/s1542-0124(12)70246-x. [DOI] [PubMed] [Google Scholar]
- 15.Pflugfelder SC, Solomon A, Dursun D, Li DQ. Dry eye and delayed tear clearance: “a call to arms. Adv Exp Med Biol. 2002;506:739–43. doi: 10.1007/978-1-4615-0717-8_104. [DOI] [PubMed] [Google Scholar]
- 16.Corrales RM, Narayanan S, Fernandez I, et al. Ocular mucin gene expression levels as biomarkers for the diagnosis of dry eye syndrome. Invest Ophthalmol Vis Sci. 2011;52:8363–9. doi: 10.1167/iovs.11-7655. [DOI] [PubMed] [Google Scholar]
- 17.Narayanan S, Corrales RM, Farley W, et al. Interleukin-1 receptor-1-deficient mice show attenuated production of ocular surface inflammatory cytokines in experimental dry eye. Cornea. 2008;27:811–7. doi: 10.1097/ICO.0b013e31816bf46c. [DOI] [PubMed] [Google Scholar]
- 18.Kimura K. Molecular mechanism of the disruption of barrier function in cultured human corneal epithelial cells induced by tumor necrosis factor-alpha, a proinflammatory cytokine. Nihon Ganka Gakkai Zasshi. 2010;114:935–43. Japanese. [PubMed] [Google Scholar]
- 19.Klocke J, Barcia RN, Heimer S, et al. Spontaneous bacterial keratitis in CD36 knockout mice. Invest Ophthalmol Vis Sci. 2011;52:256–63. doi: 10.1167/iovs.10-5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cavanagh HD, Robertson DM, Petroll WM, Jester JV. Castroviejo Lecture 2009: 40 years in search of the perfect contact lens. Cornea. 2010;29:1075–85. doi: 10.1097/ICO.0b013e3181d103bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medzhitov R, Janeway CA., Jr An ancient system of host defense. Curr Opin Immunol. 1998;10:12–5. doi: 10.1016/s0952-7915(98)80024-1. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R, Janeway CA., Jr Innate immune recognition and control of adaptive immune responses. Semin Immunol. 1998;10:351–3. doi: 10.1006/smim.1998.0136. [DOI] [PubMed] [Google Scholar]
- 23.Weissman BA, Giese MJ, Mondino BJ. An introduction to ocular immunology. Optom Clin. 1994;3:1–22. [PubMed] [Google Scholar]
- 24.Dudley DJ. The immune system in health and disease. Baillieres Clin Obstet Gynaecol. 1992;6:393–416. doi: 10.1016/s0950-3552(05)80003-3. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130:90–100. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pflugfelder SC, de Paiva CS, Li DQ, Stern ME. Epithelial-immune cell interaction in dry eye. Cornea. 2008;27 (Suppl 1):S9–11. doi: 10.1097/ICO.0b013e31817f4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Research in dry eye: report of the Research Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:179–93. doi: 10.1016/s1542-0124(12)70086-1. (No authors listed) [DOI] [PubMed] [Google Scholar]
- 28.Yanoff M, Duker JS, Augsburger JJ. Ophthalmology. St. Louis, MO: Mosby; 2004. p. xxii.p. 1652. [Google Scholar]
- 29.Kwong MS, Evans DJ, Ni M, et al. Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model. Infect Immun. 2007;75:2325–32. doi: 10.1128/IAI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bialasiewicz AA, Shenoy R, Al-Muniri A, Thakral A. Diseases of the adnexa in the tropics: amnion membrane transplantation for noninfectious trachoma-associated corneal ulcers. Ophthalmologe. 2006;103:940–4. doi: 10.1007/s00347-006-1377-9. German. [DOI] [PubMed] [Google Scholar]
- 31.Ormerod LD, Collin HB, Dohlman CH, et al. Paraproteinemic crystalline keratopathy. Ophthalmology. 1988;95:202–12. doi: 10.1016/s0161-6420(88)33200-8. [DOI] [PubMed] [Google Scholar]
- 32.Narayanan S, Miller WL, Prager TC, et al. The diagnosis and characteristics of moderate dry eye in non-contact lens wearers. Eye Contact Lens. 2005;31:96–104. doi: 10.1097/01.icl.0000140907.45705.e2. [DOI] [PubMed] [Google Scholar]
- 33.Morgan PB, Maldonado-Codina C. Corneal staining: do we really understand what we are seeing? Cont Lens Anterior Eye. 2009;32:48–54. doi: 10.1016/j.clae.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabery HM. Dual appearance of fluorescein staining in vivo of diseased human corneal epithelium. A non-contact photomicrographic study. Br J Ophthalmol. 1992;76:43–4. doi: 10.1136/bjo.76.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mokhtarzadeh M, Casey R, Glasgow BJ. Fluorescein punctate staining traced to superficial corneal epithelial cells by impression cytology and confocal microscopy. Invest Ophthalmol Vis Sci. 2011;52:2127–35. doi: 10.1167/iovs.10-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleiszig SM, Evans DJ. Pathogenesis of contact lens-associated microbial keratitis. Optom Vis Sci. 2010;87:225–32. doi: 10.1097/OPX.0b013e3181d408ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tam C, LeDue J, Mun JJ, et al. 3D quantitative imaging of unprocessed live tissue reveals epithelial defense against bacterial adhesion and subsequent traversal requires MyD88. PLoS One. 2011;6:e24008. doi: 10.1371/journal.pone.0024008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:108–52. doi: 10.1016/s1542-0124(12)70083-6. (No authors listed) [DOI] [PubMed] [Google Scholar]
- 40.Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–9. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jhanji V, Constantinou M, Taylor HR, Vajpayee RB. Microbiological and clinical profiles of patients with microbial keratitis residing in nursing homes. Br J Ophthalmol. 2009;93:1639–42. doi: 10.1136/bjo.2008.154468. [DOI] [PubMed] [Google Scholar]
- 42.Van Der Beek MT, Bernards AT, Lapid-Gortzak R. Mycobacterium chelonae keratitis in a patient with Sjogren’s syndrome. Eur J Ophthalmol. 2008;18:294–6. doi: 10.1177/112067210801800221. [DOI] [PubMed] [Google Scholar]
- 43.Boiko EV, Chernysh VF, Pozniak AL, Ageev VS. To the role of Chlamydia infection in the development of dry eye. Vestn Oftalmol. 2008;124:16–9. Russian. [PubMed] [Google Scholar]
- 44.Krasny J, Hruba D, Netukova M, et al. Chlamydia pneumoniae in the etiology of the keratoconjunctivitis sicca in adult patients (a pilot study) Cesk Slov Oftalmol. 2009;65:102–6. Czech. [PubMed] [Google Scholar]
- 45.Petroutsos G, Paschides CA, Kitsos G, et al. Sterile corneal ulcers in dry eye. II. Treatment, complications and course. J Fr Ophtalmol. 1992;15:106–11. French. [PubMed] [Google Scholar]
- 46.Petroutsos G, Paschides CA, Kitsos G, et al. Sterile corneal ulcers in dry eye. Incidence and factors of occurrence. J Fr Ophtalmol. 1992;15:103–5. French. [PubMed] [Google Scholar]
- 47.Hemady R, Chu W, Foster CS. Keratoconjunctivitis sicca and corneal ulcers. Cornea. 1990;9:170–3. [PubMed] [Google Scholar]
- 48.Pfister RR, Murphy GE. Corneal ulceration and perforation associated with Sjogren’s syndrome. Arch Ophthalmol. 1980;98:89–94. doi: 10.1001/archopht.1980.01020030091006. [DOI] [PubMed] [Google Scholar]
- 49.Vivino FB, Minerva P, Huang CH, Orlin SE. Corneal melt as the initial presentation of primary Sjogren’s syndrome. J Rheumatol. 2001;28:379–82. [PubMed] [Google Scholar]
- 50.Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–8. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113:109–16. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Green M, Apel A, Stapleton F. A longitudinal study of trends in keratitis in Australia. Cornea. 2008;27:33–9. doi: 10.1097/ICO.0b013e318156cb1f. [DOI] [PubMed] [Google Scholar]
- 53.Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–7. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 54.Heimer S, Mun JJ, Stern M, et al. Ocular resistance to Pseudomonas aeruginosa infection in a murine model of dry eye disease. Invest Ophthalmol Vis Sci. 2010:E-Abstract 3897. [Google Scholar]
- 55.Poggio EC, Glynn RJ, Schein OD, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med. 1989;321:779–83. doi: 10.1056/NEJM198909213211202. [DOI] [PubMed] [Google Scholar]
- 56.Schein OD, Glynn RJ, Poggio EC, et al. The relative risk of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. A case-control study. Microbial Keratitis Study Group. N Engl J Med. 1989;321:773–8. doi: 10.1056/NEJM198909213211201. [DOI] [PubMed] [Google Scholar]
- 57.Stapleton F, Dart JK, Minassian D. Risk factors with contact lens related suppurative keratitis. CLAO J. 1993;19:204–10. [PubMed] [Google Scholar]
- 58.Stapleton F, Dart JK, Seal DV, Matheson M. Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol Infect. 1995;114:395–402. doi: 10.1017/s0950268800052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stapleton F. Contact lens-related microbial keratitis: what can epidemiologic studies tell us? Eye Contact Lens. 2003;29:S85–9. doi: 10.1097/00140068-200301001-00024. discussion S115–8, S92–4. [DOI] [PubMed] [Google Scholar]
- 60.MacRae S, Herman C, Stulting RD, et al. Corneal ulcer and adverse reaction rates in premarket contact lens studies. Am J Ophthalmol. 1991;111:457–65. doi: 10.1016/s0002-9394(14)72381-5. [DOI] [PubMed] [Google Scholar]
- 61.Dart JK, Radford CF, Minassian D, et al. Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology. 2008;115:1647–54. 54, e1–3. doi: 10.1016/j.ophtha.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Keay L, Stapleton F, Schein O. Epidemiology of contact lens-related inflammation and microbial keratitis: a 20-year perspective. Eye Contact Lens. 2007;33:346–53. doi: 10.1097/ICL.0b013e318157c49d. discussion 62–3. [DOI] [PubMed] [Google Scholar]
- 63.Alarcon I, Tam C, Mun JJ, et al. Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Invest Ophthalmol Vis Sci. 2011;52:1368–77. doi: 10.1167/iovs.10-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fleiszig SM. The Glenn A. Fry award lecture 2005. The pathogenesis of contact lens-related keratitis. Optom Vis Sci. 2006;83:866–73. doi: 10.1097/01.opx.0000250045.85499.55. [DOI] [PubMed] [Google Scholar]
- 65.Fleiszig SM, Evans DJ, Do N, et al. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun. 1997;65:2861–7. doi: 10.1128/iai.65.7.2861-2867.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tam C, Mun JJ, Evans DJ, Fleiszig SM. The impact of inoculation parameters on the pathogenesis of contact lens-related infectious keratitis. Invest Ophthalmol Vis Sci. 2010;51:3100–6. doi: 10.1167/iovs.09-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Efron N, Morgan PB. Rethinking contact lens associated keratitis. Clin Exp Optom. 2006;89:280–98. doi: 10.1111/j.1444-0938.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 68.Sack RA, Beaton A, Sathe S, et al. Towards a closed eye model of the pre-ocular tear layer. Prog Retin Eye Res. 2000;19:649–68. doi: 10.1016/s1350-9462(00)00006-9. [DOI] [PubMed] [Google Scholar]
- 69.Sack RA, Sathe S, Hackworth LA, et al. The effect of eye closure on protein and complement deposition on Group IV hydrogel contact lenses: relationship to tear flow dynamics. Curr Eye Res. 1996;15:1092–100. doi: 10.3109/02713689608995140. [DOI] [PubMed] [Google Scholar]
- 70.Sack RA, Tan KO, Tan A. Diurnal tear cycle: evidence for a nocturnal inflammatory constitutive tear fluid. Invest Ophthalmol Vis Sci. 1992;33:626–40. [PubMed] [Google Scholar]
- 71.Thakur A, Willcox MD, Stapleton F. The proinflammatory cytokines and arachidonic acid metabolites in human overnight tears: homeostatic mechanisms. J Clin Immunol. 1998;18:61–70. doi: 10.1023/a:1023291921695. [DOI] [PubMed] [Google Scholar]
- 72.Imayasu M, Moriyama T, Ichijima H, et al. The effects of daily wear of rigid gas permeable contact lenses treated with contact lens care solutions containing preservatives on the rabbit cornea. CLAO J. 1994;20:183–8. doi: 10.1097/00140068-199407000-00010. [DOI] [PubMed] [Google Scholar]
- 73.Imayasu M, Petroll WM, Jester JV, et al. The relation between contact lens oxygen transmissibility and binding of Pseudomonas aeruginosa to the cornea after overnight wear. Ophthalmology. 1994;101:371–88. doi: 10.1016/s0161-6420(94)31326-1. [DOI] [PubMed] [Google Scholar]
- 74.Jalbert I, Sweeney DF, Stapleton F. The effect of long-term wear of soft lenses of low and high oxygen transmissibility on the corneal epithelium. Eye (Lond) 2009;23:1282–7. doi: 10.1038/eye.2008.307. [DOI] [PubMed] [Google Scholar]
- 75.Ladage PM, Yamamoto K, Ren DH, et al. Effects of rigid and soft contact lens daily wear on corneal epithelium, tear lactate dehydrogenase, and bacterial binding to exfoliated epithelial cells. Ophthalmology. 2001;108:1279–88. doi: 10.1016/s0161-6420(01)00639-x. [DOI] [PubMed] [Google Scholar]
- 76.O’Leary DJ, Madgewick R, Wallace J, Ang J. Size and number of epithelial cells washed from the cornea after contact lens wear. Optom Vis Sci. 1998;75:692–6. doi: 10.1097/00006324-199809000-00025. [DOI] [PubMed] [Google Scholar]
- 77.Ren DH, Petroll WM, Jester JV, et al. Short-term hypoxia downregulates epithelial cell desquamation in vivo, but does not increase Pseudomonas aeruginosa adherence to exfoliated human corneal epithelial cells. CLAO J. 1999;25:73–9. [PubMed] [Google Scholar]
- 78.Yamamoto N, Petroll MW, Cavanagh HD, Jester JV. Internalization of Pseudomonas aeruginosa is mediated by lipid rafts in contact lens-wearing rabbit and cultured human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:1348–55. doi: 10.1167/iovs.04-0542. [DOI] [PubMed] [Google Scholar]
- 79.Yamamoto N, Petroll MW, Jester JV, Cavanagh HD. Regulation of Pseudomonas aeruginosa internalization after contact lens wear in vivo and in serum-free culture by ocular surface cells. Invest Ophthalmol Vis Sci. 2006;47:3430–40. doi: 10.1167/iovs.05-1332. [DOI] [PubMed] [Google Scholar]
- 80.Fleiszig SM, Efron N, Pier GB. Extended contact lens wear enhances Pseudomonas aeruginosa adherence to human corneal epithelium. Invest Ophthalmol Vis Sci. 1992;33:2908–16. [PubMed] [Google Scholar]
- 81.Lin MC, Polse KA. Hypoxia, overnight wear, and tear stagnation effects on the corneal epithelium: data and proposed model. Eye Contact Lens. 2007;33:378–81. doi: 10.1097/ICL.0b013e318157d7c9. discussion 82. [DOI] [PubMed] [Google Scholar]
- 82.Sweeney D. Silicone hydrogels : the rebirth of continuous wear contact lenses. Oxford; Boston: Butterworth Heinemann; 2000. p. xiv.p. 279. [Google Scholar]
- 83.Begley CG, Caffery B, Nichols KK, Chalmers R. Responses of contact lens wearers to a dry eye survey. Optom Vis Sci. 2000;77:40–6. doi: 10.1097/00006324-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 84.Begley CG, Chalmers RL, Mitchell GL, et al. Characterization of ocular surface symptoms from optometric practices in North America. Cornea. 2001;20:610–8. doi: 10.1097/00003226-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 85.Doughty MJ, Fonn D, Richter D, et al. A patient questionnaire approach to estimating the prevalence of dry eye symptoms in patients presenting to optometric practices across Canada. Optom Vis Sci. 1997;74:624–31. doi: 10.1097/00006324-199708000-00023. [DOI] [PubMed] [Google Scholar]
- 86.Nichols JJ, Mitchell GL, Nichols KK, et al. The performance of the contact lens dry eye questionnaire as a screening survey for contact lens-related dry eye. Cornea. 2002;21:469–75. doi: 10.1097/00003226-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 87.Nichols JJ, Ziegler C, Mitchell GL, Nichols KK. Self-reported dry eye disease across refractive modalities. Invest Ophthalmol Vis Sci. 2005;46:1911–4. doi: 10.1167/iovs.04-1294. [DOI] [PubMed] [Google Scholar]
- 88.Schafer J, Mitchell GL, Chalmers RL, et al. The stability of dryness symptoms after refitting with silicone hydrogel contact lenses over 3 years. Eye Contact Lens. 2007;33:247–52. doi: 10.1097/ICL.0b013e3180587e21. [DOI] [PubMed] [Google Scholar]
- 89.Pritchard N, Fonn D. Dehydration, lens movement and dryness ratings of hydrogel contact lenses. Ophthalmic Physiol Opt. 1995;15:281–6. [PubMed] [Google Scholar]
- 90.Richdale K, Sinnott LT, Skadahl E, Nichols JJ. Frequency of and factors associated with contact lens dissatisfaction and discontinuation. Cornea. 2007;26:168–74. doi: 10.1097/01.ico.0000248382.32143.86. [DOI] [PubMed] [Google Scholar]
- 91.Schlanger JL. A study of contact lens failures. J Am Optom Assoc. 1993;64:220–4. [PubMed] [Google Scholar]
- 92.Young G, Veys J, Pritchard N, Coleman S. A multi-centre study of lapsed contact lens wearers. Ophthalmic Physiol Opt. 2002;22:516–27. doi: 10.1046/j.1475-1313.2002.00066.x. [DOI] [PubMed] [Google Scholar]
- 93.Nichols JJ, Sinnott LT. Tear film, contact lens, and patient-related factors associated with contact lens-related dry eye. Invest Ophthalmol Vis Sci. 2006;47:1319–28. doi: 10.1167/iovs.05-1392. [DOI] [PubMed] [Google Scholar]
- 94.Fonn D, Situ P, Simpson T. Hydrogel lens dehydration and subjective comfort and dryness ratings in symptomatic and asymptomatic contact lens wearers. Optom Vis Sci. 1999;76:700–4. doi: 10.1097/00006324-199910000-00021. [DOI] [PubMed] [Google Scholar]
- 95.Chalmers R, Long B, Dillehay S, Begley C. Improving contact-lens related dryness symptoms with silicone hydrogel lenses. Optom Vis Sci. 2008;85:778–84. doi: 10.1097/OPX.0b013e318181a90d. [DOI] [PubMed] [Google Scholar]
- 96.Dillehay SM, Miller MB. Performance of Lotrafilcon B silicone hydrogel contact lenses in experienced low-Dk/t daily lens wearers. Eye Contact Lens. 2007;33:272–7. doi: 10.1097/ICL.0b013e31802f78c2. [DOI] [PubMed] [Google Scholar]
- 97.Kojima T, Matsumoto Y, Ibrahim OM, et al. Effect of controlled adverse chamber environment exposure on tear functions in silicon hydrogel and hydrogel soft contact lens wearers. Invest Ophthalmol Vis Sci. 2011;52:8811–7. doi: 10.1167/iovs.10-6841. [DOI] [PubMed] [Google Scholar]
- 98.Jacobs DS, Rosenthal P. Boston scleral lens prosthetic device for treatment of severe dry eye in chronic graft-versus-host disease. Cornea. 2007;26:1195–9. doi: 10.1097/ICO.0b013e318155743d. [DOI] [PubMed] [Google Scholar]
- 99.Takahide K, Parker PM, Wu M, et al. Use of fluid-ventilated, gas-permeable scleral lens for management of severe keratoconjunctivitis sicca secondary to chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:1016–21. doi: 10.1016/j.bbmt.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cole N, Bao S, Stapleton F, et al. Pseudomonas aeruginosa keratitis in IL-6-deficient mice. Int Arch Allergy Immunol. 2003;130:165–72. doi: 10.1159/000069006. [DOI] [PubMed] [Google Scholar]
- 101.Dogru M, Ward SK, Wakamatsu T, et al. The effects of 2 week senofilcon-A silicone hydrogel contact lens daily wear on tear functions and ocular surface health status. Cont Lens Anterior Eye. 2011;34:77–82. doi: 10.1016/j.clae.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 102.Nichols JJ, Green-Church KB. Mass spectrometry-based proteomic analyses in contact lens-related dry eye. Cornea. 2009;28:1109–17. doi: 10.1097/ICO.0b013e3181a2ad81. [DOI] [PubMed] [Google Scholar]
- 103.Chalmers RL, Keay L, Long B, et al. Risk factors for contact lens complications in US clinical practices. Optom Vis Sci. 2010;87:725–35. doi: 10.1097/OPX.0b013e3181f31f68. [DOI] [PubMed] [Google Scholar]
- 104.Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye (Lond) 2012;26:185–93. doi: 10.1038/eye.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stapleton F, Edwards K, Keay L, et al. Risk factors for moderate and severe microbial keratitis in daily wear contact lens users. Ophthalmology. 2012;119:1516–21. doi: 10.1016/j.ophtha.2012.01.052. [DOI] [PubMed] [Google Scholar]
- 106.Miller D, Iovieno A. The role of microbial flora on the ocular surface. Curr Opin Allergy Clin Immunol. 2009;9:466–70. doi: 10.1097/ACI.0b013e3283303e1b. [DOI] [PubMed] [Google Scholar]
- 107.Dong Q, Brulc JM, Iovieno A, et al. Diversity of bacteria at healthy human conjunctiva. Invest Ophthalmol Vis Sci. 2011;52:5408–13. doi: 10.1167/iovs.10-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Willamson J, Doig WM, Forrester JV, et al. Studies of the viral flora in keratoconjunctivitis sicca. Br J Ophthalmol. 1975;59:45–6. doi: 10.1136/bjo.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Robert PY, Traccard I, Adenis JP, et al. Multiplex detection of herpesviruses in tear fluid using the “stair primers” PCR method: prospective study of 93 patients. J Med Virol. 2002;66:506–11. doi: 10.1002/jmv.2173. [DOI] [PubMed] [Google Scholar]
- 110.Albietz JM, Lenton LM. Effect of antibacterial honey on the ocular flora in tear deficiency and meibomian gland disease. Cornea. 2006;25:1012–9. doi: 10.1097/01.ico.0000225716.85382.7b. [DOI] [PubMed] [Google Scholar]
- 111.Graham JE, Moore JE, Jiru X, et al. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest Ophthalmol Vis Sci. 2007;48:5616–23. doi: 10.1167/iovs.07-0588. [DOI] [PubMed] [Google Scholar]
- 112.Dougherty JM, McCulley JP. Comparative bacteriology of chronic blepharitis. Br J Ophthalmol. 1984;68:524–8. doi: 10.1136/bjo.68.8.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Groden LR, Murphy B, Rodnite J, Genvert GI. Lid flora in blepharitis. Cornea. 1991;10:50–3. [PubMed] [Google Scholar]
- 114.Seal DV, McGill JI, Mackie IA, et al. Bacteriology and tear protein profiles of the dry eye. Br J Ophthalmol. 1986;70:122–5. doi: 10.1136/bjo.70.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shine WE, Silvany R, McCulley JP. Relation of cholesterol-stimulated Staphylococcus aureus growth to chronic blepharitis. Invest Ophthalmol Vis Sci. 1993;34:2291–6. [PubMed] [Google Scholar]
- 116.Sharma S. Diagnosis of external ocular infections: microbiological processing and interpretation. Br J Ophthalmol. 2000;84:229. doi: 10.1136/bjo.84.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ta CN, Chang RT, Singh K, et al. Antibiotic resistance patterns of ocular bacterial flora: a prospective study of patients undergoing anterior segment surgery. Ophthalmology. 2003;110:1946–51. doi: 10.1016/s0161-6420(03)00735-8. [DOI] [PubMed] [Google Scholar]
- 118.Cuello OH, Caorlin MJ, Reviglio VE, et al. Rhodococcus globerulus keratitis after laser in situ keratomileusis. J Cataract Refract Surg. 2002;28:2235–7. doi: 10.1016/s0886-3350(01)01347-5. [DOI] [PubMed] [Google Scholar]
- 119.Fleiszig SM, Evans DJ. Contact lens infections: can they ever be eradicated? Eye Contact Lens. 2003;29:S67–71. doi: 10.1097/00140068-200301001-00019. discussion S83–4, S192–4. [DOI] [PubMed] [Google Scholar]
- 120.Hori Y, Maeda N, Sakamoto M, et al. Bacteriologic profile of the conjunctiva in the patients with dry eye. Am J Ophthalmol. 2008;146:729–34. doi: 10.1016/j.ajo.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 121.Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008;158:442–55. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berry M, Corfield AP, Harris A, Khan-Lim D. Functional processing of ocular mucins. Adv Exp Med Biol. 2002;506:283–8. doi: 10.1007/978-1-4615-0717-8_39. [DOI] [PubMed] [Google Scholar]
- 123.Berry M, Pult H, Purslow C, Murphy PJ. Mucins and ocular signs in symptomatic and asymptomatic contact lens wear. Optom Vis Sci. 2008;85:E930–8. doi: 10.1097/OPX.0b013e318188896b. [DOI] [PubMed] [Google Scholar]
- 124.Aronowicz JD, Shine WE, Oral D, et al. Short term oral minocycline treatment of meibomianitis. Br J Ophthalmol. 2006;90:856–60. doi: 10.1136/bjo.2006.091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iovieno A, Lambiase A, Micera A, et al. In vivo characterization of doxycycline effects on tear metalloproteinases in patients with chronic blepharitis. Eur J Ophthalmol. 2009;19:708–16. doi: 10.1177/112067210901900504. [DOI] [PubMed] [Google Scholar]
- 126.Pfeffer I, Borelli C, Zierhut M, Schaller M. Treatment of ocular rosacea with 40 mg doxycycline in a slow release form. J Dtsch Dermatol Ges. 2011;9:904–7. doi: 10.1111/j.1610-0387.2011.07723.x. [DOI] [PubMed] [Google Scholar]
- 127.Shine WE, McCulley JP, Pandya AG. Minocycline effect on meibomian gland lipids in meibomianitis patients. Exp Eye Res. 2003;76:417–20. doi: 10.1016/s0014-4835(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 128.Ta CN, Shine WE, McCulley JP, et al. Effects of minocycline on the ocular flora of patients with acne rosacea or seborrheic blepharitis. Cornea. 2003;22:545–8. doi: 10.1097/00003226-200308000-00011. [DOI] [PubMed] [Google Scholar]
- 129.Williamson J, Gordon AM, Wood R, et al. Fungal flora of the conjunctival sac in health and disease. Influence of topical and systemic steroids. Br J Ophthalmol. 1968;52:127–37. doi: 10.1136/bjo.52.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan Z, Young AL, Hua H, Xu Y. Multiple oral Candida infections in patients with Sjogren’s syndrome -- prevalence and clinical and drug susceptibility profiles. J Rheumatol. 2011;38:2428–31. doi: 10.3899/jrheum.100819. [DOI] [PubMed] [Google Scholar]
- 131.Koh S, Maeda N, Soma T, et al. Development of methicillin-resistant Staphylococcus aureus keratitis in a dry eye patient with a therapeutic contact lens. Eye Contact Lens. 2012;38:200–2. doi: 10.1097/ICL.0b013e31823ff1f4. [DOI] [PubMed] [Google Scholar]
- 132.Gregory MS. Encyclopedia of the eye. Elsevier; 2010. Innate immune systems and the eye; pp. 439–45. [Google Scholar]
- 133.McDermott AM. Defense mechanisms of tears and ocular surface. In: Dartt DA, Besharse J, Dana MR, editors. Encyclopedia of the eye. Elsevier; 2010. pp. 1–8. [Google Scholar]
- 134.Pearlman E, Johnson A, Adhikary G, et al. Toll-like receptors at the ocular surface. Ocul Surf. 2008;6:108–16. doi: 10.1016/s1542-0124(12)70279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Redfern RL, McDermott AM. Toll-like receptors in ocular surface disease. Exp Eye Res. 2010;90:679–87. doi: 10.1016/j.exer.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barabino S, Rolando M, Bonini S, et al. Toll–like receptor (TLR) 2 and TLR4 expression in dry eye (abstract) Invest Ophthalmol Vis Sci. 2006:E-Abstract 5594. [Google Scholar]
- 137.Redfern RL, Patel N, Hanlon S, et al. Toll-like receptor expression and activation in mice with experimental dry eye. Invest Ophthalmol Vis Sci. 2013 doi: 10.1167/iovs.12-10739. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee HS, Hattori T, Park EY, et al. Expression of toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Invest Ophthalmol Vis Sci. 2012;53:5632–40. doi: 10.1167/iovs.12-9547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Janssen PT, van Bijsterveld OP. Origin and biosynthesis of human tear fluid proteins. Invest Ophthalmol Vis Sci. 1983;24:623–30. [PubMed] [Google Scholar]
- 140.Bezkorovainy A, Topouzian N. The effect of metal chelators and other metabolic inhibitors on the growth of Bifidobacterium bifidus var. Pennsylvanicus. Clin Biochem. 1981;14:135–41. doi: 10.1016/s0009-9120(81)90281-2. [DOI] [PubMed] [Google Scholar]
- 141.Wiesner J, Vilcinskas A. Antimicrobial peptides: the ancient arm of the human immune system. Virulence. 2010;1:440–64. doi: 10.4161/viru.1.5.12983. [DOI] [PubMed] [Google Scholar]
- 142.Garcia-Montoya IA, Cendon TS, Arevalo-Gallegos S, Rascon-Cruz Q. Lactoferrin a multiple bioactive protein: An overview. Biochim Biophys Acta. 2012;182:226–36. doi: 10.1016/j.bbagen.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Albert DM, Jakobiec FA. Principles and practice of ophthalmology. 2 Philadelphia: WB Saunders; 2000. [Google Scholar]
- 144.Aho HJ, Saari KM, Kallajoki M, Nevalainen TJ. Synthesis of group II phospholipase A2 and lysozyme in lacrimal glands. Invest Ophthalmol Vis Sci. 1996;37:1826–32. [PubMed] [Google Scholar]
- 145.Boukes RJ, Boonstra A, Breebaart AC, et al. Analysis of human tear protein profiles using high performance liquid chromatography (HPLC) Doc Ophthalmol. 1987;67:105–13. doi: 10.1007/BF00142704. [DOI] [PubMed] [Google Scholar]
- 146.McCollum CJ, Foulks GN, Bodner B, et al. Rapid assay of lactoferrin in keratoconjunctivitis sicca. Cornea. 1994;13:505–8. [PubMed] [Google Scholar]
- 147.Ohashi Y, Ishida R, Kojima T, et al. Abnormal protein profiles in tears with dry eye syndrome. Am J Ophthalmol. 2003;136:291–9. doi: 10.1016/s0002-9394(03)00203-4. [DOI] [PubMed] [Google Scholar]
- 148.Zhou L, Beuerman RW, Chan CM, et al. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. 2009;8:4889–905. doi: 10.1021/pr900686s. [DOI] [PubMed] [Google Scholar]
- 149.Grus FH, Podust VN, Bruns K, et al. SELDI-TOF-MS ProteinChip array profiling of tears from patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46:863–76. doi: 10.1167/iovs.04-0448. [DOI] [PubMed] [Google Scholar]
- 150.Caffery B, Joyce E, Boone A, et al. Tear lipocalin and lysozyme in Sjogren and non-Sjogren dry eye. Optom Vis Sci. 2008;85:661–7. doi: 10.1097/OPX.0b013e318181ae4f. [DOI] [PubMed] [Google Scholar]
- 151.Versura P, Nanni P, Bavelloni A, et al. Tear proteomics in evaporative dry eye disease. Eye (Lond) 2010;24:1396–402. doi: 10.1038/eye.2010.7. [DOI] [PubMed] [Google Scholar]
- 152.Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–11. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Franklin RM, Remus LE. Conjunctival-associated lymphoid tissue: evidence for a role in the secretory immune system. Invest Ophthalmol Vis Sci. 1984;25:181–7. [PubMed] [Google Scholar]
- 154.Wieczorek R, Jakobiec FA, Sacks EH, Knowles DM. The immunoarchitecture of the normal human lacrimal gland. Relevancy for understanding pathologic conditions. Ophthalmology. 1988;95:100–9. doi: 10.1016/s0161-6420(88)33228-8. [DOI] [PubMed] [Google Scholar]
- 155.Wehmeyer A, Das PK, Swaak T, et al. Sjogren syndrome: comparative studies in local ocular and serum immunoglobulin concentrations with special reference to secretory IgA. Int Ophthalmol. 1991;15:147–51. doi: 10.1007/BF00153916. [DOI] [PubMed] [Google Scholar]
- 156.Lan J, Willcox MD, Jackson GD. Effect of tear-specific immunoglobulin A on the adhesion of Pseudomonas aeruginosa I to contact lenses. Aust N Z J Ophthalmol. 1999;27:218–20. doi: 10.1046/j.1440-1606.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- 157.Willcox MD, Lan J. Secretory immunoglobulin A in tears:functions and changes during contact lens wear. Clin Exp Optom. 1999;82:1–3. doi: 10.1111/j.1444-0938.1999.tb06777.x. [DOI] [PubMed] [Google Scholar]
- 158.Pearce DJ, Demirci G, Willcox MD. Secretory IgA epitopes in basal tears of extended-wear soft contact lens wearers and in non-lens wearers. Aust N Z J Ophthalmol. 1999;27:221–3. doi: 10.1046/j.1440-1606.1999.00206.x. [DOI] [PubMed] [Google Scholar]
- 159.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 160.Buckland AG, Wilton DC. The antibacterial properties of secreted phospholipases A(2) Biochim Biophys Acta. 2000;1488:71–82. doi: 10.1016/s1388-1981(00)00111-6. [DOI] [PubMed] [Google Scholar]
- 161.Nevalainen TJ, Graham GG, Scott KF. Antibacterial actions of secreted phospholipases A2. Review. Biochim Biophys Acta. 2008;1781:1–9. doi: 10.1016/j.bbalip.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 162.Qu XD, Lehrer RI. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect Immun. 1998;66:2791–7. doi: 10.1128/iai.66.6.2791-2797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aho V, Nevalainen T, Saari M. Group IIA phospholipase A2 content of tears in patients with keratoconjunctivitis sicca. Graefes Arch Clin Experl Ophthalmol. 2002;240:521–23. doi: 10.1007/s00417-002-0477-8. [DOI] [PubMed] [Google Scholar]
- 164.Chen D, Wei Y, Li X, et al. sPLA2-IIa is an inflammatory mediator when the ocular surface is compromised. Exp Eye Res. 2009;88:880–8. doi: 10.1016/j.exer.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wei Y, Epstein SP, Fukuoka S, et al. sPLA2-IIa amplifies ocular surface inflammation in the experimental dry eye (DE) BALB/c mouse model. Invest Ophthalmol Vis Sci. 2011;52:4780–8. doi: 10.1167/iovs.10-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Debailleul V, Laine A, Huet G, et al. Human mucin genes MUC2, MUC3, MUC4, MUC5AC, MUC5B, and MUC6 express stable and extremely large mRNAs and exhibit a variable length polymorphism. An improved method to analyze large mRNAs. J Biol Chem. 1998;273:881–90. doi: 10.1074/jbc.273.2.881. [DOI] [PubMed] [Google Scholar]
- 167.Argueso P, Balaram M, Spurr-Michaud S, et al. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–11. [PubMed] [Google Scholar]
- 168.Argueso P, Spurr-Michaud S, Russo CL, et al. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44:2487–95. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 169.Berry M, Ellingham RB, Corfield AP. Polydispersity of normal human conjunctival mucins. Invest Ophthalmol Vis Sci. 1996;37:2559–71. [PubMed] [Google Scholar]
- 170.Corrales RM, Calonge M, Herreras JM, et al. Human epithelium from conjunctival impression cytology expresses MUC7 mucin gene. Cornea. 2003;22:665–71. doi: 10.1097/00003226-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 171.Corrales RM, Galarreta DJ, Herreras JM, et al. Normal human conjunctival epithelium expresses MUC13, MUC15, MUC16 and MUC17 mucin genes. Arch Soc Esp Oftalmol. 2003;78:375–81. Spanish. [PubMed] [Google Scholar]
- 172.Diebold Y, Corrales RM, Calonge M, et al. Human conjunctival epithelium in culture: a tool to assay new therapeutic strategies for dry eye. Adv Exp Med Biol. 2002;506:307–11. doi: 10.1007/978-1-4615-0717-8_43. [DOI] [PubMed] [Google Scholar]
- 173.Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Invest Ophthalmol Vis Sci. 1995;36:1818–27. [PubMed] [Google Scholar]
- 174.Inatomi T, Spurr-Michaud S, Tisdale AS, et al. Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996;37:1684–92. [PubMed] [Google Scholar]
- 175.Jumblatt MM, McKenzie RW, Jumblatt JE. MUC5AC mucin is a component of the human precorneal tear film. Invest Ophthalmol Vis Sci. 1999;40:43–9. [PubMed] [Google Scholar]
- 176.Jumblatt MM, McKenzie RW, Steele PS, et al. MUC7 expression in the human lacrimal gland and conjunctiva. Cornea. 2003;22:41–5. doi: 10.1097/00003226-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 177.McKenzie RW, Jumblatt JE, Jumblatt MM. Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Invest Ophthalmol Vis Sci. 2000;41:703–8. [PubMed] [Google Scholar]
- 178.Pflugfelder SC, Liu Z, Monroy D, et al. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest Ophthalmol Vis Sci. 2000;41:1316–26. [PubMed] [Google Scholar]
- 179.Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res. 2010;90:655–63. doi: 10.1016/j.exer.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Blalock TD, Spurr-Michaud SJ, Tisdale AS, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:4509–18. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 181.Imbert Y, Darling DS, Jumblatt MM, et al. MUC1 splice variants in human ocular surface tissues: possible differences between dry eye patients and normal controls. Exp Eye Res. 2006;83:493–501. doi: 10.1016/j.exer.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 182.Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Release of membrane-associated mucins from ocular surface epithelia. Invest Ophthalmol Vis Sci. 2008;49:1864–71. doi: 10.1167/iovs.07-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Enriquez-de-Salamanca A, Castellanos E, Stern ME, et al. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol Vis. 2010;16:862–73. [PMC free article] [PubMed] [Google Scholar]
- 184.Caffery B, Heynen ML, Joyce E, et al. MUC1 expression in Sjogren’s syndrome, KCS, and control subjects. Mol Vis. 2010;16:1720–7. [PMC free article] [PubMed] [Google Scholar]
- 185.Yu DF, Chen Y, Han JM, et al. MUC19 expression in human ocular surface and lacrimal gland and its alteration in Sjogren syndrome patients. Exp Eye Res. 2008;86:403–11. doi: 10.1016/j.exer.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 186.Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379–88. doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 187.Danjo Y, Watanabe H, Tisdale AS, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–9. [PubMed] [Google Scholar]
- 188.Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008;8:477–83. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lievin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–37. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McDermott AM. The role of antimicrobial peptides at the ocular surface. Ophthalmic Res. 2009;41:60–75. doi: 10.1159/000187622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Huang LC, Jean D, Proske RJ, et al. Ocular surface expression and in vitro activity of antimicrobial peptides. Curr Eye Res. 2007;32:595–609. doi: 10.1080/02713680701446653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Huang LC, Redfern RL, Narayanan S, et al. In vitro activity of human beta-defensin 2 against Pseudomonas aeruginosa in the presence of tear fluid. Antimicrob Agents Chemother. 2007;51:3853–60. doi: 10.1128/AAC.01317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.McDermott AM. Cationic antimicrobial peptides. A future therapeutic option? Arch Soc Esp Oftalmol. 2007;82:467–70. [PubMed] [Google Scholar]
- 194.McDermott AM. Defensins and other antimicrobial peptides at the ocular surface. Ocul Surf. 2004;2:229–47. doi: 10.1016/s1542-0124(12)70111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Narayanan S, Manning J, Proske R, McDermott AM. Effect of hyperosmolality on beta-defensin gene expression by human corneal epithelial cells. Cornea. 2006;25:1063–8. doi: 10.1097/01.ico.0000228785.84581.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McDermott AM, Rich D, Cullor J, et al. The in vitro activity of selected defensins against an isolate of Pseudomonas in the presence of human tears. Br J Ophthalmol. 2006;90:609–11. doi: 10.1136/bjo.2005.083428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gordon YJ, Huang LC, Romanowski EG, et al. Human cathelicidin (LL-37), a multifunctional peptide, is expressed by ocular surface epithelia and has potent antibacterial and antiviral activity. Curr Eye Res. 2005;30:385–94. doi: 10.1080/02713680590934111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Narayanan S, Miller WL, McDermott AM. Expression of human beta-defensins in conjunctival epithelium: relevance to dry eye disease. Invest Ophthalmol Vis Sci. 2003;44:3795–801. doi: 10.1167/iovs.02-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.McDermott AM, Redfern RL, Zhang B, et al. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–65. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McDermott AM, Redfern RL, Zhang B. Human beta-defensin 2 is up-regulated during re-epithelialization of the cornea. Curr Eye Res. 2001;22:64–7. doi: 10.1076/ceyr.22.1.64.6978. [DOI] [PubMed] [Google Scholar]
- 201.Narayanan S, Miller WL, McDermott AM. Expression of human b-Defensins in conjunctival epithelium: relevance to dry eye disease. Invest Ophthalmol Vis Sci. 2003;44:3795–801. doi: 10.1167/iovs.02-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Huang LC, Reins RY, Gallo RL, McDermott AM. Cathelicidin-deficient (Cnlp −/−) mice show increased susceptibility to Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2007;48:4498–508. doi: 10.1167/iovs.07-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Haynes RJ, Tighe PJ, Dua HS. Antimicrobial defensin peptides of the human ocular surface. Br J Ophthalmol. 1999;83:737–41. doi: 10.1136/bjo.83.6.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Garreis F, Schlorf T, Worlitzsch D, et al. Roles of human beta-defensins in innate immune defense at the ocular surface: arming and alarming corneal and conjunctival epithelial cells. Histochem Cell Biol. 2010;134:59–73. doi: 10.1007/s00418-010-0713-y. [DOI] [PubMed] [Google Scholar]
- 205.Huang LC, Petkova TD, Reins RY, et al. Multifunctional roles of human cathelicidin (LL-37) at the ocular surface. Invest Ophthalmol Vis Sci. 2006;47:2369–80. doi: 10.1167/iovs.05-1649. [DOI] [PubMed] [Google Scholar]
- 206.Augustin DK, Heimer SR, Tam C, et al. Role of defensins in corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Infect Immun. 2011;79:595–605. doi: 10.1128/IAI.00854-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wu M, McClellan SA, Barrett RP, et al. Beta-defensins 2 and 3 together promote resistance to Pseudomonas aeruginosa keratitis. J Immunol. 2009;183:8054–60. doi: 10.4049/jimmunol.0902140. [DOI] [PubMed] [Google Scholar]
- 208.Kawasaki S, Kawamoto S, Yokoi N, et al. Up-regulated gene expression in the conjunctival epithelium of patients with Sjogren’s syndrome. Exp Eye Res. 2003;77:17–26. doi: 10.1016/s0014-4835(03)00087-3. [DOI] [PubMed] [Google Scholar]
- 209.Kaneda Y, Yamaai T, Mizukawa N, et al. Localization of antimicrobial peptides human beta-defensins in minor salivary glands with Sjogren’s syndrome. Eur J Oral Sci. 2009;117:506–10. doi: 10.1111/j.1600-0722.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- 210.Abedin A, Mohammed I, Hopkinson A, Dua HS. A novel antimicrobial peptide on the ocular surface shows decreased expression in inflammation and infection. Invest Ophthalmol Vis Sci. 2008;49:28–33. doi: 10.1167/iovs.07-0645. [DOI] [PubMed] [Google Scholar]
- 211.Guani-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Teran LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135:1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 212.Howl J, Jones S. Bioactive peptides. Boca Raton: CRC Press; 2009. p. xv.p. 479. 8 pp of plates. [Google Scholar]
- 213.Kolar SS, McDermott AM. Role of host-defence peptides in eye diseases. Cell Mol Life Sci. 2011;68:2201–13. doi: 10.1007/s00018-011-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McGinnigle S, Naroo SA, Eperjesi F. Evaluation of dry eye. Surv Ophthalmol. 2012;57:293–316. doi: 10.1016/j.survophthal.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 215.Radfar L, Shea Y, Fischer SH, et al. Fungal load and candidiasis in Sjogren’s syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:283–7. doi: 10.1016/s1079-2104(03)00224-5. [DOI] [PubMed] [Google Scholar]
- 216.Labbe A, Alalwani H, Van Went C, et al. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–31. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- 217.McMonnies CW. Blink efficiency: a neglected area of ocular surface disease management? Invest Ophthalmol Vis Sci. 2011;52:4484. doi: 10.1167/iovs.11-7751. [DOI] [PubMed] [Google Scholar]
- 218.Alarcon I, Kwan L, Yu C, et al. Role of the corneal epithelial basement membrane in ocular defense against Pseudomonas aeruginosa. Infect Immun. 2009;77:3264–71. doi: 10.1128/IAI.00111-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens. 2005;31:186–93. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- 220.Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 221.Ni M, Evans DJ, Hawgood S, et al. Surfactant protein D is present in human tear fluid and the cornea and inhibits epithelial cell invasion by Pseudomonas aeruginosa. Infect Immun. 2005;73:2147–56. doi: 10.1128/IAI.73.4.2147-2156.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Maltseva IA, Fleiszig SM, Evans DJ, et al. Exposure of human corneal epithelial cells to contact lenses in vitro suppresses the upregulation of human beta-defensin-2 in response to antigens of Pseudomonas aeruginosa. Exp Eye Res. 2007;85:142–53. doi: 10.1016/j.exer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 223.Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:163–78. doi: 10.1016/s1542-0124(12)70085-x. (No authors listed) [DOI] [PubMed] [Google Scholar]
- 224.Narayanan S. Which drop for dry eye? Review of Optometry. 2009;146:4. [Google Scholar]
- 225.Ryan G, Jr, Fain JM, Lovelace C, Gelotte KM. Effectiveness of ophthalmic solution preservatives: a comparison of latanoprost with 0.02% benzalkonium chloride and travoprost with the sofZia preservative system. BMC Ophthalmol. 2011;11:8. doi: 10.1186/1471-2415-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hyon JY, Eser I, O’Brien TP. Kill rates of preserved and preservative-free topical 8-methoxy fluoroquinolones against various strains of Staphylococcus. J Cataract Refract Surg. 2009;35:1609–13. doi: 10.1016/j.jcrs.2009.04.036. [DOI] [PubMed] [Google Scholar]
- 227.Kowalski RP, Kowalski BR, Romanowski EG, et al. The in vitro impact of moxifloxacin and gatifloxacin concentration (0.5% vs 0.3%) and the addition of benzalkonium chloride on antibacterial efficacy. Am J Ophthalmol. 2006;142:730–5. doi: 10.1016/j.ajo.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 228.Gobbels M, Spitznas M. Corneal epithelial permeability of dry eyes before and after treatment with artificial tears. Ophthalmology. 1992;99:873–8. doi: 10.1016/s0161-6420(92)31879-2. [DOI] [PubMed] [Google Scholar]
- 229.Kimura K, Teranishi S, Kawamoto K, Nishida T. Protection of human corneal epithelial cells from hypoxia-induced disruption of barrier function by hepatocyte growth factor. Exp Eye Res. 2010;90:337–43. doi: 10.1016/j.exer.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 230.Huang LC, Jean D, McDermott AM. Effect of preservative-free artificial tears on the antimicrobial activity of human beta-defensin-2 and cathelicidin LL-37 in vitro. Eye Contact Lens. 2005;31:34–8. doi: 10.1097/01.ICL.0000146320.64438.8C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137:337–42. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 232.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107:631–9. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 233.Clipstone NA, Fiorentino DF, Crabtree GR. Molecular analysis of the interaction of calcineurin with drug-immunophilin complexes. J Biol Chem. 1994;269:26431–7. [PubMed] [Google Scholar]
- 234.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–47. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 235.Turner K, Pflugfelder SC, Ji Z, et al. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea. 2000;19:492–6. doi: 10.1097/00003226-200007000-00018. [DOI] [PubMed] [Google Scholar]
- 236.Brignole F, Pisella PJ, De Saint Jean M, et al. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol Vis Sci. 2001;42:90–5. [PubMed] [Google Scholar]
- 237.Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118:1489–96. doi: 10.1001/archopht.118.11.1489. [DOI] [PubMed] [Google Scholar]
- 238.Guzey M, Satici A, Karaman SK, et al. The effect of topical cyclosporine A treatment on corneal thickness in patients with trachomatous dry eye. Clin Exp Optom. 2009;92:349–55. doi: 10.1111/j.1444-0938.2009.00369.x. [DOI] [PubMed] [Google Scholar]
- 239.Hara Y, Shiraishi A, Kobayashi T, et al. Alteration of TLR3 pathways by glucocorticoids may be responsible for immunosusceptibility of human corneal epithelial cells to viral infections. Mol Vis. 2009;15:937–48. [PMC free article] [PubMed] [Google Scholar]
- 240.Sheppard JD, Wertheimer ML, Scoper SV. Modalities to decrease stromal herpes simplex keratitis reactivation rates. Arch Ophthalmol. 2009;127:852–6. doi: 10.1001/archophthalmol.2009.163. [DOI] [PubMed] [Google Scholar]
- 241.Stevenson D, Tauber J, Reis BL. Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. The Cyclosporin A Phase 2 Study Group. Ophthalmology. 2000;107:967–74. doi: 10.1016/s0161-6420(00)00035-x. [DOI] [PubMed] [Google Scholar]
- 242.Salisbury MA, Kaswan RL, Brown J. Microorganisms isolated from the corneal surface before and during topical cyclosporine treatment in dogs with keratoconjunctivitis sicca. Am J Vet Res. 1995;56:880–4. [PubMed] [Google Scholar]
- 243.Bell NP, Karp CL, Alfonso EC, et al. Effects of methylprednisolone and cyclosporine A on fungal growth in vitro. Cornea. 1999;18:306–13. doi: 10.1097/00003226-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 244.Onyewu C, Afshari NA, Heitman J. Calcineurin promotes infection of the cornea by Candida albicans and can be targeted to enhance fluconazole therapy. Antimicrob Agents Chemother. 2006;50:3963–5. doi: 10.1128/AAC.00393-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Djalilian AR, Nagineni CN, Mahesh SP, et al. Inhibition of inflammatory cytokine production in human corneal cells by dexamethasone, but not cyclosporin. Cornea. 2006;25:709–14. doi: 10.1097/01.ico.0000208815.02120.90. [DOI] [PubMed] [Google Scholar]
- 246.Flueckiger F, Kodjikian L, Halberstadt M, et al. An ex-vivo, whole-globe porcine model of corneoepithelial wound healing tested using immunomodulatory drugs. J Ocul Pharmacol Ther. 2005;21:367–75. doi: 10.1089/jop.2005.21.367. [DOI] [PubMed] [Google Scholar]
- 247.Garweg JG, Wegmann-Burns M, Goldblum D. Effects of daunorubicin, mitomycin C, azathioprine and cyclosporin A on human retinal pigmented epithelial, corneal endothelial and conjunctival cell lines. Graefes Arch Clin Exp Ophthalmol. 2006;244:382–9. doi: 10.1007/s00417-005-0017-4. [DOI] [PubMed] [Google Scholar]
- 248.Terai K, Sano Y, Kawasaki S, et al. Effects of dexamethasone and cyclosporin A on human beta-defensin in corneal epithelial cells. Exp Eye Res. 2004;79:175–80. doi: 10.1016/j.exer.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 249.Abelson MB, Schaefer K. Conjunctivitis of allergic origin: immunologic mechanisms and current approaches to therapy. Surv Ophthalmol. 1993;38 (Suppl):115–32. doi: 10.1016/0039-6257(93)90036-7. [DOI] [PubMed] [Google Scholar]
- 250.Tinkelman DG, Rupp G, Kaufman H, et al. Double-masked, paired-comparison clinical study of ketorolac tromethamine 0.5% ophthalmic solution compared with placebo eyedrops in the treatment of seasonal allergic conjunctivitis. Surv Ophthalmol. 1993;38 (Suppl):133–40. doi: 10.1016/0039-6257(93)90037-8. [DOI] [PubMed] [Google Scholar]
- 251.Ilyas H, Slonim CB, Braswell GR, et al. Long-term safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis. Eye Contact Lens. 2004;30:10–3. doi: 10.1097/01.ICL.0000092071.82938.46. [DOI] [PubMed] [Google Scholar]
- 252.Bodor N, Loftsson T, Wu WM. Metabolism, distribution, and transdermal permeation of a soft corticosteroid, loteprednol etabonate. Pharm Res. 1992;9:1275–8. doi: 10.1023/a:1015849132396. [DOI] [PubMed] [Google Scholar]
- 253.Comstock TL, Decory HH. Advances in corticosteroid therapy for ocular inflammation: loteprednol etabonate. Int J Inflam. 2012;2012:789623. doi: 10.1155/2012/789623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Suto C, Morinaga M, Yagi T, et al. Conjunctival sac bacterial flora isolated prior to cataract surgery. Infect Drug Resist. 2012;5:37–41. doi: 10.2147/IDR.S27937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Driver PJ, Lemp MA. Meibomian gland dysfunction. Surv Ophthalmol. 1996;40:343–67. doi: 10.1016/s0039-6257(96)80064-6. [DOI] [PubMed] [Google Scholar]
- 256.Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–92. [PubMed] [Google Scholar]
- 257.De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–35. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 258.Burns FR, Stack MS, Gray RD, Paterson CA. Inhibition of purified collagenase from alkali-burned rabbit corneas. Invest Ophthalmol Vis Sci. 1989;30:1569–75. [PubMed] [Google Scholar]
- 259.Abdel-Khalek LM, Williamson J, Lee WR. Morphological changes in the human conjunctival epithelium. II. In keratoconjunctivitis sicca. Br J Ophthalmol. 1978;62:800–6. doi: 10.1136/bjo.62.11.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Li DQ, Luo L, Chen Z, et al. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82:588–96. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hoeprich PD, Warshauer DM. Entry of four tetracyclines into saliva and tears. Antimicrob Agents Chemother. 1974;5:330–6. doi: 10.1128/aac.5.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Krakauer T, Buckley M. Doxycycline is anti-inflammatory and inhibits staphylococcal exotoxin-induced cytokines and chemokines. Antimicrob Agents Chemother. 2003;47:3630–3. doi: 10.1128/AAC.47.11.3630-3633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Dougherty JM, McCulley JP, Silvany RE, Meyer DR. The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest Ophthalmol Vis Sci. 1991;32:2970–5. [PubMed] [Google Scholar]
- 264.Foulks GN, Borchman D, Yappert M, Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. 2013;32:44–53. doi: 10.1097/ICO.0b013e318254205f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Foulks GN, Borchman D, Yappert M, et al. Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010;29:781–8. doi: 10.1097/ICO.0b013e3181cda38f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Luchs J. Azithromycin in DuraSite for the treatment of blepharitis. Clin Ophthalmol. 2010;4:681–8. doi: 10.2147/opth.s6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Nichols JJ, Bickle KM, Zink RC, et al. Safety and efficacy of topical azithromycin ophthalmic solution 1.0% in the treatment of contact lens-related dry eye. Eye Contact Lens. 2012;38:73–9. doi: 10.1097/ICL.0b013e31823ff229. [DOI] [PubMed] [Google Scholar]