Abstract
It is unclear why the histology of pediatric and adult nonalcoholic fatty liver disease sometimes differs. In adults, severity of portal inflammation and fibrosis correlate with Hedgehog pathway activity. Hedgehog (Hh) signaling regulates organogenesis, but is silent in adult livers until injury re-induces Hh ligand production. During adolescence, liver development is completed and children’s livers normally lose cells that produce and/or respond to Hh ligands. We postulated that fatty liver injury interferes with this process by increasing Hh ligand production, and theorized that hepatic responses to Hh ligands might differ among children according to age, gender, and/or puberty status. Using unstained liver biopsy slides from 56 children with nonalcoholic liver disease (NAFLD), we performed immunohistochemistry to assess Hh pathway activation and correlated results with clinical information obtained at biopsy. Fibrosis stage generally correlated with Hh pathway activity, as demonstrated by numbers of Hh-ligand producing cells (P < 0.0001) and Hh-responsive (glioma-associated oncogene 2-positive [Gli2]) cells (P = 0.0013). Numbers of Gli2 (+) cells also correlated with portal inflammation grade (P = 0.0012). Two distinct zonal patterns of Hh-ligand production, portal/peri-portal vs. lobular, were observed. Higher portal/peri-portal Hh-ligand production was associated with male gender. Male gender and pre-puberty were also associated with ductular proliferation (P<0.05), increased numbers of portal Gli2 (+) cells (P<0.017) and portal fibrosis. Conclusion: The portal/peri-portal (progenitor) compartment of pre-pubescent male livers exhibits high Hh pathway activity. This may explain unique histologic features of pediatric NAFLD because Hedgehog signaling promotes the fibro-ductular response.
Keywords: nonalcoholic steatohepatitis, ductular proliferation, sonic Hedgehog, glioblastoma family transcription factor 2, keratin 7
INTRODUCTION
With the rise in obesity, nonalcoholic fatty liver disease (NAFLD) has been rapidly emerging among children and adolescent children and is now the most common cause of chronic liver disease in the pediatric population in the United States (1, 2). Of concern, several recent studies have demonstrated that advanced forms of NAFLD (i.e. bridging fibrosis and/or cirrhosis) can occur in children despite their relatively brief exposure to fatty liver damage (3–6).
The histologic features of NAFLD observed among children are often different from those observed in adults. Children tend to have less hepatocyte ballooning and more portal inflammation and fibrosis, showing a distinct pattern of steatohepatitis (i.e., pediatric pattern, zone 1 dominant, or Type 2) (4, 7). We recently reported that variability in pubertal stages and/or gender among the pediatric population could account for some of the histologic differences in pediatric patients with NAFLD (8). Potential mechanisms explaining the distinct histologic patterns observed in children with NAFLD, however, remain uncertain.
A growing body of evidence supports a role for deregulation of the Hedgehog (Hh) pathway in the pathogenesis and progression of adult NAFLD. Hh is a morphogenic signaling pathway that orchestrates organogenesis during development. Hh ligand family members (Sonic Hh [SHh], Indian Hh and Desert Hh) activate Hh signaling by engaging Patched (Ptc), their transmembrane receptor on the surface of Hh-responsive cells. Binding of Hh ligands to Ptc prevents Ptc from inhibiting Smoothened (Smo). Activated Smo controls cellular accumulation and nuclear localization of Glioblastoma (Gli) family transcription factors (Gli1, Gli2 and Gli3) that regulate the expression of Hh-regulated genes which modulate the proliferation, differentiation, and survival of Hh-responsive cells (9). The Hh pathway is typically silent in healthy adult livers but becomes reactivated when injury stimulates production of Hh ligands (9, 10). Increased exposure to Hh ligands triggers the growth of various cell types involved in wound healing responses, including resident hepatic immune cells, stellate cells, and progenitors. While Hh signaling is necessary for injured adult livers to regenerate, chronic inflammation, fibrosis and cancer result when pathway activation is excessive and/or prolonged (10, 11). Our group demonstrated that ballooned hepatocytes produce Hh ligands in adults with nonalcoholic steatohepatitis (NASH) (12), and reported significant correlations between the hepatic content of Hh ligands, numbers of Hh-responsive (Gli2-positive cells), and the severity of inflammation and fibrosis in adult NAFLD (13). Whether or not similar mechanisms are involved in pediatric NAFLD is not known.
The livers of children generally harbor greater numbers of Hh-producing cells and Hh-responsive cells than adults (14), in keeping with evidence that Hh regulates organogenesis. This suggested to us that children might be particularly vulnerable to insults that stimulate Hh ligand production. Moreover, because human liver development is not completed until adolescence (15, 16) we postulated that children remain in this Hh-vulnerable state for years, reverting to adult levels of vulnerability only as Hh pathway activity becomes down-regulated during adolescence and completion of hepatic maturation. This reasoning led us to hypothesize that age, gender, and/or puberty status might influence Hh pathway activity in children, thereby modulating hepatic responses to fatty liver injury and hence, histologic features of NAFLD. To evaluate this hypothesis, we investigated the associations between Hh pathway activity and clinicopathologic characteristics of NAFLD in a well-characterized pediatric population.
MATERIAL AND METHODS
Study Design and Population
We performed a cross-sectional analysis using core liver biopsy sections and clinical data from 56 consecutive patients diagnosed with NAFLD at the Division of Pediatric Gastroenterology and Nutrition, the University of California, San Diego. All cases met the following criteria: 1) <18 years of age, 2) absence of other liver diseases or other causes of fatty liver according to the medical history, laboratory tests and histologic evaluation, 3) liver biopsy sample of >20 mm and 4) clinical information was available at the time of liver biopsy. One formalin-fixed, paraffin-embedded unstained section was obtained from each biopsy, along with the paired hematoxylin and eosin (H&E) stained and Masson’s trichrome stained slides. Information on age, gender, Tanner stage and body mass index (BMI) at the time of liver biopsy was also obtained.
This study was conducted using only de-identified slides and clinical information provided from the University of California, San Diego (UCSD) and did not directly involve human subjects [45 CFR 46.102(f)]. The prior UCSD study was approved by the Institutional Review Board (IRB) and informed consent and assent were obtained. This study was approved by the Duke IRB.
Histologic Evaluation of NAFLD
Histologic features of NAFLD were scored by a single hepatopathologist (CG) using the H&E and Masson’s trichrome stained slides according to the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) scoring system (17). Due to limited numbers of study subjects in each category, grades (G) and stages (S) were combined for the analyses as follows: steatosis grade, G0–1, G2 and G3 or G0–1 and G2–3; lobular inflammation grade, G0–1 and G2 (no case had G3); hepatocyte ballooning grade, G0 and G1–2; portal inflammation, G0, G1 and G2 or G0–1 and G2; and fibrosis stage, S0, S1–2 and S3–4 or S0–2 and S3–4. An overall diagnosis was also recorded using the following diagnostic categories: steatosis (NAFLD but not NASH), suspicious for steatohepatitis (SH) adult pattern, which is also referred to zone 3 or Type 1 pattern, suspicious for SH pediatric pattern, which is also referred to zone 1 or Type 2 pattern, definite SH adult pattern and definite SH pediatric pattern.
Immunohistochemical Staining and Evaluations
Initially, 30 of the 56 slides were stained for SHh (N=20), vimentin (Vim, N=6) or alpha-smooth muscle actin (α-SMA, N=4), and the remaining slides were co-stained for Gli2 and keratin 7 (Gli2/K7, N=26). To buttress sample size, 2 of the Gli2/K7 co-stained slides were additionally stained for Vim, 2 of the Gli2/K7 co-stained slides were additionally stained for α-SMA and 5 of the SHh stained slides were additionally stained for K7. Thus, in total, 20 slides were evaluated for SHh, 26 for Gli2, 31 for K7, 8 for Vim and 6 for α-SMA. This approach was necessary because the number of available slides was limited. In order to determine which slides were used for which stain, cases were grouped according to histologic severity and then slides were randomly selected from each group for immunohistochemistry (Ihc) in order to have roughly equivalent numbers of cases within each category of histologic severity. Details of the Ihc methods and antibodies have been published (18, 19).
Positive staining for SHh, Vim and αSMA was semi-quantified as a percentage of the total surface area in low power fields (x100 magnification) and graded into 5 ranked categories: G1 (less than 20%), G2 (20–39%), G3 (40–59%), G4 (60–79%) and G5 (≥80%). Nuclear positivity for Gli2 and cellular positivity for K7 were counted in 5 randomly-selected high power fields (HPFs, x200 magnification). The average counts of K7+, Gli2+, K7+/Gli2− and K7+/Gli2+ cells per HPF were calculated and used for the analyses.
Furthermore, the intensity and histologic location of SHh and Gli2/K7 staining was evaluated by a hepatopathologist (CG). After excluding fragmented samples, 16 SHh slides, 18 Gli2 slides, and 25 K7 slides were used in this sub-analysis. The following features were scored: 1) percentage of portal tracts with SHh+ periportal hepatocytes ([the number of portal tracts with SHh+ periportal hepatocytes / the total number of portal tracts in the biopsy]×100); 2) intensity of periportal hepatocellular SHh staining (G0, none; G1, minimal; G2, mild; G3, moderate); 3) number of SHh+ ballooned hepatocytes (the average number of SHh+ ballooned hepatocytes per x100 magnification field); 4) Gli2+positive portal tract cells reflecting a composite of intensity of staining and quantity (G0, none; G1, minimal; G2, mild; G3, moderate); 5) Gli2+ hepatocytes reflecting location (periportal versus central) and a composite of intensity of staining and quantity (G0–3); and 6) K7+ ductular cells/progenitor cells in portal and periportal areas reflecting a composite of intensity of staining and quantity (G0, none; G1, minimal; G2, mild; G3, moderate). These scored features were analyzed to assess correlations among each other, the H&E and trichrome histologic features and clinical information (age, gender, pubertal stages, and BMI).
To map changes in Hh pathway activity, progenitors, and related stromal cells during normal liver maturation, immunohistochemistry for Indian Hh (IHh), Gli2, Sex-determining region Y-box 9 (Sox9), alpha fetoprotein (AFP), and aSMA was performed on banked formalin-fixed/paraffin-embedded liver sections that had been obtained from healthy male mice on embryonic days 13 and 14, and post-natal weeks 3 and 12 (n=3 mice/time point). Details of the Ihc methods and antibodies have been published (18, 19). Results were quantified as described in the Supplementary Figure Legends.
Statistical Analyses
Clinical characteristics are reported as the mean ± SD for continuous variables, the median and inter-quartile range (IQR, 25th and 75th) for scored variables, or as a proportion with a condition for categorical variables.
Associations of the histologic features with age, gender, and pubertal stages (Tanner stage 1, pre-pubertal; stage 2–4, pubertal; stage 5, post-pubertal) were assessed using Chi-square tests, Kruskal-Wallis tests or ANOVA with Tukey test as appropriate. Due to the small number of patients, puberty and post-puberty were combined into one category.
To assess associations between the Ihc scores and histologic features, we performed Wilcoxon rank sum tests, Kruskal-Wallis tests, Chi-square tests, or Fisher’s exact tests as appropriate. For post-hoc comparison, Wilcoxon rank sum tests were used. To adjust for other factors, multiple ordinal or linear regression models were also used. For the sub-analysis on the intensity and location of SHh+, Gli2+, and K7+ cells, we presented the data in a descriptive manner due to the limited sample size.
For all the analyses, we used JMP statistical software version 8.0 (SAS institute Inc., Cary, NC) and considered differences statistically significant when the P-values were equal to or less than 0.05, except for the post-hoc comparisons in which alpha-levels were adjusted by 0.05/a number of pairs in a comparison. All P values presented are two-sided.
RESULTS
Clinical Characteristics of the Study Population
Clinical characteristics of this study population are summarized in Table 1. The mean age and BMI of the study population were 13±2 years and 34±8 kg/m2, respectively. Seventy five percent were boys. Nineteen point six percent were prepubertal (Tanner stage 1), 65.2% were in puberty (Tanner stage 2–4) and 15.2% were post-pubertal (Tanner stage 5). The distribution of histologic features is also summarized in Table 1. Briefly, the prevalence of severe steatosis (G3) was 39.3%, severe lobular inflammation (G2–3) was 8.9%, hepatocyte ballooning (G1–2) was 23.2%, more than mild portal inflammation (G2) was 23.6% and advanced fibrosis was 37.8%. Simple steatosis was seen in 20 cases (35.7%). Features of SH were noted in the remaining 36 cases (64.3%). Histology was suspicious for SH in 26 cases, including 9 cases (35%) with the pediatric pattern and 17 cases (65%) with the adult pattern. Definite SH was diagnosed in 10 cases, 2 of which (20%) demonstrated the pediatric pattern; the 8 remaining cases of definite SH (80%) exhibited the adult pattern. Thus, simple steatosis was present in a minority of our cohort. The larger sub-group had features of SH, with roughly one third of those individuals demonstrating pediatric pattern SH and the remaining two-thirds exhibiting the more classical, adult SH pattern.
Table 1.
Clinical characteristics of the study population
| Summary statistics | |
|---|---|
| Age, years | 13±2 |
| Gender, male % | 75% |
| Tanner stage | Tanner stage 1, 19.6%; Tanner stages 2–4, 65.2%; Tanner stage 5, 15.2% |
| BMI | 34±8 |
| Steatosis | G0: 1.8%; G1: 37.5%; G2: 21.4%; G3: 39.3% |
| Lobular inflammation | G0: 3.6%; G1: 87.5%; G2: 8.9%; G3: 0% |
| Hepatocyte ballooning | G0: 76.8%; G1:17.9%; G2: 5.3% |
| Portal inflammation | G0: 30.9%; G1:45.5%; G2: 23.6 |
| Diagnostic category | Steatosis: 35.7%; Suspicious SH:46.5%; SH adult pattern:14.3%; SH pediatric pattern: 2:3.6% |
| Fibrosis | S0: 30.2%; S1: 24.5%; S2: 7.5%; S3: 34.0%; S4: 3.8% |
| NAS score | 3[2, 4] |
G: histologic grade; SH: steatohepatitis; S: stage
When comparing the severity of the histologic features between boys and girls, we noted that steatosis grade and fibrosis stage were significantly higher in boys (Wilcoxon rank sum test: P=0.037 for steatosis and P=0.02 for fibrosis). Regardless of gender, age was found to correlate with portal inflammation (ANOVA: P=0.0005); patients with G1–2 portal inflammation were younger than patients with no portal inflammation (G0) (Tukey test: p<0.05). Although univariate analyses did not show significant associations between pubertal stages and histologic features, there was a trend toward higher grades of portal inflammation in earlier pubertal stages (Wilcoxon rank sum test, P=0.08).
Associations Between SHh Expression and Severity of Histologic Features
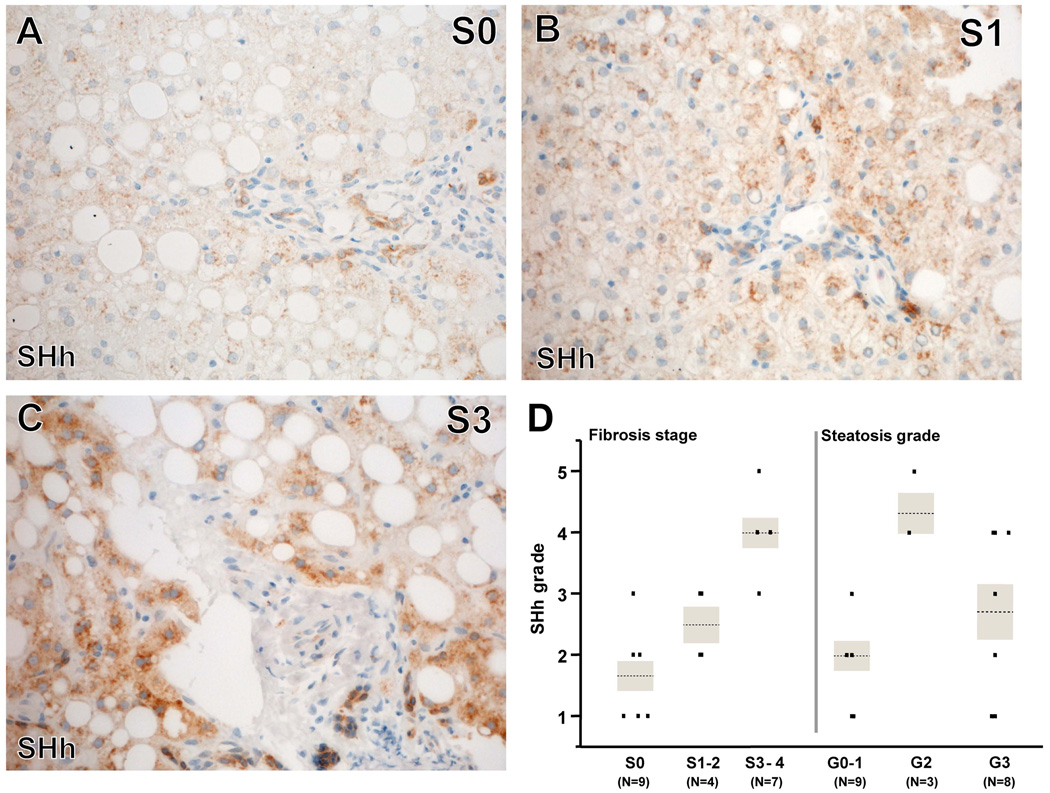
The median SHh grade was 2.5 [2, 4] in the available cohort (N=20). Univariate analysis showed that SHh grade was significantly associated with fibrosis stage (P=0.0008, Figure 1A-D) and steatosis grade (P=0.022, Figure 1D). SHh grades were higher in cases with advanced fibrosis (S3–4) compared to cases with no fibrosis (S0, P=0.0007) and cases with mild to moderate fibrosis (S1–2, P=0.009) (Figure 1A-D). SHh grades were higher in cases with moderate steatosis (G2) than in cases with no to mild steatosis (G0–1, P=0.0091, Figure 1D). In the ordinal logistic regression model including steatosis grade, fibrosis stage, and gender, the association between SHh grade and fibrosis stage remained significant (P=0.002), although steatosis grade did not (P=0.241). There were no significant associations between SHh expression and grades of lobular inflammation, ballooned hepatocytes, or portal inflammation.
Figure 1. SHh immunohistochemistry correlated with fibrosis stage and increased steatosis grade.
Photomicrographs of SHh immunohistochemistry (brown) in patients with S0 (A), S1 (B) and S3 (C) fibrosis show increased numbers of portal and periportal SHh+ cells with increased fibrosis stage. Correlations between SHh staining and severity of fibrosis or steatosis are graphed (D). Severity of fibrosis stage correlated with SHh grade. P=0.0008 (Kruskal Wallis test). *: P<0.017 (adjusted alpha levels in this comparison). Severity of steatosis grade and SHh grade also correlated. P=0.022 (Kruskal Wallis test). *: P<0.017 (adjusted alpha levels in these comparisons). After adjusting for fibrosis stages and gender, the association with fibrosis stage, but not steatosis grades, was still significant (multiple ordinal logistic regression, likelihood ratio test: P=0.24 for steatosis and P=0.006 for fibrosis).
Associations of Gli2 and K7 Expression and Severity of Histologic Features
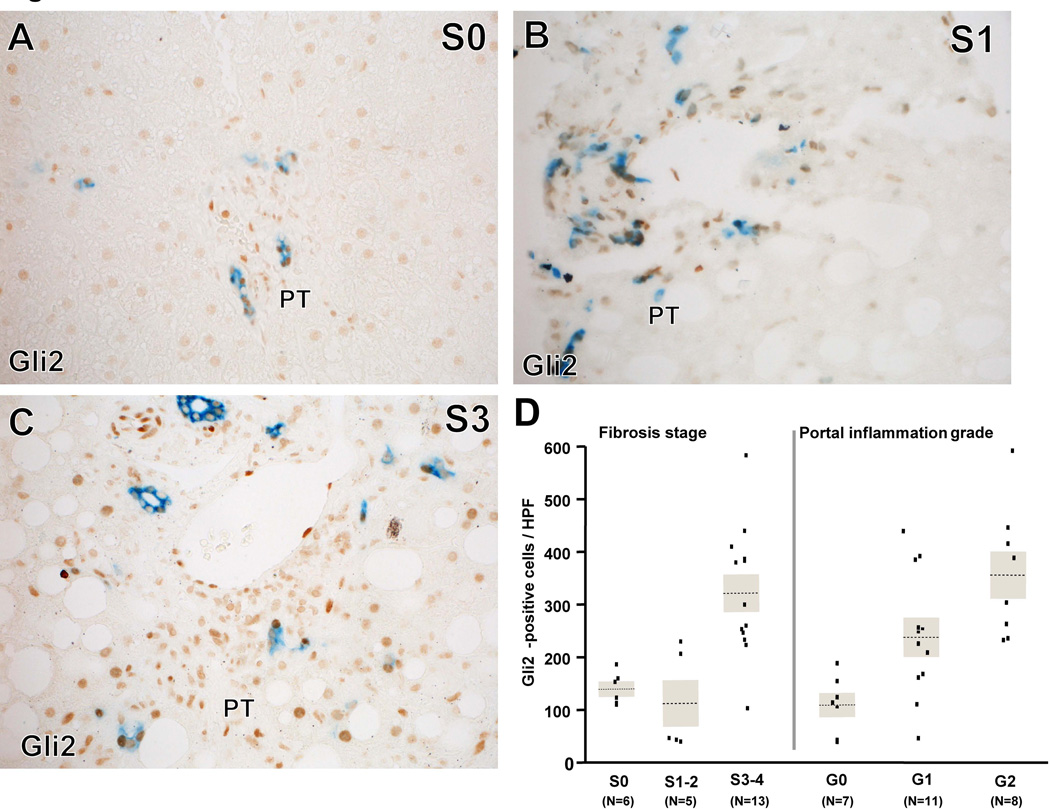
Liver progenitor cells are known to be Hh-responsive. Therefore, we stained liver sections for the progenitor cell marker, keratin 7 (K7), and Gli2 (a marker of Hh-responsiveness). The median numbers of Gli2+ and K7+ cells per HPF were 227 [121, 380] and 17 [14, 25], respectively (N=26 for both stains). On average, 44% of the K7+ cells were also positive for Gli2 (range, 11–73%). Gli2+ cell counts were associated with fibrosis stage (P=0.0013); numbers of Gli2+ cells were higher in cases with advanced fibrosis (S3–4) than in cases with no fibrosis (S0, P=0.004) and cases with mild to moderate fibrosis (S1–2, P=0.004) (Figure 2A - D). The number of Gli2+ cells was also significantly associated with the severity of portal inflammation (P=0.0012, Figure 2D) and tended to be associated with the severity of hepatocyte ballooning (P=0.073). In the ordinal logistic regression model including portal inflammation grade, fibrosis stage and gender, portal inflammation and fibrosis showed independent associations with the number of Gli2+ cells (multiple linear regression model, effect test: P=0.022 for portal inflammation and P=0.045 for fibrosis). There were no significant associations between Gli2+cells and grade of steatosis or lobular inflammation, while a borderline positive association with ballooning grade was noted (P=0.074).
Figure 2. Gli2 and K7 immunohistochemistry correlated with fibrosis stage.
Immunohistochemistry for Gli2 (brown, Hh-responsive cells) and K7 (blue, progenitors) in patients with S0 (A), S1 (B) and S3 (C) fibrosis show increased numbers of Gli2 and K7 positive portal tract (PT) cells with increased fibrosis stage. Correlations between numbers of Gli2+ cells and fibrosis stage or severity of portal inflammation are graphed (D). Severity of fibrosis stage and numbers of Gli2+ cells correlated. P=0.0013 (Kruskal Wallis test). *: P<0.017 (adjusted alpha levels in the comparison). Severity of portal inflammation grade and numbers of Gli2+ cells also correlated. P=0.0012 (Kruskal Wallis test). *: P<0.017 (adjusted alpha levels in this comparison). Fibrosis stages and portal inflammation grades were independently correlated with numbers of Gli2+ cells in the model including gender, fibrosis stages, and portal inflammation grades (multiple linear regression, effect tests: P=0.022 for portal inflammation and P=0.045 for fibrosis).
K7+ cell counts were significantly positively associated with the severity of portal inflammation (P=0.0185) and tended to be associated with the severity of hepatocyte ballooning (P=0.074). In the multiple linear regression model including both grade of portal inflammation and ballooning, portal inflammation, but not hepatocyte ballooning, tended to be associated with the number of K7+ cells (effect test: P=0.082 for portal inflammation and P=0.546 for hepatocyte ballooning). Further, advanced fibrosis (S3–4) tended to be associated with a higher number of K7+ cells versus none to moderate fibrosis (S0–2, P=0.064). There were no significant associations between the number of K7+cells and grade of steatosis or lobular inflammation.
The percentage of Gli2+ cells among the K7+ cells (percentage of Gli2+/K7+ cells) did not show a significant association with the severity of any of the histologic features.
Associations of Vim and αSMA Expression with Severity of Histologic Features
Because Hh signaling promotes fibrogenesis, remaining available liver sections were stained for Vim, a general marker of mesenchymal cells, and α-SMA, a marker of myofibroblasts. The median grades of Vim and αSMA staining were 3 [IQR 1.8, 4.3] (N=8) and 3 [IQR 1.3, 4.5] (N=6), respectively. Vim expression was significantly associated with advanced fibrosis (P=0.038). α-SMA expression showed borderline association with fibrosis and portal inflammation (P=0.0603).
Analysis of Associations of the Intensity and Histologic Location of SHh and Gli2/K7 Ihc staining
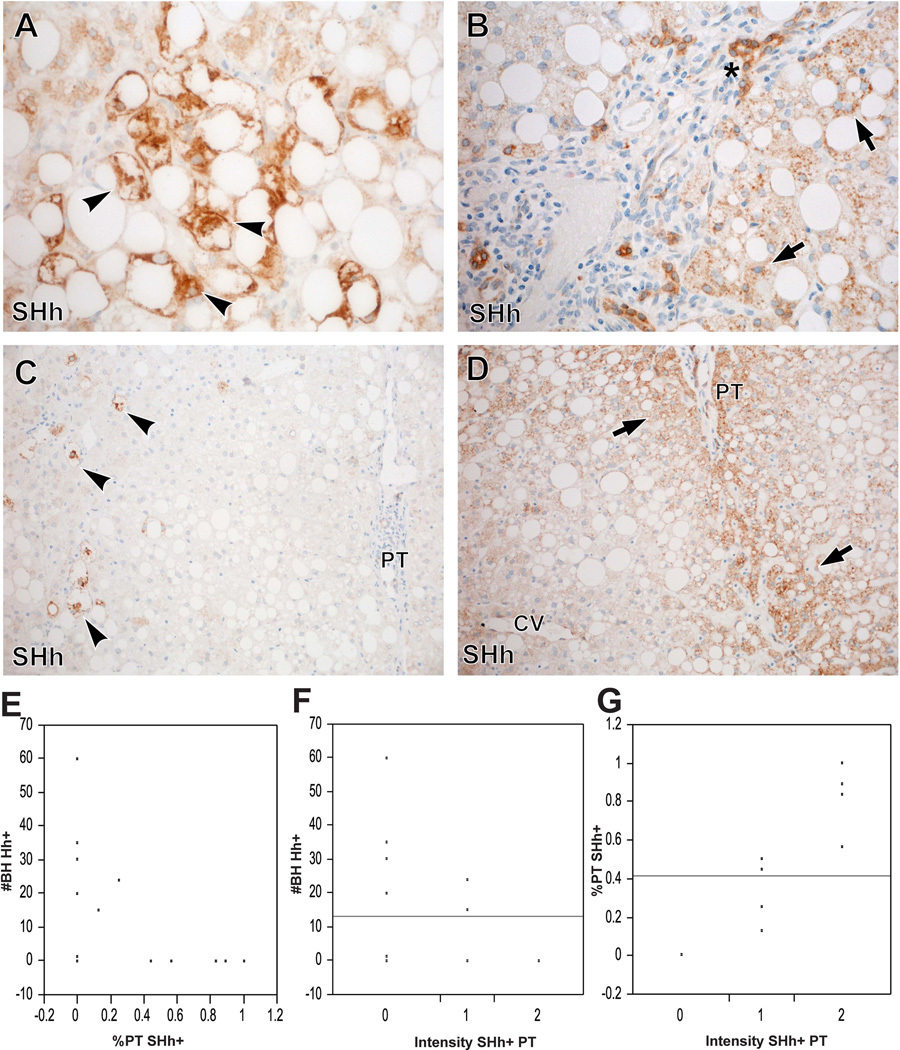
SHh positivity was visualized as three, relatively discrete, patterns: SHh+ ballooned hepatocytes, SHh+ bile ducts and ductules, and SHh+ periportal non-ballooned hepatocytes (Figure 3A-D). SHh+ ballooned hepatocytes, periportal non-ballooned hepatocytes and bile duct/ductular cells were noted in 46.7%, 62.5% and 86.7% of the cases, respectively. Interestingly, the presence of SHh+ ballooned hepatocytes and SHh+ periportal non-ballooned hepatocytes were mutually exclusive (Figure 3C-E, Chi-square test for the presence or absence of these SHh+ patterns, P=0.03). That is, most cases with SHh+ ballooned hepatocytes showed no SHh+ periportal hepatocytes. More specifically, of the 8 cases with SHh+ ballooned hepatocytes, only 2 showed SHh+ periportal hepatocytes and in these 2 cases, less than 25% of the portal tracts showed periportal hepatocellular SHh positivity. Conversely, most of the cases with SHh+ periportal hepatocytes showed no SHh+ ballooned hepatocytes. Of the 2 cases with SHh+ periportal hepatocytes and SHh+ ballooned hepatocytes, 3 or fewer SHh+ ballooned hepatocytes were identified per x100 magnification. On the other hand, SHh+ bile duct/ductular cells tended to be associated with SHh+ periportal hepatocytes, and (like SHh+ periportal hepatocytes) were rarely noted in livers with prevalent SHh+ ballooned hepatocytes. The intensity of SHh+ periportal hepatocellular staining was significantly positively associated with the percentage of portal tracts showing SHh+ periportal hepatocytes (P<0.0009) and negatively associated with numbers of SHh+ ballooned hepatocytes (Figures 3F-G).
Figure 3. Patterns of SHh staining.
Ballooned hepatocytes (arrow heads) strongly express SHh (A). Most ductular cells (asterisks) and some periportal hepatocytes (arrows) also express SHh (B). Cases with ballooned hepatocytes (arrow heads) typically show no SHh+ periportal hepatocytes (C). Cases with no ballooning typically show strong SHh expression in periportal hepatocytes (arrows) in contrast to hepatocytes around the central vein (CV) (D). The percentage of PTs with SHh+ periportal hepatocytes shows an inverse relationship with the number of SHh+ ballooned hepatocytes (E). The intensity of SHh+ periportal hepatocytes also shows an inverse relationship with the numbers of SHh+ ballooned hepatocytes (F). The intensity of S Hh+ periportal hepatocytes was significantly positively associated with the percentage of portal tracts showing SHh+ periportal hepatocytes (G).
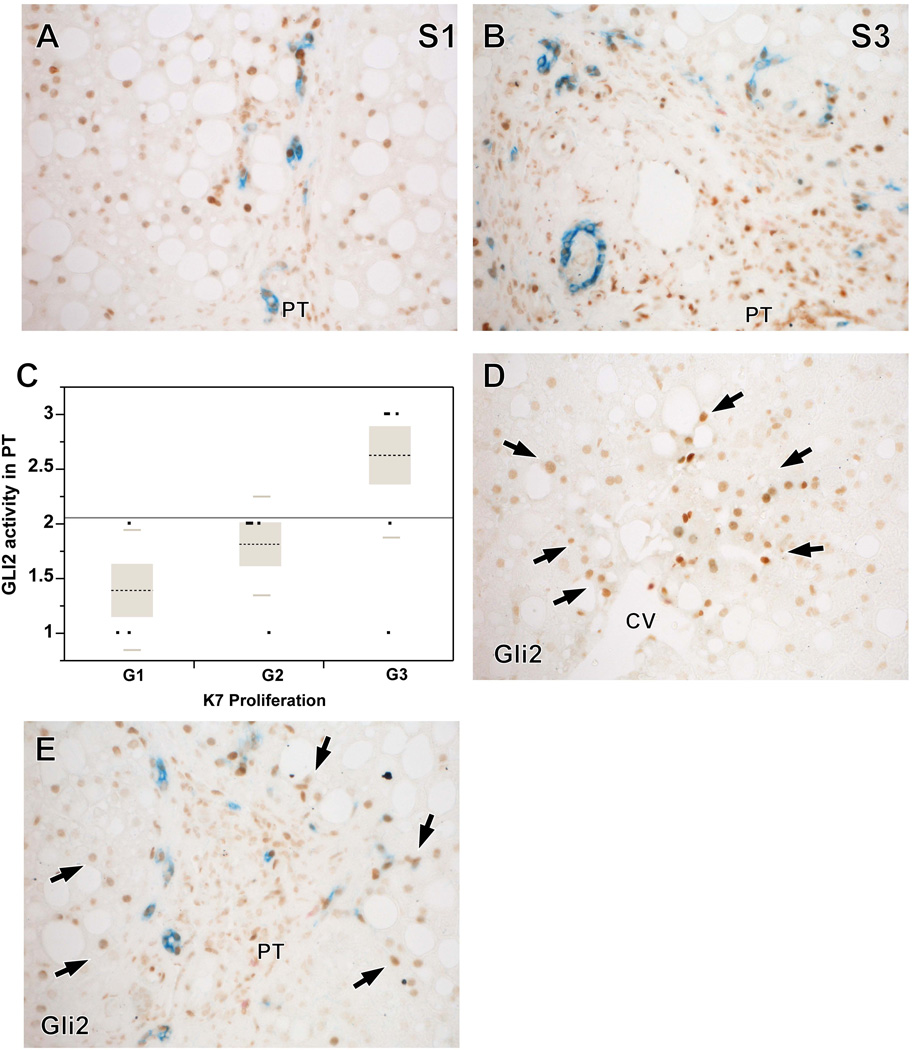
Gli2+ staining in portal tracts cells was observed in all cases examined (N=18). The distribution of the grades of Gli2+ portal tract staining was: G1, 27.8%; G2, 38.9% and G3, 33.3%. K7+ ductular cells (i.e., liver progenitor cells) were also identified in all cases evaluated (N=25). The distribution of the grades of K7+ positivity was: G1, 27.8%; G2, 27.8% and G3, 44.4%. Gli2+ staining and K7+ staining increased with fibrosis stage (Figures 4 A-B).There was a significant positive association between grades of Gli2 portal tract staining and grades of K7 staining (P<0.017, Figure 4C). Gli2+ cells were also located in the hepatic lobule in 13 out of the 18 cases, showing either a zone 3-dominant pattern (Figure 4D, N=4), or a zone 1 dominant pattern (Figure 4E, N=1) or a combination of zone 1- and zone 3-positivity (N=8). The pattern of Gli2 staining in the lobule did not show an association with any of the histologic features.
Figure 4. Patterns of Gli2 and K7 staining.
Higher fibrosis stages showed higher numbers of K7+ cells (progenitors/ductular cells, blue) and Gli2+ cells (Hh-responsive cells, brown) (A-B). There was a significant positive association between grades of Gli2+ PT cells and grades of K7+ PT cells (P <0.017) (C). Gli2+ hepatocytes (arrows) were also identified in the lobular parenchyma and showed a zone 3 dominant pattern (D) or a zone 1 dominant pattern (E) or a combined pattern of Gli2 staining.
In a small number of cases (N=5), we co-stained for SHh ligand and the liver progenitor marker, K7. Interesting relationships between SHh positivity and K7 positivity were revealed. All the cases with more than minimal K7 staining (n = 4) showed SHh+ bile duct cells and mild to moderate SHh+ periportal hepatocytes, while the 1 case with minimal K7 positivity did not show any SHh+ bile duct cells or periportal hepatocytes. The aggregate data, therefore, link portal/peri-portal production of Hh ligands with accumulation of immature liver cells in the portal/peri-portal progenitor niche (e.g., ductal plate remnant).
Because it is difficult to acquire liver tissue from healthy children to map development-related changes in Hh pathway activity, we performed this analysis in liver sections harvested from healthy male mice at different time points during development. Samples were collected at around the time of ductal plate formation (embryonic day 13–14, when liver cells are known to be highly proliferative), at weaning (post-natal week 3, when mice are pre-pubescent and the liver becomes dramatically less proliferative), and during early adulthood (post-natal week 12, by which time the mice have sexually matured and liver cells have acquired the low proliferative activity typical of healthy adults) (20). Immunohistochemistry demonstrated that numbers of Hh ligand-producing cells, Gli2(+) Hh-responsive cells, myofibroblasts (αSMA-positive cells), and progenitors (Sox9- and AFP-positive cells) all decrease significantly as development progresses (Supplementary Figures 1–2). These results support the concept that Hh pathway activity is normally silenced as the hepatic progenitor compartment shrinks to its adult size.
Analysis of Associations of the Histologic Localization and Intensity of SHh and Gli2/K7 Ihc staining with Gender and Puberty
SHh+ periportal non-ballooned hepatocytes, SHh+ ballooned hepatocytes, Gli2+ portal tract cells, and K7+ cells are associated with gender and puberty (Table 2). Boys had a higher percentage of portal tracts with SHh+ periportal hepatocytes (P<0.05), higher intensity of SHh+ periportal hepatocellular staining (P<0.04), higher grade of K7+cells (P<0.005) and lower numbers of SHh+ ballooned hepatocytes (P<0.04). These results indicate that the progenitor response to fatty liver injury differs between boys and girls, with boys demonstrating more robust expansion of the portal/peri-portal progenitor compartment despite seemingly milder parenchymal injury (evidenced by fewer ballooned hepatocytes). Regardless of gender, children in pre-puberty showed higher grades of K7+ positivity (P<0.008), and tended to show higher grades of Gli2 positivity (P=0.055). These findings are consistent with other evidence that children’s livers harbor greater numbers of Hh-responsive progenitors than adult livers (14), and suggest that the “switch” from the childhood liver progenitor compartment to the adulthood liver progenitor compartment occurs during puberty. Pre-pubertal children also demonstrated lower numbers of SHh+ ballooned hepatocytes (P=0.056). In fact, there was no case in pre-puberty (N=4) which showed SHh+ ballooned hepatocytes while 6 out of 11 cases in puberty or post-puberty showed SHh+ ballooned hepatocytes. Although independent associations adjusting for gender and pubertal stages could not be assessed in this population due to the small sample size, these findings support the concept that young children efficiently mobilize Hh-responsive liver progenitors during fatty liver injury.
Table 2.
Associations of SHH, GLI2, and K7 staining with gender and puberty
| N | Gender | Puberty | |||||
|---|---|---|---|---|---|---|---|
| Girls | Boys | P-value | Pre-puberty | Post-puberty | P-value | ||
| SHH+ bile ducts* | 15 | 2[0, 2] | 2[1, 2] | NS | 2[2, 2] | 2[1, 2] | NS |
| % of portal tracts with periportal SHH+ hepatocytes | 16 | 0%[0%, 0%] | 50% [6%, 94%] | 0.045 | 56.5%[0%, 100%] | 25%[0%, 86%] | NS |
| Intensity of SHH+ staining in periportal hepatocytes* | 16 | 0[0, 0] | 1[0.5, 2] | 0.038 | 2[0, 2] | 1[0, 2] | NS |
| K7 proliferation score* | 25 | 1[1, 1.25] | 2[2, 3] | 0.005 | 3[3, 3] | 2[1, 2] | 0.009 |
| GLI2 activity in portal tract cells* | 18 | 1.5[1, 2] | 2[1.75, 3] | 0.128 | 3[2.25, 3] | 2[1, 2.25] | 0.055 |
| SHH+ ballooned hepatocytes | 15 | 5[0.08, 8.6] | 0[0, 2.2] | 0.035 | 0[0, 0] | 2.1[0, 3] | 0.056 |
Data are expressed as median [interquartile range]. NS (not significant): P>0.1
histologic scores (see methods)
Analysis of Associations of the Histologic Localization and Intensity of SHh and Gli2/K7 Ihc staining with Routine Histologic Features
In adults with NAFLD, ductular-appearing progenitor cells typically intermingle with fibro-inflammatory cells along fibrous septae, and the intensity of this fibro-ductular reaction correlates with the severity of liver fibrosis (21). Therefore, we assessed associations of the patterns of Ihc with grades/stages of the routine histologic features. Due to the limited sample size for this analysis, we combined historic grades/stages to create binary variables and compared the patterns of Ihc. The percent of portal tracts showing SHh+ periportal hepatocytes tended to be positively associated with advanced fibrosis (S3–4) (26.9%±32.7% vs. 63.7%±44.0%, P=0.076) and negatively associated with hepatocyte ballooning (53.0%±41.6% vs. 6.3%±12.5%, P=0.053). In contrast, the numbers of SHh+ ballooned hepatocytes were negatively associated with advanced fibrosis (S3–4) (2.7±3.0 vs. 0.4±0.9, P=0.054) and positively associated with hepatocyte ballooning as identified on routine H&E stain (0.4±0.9 vs. 4.8±2.7, P<0.002). Therefore, in children (as in adults), the intensity of the ductular (i.e., progenitor) response correlates with the severity of fibrosis. However, while adults generally require substantial parenchymal injury (evidenced by accumulation of ballooned hepatocytes) to provoke a progenitor-based wound healing response, children whose livers have not fully matured mount robust wound healing responses to much milder liver injury. Consistent with this concept, no significant (or borderline) associations were observed between the patterns and intensity of Ihc staining and grades of steatosis, portal inflammation, or lobular inflammation. Moreover, the cases of definite adult pattern SH (N=2) showed higher numbers of SHh+ ballooned hepatocytes compared to the cases of simple steatosis and suspicious for steatohepatitis cases (5.6±4.2 vs. 0.5±1.0 vs. 1.3±1.9). The same two cases of SH adult pattern showed no SHh+ periportal hepatocytes, while in the cases of steatosis and suspicious for SH, about a half of portal tracts showed SHh+ periportal hepatocytes (0% vs. 48.0%±46.5% vs. 46.8%±41%).
DISCUSSION
Using liver sections from a well-characterized pediatric population with NAFLD, we performed immunohistochemical evaluations of SHh ligand-producing cells, Hh-responsive (Gli2+) cells, K7-expressing ductular progenitors, and cells marked by Vim or a-SMA (indicators of fibrogenesis), and assessed their associations with clinical characteristics and severity of NAFLD histologic features. As we have previously reported in adult NAFLD (13), in pediatric NAFLD total Hh pathway activity (demonstrated by both ligand-producing cells (SHh+) and Hh-responsive (Gli2+) progenitor and stromal cells) increased in parallel with fibrosis stage, and numbers of Gli2+ cells correlated with the severity of portal inflammation. The new data in children complement findings in adults with NAFLD (13), as well as similar data generated by studying culture cells and animal models of NAFLD (10,11), and together strongly support the concept that activation of the Hh pathway during fatty liver injury is one of the dominant mechanisms driving fibro-inflammatory repair responses in NAFLD. Therefore, variations in NAFLD outcomes are likely to result from differences in Hh pathway activation among individuals, or within a given individual at different points in time.
A portal-based fibro-inflammatory response (dubbed pediatric pattern or Type 2 NAFLD) occurs in some children with NAFLD, while other children manifest typical features of adult NAFLD. The basis for these differences in the response to fatty liver injury are not known, although it has been noted that young boys with NAFLD are particularly likely to demonstrated zone 1-based pathology (7). Our results identify a role for maturation-related differences in the Hh pathway in this variability. Hh pathway activity is generally low in healthy adult livers, but robust during embryogenesis. Here we demonstrate that over the course of mouse liver development, Hh signaling is gradually down-regulated as organogenesis is completed. Hence, cells that produce and/or respond to Hh are largely restricted to tissue progenitor compartments in adulthood. The main hepatic progenitor compartment in adults is based peri-portally within the vestiges of the fetal liver ductal plate (dubbed the canals of Hering) (20, 21, 22). Human liver progenitors are Hh-responsive, and rare Gli2-positive cells have been demonstrated in the livers of healthy adults (10). The livers of healthy children harbor greater numbers of Hh-responsive cells than the livers of healthy adults (14). Our new findings support the concept that the transition from a childhood complement of Hh-responsive liver progenitors to an adult complement of Hh-responsive progenitors occurs during puberty. This interpretation is supported by our new evidence that in healthy, pre-pubescent male mice, Hh-responsive progenitors decline to adult levels during post-weaning sexual maturation. It is also consistent with the fact that human liver development is completed during adolescence (15, 16).
An important, disease-pertinent, implication of our discovery is that Hh-mediated, progenitor-based repair responses to liver injury are much more robust in pre-pubertal children than in adults. Hh pathway activation has been shown to stimulate outgrowth of immature ductular-type progenitors and myofibroblasts (the fibroductular reaction) and consequent liver fibrosis. In adults, the intensity of these Hh-mediated repair responses generally parallels the severity of liver injury because wounded hepatocytes produce Hh ligands, and release them as they die. Thus, hepatocyte ballooning, Hh pathway activity, portal inflammation, and liver fibrosis are all tightly correlated in adults with NAFLD (13). In young children with NAFLD, however, we demonstrated that cells in the progenitor compartment (ductular cells and peri-portal hepatocytes) produce Hh ligands and showed that large numbers of Hh-responsive (Gli2-positive) cells accumulate there even when parenchymal liver injury is relatively minor (as evidenced by relatively rare ballooned hepatocytes). These findings suggest that the relatively primitive, childhood progenitor compartment is readily mobilized in response to fatty liver injury. As in adults, Hh pathway activation in children provoked portal-based inflammation, and a fibroductular reaction that resulted in local accumulation of fibrous scar. In pre-pubertal children, this portal-based response was the dominant manifestation of fatty liver damage, resulting in Type 2 NAFLD. As puberty progresses and liver maturation is finalized, the progenitor compartment gradually acquires adult characteristics. Therefore, children in later stages of puberty manifest an adult-like pattern of NAFLD. Our data also suggest that male gender impacted this process, because Hh-mediated repair responses tends to be more robust in boys with NAFLD.
This novel model for pediatric NAFLD progression predicts that pre-pubertal children are uniquely capable of mobilizing wound healing responses to liver injury, and thus, are more vulnerable to the adverse consequences of those processes (e.g., fibrosis) than adults. This may explain why advanced fibrosis/cirrhosis ensues relatively rapidly in many types of pediatric liver injury. Additional research is needed to examine this issue, particularly in light of recent studies of mouse hepatic stellate cells (HSC). Liver injury typically stimulates resident HSC to become myofibroblastic (MF). Such MF-HSC are major producers of collagen matrix in many types of liver injury. Although early work showed that resolution of liver injury results in apoptosis of MF-HSC (23, 24), more recent studies demonstrate that some MF-HSC survive and revert to a more quiescent phenotype when injury dissipates. These “reverted” MF-HSC, however, appear to be “primed” to re-acquire myofibroblastic, fibrogenic characteristics when the liver experiences subsequent injury (23, 24). Because Hh pathway activation stimulates the accumulation of MF-HSC (10, 11) and Hh-mediated repair responses tend to be aggressive in children, even transient liver injury during childhood may expand myofibroblast populations, thereby enhancing the lifelong risk for liver fibrosis. Given the emerging epidemic of childhood NAFLD, this prediction has ominous public health implications, and underscores the importance of efforts to prevent, diagnose and treat childhood obesity and its end-organ consequences.
Supplementary Material
Table 3.
Associations of SHH, GLI2, and K7 staining with histologic features of NAFLD
| N | Ballooning grade | Fibrosis stage | |||||
|---|---|---|---|---|---|---|---|
| G0 | G1–2 | P-value | S0–2 | S3–4 | P-value | ||
| SHH+ bile ducts/ductules | 15 | 2[1, 2] | 1[0, 2] | NS | 2[0.5, 2] | 1.5[1, 2] | NS |
| % PTs with SHH+ periportal hepatocytes | 16 | 53%[3%, 97%] | 0%[0%, 19%] | 0.061 | 12.5%[0%, 55%] | 89%[13%, 100%] | 0.086 |
| Intensity of SHH+ staining in periportal hepatocytes | 16 | 1.5[0.3, 2] | 0[0, 0.8] | 0.061 | 0.5[0, 1.8] | 2[1, 2] | NS |
| K7 proliferation score | 25 | 2[1, 3] | 2.5[1.3, 3] | NS | 1[1, 2.5] | 2[2, 3] | NS |
| GLI2 activity in PT cells | 18 | 2[1, 3] | 2[2, 3] | NS | 2[1, 2.3] | 2[1.8, 3] | NS |
| SHH+ ballooned hepatocytes | 15 | 0[0, 0.08] | 4[2.8, 7.8] | 0.003 | 2.5[0, 4.5] | 0[0, 0.5] | 0.063 |
No associations were noted with steatosis, portal inflammation, and lobular inflammation. NS (not significant): p>0.1
G, grade; S, stage; PT, portal tract
Acknowledgments
Sources of funding:
Dr. Anna Mae Diehl is supported by NIH (UO1DK061713, R01-DK053792 and R01-DK077794)
Dr. Jeffrey Schwimmer is supported by NIH (U01DK061734)
Dr. Manal Abdelmalek is supported by NIH (UO1DK061713)
Dr. Joel Lavine is supported by NIH (U01DK061734 and UL1TR000040)
List of Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- Hh
Hedgehog
- Patched
Ptc
- Smo
Smoothened (Smo)
- Gli
Glioblastoma family transcription factors
- Gli2
Glioblastoma 2 transcription factor
- H&E
hematoxylin and eosin
- BMI
body mass index (weight in kg/height in square meters)
- UCSD
University of California San Diego
- IRB
Institutional Review Board
- NASH CRN
NASH Clinical Research Network
- G
histologic grade
- S
fibrosis stage
- NASH
nonalcoholic steatohepatitis
- SH
steatohepatitis
- SHh
sonic Hedgehog
- Vim
vimentin
- α-SMA
alpha-smooth muscle actin
- K7
keratin 7
- Ihc
immunohistochemistry
- HPF
high power field
- IHh
Indian Hedgehog
- Sox9
Sex-determining region Y-box 9
- AFP
alpha fetoprotein
- IQR
interquartile range
- PT
portal tract
- CV
central vein
Footnotes
Conflict of Interest: The authors report no conflict of interest.
Financial Disclosure: The authors have no financial disclosures.
Author Contributions:
Marzena Swiderska-Syn optimized and performed the immunohistochemistry of liver biopsy slides, performed immunohistochemical evaluations, contributed to data analyses, manuscript writing and critical review of manuscript for final submission.
Ayako Suzuki contributed to the generation of the research idea, analysis and interpretation of data, manuscript writing and critical review of manuscript for final submission.
Cynthia D Guy contributed to the generation of the research idea, performed histochemical and immunohistochemical evaluations, interpretation of histologic data, analysis and interpretation of data, manuscript writing and critical review of manuscript for final submission.
Jeffrey B. Schwimmer contributed to the generation of the research idea, data acquisition, data interpretation, manuscript writing and critical review of manuscript for final submission.
Manal F Abdelmalek contributed to interpretation of data, manuscript writing and critical review of the manuscript for final submission.
Joel E. Lavine contributed to the generation of the research idea, data acquisition, data interpretation, manuscript writing and critical review of manuscript for final submission.
Anna Mae Diehl contributed to the generation of the research idea, funded and supervised data acquisition, performed data analysis and interpretation, assisted with manuscript writing and critical review and revision of the manuscript for important intellectual content.
Contributor Information
Marzena Swiderska-Syn, Email: kzdanow@notes.duke.edu.
Ayako Suzuki, Email: ayako.suzuki@duke.edu.
Cynthia D. Guy, Email: cynthia.guy@duke.edu.
Jeffrey B. Schwimmer, Email: jschwimmer@ucsd.edu.
Manal F. Abdelmalek, Email: manal.abdelmalek@duke.edu.
Joel E. Levine, Email: jl3553@columbia.edu.
Anna Mae Diehl, Email: annamae.diehl@duke.edu.
REFERENCES
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 3.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter-Kent C, Yerian LM, Brunt EM, Angulo P, Kohli R, Ling SC, et al. Nonalcoholic steatohepatitis in children: a multicenter clinicopathological study. Hepatology. 2009;50:1113–1120. doi: 10.1002/hep.23133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol. 2002;97:2460–2462. doi: 10.1111/j.1572-0241.2002.06003.x. [DOI] [PubMed] [Google Scholar]
- 6.Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2000;30:48–53. doi: 10.1097/00005176-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki A, Abdelmalek MF, Schwimmer JB, Lavine JE, Scheimann AO, Unalp-Arida A, et al. Association between puberty and features of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2012;10:786–794. doi: 10.1016/j.cgh.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatol. 2011;54:366–373. doi: 10.1016/j.jhep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi SS, Omenetti A, Syn WK, Diehl AM. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol. 2011;43:238–244. doi: 10.1016/j.biocel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bohinc BN, Diehl AM. Mechanisms of Disease Progression in NASH: New Paradigms. Clin Liver Dis. 2012;16:549–565. doi: 10.1016/j.cld.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Rangwala F, Guy CD, Lu J, Suzuki A, Burchette JL, Abdelmalek MF, et al. Increased production of sonic hedgehog by ballooned hepatocytes. J Pathol. 2011;224:401–410. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–1721. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omenetti A, Bass LM, Anders RA, Clemente MG, Francis H, Guy CD, et al. Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology. 2011;53:1246–1258. doi: 10.1002/hep.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libbrecht L, Spinner NB, Moore EC, Cassiman D, Van Damme-Lombaerts R, Roskams T. Peripheral bile duct paucity and cholestasis in the liver of a patient with Alagille syndrome: further evidence supporting a lack of postnatal bile duct branching and elongation. Am J Surg Pathol. 2005;29:820–826. doi: 10.1097/01.pas.0000161325.36348.25. [DOI] [PubMed] [Google Scholar]
- 16.Balcha HH, Admassi D. Normal liver size measurement in Ethiopian children below thirteen years of age, Black Lion Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2012;50:153–158. [PubMed] [Google Scholar]
- 17.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 18.Jung Y, McCall SJ, Li YX, Diehl AM. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology. 2007;45:1091–1096. doi: 10.1002/hep.21660. [DOI] [PubMed] [Google Scholar]
- 19.Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, et al. Repair-related activation of hedgehog signaling promotes cholangiocyte chemokine production. Hepatology. 2009;50:518–527. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpentier R, Suner RE, van Hul N, Kopp JL, Beaudry JB, Cordi S, et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–1438. doi: 10.1053/j.gastro.2011.06.049. 1438 e1431-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, et al. Hedgehogmediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–1488 e1478. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 24.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.