Abstract
Background
Drug-induced liver injury (DILI) is an uncommon adverse drug reaction of increasing importance to the medical community, pharmaceutical industry, regulatory agencies and the general public.
Objectives
The Drug-Induced Liver Injury Network (DILIN) was established to advance understanding and research into DILI by initiating a prospective registry of patients with bona fide DILI for future studies of host clinical, genetic, environmental and immunological risk factors. The DILIN was also charged with developing standardized nomenclature, terminology and causality assessment instruments.
Methods
Five clinical sites, a data coordinating centre and senior scientists from the National Institute of Diabetes and Digestive and Kidney Diseases initiated the DILIN prospective study in September 2004. Eligible patients are required to meet minimal laboratory or histological criteria within 6 months of DILI onset and have other competing causes of liver injury excluded. Patients in the general community setting with pre-existing HIV, hepatitis B virus or hepatitis C virus infections and/or abnormal baseline liver biochemistries are eligible for enrolment. In addition, subjects with liver injury due to herbal products are eligible to participate. Control patients without DILI are also to be recruited in the future.
Results
All referred subjects undergo an extensive review of available laboratory, pathology and imaging studies. Subjects who meet pre-defined eligibility criteria at the 6-month study visit are followed for 2 years to better define the natural history of chronic DILI. Causality assessment is determined by a panel of three hepatologists who independently assign a causality score ranging from 1 (definite) to 5 (unlikely) as well as a severity score ranging from 1 (mild) to 5 (fatal). During the first 3 years, 367 subjects were enrolled into the DILIN prospective study.
Conclusion
DILIN is a multicentre research network charged with improving our understanding of the aetiologies, risk factors and outcomes of DILI in the US. The network is meeting the targeted enrolment of ten patients per month and is developing a repository of clinical data and biological samples for future studies of DILI pathogenesis and outcome.
Background
Drug-induced liver injury (DILI) is the leading cause of acute liver failure in the US and the most common reason for US FDA regulatory actions regarding approved medications.[1,2] Implicated drugs include not only prescription medications but also herbal products and over-the-counter dietary supplements and medications.[3,4] Persons who develop hepatocellular DILI with jaundice have at least a 10% chance of dying from the injury and DILI patients that progress to acute liver failure have only a 25% chance of spontaneous recovery.[1,5] While DILI caused by a particular agent can be serious, it is relatively uncommon, with an estimated frequency that ranges from 1 per 10 000 to 1 per 10 000 000 patient-years of exposure. Therefore, the low incidence of DILI coupled with the limited knowledge of the biochemical mechanism(s) or pathways responsible for this ‘idiosyncratic’ adverse event make it difficult to identify high-risk patients.[6] Some investigators have postulated the importance of reactive metabolites or aberrant host metabolism and immune responses in the pathogenesis of DILI but the precise molecular mechanism(s) involved are largely unknown.[6,7] Furthermore, pre-clinical testing does not provide a reliable assessment of the hepatotoxic risk of new medications, and because DILI is a rare event, pre-marketing clinical studies conducted in highly selected populations over a relatively short period of time also may not detect a potential for liver injury. Therefore, the hepatotoxicity of a specific medication often becomes apparent only after regulatory approval and when the drug is used by large numbers of unselected patients in the general population.[2,6] Even then, voluntary post-marketing surveillance systems such as the FDA MedWatch programme frequently lag behind in detecting ‘hepatotoxic signals’ from newly approved drugs.[8] Finally, the biochemical, clinical and histological features of DILI from initial onset to clinical presentation can not only mimic most other known forms of acute and chronic liver disease but can also vary substantially with a single agent.[9,10] Thus, at present, there is no objective ‘gold standard’ for diagnosing DILI and its identification largely relies on excluding other more common causes of liver disease and having a ‘compatible’ time of onset and evolution.[2,10]
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) established the Drug-Induced Liver Injury Network (DILIN) in 2003 to advance understanding and research into DILI by selecting five clinical sites and a data coordinating centre (DCC) that had submitted competitive grant applications.[11,12] In this article we present the rationale, design and methods of the prospective DILIN study.
Aims and Objective
The primary aim of the prospective DILIN study is to identify a large number of patients with bona fide DILI that will allow for collection of epidemiological data and biological samples for future mechanistic studies. The DILIN study aims to do this by creating within 6 months of onset a registry and tissue bank from people who have experienced liver injury due to prescription and over-the-counter drugs as well as herbal products. A prospective registry will allow investigation into clinical, immunological, environmental and genetic risk factors by comparing DILI cases with matched control patients with a similar drug exposure history but without evidence of liver injury. Since the natural history of DILI is not well understood, all of the DILI cases are to be re-evaluated 6 months after enrolment. All participants agree to be re-contacted for up to 20 years for potential participation in additional studies, such as those that explore the relationship between genotype and phenotype or pedigree studies. Lastly, the DILIN is charged with developing and testing causality assessment instruments for drug, herbal and over-the-counter medication-induced liver injury.
Methods
Prospective Study Design
Eligible patients with suspected DILI are referred to one of the five DILIN sites or their satellite affiliates. Patients may be referred by a physician/medical provider at one of the clinical sites, a physician/medical provider at a nearby location or hospital or may refer themselves. The DILI event must be attributable to one or more prescription or over-the-counter medications or herbal products. Subjects are categorized as having ‘standard DILI’ if they did not have known liver disease prior to starting the suspect medication. In contrast, subjects with known liver disease prior to DILI onset, such as chronic hepatitis B virus (HBV) or chronic hepatitis C virus (HCV) infection or fatty liver disease, are referred to as ‘liver DILI’ patients.
Baseline Study Visit
After an informed consent document is signed, all relevant laboratory, pathology and imaging studies are reviewed to ensure that referred patients meet entry criteria. A complete history and physical examination are undertaken and detailed information regarding medications, past medical history, drug intolerances and symptoms at DILI onset is obtained (table I). In particular, the patient is questioned about the lifetime use of the suspect medication and agents in the same therapeutic class identified from an electronic database (Lexi-Comp®, Lexi-Comp Inc., Hudson, OH, USA). In addition, recent alcohol consumption, medication compliance and smoking history are obtained using interviewer-administered questionnaires. Adult participants also complete the Short Form-36 quality-of-life form (Rand Corporation, Arlington, VA, USA) and children complete the Pediatric quality-of-life form (MAPI Research Institute, Lyon, France).[13,14] These instruments were selected because of their established validity, brevity and frequent use in other studies of patients with liver disease.
Table I.
Visit schedule in the Drug-Induced Liver Injury Network (DILIN) prospective study
| Item | Data source | Baseline visit | 6-mo study visit | 12- and 24-mo study visit |
|---|---|---|---|---|
| Eligible patients | Standard DILIa, liver DILIb, control |
Standard DILI, liver DILI |
Chronic DILIc | |
| Demographics | Interview | ✓ | ||
| Pharmacy use | Interview | ✓ | ||
| Medication compliance | Interview | ✓ | ||
| Family history | Interview | ✓ | ||
| Diagnostic laboratory testsd | Records/visit | ✓ | ||
| Suspect medication use | Interview/records | ✓ | ||
| HCV/HBV laboratory testse | Records/visit | ✓ | ✓ | |
| Concomitant medication and CAM use history |
Interview/records | ✓ | ✓ | ✓ |
| Alcohol and smoking | Interview | ✓ | ✓ | ✓ |
| Physical examination | Visit | ✓ | ✓ | ✓ |
| Symptom score | Interview | ✓ | ✓ | ✓ |
| Quality-of-life survey | Interview | ✓ | ✓ | ✓ |
| Medical history | Interview/records | ✓ | ✓ | ✓ |
| Standard laboratory tests | Records/visit | ✓ | ✓ | ✓ |
| Research blood sample | Visit | ✓ | ✓ | ✓ |
| Research urine sample | Visit | ✓ | ✓ | ✓ |
| Liver imaginga | Records | ✓ | ✓ | ✓ |
Standard DILI case = normal liver biochemistries or no known liver disease before starting the suspect medication.
Liver DILI case = known chronic liver disease such as chronic HCV or HBV or fatty liver before starting the suspect medication.
Chronic DILI case = evidence of persistent laboratory, radiological or pathological abnormalities 6mo after DILI onset.
Not done in control patients.
Only in HCV/HBV liver DILI patients.
CAM=complementary and alternative medicine; DILI=drug-induced liver injury; HBV=hepatitis B virus; HCV=hepatitis C virus.
The type of liver injury at DILI onset is classified as hepatocellular, cholestatic or mixed by the R ratio, which compares ALT and alkaline phosphatase levels in multiples of their upper limit of normal (ULN) based upon the first available values after DILI onset. The R ratio is calculated by the formula R = (ALT/ULN)/(alkaline phosphatase/ULN). For eligible patients with acute hepatocellular injury (i.e. R > 5), diagnostic tests required at enrolment include: total and, if positive, IgM antibody to hepatitis A virus, hepatitis B surface antigen (HBsAg), antibody to hepatitis B core antigen, anti-HCV and if positive HCV RNA, anti-nuclear antibody, anti-smooth muscle antibody, IgM antibody to cytomegalovirus, Epstein Barr-virus (EBV) serologies or a monospot test and ceruloplasmin if the patient is aged <50 years. A cross-sectional abdominal imaging study such as an ultrasound, abdominal CT or MRI is also required. Subjects with a mixed (i.e. 5 > R > 2) or cholestatic (R < 2) biochemical profile at DILI onset are also required to have all of the above completed as well as an anti-mitochondrial antibody test. Serum α1-antitrypsin and iron indices are recorded, if available, but are not mandatory. Available liver biochemistry results starting 8 weeks prior to initiation of the suspect drug through the baseline study visit are recorded as well as the results of other selected laboratory tests (e.g. haemoglobin, white blood cell count and serum creatinine).
Urine and whole blood samples are collected at the baseline study visit. Plasma, serum and lymphocytes for DNA extraction, EBV immortalization and proliferation assays are obtained and stored at the NIDDK biosample repository (Rutgers University, Piscataway, NJ, USA). Routine laboratory tests obtained at each study visit include a urinalysis, complete blood count, liver biochemistries, international normalized ratio (INR), serum amylase, lipase, creatinine phosphokinase, γ-glutamyl transpeptidase and lactate dehydrogenase levels. For HIV-infected patients and those with known chronic HBV or HCV, detailed data regarding prior disease status including available CD4 counts and viral test results as well as antiviral treatment are collected. The site investigator also generates a written clinical narrative summarizing the DILI event for causality assessment. For patients with DILI due to herbal products, the brand name, manufacturer and ingredients of each product are recorded. In addition, samples of the suspect herbal product are collected and forwarded to the US National Institutes of Health (NIH) for future analysis of ingredient content.
Inclusion and Exclusion Criteria
Participants are required to have experienced a DILI event within 6 months of the baseline visit (table II). The DILI onset date is defined as the first date after starting the suspect medication wherein the subject met the laboratory entry criteria. Both children and adults are eligible to participate, the minimum subject age being 2 years. Inclusion laboratory criteria are a serum AST or ALT >5 times ULN (or pretreatment baseline if baseline levels are elevated) on two separate occasions. Alternatively, subjects with serum alkaline phosphatase levels >2 times ULN (or baseline if the baseline level is abnormal) on two consecutive occasions may qualify. In addition, subjects who develop a serum total bilirubin of greater than 2.5 mg/dL or an INR above 1.5 in the absence of a competing cause of hyperbilirubinaemia or hypoprothrombinaemia, respectively, are eligible.
Table II.
Entry criteria for the Drug-Induced Liver Injury Network (DILIN) prospective study
| Inclusion criteria |
| Age >2ya |
| Enrolled within 6 mo of liver injury onset due to a drug or herbal product |
| Any one of the following laboratory criteria must be present |
|
| Exclusion criteria |
| Paracetamol (acetaminophen) hepatotoxicity |
| Pre-existing liver disease such as primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune hepatitis or other chronic biliary tract disease that may confound diagnosis |
| Liver or bone marrow transplant prior to enrolment |
| Identifiable competing cause of liver injury felt to be responsible for observed liver injury |
Age restriction due to need for blood withdrawal.
DILI=drug-induced liver injury; HBV=hepatitis B virus; HCV=hepatitis C virus; INR=international normalized ratio; ULN=upper limit of normal.
Exclusion criteria are known or suspected paracetamol (acetaminophen) overdose as well as a history of bone marrow or liver transplant prior to DILI onset. Subjects with pre-existing immune-mediated liver disease such as autoimmune hepatitis are also excluded. However, subjects with unexplained abnormal liver biochemistries or with pre-existing chronic HBV, chronic HCV and HIV infection are allowed to participate in light of the high prevalence of these conditions in the general population.[15-17]
Six-Month Study Visit
At the 6-month study visit, interval medical history, laboratory tests, medication use and liver imaging are reviewed (table I). In subjects with pre-existing chronic HBV, a quantitative HBV DNA, hepatitis B e antigen and antibody measurements are obtained. Similarly, in subjects with pre-existing chronic HCV, a quantitative HCV RNA measurement is obtained and an HIV RNA and CD4 count are obtained in patients with HIV infection. Subjects with evidence of ‘chronic DILI’ undergo a cross-sectional imaging study while those without evidence of persistent liver injury are asked to provide contact information for future studies.[18] A written narrative summarizing any data that may influence causality assessment such as re-challenge, new medical diagnoses or liver biopsy findings is generated.
Chronic Drug-Induced Liver Injury
Chronic DILI is defined at 6 months after DILI onset as one of the following: (i) for subjects with normal or unknown baseline liver biochemistries, a serum AST, ALT, alkaline phosphatase, INR or total bilirubin that is persistently elevated on two separate occasions; (ii) for liver DILI subjects, a serum AST, ALT, alkaline phosphatase, INR or total bilirubin level that exceeds 1.25 times the baseline value on two separate occasions; (iii) any evidence of portal hypertension such as ascites on imaging, varices on upper endoscopy or clinical evidence of hepatic encephalopathy; (iv) any histological evidence of persistent liver injury at least 6 months after DILI onset; or (v) any radiological evidence of chronic liver disease such as ascites, hepatomegaly, nodular liver or intra-abdominal varices. Subjects with pre-existing chronic HBV or HCV infection, liver transplant recipients since the baseline visit and patients with cirrhosis or clinical evidence of portal hypertension before starting the suspect medication are excluded from the chronic DILI protocol.
Subjects with chronic DILI are seen at 12 and 24 months after the baseline visit wherein incremental medical history, medication use, laboratory and imaging studies and questionnaires are completed. A final written narrative is also generated by the site investigator summarizing the course of the DILI episode. For subjects who die during follow-up, a death narrative recording whether the death was attributable to a liver or non-liver related cause is generated by the site investigator for review by the causality committee.
Liver Histopathology Review
Liver biopsy is not part of the protocol. When performed as part of routine care, the written pathology report is incorporated into the case report and considered during causality assessment. When available, six unstained slides are also sent to the DILIN pathologist, Dr David Kleiner, for formal scoring and assessment of histopathological features of DILI.[19] The centralized histological assessment is not included in the causality review process but is retained in the database along with representative photomicrographs.
Causality Assessment
Because DILI is a clinical diagnosis of exclusion, a formal and standardized approach to causality assessment was established in the DILIN.[5,20,21] Causality is assessed with the widely used Roussel Uclaf Causality Assessment Method (RUCAM) as well as by ‘expert consensus’.[22] The RUCAM provides a semi-quantitative assessment of causality by assigning −3 to +3 points to each of six domains. A final summary score allows categorization of causality as highly probable (>8), probable (6–8), possible (3–5), unlikely (1–2) or excluded (<0). For expert consensus, a DILIN causality committee was established that consists of seven voting members including the five clinical site investigators (or their designees) and a representative from the DCC and the NIDDK. Three causality committee members (the clinical site principal investigator and two others) receive key clinical, laboratory and diagnostic data abstracted from the DILIN baseline visit and clinical narrative (table I). The three committee members each independently calculate a RUCAM score using forms developed for this purpose and also assign a DILIN causality score ranging from 1 (definite) to 5 (unlikely). To standardize terminology and attempt to make causality assessment by expert opinion more objective, definitions were developed for each causality level (table III). These definitions included three elements: (i) a percentage likelihood that the drug caused the liver injury (>95%, 75–95%, 50–74%, 25–49% and <25%); (ii) standard legal terms to help define the weight of the evidence for causality (i.e. ‘beyond a reasonable doubt’, ‘clear and convincing evidence’ and ‘the preponderance of evidence’); and (iii) a set of criteria describing the clinical features that match the known timing and pattern of injury. These definitions were developed during the initial years of the DILIN study in an attempt to provide guidance for the expert opinions.
Table III.
Causality assessment scoring in the Drug-Induced Liver Injury Network (DILIN) prospective study
| Causality score |
Likelihood (%) |
Description |
|---|---|---|
| 1 = definite | >95 | Liver injury is typical for the drug or herbal product (‘signature’ or pattern of injury, timing of onset, recovery). The evidence for causality is ‘beyond a reasonable doubt’ |
| 2 = highly likely | 75–95 | The evidence for causality is ‘clear and convincing’ but not definite |
| 3 = probable | 50–74 | The causality is supported by ‘the preponderance of evidence’ as implicating the drug but the evidence cannot be considered definite or highly likely |
| 4 = possible | 25–49 | The causality is not supported by ‘the preponderance of evidence’; however, one cannot definitively exclude the possibility |
| 5 = unlikely | <25 | The evidence for causality is ‘highly unlikely’ based upon the available information |
| 6 = insufficient data |
Not applicable |
Key elements of the drug exposure history, initial presentation, alternative diagnoses and/or diagnostic evaluation prevent one from determining a causality score |
In cases involving more than one implicated drug, an overall causality score for the DILI event is assigned as well as scores for up to three different medications. When the DILIN causality scores of the three assigned reviewers do not agree, the case is discussed by the entire committee on a teleconference wherein a final consensus score is assigned. Finally, the severity of the DILI episode is categorized on a scale of 1–5 as summarized in table IV.
Table IV.
Drug-induced liver injury (DILI) severity index definitionsa
| Score | Grade | Definition |
|---|---|---|
| 1 | Mild | Patient has elevation in ALT and/or alkaline phosphatase levels but total serum bilirubin is <2.5mg/dL and INR is <1.5 |
| 2 | Moderate | Patient has elevation in ALT and/or alkaline phosphatase levels and serum bilirubin is ≥2.5mg/dL or INR is ≥1.5 |
| 3 | Moderate– severe |
Patient has elevation in ALT, alkaline phosphatase, bilirubin and/or INR levels and patient is hospitalized or an ongoing hospitalization is prolonged because of DILI |
| 4 | Severe | Patient has elevation in ALT and/or alkaline phosphatase levels and total serum bilirubin is 2.5mg/dL or greater and there is at least one of the following: (i) hepatic failure (INR ≥1.5, ascites or encephalopathy); (ii) other organ failure believed to be due to DILI event |
| 5 | Fatal | Patient dies or undergoes liver transplantation because of DILI event |
In addition, cases are rated for presence or absence of symptoms (A = asymptomatic; S = symptomatic). Symptoms include fatigue, nausea, vomiting, right upper quadrant pain, itching, skin rash, jaundice, weakness, anorexia or weight loss, which in the opinion of the investigator are due to DILI.
INR = international normalized ratio.
Controls
Once a minimum of 15 standard DILI cases due to a single medication are enrolled, up to three age-matched control subjects per case will be identified to test for differences in clinical risk factors. A minimum of 15 cases are required to have adequate power (i.e. 80%) to detect a difference in a risk factor with a prevalence of 20% in the control subjects that is strongly associated with DILI and has an odds ratio of 12 or greater. Controls for clinical and environmental risk factors may consist of individuals who were exposed to the suspect medication for the same or longer period of time than the index case but did not develop liver injury. However, it may not be possible to identify controls for cases with more than one suspect medication, which has been noted in 20% of the patients enrolled to date.[23] Therefore, recruitment of controls is being deferred until an adequate number of bona fide cases due to a specific medication are enrolled. Furthermore, it is possible that population controls matched by age and ethnicity may be selected for DILI cases if genetic association studies for rare phenotypes such as DILI can be accurately done using population controls.[24] By pooling the DILI cases together for genomic studies, there will be a greater likelihood of detecting significant associations between genetic polymorphisms and DILI susceptibility. For example, if 1000 DILI cases and 3000 controls were analysed, we would have adequate power (i.e. 80%) to detect a polymorphism that occurs in 1% of the controls with an odds ratio of 2.75 or greater or a polymorphism that occurs in 0.1% of the controls with an odds ratio of 8.0 or greater. A DILIN genomics committee will be developed to help select the optimal genomics platform to use and develop a strategy to recruit and enrol control subjects.
Data Coordinating Centre
In addition to arranging study meetings and conference calls, the DCC is responsible for the development of case report forms and maintenance of a computerized study database. Double data entry is used at the DCC and data queries are generated to clarify potential errors or missing values on the case report forms along with annual site audits to verify source documents and protocol adherence. Enrolment and specimen tracking reports are prepared by the DCC as well as data analyses for various abstracts and presentations. The DCC also submits de-identified information on adjudicated cases to the FDA by completing a MEDWATCH form that includes data regarding the suspect drug, concomitant medications, medical history, diagnostic evaluation and clinical outcome. A certificate of confidentiality from the National Institute of Mental Health (NIMH) was obtained for the study to assure the confidentiality of the acquired research data.
Results
Recruitment and Retention Procedures
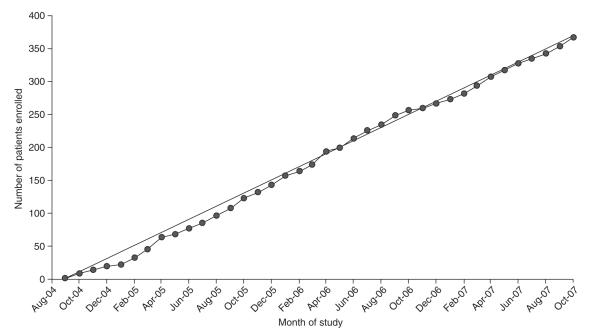
The projected enrolment rate of two DILI patients per centre per month has been achieved (figure 1). Most centres sent recruitment brochures to local physicians and gastroenterology specialists in their regions. In addition, the site investigators have given educational seminars on DILI at various venues including medical grand rounds, divisional conferences and regional and national continuing medical education and professional society meetings. The DILIN steering committee has also developed a central website (http://dilin.dcri.duke.edu) and co-sponsored several research meetings on DILI including the American Association of the Study of Liver Disease’s Single Topic Clinical Research conference on DILI. An international workshop on standardization of nomenclature and causality assessment of DILI is also being planned for December 2008 (see http://www3.niddk.nih.gov/fund/other/conferences.shtml).
Fig. 1.
Cumulative enrolment into the Drug-Induced Liver Injury Network (DILIN) prospective study. The observed enrolment of 367 patients has paralleled the projected rate of enrolment of two cases per centre each month or ten cases total for the five clinical sites every month from September 2004 to October 2007.
All clinical sites have attempted to develop collaborations with other investigators in their region. For example, the University of Connecticut (principal investigator [PI]: Dr Herbert Bonkovsky) established ties with four other regional academic medical centres wherein patients can be recruited and enrolled into the study at those approved satellite sites. As of October 2007, these four satellite sites have contributed 16 of the 64 (25%) patients enrolled through the University of Connecticut. At the University of North Carolina (PI: Dr Paul Watkins), the DILIN study staff travel to other academic institutions in the state (Duke University Medical Center in Durham, Carolinas Medical Center in Charlotte) or elsewhere (Georgetown University Hospital in Washington, DC, USA) to recruit and enrol patients. This effort has provided 12 of the 85 (14%) patients enrolled through this site. A third recruitment strategy at the University of Michigan (PI: Dr Robert J. Fontana) has been to develop a multi-institutional statewide DILIN with regularly scheduled face-to-face meetings, electronic newsletters and educational exercises for participants. In this model, all of the subjects are seen at the primary clinical site although investigators at other nearby institutions are intimately involved in screening and enrolling subjects. Recruitment at the University of California, San Francisco, CA, USA (PI: Dr Timothy Davern) and Indiana University, Indianapolis, Indiana, USA (PI: Dr Naga Chalasani) has relied upon referrals to their medical centres but all patients are seen at the central site under the auspices of a general clinical research centre.
The most common reason for non-participation in the study has been subject refusal in 30% of individuals (e.g. due to travel distance or illness), followed by failure to meet laboratory entry criteria or a competing cause of liver injury identified in 19% (table V). Some referred patients with suspected DILI did not qualify for the study based upon the laboratory entry criteria or the strict requirement for referral within 6 months of DILI onset. As a result, an exemptions committee was formed consisting of an NIH representative, a member of the DCC and a rotating site investigator. To request an exemption, a site investigator submits a brief narrative and rationale to allow the patient into the study. From June 2005 to October 2007, there were 40 protocol exemptions submitted, of which 34 were approved and enrolled in the study and six were denied (table VI).
Table V.
Reasons for patient exclusion from the Drug-Induced Liver Injury Network (DILIN) prospective study from September 2004 to October 2007
| Site | UConn | Indiana | UCSF | UMich | UNC | Total |
|---|---|---|---|---|---|---|
| Enrolled into the study | 64 | 83 | 46 | 89 | 85 | 367 |
| Excluded patients (%) | ||||||
| refused to participate | 24 (35) | 5 (25) | 16 (44) | 22 (39) | 6 (9) | 73 (30) |
| did not meet laboratory entry criteria on two consecutive occasions |
7 (10) | 6 (32) | 2 (6) | 11 (20) | 21 (32) | 47 (19) |
| competing cause identified | 15 (22) | 4 (21) | 3 (8) | 13 (23) | 11 (17) | 46 (19) |
| DILI onset >6 mo from enrolment | 10 (15) | 0 (0) | 5 (14) | 0 (0) | 17 (26) | 32 (13) |
| travel distance too far | 6 (9) | 2 (11) | 8 (22) | 4 (7) | 1 (2) | 21 (8) |
| paracetamol (acetaminophen) hepatoxicity | 0 (0) | 0 (0) | 1 (3) | 1 (2) | 8 (12) | 10 (4) |
| died prior to enrolment | 5 (7) | 2 (11) | 0 (0) | 3 (5) | 0 (0) | 10 (5) |
| immune-mediated chronic liver disease | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 0 (0) | 2 (1) |
| liver or bone marrow transplant prior to DILI onset | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (2) | 2 (1) |
| age <2 y | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (0.5) |
| other (regulatory) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 | 1 (0.5) |
| Total | 68 | 19 | 36 | 56 | 66 | 245 |
DILI = drug-induced liver injury; Indiana = Indiana University; UConn = University of Connecticut; UCSF= University of California San Francisco; UMich = University of Michigan; UNC= University of North Carolina.
Table VI.
Drug-Induced Liver Injury Network (DILIN) exemption committee activity June 2005 to September 2007
| Exemption category | No. submitted |
No. approved (%) |
|---|---|---|
| Age <2 y | 2 | 2 (100) |
| DILI onset >6mo prior | 20 | 17 (85) |
| Failed to meet biochemical criteria |
18 | 15 (83) |
| Total | 40 | 34 (85) |
DILI = drug-induced liver injury.
Discussion
DILI is an infrequent but increasingly recognized adverse event that affects the current and future practice of medicine. DILI is the single most common reason for the FDA to remove or restrict medications in the marketplace.[25-27] In addition, DILI is a frequent reason for drugs in development not to receive FDA approval and a leading reason to stop investigating drug candidates in early clinical development.[28,29] Unfortunately, preclinical testing in animal models and in vitro toxicology systems have provided minimal assistance in identifying potentially hepatotoxic drugs.[2] Clearly, an improved understanding of the risk factors and mechanisms of idiosyncratic DILI in humans is needed to allow otherwise safe and effective medications to remain available to the general population.
Challenges to performing clinical studies of DILI include the need to exclude more common causes of liver injury and the lack of standardized diagnostic criteria. Furthermore, the generally low frequency of DILI with most available drugs has made it difficult to recruit patients even in referral centres.[30,31] In Northern France, a prospective study identified 95 suspected DILI subjects from a population of 81 000 inhabitants over a 3-year period.[32] However, after alternative causes of liver injury were excluded, only 34 patients (36%) with presumed DILI were enrolled giving an estimated annual incidence of DILI of 14 per 100 000 patient years. Importantly, 82% of the DILI patients were never hospitalized and <30% were referred to a gastroenterologist. Similarly, in studies from England and Spain, most suspected DILI cases were reported by general physicians and a large proportion were ultimately judged to be unlikely providing an estimated DILI incidence of only 1–3 per 100 000 patient years of exposure.[33,34] These observations suggest that prospective studies of DILI need to involve a large number of practicing physicians in the community to identify the full spectrum of DILI in the general population via a multicentre, surveillance network.[2,11]
The DILIN prospective protocol was initiated with the intent to enrol consecutive patients with suspected DILI at the five clinical sites in the US.[12] Cases of paracetamol hepatotoxicity were excluded in light of multiple prior studies on the mechanism and natural history of this common cause of acute hepatocellular injury.[35] In addition, subjects with pre-existing immune-mediated chronic liver disease such as autoimmune hepatitis were excluded in light of the difficulty of distinguishing a DILI episode from a disease flare.[36] However, HIV-positive patients were targeted for enrolment into the DILIN prospective study in light of the increasing recognition of liver-related morbidity and mortality in subjects receiving highly active antiretroviral therapy (HAART).[15,16] Furthermore, since up to 30% of HIV-positive patients have concomitant viral hepatitis, subjects with pre-existing chronic HBV or HCV were also targeted for enrolment into the DILIN prospective protocol. The generalizability of the findings from DILIN will require comparison with other population-based studies of DILI in the US and abroad that may employ similar or alternative recruitment methods.
The five DILIN clinical sites have enrolled ~2 cases per month at each centre with 367 total cases enrolled into the DILIN prospective study through October 2007 (figure 1). However, screen failures have been encountered at each site due to a multitude of reasons (table IV). Although clinical data are not collected on screen failures, it is possible that these patients intrinsically differ from the patients who enrol. To recruit the largest number of DILI cases, most of the DILIN sites have developed local or regional referral networks. The two models of satellite site development with either an off-site research team (University of Connecticut) or use of the main site study staff at distant locations (University of North Carolina) have had varied results. Over the next 5 years, it is expected that the number of DILIN clinical sites will be increased to eight (see http://grants.nih.gov/grants/guide/rfa-files/RFA-DK-07-012). If the current rate of enrolment was to continue following expansion of the network, it is anticipated that over 1000 DILI cases would be enrolled in the next 5 years.
Criteria for a diagnosis of DILI include identification of a suspect medication with a plausible temporal association to the liver injury episode, exclusion of competing causes of liver injury and comparison of the case phenotype with published reports of liver injury due to the suspect medication. In addition, improvement of the liver injury with drug cessation is typically observed. Finally, intentional or inadvertent re-challenge can be helpful but is infrequently undertaken and does not always lead to reproducible liver injury.[10] In the DILIN prospective study, extensive diagnostic serologies, laboratory tests and liver imaging are required to exclude other more common causes of liver injury (table I). In addition, a detailed narrative is generated to provide clinical information on other contributing factors such as systemic illnesses and concomitant medications. Finally, the 6-month follow-up visit allows other disease processes to be diagnosed such as occult pancreaticobiliary cancer. Subject retention thus far has been excellent with over 82% of eligible subjects completing the 6-month study visit.
A final goal for DILIN is to develop standardized terminology, definitions and causality assessment instruments to be used by other research groups as well as practicing clinicians.[12] In the DILIN prospective study, subjects are classified as ‘standard DILI’ or ‘liver DILI’ cases. The reason to include the latter group is because of the high prevalence of fatty liver disease, abnormal liver biochemistries and viral hepatitis in the general US population. Furthermore, inclusion of these patients may help determine if pre-existing liver disease increases susceptibility or influences outcomes with DILI.[37,38] It is anticipated that the level of diagnostic certainty for a DILI episode may be lower in subjects with pre-existing liver disease due to the difficulty in distinguishing a disease flare from the effect of a suspect medication. However, only prospective determination of causality assessment will allow us to determine if the diagnostic criteria for ‘standard DILI’ and ‘liver DILI’ cases should be different. Finally, multivariate modelling is planned to help identify the key components from the extensive data collected in enrolled subjects. Development of an evidence-based causality assessment instrument will enable the more confident diagnosis of DILI.
An international consensus group proposed the persistence of laboratory abnormalities for more than 3 months in subjects with acute hepatocellular DILI and 6 months in subjects with cholestatic DILI as criteria for ‘chronic DILI’.[39,40] However, in other liver diseases such as acute HBV or HCV infection persistence of detectable HBsAg or HCV RNA for at least 6 months is used to identify subjects with chronic infection.[41,42] Therefore, a 6-month persistence of biochemical abnormalities in a subject with previously normal aminotransferase levels was felt to be a reasonable criterion for ‘chronic DILI’. In addition, clinical, histopathological or radiological abnormalities that persist 6 months after DILI onset are proposed as diagnostic criteria for ‘chronic DILI’.[18,43,44]
Although the RUCAM is the most frequently used causality assessment instrument, limitations include difficulty in interpreting definitions, a lack of accounting for HCV infection and a low level of inter-observer reproducibility.[45,46] The causality categories in the DILIN prospective study are largely clinical in nature and are designed to convey varying levels of confidence in the diagnosis of DILI. This methodology relies on the expert opinion of three experienced hepatologists who are provided with extensive clinical, laboratory and imaging data from the baseline visit. In order to decrease the subjective nature of causality assessment, a set of definitions was developed for each category using three approaches: percentage likelihood, legal phrases and a set of conditions to support each level of causality (table III). Although these terms are widely used in assessing causality in various adverse drug events, their meanings have not been standardized. The terms of ‘definite’, ‘highly likely’ and ‘probable’ were used to define cases that were convincingly related to the medication, whereas ‘possible’ and ‘unlikely’ were used to define cases that were not convincingly related. Whether this approach will be generalisable to all patients presenting with DILI remains unclear and these terms and definitions will be prospectively evaluated and compared with more conventional approaches such as the RUCAM.
Causality assessment is also hampered by the lack of accurate and reliable biomarkers of DILI. Although lymphocyte stimulation or transformation assays have been proposed, they are currently not well standardized, validated nor widely available. Thus, their role in diagnosis amongst individual patients remains uncertain.[47] The prospective collection of biological samples in DILIN at baseline and 6 months will hopefully provide the materials to develop improved biomarkers. These samples will also provide invaluable resources for the investigation of genomic, proteomic and metabolomic approaches to establishing causality and identifying underlying mechanisms of liver DILI. Finally, DILIN is interested in developing a web-based causality assessment instrument and comprehensive database on DILI in collaboration with the National Library of Medicine to assist investigators interested in DILI as well as practicing clinicians.
Conclusions
The DILIN prospective study is designed to improve our understanding of the mechanisms, risk factors and natural history of DILI amongst children and adults in the US. Criteria for patient enrolment, standardized terminology and causality assessment methods have been developed as part of the DILIN prospective study. The five clinical sites have enrolled 367 subjects thus far, using a variety of recruitment methods. The study entry criteria have been adapted and modified via the development of an exemptions committee. Causality assessment is continuously being refined with the ultimate goal of developing a user-friendly, reproducible and accurate causality assessment instrument. Finally, the collection of biological samples from cases and controls will allow for genetic association studies as well as identification of clinical, immunological and environmental risk factors for DILI susceptibility and outcome.
Acknowledgements
Funding for the DILIN is provided by the NIDDK under cooperative agreements: 1U01DK065201, 1U01DK065193, 1U01DK065184, 1U01DK065211, 1U01DK065238 and 1U01DK065176.
Footnotes
The authors have the following disclosures: Robert J. Fontana: Speaker’s Bureau for Roche Laboratories, Bristol-Meyers Squibb, consultant for Vertex Pharmaceuticals; Naga Chalasani: consultant for or grant funding from Takeda, Atherogenics, Eli-Lilly Company, Merck, Abbott, Ortho-McNeill, Metabasis Advanced Life Sciences, Sanof-Aventis, Deviousion; Herbert L. Bonkovsky: served as a paid advisor to Infracare, Novartis and Ovation Pharmaceuticals and is on the Speaker’s Bureau for Ovation Pharmaceuticals, grant support from Merck, Novartis, Roche and Vertex, during the past 12 months he served as an expert witness for plaintiffs in litigation regarding potential DILI; Paul B. Watkins: served as a paid consultant in the preceding 12 months to the following pharmaceutical companies: Actellion, Aldus, Astellas, Bristol-Myers Squibb, Boerringer-Ingleheim, Danube, Endo, Fibrogen, GlaxoSmithKline, Hoffman-LaRoche, Imtech, King, Mellenium, Merck, Novartis, Nuon, Orion, Pfizer, Pharmasset, Schering Plough, TAP, Valiant, VIA and Wyeth, he served as both a plaintiff and defence expert in litigation involving suspected DILI involving some of these companies. Drs Rochon, Davern and Serrano have no financial disclosures relating to this work.
The following is a list of the principal investigators (co-investigators) and [study coordinators] participating in the DILIN: University of North Carolina at Chapel Hill: Paul Watkins, Chair of DILIN Steering Committee (Paul Hayashi, Mark Russo) [Anne Criss, Susan Pusek, Gale Groseclose]; University of Connecticut Health Center: Herbert Bonkovsky (James Freston, Robert Levine, Benedict Maliakkal, Petr Protiva, Robert Rosson) [Jane Bulger, Mariola Smialek, Paul Appleton]; Indiana University School of Medicine: Naga Chalasani (Lawrence Lumeng, Raj Vuppalanchi) [Audrey Corne]; University of California San Francisco: Tim Davern (Maurizio Bonacini) [Dalia Moawad, Kristine Partovi]; University of Michigan: Robert Fontana (Hari Conjeeveram) [Suzanne Welch, Jordan Kridler]; Data Coordinating Center, Duke Clinical Research Institute: James Rochon (John McHutchison, Don C. Rockey) [Carla Ange, Katherine Berezny, Michelle Crowder, Satarah Latiker, Mary Moggio, Lauren Ruffrage, Carmel Scharenbroich, June Wampole]; NIDDK: José Serrano, Leonard Seeff, Jay Hoofnagle; NIDDK Biosample Repository: David Toke (Dana Witt), Rutgers University; Rich Frome, Fisher BioServices; Liver Histopathology Review Center: David Kleiner, National Cancer Institute.
References
- 1.Ostapowicz G, Fontana RJ, Schiodt RV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 2.Lee WM, Senior JR. Recognizing drug-induced liver injury: current problems, possible solutions. Toxciol Pathol. 2005;33:155–64. doi: 10.1080/01926230590522356. [DOI] [PubMed] [Google Scholar]
- 3.Favreau JT, Ryu ML, Braunstein G, et al. Severe hepatoxicity associated with the dietary supplement Lipokinetix. Ann Intern Med. 2002;136:590–5. doi: 10.7326/0003-4819-136-8-200204160-00008. [DOI] [PubMed] [Google Scholar]
- 4.Stickel F, Baumuller HM, Seitz K, et al. Hepatitis induced by Kava (Piper methysticum rhizome) J Hepatol. 2003;39:62–7. doi: 10.1016/s0168-8278(03)00175-2. [DOI] [PubMed] [Google Scholar]
- 5.Bjornson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–9. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 6.Watkins PB, Seeff LB. Drug induced liver injury: summary of a single topic clinical research conference. Hepatology. 2006;43:618–31. doi: 10.1002/hep.21095. [DOI] [PubMed] [Google Scholar]
- 7.Walgren JL, Mitchell MD, Thompson DC. Role of metabolism in drug-induced idiosyncratic hepatotoxicity. Crit Rev Toxicol. 2005;35:325–61. doi: 10.1080/10408440590935620. [DOI] [PubMed] [Google Scholar]
- 8.Navarro VJ, Senior JR. Drug related hepatotoxicity. N Engl J Med. 2006;354:731–9. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 9.Andrade RJ, Lucena I, Alonso A, et al. HLA class II genotype influences the type of liver injury in drug-induced idiosyncratic liver disease. Hepatology. 2004;39:1603–12. doi: 10.1002/hep.20215. [DOI] [PubMed] [Google Scholar]
- 10.Kaplowitz N. Causality assessment versus guilt-by-association in drug hepatotoxicity. Hepatology. 2001;33:308–10. doi: 10.1053/jhep.2001.21083. [DOI] [PubMed] [Google Scholar]
- 11.Verma A, Lilienfeld DE. The need for a population-based surveillance system for liver disease in the United States. Pharmacoepidemiol Drug Saf. 2004;13:821–4. doi: 10.1002/pds.1022. [DOI] [PubMed] [Google Scholar]
- 12.Hoofnagle JH. Drug induced liver injury network (DILIN) Hepatology. 2004;40:773. doi: 10.1002/hep.20445. [DOI] [PubMed] [Google Scholar]
- 13.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 14.Varni JW, Burwinkle TM, Seid M, et al. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–41. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Nunez M, Soriano V. Hepatotoxicity of antiretrovirals: incidence, mechanisms, and management. Drug Saf. 2005;38:53–66. doi: 10.2165/00002018-200528010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Ogedegbe AO, Sulkowski MS. Antiretroviral-associated liver injury. Clin Liver Dis. 2003;7:475–99. doi: 10.1016/s1089-3261(03)00023-0. [DOI] [PubMed] [Google Scholar]
- 17.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 18.Aithal PG, Day CP. The natural history of histologically proved drug induced liver disease. Gut. 1999;44:731–5. doi: 10.1136/gut.44.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleiner DA, Chalasani NP, Conjeevaram HS, et al. Relationship of biochemical to histologic findings and the pathological pattern of injury among cases identified in the NIH drug induced liver injury network (DILIN) study [abstract] Gastroenterology. 2007;132:M1775. [Google Scholar]
- 20.Andrade RJ, Lucerna MI, Fernandez MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro MZ, Lewis JH. Causality assessment of drug-induced hepatotoxicity: promises and pitfalls. Clin Liver Dis. 2007;11:477–505. doi: 10.1016/j.cld.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Danan G, Benichou C. Causality assessment of adverse reactions to drugs: a novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–30. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 23.Chalasani N, Fontana RJ, Bonkovsky HL, for the Drug Induced Liver Injury Network (DILIN) Causes, clinical features, and outcomes from a prospective study of drug induced liver injury in the United States. Gastroenterology. doi: 10.1053/j.gastro.2008.09.011. Epub 2008 Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunter DJ, Kraft P. Drinking from the fire hose: statistical issues in genome-wide association studies. N Engl J Med. 2007;357:436–9. doi: 10.1056/NEJMp078120. [DOI] [PubMed] [Google Scholar]
- 25.Ballet F. Hepatotoxicity in drug development: detection, significance and solutions. J Hepatol. 1997;26(Suppl. 2):26–36. doi: 10.1016/s0168-8278(97)80494-1. [DOI] [PubMed] [Google Scholar]
- 26.Fontana RJ, McCashland TM, Benner KG, et al. Acute liver failure associated with prolonged use of bromfenac leading to liver transplantation. Liver Transpl Surg. 1999;5:480–4. doi: 10.1002/lt.500050607. [DOI] [PubMed] [Google Scholar]
- 27.Graham DJ, Green L, Senior JR, et al. Troglitazone-induced liver failure: a case study. Am J Med. 2003;114:299–306. doi: 10.1016/s0002-9343(02)01529-2. [DOI] [PubMed] [Google Scholar]
- 28.Lee WM, Larrey D, Olsson R, et al. Hepatic findings in long-term clinical trials of ximelagatran. Drug Saf. 2005;28:351–70. doi: 10.2165/00002018-200528040-00006. [DOI] [PubMed] [Google Scholar]
- 29.Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969-2002. Arch Intern Med. 2005;165:1363–9. doi: 10.1001/archinte.165.12.1363. [DOI] [PubMed] [Google Scholar]
- 30.Devalle MB, Klinteberg AV, Alem N, et al. Drug induced liver injury in a Swedish University hospital outpatient hepatology clinic. Aliment Pharmacol Ther. 2006;24:1187–95. doi: 10.1111/j.1365-2036.2006.03117.x. [DOI] [PubMed] [Google Scholar]
- 31.Galan M, Potts J, Silverman AL, et al. The burden of acute non-fulminant drug-induced hepatitis in a United States Tertiary care center. J Clin Gastroenterol. 2005;39:64–7. [PubMed] [Google Scholar]
- 32.Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–5. doi: 10.1053/jhep.2002.34857. [DOI] [PubMed] [Google Scholar]
- 33.Whitehead MW, Hainsworth I, Kingham JGC. The cause of obvious jaundice in South West Wales: perceptions versus reality. Gut. 2001;48:409–13. doi: 10.1136/gut.48.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ibanez L, Perez E, Vidal X, et al. Prospective surveillance of acute serious liver disease unrelated to infectious obstructive or metabolic diseases: epidemiological and clinical features, and exposure to drugs. J Hepatology. 2002;37:592–600. doi: 10.1016/s0168-8278(02)00231-3. [DOI] [PubMed] [Google Scholar]
- 35.Rumack BH. Acetaminophen hepatotoxicity: the first 35 years. J Toxicol Clin Toxicol. 2002;40:3–20. doi: 10.1081/clt-120002882. [DOI] [PubMed] [Google Scholar]
- 36.LaRusso NF, Schneider BL, Black D, et al. Primary sclerosing cholangitis: summary of a workshop. Hepatology. 2006;44:746–64. doi: 10.1002/hep.21337. [DOI] [PubMed] [Google Scholar]
- 37.Patel PA, Voigt MD. Prevalence and interaction of hepatitis B and latent tuberculosis in Vietnamese immigrants to the United States. Am J Gastroenterol. 2002;97:1198–203. doi: 10.1111/j.1572-0241.2002.05704.x. [DOI] [PubMed] [Google Scholar]
- 38.Mok MY, Ng WL, Yuen MF, et al. Safety of disease modifying anti-rheumatic agents in rheumatoid arthritis patients with chronic viral hepatitis. Clin Exp Rheumatol. 2000;18:363–8. [PubMed] [Google Scholar]
- 39.Benichou C. Criteria of drug-induced liver disorders: report of an international consensus meeting. J Hepatol. 1990;11:272–6. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 40.Andrade RJ, Lucena MI, Kaplowitz N, et al. Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–8. doi: 10.1002/hep.21424. [DOI] [PubMed] [Google Scholar]
- 41.Lok ASF, McMahon B. AASLD practice guideline: chronic hepatitis B. Hepatology. 2007;45:507–39. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 42.Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231–64. doi: 10.1053/j.gastro.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Sharp JR, Ishak KG, Zimmerman HJ. Chronic active hepatitis and severe hepatic necrosis associated with nitrofurantoin. Ann Intern Med. 1980;92:14–9. doi: 10.7326/0003-4819-92-1-14. [DOI] [PubMed] [Google Scholar]
- 44.Shergy WJ, Polisson RP, Caldwell DS, et al. Methotrexate-associated hepatotoxicity: retrospective analysis of 210 patients with rheumatoid arthritis. Am J Med. 1988;85:771–4. doi: 10.1016/s0002-9343(88)80019-6. [DOI] [PubMed] [Google Scholar]
- 45.Rockey DC, Seeff LB, Freston JW, et al. Comparison between expert opinion and RUCAM for assignment of causality in drug induced liver injury [abstract] Gastroenterology. 2007;132:M1777. [Google Scholar]
- 46.Rochon J, Protiva B, Seeff LB, for the Drug Induced Liver Injury Network The reliability of the RUCAM for assessing causality in drug-induced liver injury. Hepatology. 2008;48:1175–83. doi: 10.1002/hep.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krivoy N, Taeri M, Neuman MG. Antiepileptic drug-induced hypersensitivity syndrome reactions. CNS Drugs. 2004;18:5–25. doi: 10.2174/157488606777934459. [DOI] [PubMed] [Google Scholar]