Abstract
Background
Cardiovascular disease is the leading cause of increased mortality for adolescents with advanced kidney disease. The quality of preventive cardiovascular care may impact long-term outcomes for these patients.
Methods
We reviewed records of 196 consecutive adolescents from 8 centers with pre-dialysis chronic kidney disease, on dialysis, or with a kidney transplant who transferred to adult-focused providers. We compared cardiovascular risk assessment and therapy within and across centers. Predictors of care were assessed using multilevel models.
Results
Overall, 58% (range: 44%–86%, p=0.08 for variance) of five recommended cardiovascular risk assessments were documented. Recommended therapy for six modifiable cardiovascular risk-factors was documented 57% (26%–76%, p=0.09) of the time. Thirty-percent of patients (n=59) were reported to go through formal transition which was independently associated with a 21% increase in composite cardiovascular risk assessment (p<0.001). Transfer after 2006 and kidney transplant status were also associated with increased cardiovascular risk assessment (p<0.01 and p=0.045, respectively).
Conclusions
Adolescents with kidney disease receive sub-optimal preventive cardiovascular care, which may contribute to their high risk of future cardiovascular mortality. Great opportunity exists to improve outcomes for children with kidney disease by improving reliability of preventive care that may include formal transition programs.
Keywords: Cardiovascular disease, transition, quality, chronic kidney disease, kidney transplantation, dialysis
Introduction
Outcomes for children with chronic kidney disease (CKD) and end-stage renal disease (ESRD) have improved with an increasing number surviving well into adulthood.[1–2] Unfortunately, many of these patients have cardiovascular disease (CVD) mortality rates over 1,000 times that of their age-matched peers and will experience premature death due to CVD in early adulthood.[3–4] This exceedingly high CVD risk is related to a high prevalence of traditional cardiovascular risk factors (CVRFs) that predict CVD in the general population (such as hypertension, dyslipidemia, obesity, diabetes, and smoking) in addition to other non-traditional CVRFs that increase in prevalence with decreased kidney function (such as chronic inflammation, oxidative stress, abnormal mineral metabolism, hyperhomocysteinemia, endothelial dysfunction, increased burden of coronary calcification and treatment with steroids and/or calcineurin inhibitors).[5–6] Accordingly, guidelines published as early as 2003 recommend screening for, and strict control of modifiable CVRFs in these patients.[5–13] Recent publications have highlighted the suboptimal quality of care for children with chronic illnesses in the primary care setting,[14] and for adults with CKD[15] or a kidney transplant (TXP),[16] yet little is known regarding the patterns of care for adolescents with kidney disease in relation to published guidelines.
Because of a standardized approach and focus on adult-specific issues, formal transition programs have been advocated as a means to improve care for adolescents transferring to adult-focused providers1, 14–17 and may improve preventive CVD care for young adults with kidney disease. Herein we report our findings regarding the patterns of preventive cardiovascular care in such patients. We hypothesized that patterns of care would be more likely related to center-specific practice patterns than individual patient characteristics, and that patients reported to go through formal transition programs would receive more reliable assessment and treatment of modifiable CVRFs.
Patients and Methods
Study Population
After obtaining Institutional Review Board approval at eight participating pediatric nephrology centers from the Midwest Pediatric Nephrology Consortium (United States and Canada), we systematically reviewed charts of consecutive patients with CKD, ESRD on dialysis, or TXP during childhood who transferred care to an adult-focused provider between January 1, 1997 and June 30, 2009.
Selection of Measures and Evidence Base
To assess quality of CVD care, we evaluated three domains of measures: 1) recommended CVRF assessment, 2) prevalence of modifiable CVRFs and 3) recommended therapy for those with modifiable CVRFs. Several international guidelines exist for CVD management in adolescents with kidney disease.[5–13, 17] The measures evaluated in this study were selected from the most comprehensive guideline published at the time by Kavey et al. in 2006.[6] More recent guidelines make similar but even more detailed and specific recommendations.[17] While recommendations are based primarily on observational data in children or extrapolation of interventional trials in adults, given the substantial evidence regarding the early onset of atherosclerosis in childhood in high-risk populations[18], such recommendations are based on the best available evidence. In addition, even though some literature suggests an inverse relationship between traditional CVRF’s and mortality in adult dialysis patients[19–20] (in contrast to those with CKD or TXP), no such data exists for children or adolescents.
Consequently, guidelines from the American Heart Association,[6] National Kidney Foundation,[11–12] and the National Heart, Lung, and Blood Institute,[17] recommend treating adolescents on dialysis similar to those with a kidney transplant and/or CKD. Thus, we applied these guidelines to each of the three populations (ESRD, CKD, TXP). Finally, while multiple non-traditional CVRF’s (e.g. inflammation, oxidative stress, abnormal mineral metabolism, hyperhomocysteinemia, etc.) affect the cardiovascular outcomes in patients with kidney disease, we chose to focus this report on assessment and treatment of traditional CVRF’s that have been widely adopted into guidelines specific to adolescents.
Measures and Definitions
To evaluate CVRF screening, we assessed whether documentation was present for each of five CVRFs recommended by published guidelines[6, 10–12] including: family history of CVD, smoking status, lipid profile, physical activity, and echocardiography for patients with a history of hypertension. If values were missing for any CVD risk assessment in the entire medical record, we assumed that assessment had not occurred.
We determined the prevalence of six modifiable CVRFs among patients assessed. Dichotomous variables were created for smoking status (yes/no), history of elevated blood pressure (blood pressure >90th percentile for age, gender, height or >130/80 for patients ≥ 18 years old), left ventricular hypertrophy as defined by each center based on echocardiography, dyslipidemia (total cholesterol >200 mg/dl and/or low-density lipoprotein cholesterol [LDL] >130 mg/dl), glucose intolerance (fasting glucose >100 mg/dl), and obesity (body mass index [BMI] > 30kg/m2).
To evaluate treatment for modifiable CVRFs, we determined whether recommended therapy was documented for each of the above CVRFs according to the following guideline definitions:[5–6, 10, 12] 1) dietary consultation for any patient with at least one modifiable CVRF or ESRD, 2) smoking cessation counseling for smokers 3) medication prescribed for elevated blood pressure, 4) medication prescribed for elevated cholesterol, 5) endocrine referral for patients with elevated fasting glucose, 6) a formal exercise program and/or referral to obesity clinic for those with obesity. Data were missing in approximately 5% (24/478) of instances where therapy was indicated, preventing a determination of whether or not therapy was prescribed. Accordingly, these instances were excluded.
We developed composite and all or nothing measures for CVRF assessment and therapy. For composite measures we divided the number of assessments or therapies documented by the number of assessments or therapies recommended. This metric thus reflects the percentage of recommended care that was documented. For the all-or nothing measures, we determined the percentage of patients with 100% of recommended care documented in the medical record.
To assess patterns of transitional care, we recorded whether centers reported that a given patient went through a formal transition program (defined by the presence of clinic policies or tools that facilitate the transition process). While recent guidelines[21] recommend specific essential components of effective transition programs, little documentation existed over the period of this study. Thus, for this analysis, formal transition was defined for each patient according to the above definition.
Statistical Methods
We generated descriptive statistics for continuous data using means with standard deviations (normally distributed data) or medians with inter-quartile ranges (non-normal data). We described categorical data with frequencies.
To compare group differences in demographics and CVRFs we used chi-squared comparisons for categorical variables. For normally distributed continuous variables, we used t-tests. For non-normal variables, we performed a square root transformation to normalize the data prior to applying the t-test. For these analyses, we used techniques (e.g., Taylor-linearized variance estimation) that account for the clustered nature of the data (patients nested within study centers).
To analyze composite CVD care, we used a multilevel model approach. Multilevel models allowed us to include patient-level variables (e.g., race/ethnicity), and center-level variables (e.g., presence of a formal transition program) simultaneously. This allowed us to assess whether factors associated with a center predicted patient-level outcomes, as well as partition the total variance in outcomes into variation due to center characteristics (as quantified by the variance across centers) and variation due to patient characteristics. Moreover, multilevel models appropriately account for the clustered nature of the data (patients within centers) allowing for appropriate statistical inferences. In each of our multilevel models, we allowed intercepts to vary randomly across centers. Our primary outcome was the composite measure for CVRF assessment. Secondary outcomes included the composite measure of therapy for modifiable CVRFs and each recommended assessment and therapy individually. Patient-level predictors included whether each patient was reported to go through a formal transition process, race and ethnicity, gender, disease population (CKD, ESRD, TXP), insurance (private or public), and year of transfer (before 2006 vs. 2006 or later when the most comprehensive guideline was published.)[6] We performed a multi-step analysis starting with bivariate analyses of each patient-level predictor. We then developed a multivariable prediction model for each outcome that included important patient-level predictors and covariates from our bivariate analyses. We estimated variances for the outcomes by center. We treated our primary outcome for CVRF assessment as a continuous variable and we report estimates for the average percent increase in documented care with 95% confidence intervals (95% CI) for each patient level predictor. For dichotomous outcomes, we report adjusted odds ratios and 95% CI’s. We used STATA® (StataCorp. 2010, Stata Statistical Software: Release 11; StataCorp LP, College Station, TX, USA) to develop our models and considered p-values < 0.05 statistically significant.
Results
Patient Demographics and Transition
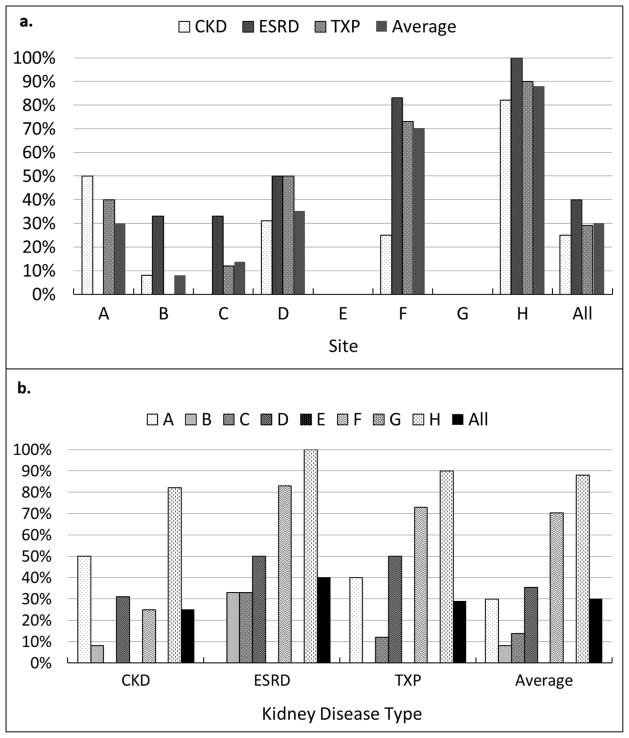
One-hundred ninety six patients (ESRD= 42, CKD=68, TXP=86,) from eight North American pediatric centers were enrolled in the study (7 in the United States, 1 in Canada; Table 1). Each center contributed 17–32 patients (median 25). Fifty-nine (30%) of 196 patients were reported to go through a formal transition, although this varied across centers from 0% to nearly 90% (Figure 1, p = 0.057). TXP patients were older at transfer than ESRD patients (median 20.6 vs. 19.2 years respectively, p=0.018). Patients who went through a formal transition pathway were older at transfer (median 20.5 vs. 19.4 years, p<0.001) and were more likely to transfer after 2006 (75% vs. 36%, p <0.001).
Table 1.
Patient Characteristics by Disease Population and Reported Transfer Type (Formal Transition vs. Other)
| Total n=196 | ESRDa n=42 | CKDb n=68 | TXPc n=86 | p-valued | No Formal Transition n=137 | Formal Transition n=59 | p-valued | |
|---|---|---|---|---|---|---|---|---|
| Female | 98 (50%) | 21 (50%) | 34 (50%) | 43 (50%) | 1.00 | 66 (48%) | 32 (54%) | 0.44 |
| Race/Ethnicity | -- | -- | -- | -- | 0.30 | -- | -- | <0.001 |
| White non-hispanic | 99 (51%) | 18 (43%) | 34 (50%) | 47 (55%) | -- | 76 (55%) | 23 (39%) | -- |
| Black non-hispanic | 55 (28%) | 15 (36%) | 16 (24%) | 24 (28%) | -- | 43 (31%) | 12 (20%) | -- |
| Other non-hispanic | 14 (7%) | 6 (14%) | 4 (6%) | 4 (5%) | -- | 9 (7%) | 5 (8%) | -- |
| Hispanic | 8 (4%) | 1 (2%) | 4 (6%) | 3 (3%) | -- | 6 (4%) | 2 (3%) | -- |
| Unknown | 20 (10%) | 2 (5%) | 10 (15%) | 8 (9%) | -- | 3 (2%) | 17 (28%) | -- |
| Primary Diagnosis | 0.03 | -- | -- | 0.49 | ||||
| Congenital | 43(22%) | 9 (21%) | 7 (10%) | 27 (31%) | -- | 30 (22%) | 13 (22%) | -- |
| Urologic | 36 (18) | 8 (19%) | 12 (18%) | 16 (19%) | -- | 27 (20%) | 9 (15%) | -- |
| Hereditary | 18 (9%) | 4 (10%) | 7 (10%) | 7 (8%) | -- | 14 (10%) | 4 (7%) | -- |
| Glomerulonephritis | 71 (36%) | 15 (36%) | 30 (44%) | 26 (30%) | -- | 50 (37%) | 21 (36%) | -- |
| Systemic | 17 (9%) | 3 (17%) | 11 (16%) | 3 (3%) | -- | 11 (8%) | 6 (10%) | -- |
| Unknown | 11 (6%) | 3 (7%) | 1 (1%) | 7 (8%) | -- | 5 (4%) | 6 (10%) | -- |
| Insurance | < 0.001 | -- | -- | 0.03 | ||||
| Private | 52 (27%) | 8 (19%) | 25 (37%) | 19 (22%) | -- | 43 (31%) | 9 (15%) | -- |
| Public | 90 (46%) | 25 (59%) | 20 (29%) | 45 (52%) | -- | 63 (46%) | 27 (46%) | -- |
| None | 3 (1%) | 3 (7%) | 0 | 0 | -- | 2 (1%) | 1 (2%) | -- |
| Uncleare | 51 (26%) | 6 (14%) | 23 (34%) | 22 (26%) | -- | 29 (21%) | 22 (37%) | -- |
| Age at transfer: median (Q1, Q3) | 19.6 (18.6, 20.9) | 19.2 (18.1, 20.8) | 19.0 (18.5, 20.4)f | 20.6 (19.1, 21)g | 19.4 (18.5, 22) | 20.5 (18.6, 21.6) | <0.001 | |
| Formal Transition Pathway | 59 (30%) | 17 (40%) | 17 (25%) | 25 (29%) | 0.21 | -- | -- | -- |
| Year of Transfer | -- | -- | -- | -- | 0.06 | -- | -- | <0.001 |
| Before 2006 | 91 (46%) | 22 (52%) | 22 (32%) | 47 (55%) | -- | 76 (55%) | 15 (25%) | -- |
| 2006 or later | 94 (48%) | 19 (45%) | 41 (60%) | 34 (40%) | -- | 50 (36%) | 44 (75%) | -- |
| Unknown | 11 (6%) | 1 (2%) | 5 (7%) | 5 (6%) | -- | 11 (8%) | 0 | -- |
End-Stage Renal Disease
Chronic Kidney Disease
Kidney Transplant
Based on Taylor-linearized estimation comparing different populations (ESRD vs. CKD vs. TXP or Formal Transition vs. No Formal Transition)
Unclear primary source of insurance coverage during period of review related to limited availability of old administrative records
NS compared to ESRD
p=0.018 compared to ESRD
ESRD – end stage renal disease; CKD – chronic kidney disease; TXP – kidney transplant
Figure 1.
The percentage of patients reported to go through a formal transition pathway at each of the eight participating centers according to disease population: chronic kidney disease (CKD), end-stage renal disease (ESRD), transplant (TXP). Note the marked within center (a) and between center (b) variation of formal transition pathways for the different kidney disease populations. The report of formal transition pathways varied across centers from 0 to 90% of patients.
CVRF Assessment and Prevalence
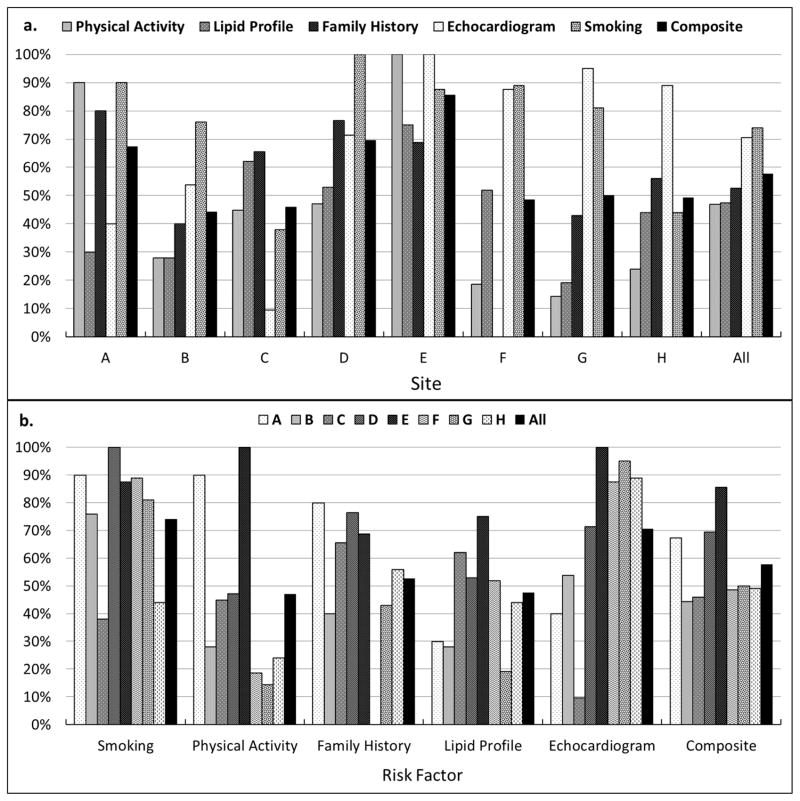
Among five CVFRs studied (Figure 2, Table 2), smoking assessment was documented most commonly (74%). An echocardiogram was documented in 70% of patients with a history of hypertension and family history of CVD was documented in 53% of all patients. Fasting lipid profiles (47%) and physical activity (47%) were assessed in fewer than half of patients. Assessment of individual CVRFs varied moderately by center, as did the composite of CVRF assessment (Table 2; mean 57%; range 44–86%; p=0.08). Overall, 58% of indicated assessments were documented in the medical record, however, only 20/196 (10%) patients received 100% of all indicated CVRF assessments.
Figure 2.
The percentage of patients who received each of five cardiovascular risk assessments before transferring to adult care. Note the variation for each assessment within centers (a) and between centers (b). On average 57% (range 44%–86%) of indicated assessment were documented in each patients chart. Smoking status was the most commonly assessed cardiovascular risk factor with 75% patients assessed, whereas less than 50% patients had a fasting lipid profile or physical activity assessment.
Table 2.
Cardiovascular Disease Care (CVD)
| Variable | Patient level: care documented/care recommended (%)
|
Center Level
|
||||||
|---|---|---|---|---|---|---|---|---|
| All Patients | Formal Transition | No Formal Transition | Odds Ratio (or regression coefficienta) | p-value | Mean % documented care across centers | Range % documented care across centers | p-valueb | |
| CVD risk assessment | ||||||||
| Smoking history | 145/196 (74%) | 48/59 (81%) | 97/137 (71%) | 24.10 | <0.01 | 80% | 38% – 100% | 0.14 |
| Echocardiogram (if hypertensive) | 105/149 (70%) | 37/48 (77%) | 68/101 (67%) | 1.30 | 0.67 | 77% | 10% – 100% | 0.13 |
| Family history of CVDc | 103/196 (53%) | 26/59 (44%) | 77/137 (56%) | 1.24 | 0.81 | 51% | 0% – 80% | 0.16 |
| Fasting lipid profile | 93/196 (47%) | 35/59 (59%) | 58/137 (42%) | 2.97 | 0.06 | 46% | 19% – 75% | 0.17 |
| Physical activity assessment | 92/196 (47%) | 25/59 (42%) | 67/137 (49%) | 4.62 | 0.04 | 51% | 14% – 100% | 0.12 |
|
| ||||||||
| Composite CVD Risk Assessment | 538/933 (58%) | 171/284 (60%) | 367/649 (57%) | 0.21a | <0.001 | 57% | 44% – 86% | 0.08 |
|
| ||||||||
| Therapy for modifiable CVD risk factors | ||||||||
| Blood pressure medication (if hypertensive) | 107/132 (81%) | 36/44 (82%) | 71/88 (81%) | 0.65 | 0.58 | 84% | 29% – 100% | 0.19 |
| Dietary counseling by dietician | 117/165 (71%) | 46/56 (82%) | 71/109 (65%) | 45.00 | 0.21 | 72% | 15% – 100% | 0.17 |
| Lipid lowering agent (if elevated total cholesterol or LDL) | 16/36 (44%) | 9/12 (75%) | 7/24 (29%) | 8.40 | 0.18 | 46% | 0% – 100% | N/Ad |
| Endocrine referral (if hyperglycemic) | 11/50 (22%) | 5/26 (19%) | 6/24 (25%) | 0.71 | 0.62 | 22% | 0% –100% | N/Ad |
| Smoking cessation counseling (if smoker) | 5/28 (18%) | 0/7 (0%) | 5/21 (24%) | N/A | N/A | 89% | 0% –100% | 0.52 |
| Exercise therapy or referral (if BMI > 30) | 4/42 (10%) | 0/15 (0%) | 4/28 (14%) | N/A | N/A | 95% | 0% –25% | N/Ad |
|
| ||||||||
| Composite CVD Therapy | 260/454 (57%) | 96/160 (60%) | 164/294 (56%) | 0.09a | 0.19 | 56% | 27% – 76% | 0.09 |
Regression estimate reflecting the percentage increase in indicated care that was documented
Based on variance estimate
Cardiovascular Disease
Insufficient numbers to compute
The prevalence of CVRFs among those who were assessed was substantial (Table 3). Nearly four-in-five had a history of hypertension and more than one-third of these had left ventricular hypertrophy. More than one-quarter had dyslipidemia and a similar number had elevated fasting glucose. Twenty-two percent were obese and 18% had a history of smoking. Prevalence of CVRFs was generally similar across groups (Table 3). However, hypertension was more common among ESRD and TXP patients than those with CKD (p=0.02) while elevated LDL was more common among ESRD patients (p<0.01). Patients reported to go through a formal transition pathway were less likely to have a family history of CVD (p<0.01) and more likely to have an elevated fasting glucose (p<0.01).
Table 3.
Prevalence of Cardiovascular Risk Factorsa by Disease Population
| Total n/N (%) | ESRDb n (%) | CKDc n (%) | TXPd n (%) | p-valuee | No Formal Transition n (%) | Formal Transition n (%) | p-valuee | |
|---|---|---|---|---|---|---|---|---|
| Family history of CVD | 40/103 (38%) | 7/16 (44%) | 19/41 (46%) | 14/46 (30%) | 0.28 | 36/77 (47%) | 4/26 (15%) | <0.01 |
| Smoking | 28/145 (18%) | 4/29 (14%) | 10/55 (18%) | 14/61 (23%) | 0.56 | 21/97 (22%) | 7/48 (15%) | 0.30 |
| Hypertension | 149/194 (77%) | 36/41 (88%) | 44/67 (66%) | 69/86 (80%) | 0.02 | 101/136 (74%) | 48/58 (83%) | 0.19 |
| Left ventricular hypertrophy by echocardiogramf | 37/103 (36%) | 13/29 (45%) | 7/33 (21%) | 17/41 (41%) | 0.1 | 24/66 (36%) | 13/37 (35%) | 0.90 |
| Total Cholesterol > 200 mg/dl | 35/121 (29%) | 10/24 (42%) | 9/32 (28%) | 16/65 (25%) | 0.29 | 23/81 (28%) | 12/40 (29%) | 0.90 |
| LDL Cholesterol > 130 mg/dl | 22/85 (26%) | 10/17 (59%) | 5/23 (22%) | 7/45 (16%) | <0.01 | 13/46 (28%) | 9/39 (23%) | 0.59 |
| Elevated fasting glucose | 50/192 (26%) | 7/41 (17%) | 20/66 (30%) | 23/85 (27%) | 0.31 | 24/133 (18%) | 26/59 (44%) | <0.01 |
| Body Mass Index > 30 | 42/189 (22%) | 5/39 (13%) | 15/67 (22%) | 22/83 (27%) | 0.24 | 27/130 (21%) | 15/59 (25%) | 0.47 |
among those who had assessment
End-Stage Renal Disease (ESRD)
Chronic Kidney Disease (CKD)
Kidney Transplant (TXP)
Based on Taylor-linearized estimation
among those with hypertension
Therapy for Modifiable CVRFs
Among the modifiable CVRFs, hypertension was the most likely to be treated (81%; Table 2), followed by dietary therapy from a dietician for any modifiable CVRF or ESRD (71%). Therapy for other modifiable CVRF’s varied (Table 2). Overall, 57% of recommended therapies were documented, though only 27% of patients (47/177) who had ≥1 modifiable CVRF received 100% of recommended therapy. Composite CVD therapy varied modestly by center (mean 56%; range 27–76%; p=0.09).
Predictors of CVD Risk Assessment and Treatment
In bivariate analyses of cumulative CVRF assessment (Table 4), formal transition was associated with the largest increase in documented care (21%, p<0.001), followed by transfer in 2006 or later (11% increase, p<0.01) and transplant recipient status (9% increase, p=0.03). In multivariable analyses including gender, disease type, race/ethnicity, formal transition and date of transfer, these associations persisted suggesting an independent association with improved documentation of care (Table 4). When evaluating the composite metric for recommended therapy, none of the patient-level factors were significantly associated in bivariate analyses (data not shown). In the multivariable model, transfer during 2006 or later was associated with an 11% increase in documented therapy for modifiable CVRFs (p=0.03)
Table 4.
Predictors of Cumulative Cardiovascular Disease Assessment
| Bivariate Analyses of CVDa Assessment
|
Multivariate Analysis of CVDa Assessment
|
|||||
|---|---|---|---|---|---|---|
| Estimate | 95% Confidence Interval | p-value | Estimate | 95% Confidence Interval | p-value | |
| Female (compared to male) | 0.04 | −0.02 to 0.10 | 0.16 | 0.01 | −0.05 to 0.06 | 0.19 |
| Disease Population | ||||||
| ESRDb (reference group) | -- | -- | -- | -- | -- | -- |
| CKDc | 0.03 | −0.05 to 0.12 | 0.47 | 0.04 | −0.04 to 0.12 | 0.47 |
| TXPd | 0.09 | 0.01 to 0.17 | 0.034 | 0.08 | 0.002 to 0.15 | 0.045 |
| Race and Ethinicity | ||||||
| Non-Hispanic White (reference group) | -- | -- | -- | -- | -- | -- |
| Non-Hispanic Black | −0.02 | −0.09 to 0.05 | 0.62 | −0.03 | −0.1 to 0.03 | 0.31 |
| Non-Hispanic Other | −0.02 | −0.15 to 0.12 | 0.79 | −0.02 | −0.1 to 0.11 | 0.78 |
| Hispanic | −0.03 | −0.18 to 0.12 | 0.66 | −0.04 | −0.17 to 0.09 | 0.53 |
| Formal Pathway | 0.21 | 0.13 to 0.28 | <0.001 | 0.20 | 0.12 to 0.28 | <0.001 |
| Transfer 2006 or later | 0.11 | 0.04 to 0.18 | 0.002 | 0.09 | 0.02 to 0.16 | 0.009 |
Cardiovascular disease (CVD)
End-Stage Renal Disease (ESRD)
Chronic Kidney Disease (CKD)
Kidney Transplant (TXP)
Discussion
The Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents from the National Heart, Lung and Blood Institute recently published its summary report indicating that children with CKD, ESRD or TXP are in the highest risk tier for CVD and recommended specific CVRF assessment and preventive therapy.[17] This study of adolescents with kidney disease transitioning to adult care highlights the large gap that exists between clinical practice and recommended guidelines.[5–13, 17] Recommended CVRF assessments and therapies were documented less than 60% of the time and a small minority of patients received all components of recommended CVD care. When evaluated, the burden of CVD risk-factors was high in this population, yet there appears to be substantial variation in care both within and between centers. Together, these results suggest that patients are not consistently receiving optimal preventive cardiology care during adolescence, which may, in part, contribute to their exceedingly high risk of cardiovascular death as adults.
The importance of CVD in adults who developed kidney disease in childhood is well established. Parekh, et al. demonstrated that CVD is the leading cause of death in patients with ESRD as children who died before the age of 30.[4] This is consistent with the high prevalence of LVH, hypertension, dyslipidemia and other traditional CVRFs observed in the present investigation and other studies[22–24] as well as the observation that the severity of asymptomatic coronary and aortic atherosclerosis increases with the number of CVRFs.[18] As such, CVD for children and adolescents with kidney disease has become a top priority condition for clinical research[25] and a target for risk reduction through clinical practice guidelines.[6–7, 9, 11–12, 17] Yet, despite these calls to action, current practice patterns appear to be sub-optimal as only a small minority of patients in our study had a complete assessment for traditional CVRFs. Even among those assessed and found to have modifiable CVRF’s, all recommended therapy was prescribed in less than one-third.
Although suboptimal care has traditionally been attributed to a lack of vigilance by care providers, it is more likely due to a lack of systems that optimize chronic disease care.[26] Fifteen years ago Wagner and colleagues[27] identified that existing systems of care were incapable of reliably delivering high-quality chronic disease management. In response, they developed the Chronic Care Model[26] to help guide greater implementation of evidence-based longitudinal care. Unfortunately, with the exception of relatively few care settings, the Chronic Care Model has not been widely implemented in routine clinical practice and sub-optimal chronic care spans across disciplines and populations.[28] From analysis of 175 quality metrics in more than 1500 children, Mangione-Smith, et al. observed that recommended preventive care, screening and diagnosis were each documented less than half of the time (41%, 38%, 47% respectively) and indicated therapy was documented only two-thirds of the time.[14] In adult studies only 42–82% of hypertensive patients with CKD were on medical therapy and only 20–60% of patients with CKD or a kidney transplant were treated for uncontrolled cholesterol.[15–16] Together with our findings these studies suggest that current systems of chronic disease management do not reliably provide optimal care. In order to improve long-term outcomes, further research should explore system redesign as a means to improve quality of care.[29]
One component of new, more reliable systems of care for adolescents transferring to adult providers may be formal transition programs. Because of their emphasis on adult medical conditions and use of pathways and coordinated care, these programs have the potential to improve care for high risk adolescents with chronic illness.[1, 30–35] Indeed, in their report “Crossing the Quality Chasm: A New Health System for the 21st Century”[36] the Institute of Medicine identified coordination of care as one of six key challenges for health system redesign. Yet there is surprisingly little research evaluating the impact of transition programs on clinical care.[1] In a recent systematic review, Crowley, et al. found only ten studies across all chronic diseases that evaluated the impact of transition programs on medical outcomes.[37] Of these, only one small, qualitative study included young people with kidney disease.[38] Six of the other nine studies demonstrated positive impacts of formal transition and two studies[39–40] in diabetes care specifically demonstrated improvements in quality indicators among patients undergoing formal transition. A key finding of our study is that formal transition was independently associated with a 21% increase in CVD assessment, thus contributing to the small but growing literature that suggests formal transition programs may have a favorable impact on quality of care.
Notably in our study, the center with the highest percentage of CVD assessments (86%) did not report any formal transitions (Center E; Figures 1a and 2a). In contrast, the center performing the most formal transitions (Center H) performed relatively few recommended CVD assessments (49%). Thus, unmeasured contextual factors, such as other clinical pathways, care preferences, priorities or presence of local experts, likely account for some variation in quality of care between centers. Although, our data did not have measures of these potential factors, our multilevel analysis allowed us to assess reported formal transition as a predictor of CVD care controlling for study center- and patient-level characteristics and partition variance into patient- and center-level variance. Despite considerable center-specific variation, we found formal transition to be the strongest independent predictor of improved CVD assessment in our population.
Both transplant status and transfer since 2006 were also associated with modest improvements in CVD assessment. The reasons for improved care for transplant patients are uncertain, but may be attributed to better adherence, greater use of protocols, more specific guidelines,[5–6, 9] improved care coordination and/or other factors. Indeed some adult literature suggests and inverse relationship between certain traditional CVRF’s and mortality in adult dialysis patients,[19–20] whereas the same is not true for CKD or TXP patients. Thus, providers may be more hesitant to monitor and treat dyslipidemia or obesity in dialysis patients in comparison to those who have had a kidney transplant. Improved CVD assessment after 2006 suggests more comprehensive care[6] or greater acceptance of guidelines over time. Alternatively, greater adoption of electronic medical records may be associated with improved documentation over time. Ultimately further research may elucidate how and why these factors improve CVD care.
This is the largest cohort study of adolescents with kidney disease who have transferred from pediatric to adult care and the only one, to our knowledge, that evaluates the quality of CVD care in this high-risk population. Nonetheless, our findings must be interpreted in light of several limitations. We sampled a diverse set of North American pediatric nephrology programs, yet the centers who chose to participate may have been motivated by a particular interest in optimizing CVD or transitional care. Thus, our results may overestimate the percentage of recommended CVD care overall. Although wide variation in care was observed, our sample has limited power to detect variations in care. The observed prevalence of traditional CVRFs such as LVH may have been increased by detection bias. We chose to focus this analysis only on traditional CVRF’s, whereas non-traditional CVRF’s (e.g. inflammation, oxidative stress, abnormal mineral metabolism, hyperhomocysteinemia, etc.) that may further impact cardiovascular outcomes in these patients and should be the focus of future studies. Our study may underestimate treatment of CVRF’s due to treatment approaches not recommended in the guidelines or captured in our analysis, such as lowering steroid or calcinuerin inhibitor exposure for patients with elevated fasting glucoses. Our analyses are based on retrospective chart reviews and are subject to biases related to documentation. Thus, documentation of formal transition may be associated with improved documentation of care, perhaps due to greater implementation of electronic medical records over time, rather than improvements in actual care delivery. Indeed, this may be one factor contributing to our observation that date of transfer after vs. before 2006 predicted improved CVD care. In addition, the components of transition programs at participating facilities varied but were not characterized in sufficient detail to determine whether more intensive or comprehensive programs were associated with better CVD care. Finally, while this study provides important initial findings, additional research is needed to assess the impact of patient-level characteristics, center-specific contextual factors, and formal transitional programs on the quality of CVD care across other pediatric nephrology centers. Nonetheless, this study provides important insight into current clinical practice and the potential positive impact of transition programs.
Conclusion
In summary, we observed that when evaluated, adolescents with kidney disease have a large burden of cardiovascular risk factors. These patients receive variable and sub-optimal preventive CVD care that may contribute to their exceedingly high risk of subsequent cardiovascular death in early adulthood. There is tremendous opportunity to lower this high CVD burden and improve long-term cardiovascular outcomes for these patients and others with chronic conditions by improving the reliability of preventive care. Formal transition programs may be an important component of such new, more reliable, chronic care systems.
Acknowledgments
This study was supported in part by the Department of Pediatrics at Duke University and a career development award to Dr. Patel from the National Institutes of Health (NIH) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; grant K23 DK075929).
We appreciate the Midwest Pediatric Nephrology Consortium, and the efforts of our outstanding coordinators and staff for their assistance with data abstraction: Patterson L (Duke University Children’s Hospital & Health Center), Kamel M (Emory University and Children’s Healthcare of Atlanta), Crumb T (DeVos Children’s Hospital), Kester J (Children’s Hospital Akron), Bickford K (University of North Carolina), Frieling M (Sick Kids Hospital, Toronto).
Abbreviations
- CKD
chronic kidney disease
- ESRD
end-stage renal disease
- CVD
cardiovascular disease
- CVRF
cardiovascular risk factor
- TXP
transplant
- LDL
low-density lipoprotein
- BMI
body mass index
Footnotes
Contributor’s statement: The following authors each contributed substantially to the conception and design, acquisition of data, and/or analysis and interpretation of the data. Each author has reviewed the manuscript and agreed to its submission.
David K. Hooper: Analysis and interpretation of data
Jason C. Williams: Acquisition of data
Adam C. Carle: Analysis and interpretation of data
Sandra Amaral: Conception and design, acquisition of data, interpretation of data
Deepa H. Chand: Acquisition of data
Maria E. Ferris: Acquisition of data
Hiren P. Patel: Acquisition of data
Christoph Licht: Acquisition of data
Gina-Marie Barletta: Acquisition of data
Veronica Bastian: Acquisition of data
Mark Mitsnefes: Analysis and interpretation of data
Uptal D. Patel: Conception and design, acquisition of data, analysis and interpretation of data
During this study Dr. Barletta was affiliated with DeVos Children’s Hospital and Veronica Bastian was affiliated with Children’s Hospital of Michigan.
Presented in part at the 42nd annual meeting of the American Society of Nephrology; October 30, 2009; San Diego, CA.
Financial Disclosure: Nothing to disclose
Conflict of Interest: None
References
- 1.Bell LE, Bartosh SM, Davis CL, Dobbels F, Al-Uzri A, Lotstein D, Reiss J, Dharnidharka VR. Adolescent Transition to Adult Care in Solid Organ Transplantation: a consensus conference report. Am J Transplant. 2008;8:2230–2242. doi: 10.1111/j.1600-6143.2008.02415.x. [DOI] [PubMed] [Google Scholar]
- 2.NAPRTCS 2008 Annual Report. North American Pediatric Renal trials and Collaborative Studies; 2009. [Google Scholar]
- 3.McDonald SP, Craig JC. Long-term survival of children with end-stage renal disease. N Engl J Med. 2004;350:2654–2662. doi: 10.1056/NEJMoa031643. [DOI] [PubMed] [Google Scholar]
- 4.Parekh RS, Carroll CE, Wolfe RA, Port FK. Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr. 2002;141:191–197. doi: 10.1067/mpd.2002.125910. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske B, Cosio FG, Beto J, Bolton K, Chavers BM, Grimm R, Jr, Levin A, Masri B, Parekh R, Wanner C, Wheeler DC, Wilson PW. Clinical practice guidelines for managing dyslipidemias in kidney transplant patients: a report from the Managing Dyslipidemias in Chronic Kidney Disease Work Group of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Am J Transplant. 2004;4(Suppl 7):13–53. doi: 10.1111/j.1600-6135.2004.0355.x. [DOI] [PubMed] [Google Scholar]
- 6.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 7.K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–290. [PubMed] [Google Scholar]
- 8.Lurbe E, Cifkova R, Cruickshank JK, Dillon MJ, Ferreira I, Invitti C, Kuznetsova T, Laurent S, Mancia G, Morales-Olivas F, Rascher W, Redon J, Schaefer F, Seeman T, Stergiou G, Wuhl E, Zanchetti A. Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens. 2009;27:1719–1742. doi: 10.1097/HJH.0b013e32832f4f6b. [DOI] [PubMed] [Google Scholar]
- 9.KDIGO . Kideney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am J Transplant. 2009;9:S1–S157. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 10.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 11.NKF. K/DOQI clinical practice guidelines for management of dyslipidemias in patients with kidney disease. Am J Kidney Dis. 2003;41:I–IV. S1–91. [PubMed] [Google Scholar]
- 12.NKF . K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S1–153. [PubMed] [Google Scholar]
- 13.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.Mangione-Smith R, DeCristofaro AH, Setodji CM, Keesey J, Klein DJ, Adams JL, Schuster MA, McGlynn EA. The quality of ambulatory care delivered to children in the United States. N Engl J Med. 2007;357:1515–1523. doi: 10.1056/NEJMsa064637. [DOI] [PubMed] [Google Scholar]
- 15.Snyder JJ, Collins AJ. KDOQI hypertension, dyslipidemia, and diabetes care guidelines and current care patterns in the United States CKD population: National Health and Nutrition Examination Survey 1999–2004. Am J Nephrol. 2009;30:44–54. doi: 10.1159/000201014. [DOI] [PubMed] [Google Scholar]
- 16.Marcen R, del Castillo D, Capdevila L, Fernandez-Fresnedo G, Rodrigo E, Cantarell C, Fernandez-Rodriguez A, Lopez-Oliva MO, Camps J, Aljama P, Ortuno J, Arias M. Achieving chronic kidney disease treatment targets in renal transplant recipients: results from a cross-sectional study in Spain. Transplantation. 2009;87:1340–1346. doi: 10.1097/TP.0b013e3181a23837. [DOI] [PubMed] [Google Scholar]
- 17.Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 19.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 20.Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol. 2003;14:2366–2372. doi: 10.1097/01.asn.0000083905.72794.e6. [DOI] [PubMed] [Google Scholar]
- 21.Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128:182–200. doi: 10.1542/peds.2011-0969. [DOI] [PubMed] [Google Scholar]
- 22.Wilson AC, Greenbaum LA, Barletta GM, Chand D, Lin JJ, Patel HP, Mitsnefes M. High prevalence of the metabolic syndrome and associated left ventricular hypertrophy in pediatric renal transplant recipients. Pediatr Transplant. 2010;14:52–60. doi: 10.1111/j.1399-3046.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 23.Mitsnefes MM, Kimball TR, Border WL, Witt SA, Glascock BJ, Khoury PR, Daniels SR. Abnormal cardiac function in children after renal transplantation. Am J Kidney Dis. 2004;43:721–726. doi: 10.1053/j.ajkd.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 24.Mitsnefes M, Flynn J, Cohn S, Samuels J, Blydt-Hansen T, Saland J, Kimball T, Furth S, Warady B. Masked hypertension associates with left ventricular hypertrophy in children with CKD. J Am Soc Nephrol. 2010;21:137–144. doi: 10.1681/ASN.2009060609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chesney RW, Brewer E, Moxey-Mims M, Watkins S, Furth SL, Harmon WE, Fine RN, Portman RJ, Warady BA, Salusky IB, Langman CB, Gipson D, Scheidt P, Feldman H, Kaskel FJ, Siegel NJ. Report of an NIH task force on research priorities in chronic kidney disease in children. Pediatr Nephrol. 2006;21:14–25. doi: 10.1007/s00467-005-2087-2. [DOI] [PubMed] [Google Scholar]
- 26.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1:2–4. [PubMed] [Google Scholar]
- 27.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74:511–544. [PubMed] [Google Scholar]
- 28.National Healthcare Quality Report 2010. Agency for Healthcare Research and Quality; Rockville, MD: 2010. [Google Scholar]
- 29.Dougherty D, Conway PH. The “3T’s” road map to transform US health care: the “how” of high-quality care. JAMA. 2008;299:2319–2321. doi: 10.1001/jama.299.19.2319. [DOI] [PubMed] [Google Scholar]
- 30.Webb N, Harden P, Lewis C, Tizzard S, Walsh G, Wray J, Watson A. Building consensus on transition of transplant patients from paediatric to adult healthcare. Arch Dis Child. 2010;95:606–611. doi: 10.1136/adc.2009.176255. [DOI] [PubMed] [Google Scholar]
- 31.Diaz-Gonzalez de Ferris ME. Adolescents and emerging adults with chronic kidney disease: their unique morbidities and adherence issues. Blood Purif. 2011;31:203–208. doi: 10.1159/000321854. [DOI] [PubMed] [Google Scholar]
- 32.Ferris ME, Mahan JD. Pediatric chronic kidney disease and the process of health care transition. Semin Nephrol. 2009;29:435–444. doi: 10.1016/j.semnephrol.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 33.A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110:1304–1306. [PubMed] [Google Scholar]
- 34.MCHB; Institute for Child Health Policy HPHaRtWTG, editor Draft 10-Year Healthy and Ready to Work Transition Plan. 2000. [Google Scholar]
- 35.Rosen DS, Blum RW, Britto M, Sawyer SM, Siegel DM. Transition to adult health care for adolescents and young adults with chronic conditions: position paper of the Society for Adolescent Medicine. J Adolesc Health. 2003;33:309–311. doi: 10.1016/s1054-139x(03)00208-8. [DOI] [PubMed] [Google Scholar]
- 36.Medicine Io. Crossing the Quality Chasm: A New Health System for the 21st Century. National Academy Press; Washington DC: 2001. [PubMed] [Google Scholar]
- 37.Crowley R, Wolfe I, Lock K, McKee M. Improving the transition between paediatric and adult healthcare: a systematic review. Arch Dis Child. 2011;96:548–553. doi: 10.1136/adc.2010.202473. [DOI] [PubMed] [Google Scholar]
- 38.Remorino R, Taylor J. Smoothing things over: the transition from pediatric to adult care for kidney transplant recipients. Prog Transplant. 2006;16:303–308. doi: 10.1177/152692480601600404. [DOI] [PubMed] [Google Scholar]
- 39.Gholap NPM, Virmani S, Lee J, James D, Morrissey J, Datta V, Patel V. The Alphabet Strategy and standards of care in young adults with type 1 diabetes. Br J Diabet Vasc Dis. 2006;6:168. [Google Scholar]
- 40.Cadario F, Prodam F, Bellone S, Trada M, Binotti M, Allochis G, Baldelli R, Esposito S, Bona G, Aimaretti G. Transition process of patients with type 1 diabetes (T1DM) from paediatric to the adult health care service: a hospital-based approach. Clin Endocrinol (Oxf) 2009;71:346–350. doi: 10.1111/j.1365-2265.2008.03467.x. [DOI] [PubMed] [Google Scholar]