Abstract
Objective
To evaluate the peer-reviewed literature on iodine deficiency and hypothyroxinemia in pregnancy.
Methods
We review published studies on isolated hypothyroxinemia in pregnancy, methodology of free thyroxine (T4) assays, impact of iodine deficiency on free T4 levels, and status of ongoing prospective randomized trials of isolated hypothyroxinemia during pregnancy.
Results
Hypothyroxinemia during pregnancy is common. Studies have demonstrated the pivotal role exerted by maternal T4 on fetal brain development and the negative impact of hypothyroxinemia on neurobehavioral performance in offspring. Two intervention studies have demonstrated a positive effect on neurodevelopment in children of mothers promptly supplemented with iodine compared with the neurodevelopment in children of nonsupplemented mothers. Free T4 assays presently in clinical use have limitations. Preliminary results of the Controlled Antenatal Thyroid Study (CATS) are somewhat mixed, and the National Institutes of Health Maternal Fetal Medicine Thyrotropin Study (TSH Study) will be completed in 2015. Knowledge regarding the impact of isolated hypothyroxinemia has progressed, but major questions remain. An optimal diagnostic test for free T4 during pregnancy (accurate, inexpensive, and widely available) remains elusive. Trimester-specific normative data and normal ranges from different geographic regions do not exist.
Conclusions
Data published to date are insufficient to recommend levothyroxine therapy in pregnant women with isolated hypothyroxinemia. Adequate iodine intake should be recommended before conception and early in pregnancy.
INTRODUCTION
The condition of isolated hypothyroxinemia in pregnancy is defined as the presence of a free thyroxine (FT4) value below the 2.5th percentile with a thyrotropin (TSH) level within the reference range. The critical questions are whether hypothyroxinemia in pregnancy is associated with negative maternal and fetal outcomes and whether treatment with levothyroxine can prevent the adverse effects. These questions remain unanswered for the following reasons: first, the definition of what constitutes a FT4 level below the 2.5th percentile is unclear because regional and iodine status reference ranges have not been established; second, and perhaps equally problematic, is that the accuracy of commonly used FT4 measurements during pregnancy is questionable; and third, research to date evaluating the effect of isolated hypothyroxinemia on maternal and fetal outcomes has yielded conflicting data.
The present review aims to examine the controversial condition of isolated hypothyroxinemia, from laboratory evaluation to studies of intervention. Clarification of the impact of isolated hypothyroxinemia is critical to the care of pregnant women, and recently completed clinical trials, as well as a National Institutes of Health Maternal Fetal Medicine multicenter trial (called the TSH trial) projected to be presented in 2015, will hopefully provide much needed clarity.
THE PROBLEM OF IODINE DEFICIENCY
Iodine deficiency and thyroid autoimmunity affect TSH and FT4 concentrations. During pregnancy, a complex combination of factors specific to the pregnant state results in profound alterations in the thyroidal economy. Moreover, in iodine-deficient pregnant women, increased thyroidal stimulation induces, in turn, a sequence of events leading from physiological adaptation of the thyroidal economy observed in healthy iodine-sufficient pregnant women to pathologic alterations affecting both thyroid function and the anatomic integrity of the thyroid gland. Even in mild iodine deficiency, signs of thyroid adaptation are represented by increased thyroid volume, increased thyroglobulin production, and elevated TSH and decreased FT4 values (1-4). The National Health and Nutrition Examination Survey 2003-2004 demonstrated that the US population was generally iodine sufficient as measured by urinary iodine concentration (160 μg/L), but showed that 11.3% of the population had a low urinary iodine concentration (<50 μg/L). Moreover, among all women of reproductive age (pregnant and nonpregnant), the median urinary iodine concentration was 139 μg/L, and 15.1% of women had a urinary iodine concentration less than 50 μg/L (5). The situation is certainly worse in Europe, especially in those countries where iodine supplementation is not mandatory. In those regions, pregnant women rarely maintain iodine intake at the levels recommended for pregnant women by the World Health Organization (250 mcg daily) (6).
The spectrum of iodine deficiency varies widely across Europe. An observational study of iodine sufficiency in pregnancy was conducted in Toulouse (southwestern France), a region thought to be iodine replete because of its relative proximity to the sea and the fish-eating habits of the population. Nevertheless, 75% of participants in a cohort of pregnant women from this region had urinary iodine excretion below 100 μg/L (7). As a component of a large, prospective, randomized trial of levothyroxine therapy in women with subclinical hypothyroidism and hypothyroxinemia (Controlled Antenatal Thyroid Study [CATS]), the iodine status of 261 hypothyroid/hypothyroxinemic women and 526 euthyroid women from Turin, Italy, and 374 hypothyroid/hypothyroxinemic women and 480 euthyroid women from Cardiff, Wales, were evaluated. The women were selected from the larger cohort on the basis of availability of stored urine samples and thyroid function data. Analysis of urine samples revealed the median urinary iodine to be low in Cardiff (98 μg/L) and extremely low in Turin (52 μg/L) (8). In 1998, a different scenario was documented in Sweden, where the mean 24-hour urinary iodine concentration was 140 μg/L in the first trimester of pregnancy—a level that still suggests lessthan-adequate iodine daily intake (9).
Sufficient iodine intake during pregnancy is sometimes adversely affected by a public health effort to limit the overall salt intake of the population, so as to decrease the incidence of hypertension, heart disease, and stroke. In a study performed in Columbia, a country that uses iodized salt to correct iodine deficiency, 50% of women had restricted salt intake during pregnancy, resulting in a significant TSH elevation at the time of delivery (10). These findings emphasize the concept that in areas where iodized salt is used to prevent iodine deficiency, dietary salt restriction, as often happens during gestation, may further increase the risk of becoming hypothyroxinemic.
DIAGNOSING HYPOTHYROXINEMIA
Evidence suggests that maternal hypothyroxinemia alone poses an increased risk for fetal neurodevelopmental deficits. This is of particular potential importance since normal serum concentrations of maternal triiodothyronine (T3) could conceivably prevent a TSH elevation, but do not compensate for low thyroxine (T4) concentrations and the resulting neurologic damage. Therefore, TSH assessment alone is insufficient in detecting hypothyroxinemia. Hypothyroxinemia is prevalent both in areas replete with iodine and regions of iodine insufficiency. Although thyroid autoimmunity carries a 5-fold increased risk of hypothyroidism, in geographic areas that are iodine deficient, iodine insufficiency seems to be a main determinant in the occurrence of thyroid underfunction (11). Prolonged iodized salt significantly improves maternal thyroid economy and reduces the risk of maternal thyroid insufficiency during gestation, probably because of the near-complete restoration of intrathyroidal iodine stores (12).
FT4 immunoassays currently available on the market are essentially FT4 estimate tests that do not measure FT4 directly and are known to be sensitive to alterations in binding proteins (13). They perform reasonably well in nonpregnant conditions by reporting low FT4 levels in thyroid hormone deficiency and high FT4 levels in thyroid hormone excess (14). As a result, pregnancy-induced elevation in T4-binding globulin and nonesterified fatty acids, as well as lower concentrations of albumin relative to serum samples of nonpregnant women, can influence FT4 measurement (14-17). A comparison of methods demonstrated that albumin-dependent immunoassays had marked negative bias with up to 50% of subnormal values, while other methods gave values above their nonpregnant reference values (18).
FT4 IMMUNOASSAYS
The uncertainty around FT4 estimates in pregnancy has called into question the wisdom of relying on FT4 immunoassays during pregnancy (15). The difficulty in measurement of FT4 is further complicated by the fact that only 0.03% of total serum T4 is unbound. It is therefore challenging to measure FT4 (measured in picomoles) in the presence of high concentrations of bound T4 (measured in nanomoles), especially during pregnancy when serum binding-protein concentrations are markedly elevated and in the presence of heterophilic antibodies (19-21). Moreover, immunoassays are prone to error by disruption of the original equilibrium, and depend on dilution, temperature, buffer composition, affinity and concentration of the T4 antibody reagent, and T4-binding capacity of the serum sample (22). These conditions make the conventional immunoassay methods unreliable with often nonreproducible results among different kits (18). Because FT4 measurement by liquid chromatography/tandem mass spectrometry (LC/MS/MS) is not readily available in most clinical laboratories, clinicians should use whichever measure for or estimate of FT4 that is available in their laboratory, being aware of the limitations of each method.
TRIMESTER-SPECIFIC REFERENCE RANGES
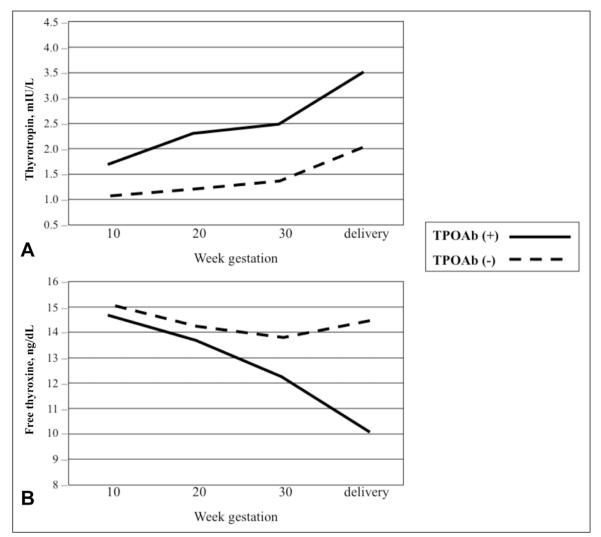
FT4 reference ranges in pregnancy vary widely among methods; therefore, interpretation of FT4 values requires method-specific ranges (16,17). Such ranges are also influenced by the iodine status of the population studied. It is customary for laboratories to adopt the reference ranges provided by the manufacturer of the test. Typically, the characteristics of these reference cohorts are not disclosed and may differ in iodine intake and ethnicity to an extent that compromises the value of adopting the manufacturer ranges across different populations. Furthermore, FT4 reference ranges provided by the manufacturers have been established using pools of serum samples from healthy, nonpregnant persons and such reference ranges are not valid in the pregnant state. Therefore, it has been proposed to adapt serum FT4 reference ranges that are laboratory-specific and trimester-specific for use during pregnancy, but, so far, no worldwide consensus has been reached on such “pregnancy-adapted” ranges (Fig. 1). Therefore, caution is recommended in the interpretation of serum FT4 levels in pregnancy.
Fig. 1.
Panel A, Thyrotropin values during gestation in thyroperoxidase antibody (TPOAb)–positive and TPOAb-negative euthyroid women. Panel B, Free thyroxine values during gestation in TPOAb-positive and TPOAb-negative euthyroid women. Adapted with permission from Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: Effects on obstetrical complications. J Clin Endocrinol Metab. 2006;91:2587-2591. Copyright 2006, The Endocrine Society.
LIQUID CHROMATOGRAPHY/TANDEM MASS SPECTROMETRY
More recently, free thyroid hormones have been measured in the dialysate or ultrafiltrate after serum online solid phase extraction by LC/MS/MS. The method is regarded as a major advancement, with higher specificity than immunoassays and great potential to be applied in the routine assessment of FT4 and free triiodothyronine (FT3). It should be noted that before FT4 hormone measurement, the free and bound T4 must be physically separated. Equilibrium dialysis and ultrafiltration are 2 methods used for physical separation of serum free T4 before analysis of the dialysate or ultrafiltrate. These separation techniques are labor-intensive, time-consuming, and costly. Compared with FT4 measured by immunoassays, free thyroid hormone concentrations measured by LC/MS/MS correlate well with inverse log TSH values (24). However, this correlation decreases during pregnancy (r = 0.11 for FT4 LC/MS/MS, and r = −0.06 for FT4 immunoassay), suggesting changes in the set point of the hypothalamuspituitary-thyroid axis during pregnancy. A working group of the International Federation of Clinical Chemistry and Laboratory Medicine recommends the use of isotope dilution LC/MS/MS as the criterion standard procedure for the measurement of serum FT4 (25). Tandem mass spectrometry is ideally suited for generating reliable and reproducible trimester-specific reference intervals for FT4 (26). Using direct equilibrium dialysis and LC/MS/MS, the 95% FT4 reference intervals during pregnancy decreased gradually with advancing gestational age: from 1.08-1.82 ng/dL in week 14 to 0.86-1.53 ng/dL in week 20 (27). In comparison, using ultrafiltration followed by isotope dilution LC/MS/MS, serum FT4 concentrations (mean ± standard error) were 1.13 ± 0.23 ng/dL in the first trimester, 0.92 ± 0.30 ng/dL in the second trimester, 0.86 ± 0.21 ng/dL in the third trimester, and 0.93 ± 0.25 ng/dL in nonpregnant women (25). Using a direct analogue immunoassay on the same serum samples, FT4 values were 1.05 ± 0.22 ng/dL, 0.88 ± 0.17 ng/dL, and 0.89 ± 0.17 ng/dL in the first, second, and third trimester, respectively. Serum FT4 by LC/MS/MS correlated very well with serum FT4 measured by classic equilibrium dialysis, but correlation with results from the FT4 immunoassay were less satisfactory (25).
COMPLICATIONS ASSOCIATED WITH MATERNAL HYPOTHYROXINEMIA
Despite the limitations of FT4 assays, multiple studies have demonstrated that low-normal FT4 concentrations are associated with adverse outcomes in the offspring. Perhaps the best-known study was published in the New England Journal of Medicine by Haddow and colleagues in 1999 (28). The Haddow et al study revealed that maternal hypothyroidism present in the second trimester of gestation, which was not adequately treated with thyroid hormone, was associated with a lower intelligence quotient (IQ) in the offspring. In that retrospective study, the authors selected 62 pregnant women with serum TSH values above the 98th percentile of the values for all pregnant women tested (n = 25 216), in combination with low FT4 values. Their children underwent IQ tests (Wechsler Intelligence Scale for Children, third edition) at ages 7 to 9 years, which revealed a mean IQ that was 4 points lower than the IQ of control children (P = .06). Furthermore, 15% of the children of the identified mothers had IQ scores of 85 or less, compared with 5% of the matched control children with euthyroid mothers. Of the 62 women with thyroid deficiency, 48 were not treated with levothyroxine during pregnancy. The full-scale IQ scores of these 48 children averaged 7 points lower than that of the control children (P = .005), with 19% of these children having IQ scores of 85 or less.
In the same year, Pop et al evaluated the relationship between FT4 values of women during gestation and psychomotor development of their infants (29). Children of women with FT4 levels below the 5th and 10th percentiles at 12 weeks’ gestation had significantly lower scores on the Bayley Psychomotor Developmental Index (PDI) scale at 10 months of age than children of mothers with higher FT4 values. A positive correlation was found between the mothers’ FT4 concentrations at 12 weeks’ gestation and children’s PDI scores. The study also demonstrated that low FT4 levels occurring in late pregnancy (32 weeks’ gestation) did not affect the children’s neurodevelopment, and that if maternal FT4 normalized by 24 and 32 weeks’ gestation, the negative effect on the PDI disappeared. A few years later, the same group of investigators evaluated the mental and motor development in 1- and 2-year-old children of mothers who experienced hypothyroxinemia (FT4 below the 10th percentile) at 12 weeks’ gestation (30). These children had PDI scores that were significantly lower (by 8 to 10 points) than scores of children born to euthyroxinemic mothers (FT4 values between 50th and 90th percentiles).
Such results have been confirmed by Vermiglio et al (31). These authors compared the neuropsychological development of children whose mothers came from a moderately iodine-deficient area and children of mothers from a marginally iodine-sufficient area. Results showed that the mothers from the iodine-deficient area experienced lower FT4 values during gestation than mothers from the iodine-sufficient area. Furthermore, the offspring of hypothyroxinemic mothers had an increased incidence of attention deficit and hyperactivity disorder, as well as a reduced IQ, compared with controls.
In 2006, Kooistra et al demonstrated that newborns of hypothyroxinemic mothers (FT4 below the 10th percentile at 12 weeks’ gestation) who were evaluated 3 weeks after delivery with the Neonatal Behavioural Assessment Scale had significantly lower scores in the orientation index than control children (children from mothers with total T4 values between the 50th and 90th percentiles) (32). A recent study from China showed that children of women with thyroid abnormalities at 16 to 20 weeks’ gestation (subclinical hypothyroidism, hypothyroxinemia, and elevated thyroperoxiase antibody titers) had mean intelligence and motor scores significantly lower than those of control children. Increased maternal serum TSH, decreased maternal serum total T4, and elevated maternal thyroperoxidase antibody titers were separately associated either with lower intelligence scores or poorer motor scores in the offspring (33).
Most recently, Henrichs et al studied associations of maternal hypothyroxinemia with cognitive functioning in early childhood. The authors conducted a population-based cohort study in the Netherlands involving 3659 children and their mothers. The mothers were defined as having mild or severe hypothyroxinemia on the basis of serum FT4 concentrations below the 10th and 5th percentile, respectively. Results showed that maternal TSH was not related to cognitive outcomes, while an increase in maternal FT4 predicted a lower risk of expressive language delay at 30 months of age. Furthermore, both mild and severe maternal hypothyroxinemia were associated with a higher risk of expressive language delay at 18 and 30 months. Severe maternal hypothyroxinemia also predicted a higher risk of nonverbal cognitive delay. On the basis of such results, the authors concluded that maternal hypothyroxinemia represents a risk factor for cognitive delay in early childhood (34). Long-term follow-up would be useful in identifying whether the neurodevelopment delay found in offspring of mothers with severe maternal hypothyroxinemia persists throughout childhood.
IN VITRO AND EXPERIMENTAL EVIDENCE FOR A ROLE OF MATERNAL T4 IN PREGNANCY
A lack of thyroid hormone during early fetal life results in irreversible brain damage The detrimental effect of iodine deficiency and maternal hypothyroxinemia on fetal brain development are probably related to reduced maternal T4 transfer to the fetus before the onset of fetal thyroid function (35,36). During the first trimester, the fetus is completely dependent on maternal T4, and although T4 in the fetal compartment is about 100 times lower than in the maternal serum, the FT4 concentrations are about one-third the maternal concentration (35,36). The development of the fetal brain is dependent on T4, as all T3 in the fetal brain is locally derived from T4 by the action of the D2 deiodinase. Maternal T3 is not used by the fetal brain (37-39). Study of the human fetal brain from 13 to 20 weeks’ gestation has revealed specific developmental patterns for thyroid hormone and D2 and D3 deiodinases, with spatial and temporal specificity and different patterns for the cerebral cortex and the cerebellum. Low maternal T4 prevents the normal T3 increases in each of the developing brain areas (40).
In rats, a mild and transient deficiency of maternal thyroid hormones for only 3 days, at the onset of corticogenesis, irreversibly deranges the migration of radial neurons and the cytoarchitecture of the cortex and hippocampus unless levothyroxine is administered within a short time span (41). Moreover, a condition of iodine deficiency is able to cause irreversible damage to the developing fetal brain, as thyroid hormones have a pivotal role in several neurobiological processes, such as neurogenesis, neuronal migration, axon and dendrite formation, myelination, synaptogenesis, and neurotransmission (42,43). In essence, strong and consistent evidence confirms that maternal hypothyroidism and hypothyroxinemia negatively affects newborn development (44). Furthermore, although early maternal hypothyroxinemia can be important in fetal brain development, iodine deficiency may be even worse than isolated maternal hypothyroxinemia/hypothyroidism because of the additional problem of fetal hypothyroidism due to the lack of iodine availability for fetal thyroid hormone synthesis (45).
STUDIES OF INTERVENTION
Two studies conducted in Spain have demonstrated the deleterious effects of iodine deficiency and the positive role exerted by adequate iodine supplementation initiated early in pregnancy. Berbel et al showed that a delay of 6 to 10 weeks in iodine supplementation (200 mcg daily of potassium iodide) in hypothyroxinemic mothers at the beginning of gestation increased the risk of neurodevelopmental delay in the progeny (46). In particular, children from hypothyroxinemic mothers had lower scores in gross and fine motor coordination and socialization performances, while no significant differences were found on language quotients. Velasco et al compared the psychological development of infants aged 3 to 18 months whose mothers had received potassium iodide, 300 mcg daily, during the first trimester of pregnancy (133 cases) with the psychological development of infants whose mothers had not received iodine supplementation (61 controls). Although collected data were not controlled for confounding factors and the study was not a randomized controlled trial, results demonstrated that those children whose mothers had received an iodine supplement had a more favorable psychometric assessment than control children (47).
Data are beginning to emerge from the largest prospective trial of levothyroxine intervention in pregnant women. Preliminary results of the CATS study were presented at the International Thyroid Congress held in Paris in September 2010. The CATS trial was a prospective randomized study that screened 22 000 women for thyroid status (TSH and FT4 were measured) within the 16th week of gestation. In the intervention group, levothyroxine was initiated during pregnancy in women with FT4 values lower than the 2.5th percentile and/or TSH values above the 97.5th percentile. The control group received no intervention. The offspring of both groups had neuropsychological development testing (Wechsler Preschool and Primary Scale of Intelligence [WPPSI-III]) performed at 3 years of age. Data from the study revealed that only 4.5% of women had both an elevated TSH level and a decreased FT4 level, with the rest of the women with thyroid dysfunction equally divided into 2 populations with either high TSH or low FT4. The goal of the study was to ascertain whether the offspring of mothers with abnormal thyroid function had delayed neuropsychological development and whether this condition was prevented by levothyroxine treatment. The primary outcome was the mean WPPS-III score and the percentage of offspring with an IQ lower than 85 points. The primary analysis, which was intention-to-treat analysis, revealed no significant differences. A secondary analysis, which excluded women nonadherent to the levothyroxine intervention, revealed a significantly higher percentage of offspring with IQs lower than 85 in the untreated group. Dr. John Lazarus, the principle investigator of the CATS trial, stated that these data should be regarded as preliminary and that further data will be forthcoming. In particular, data were not presented on whether the 3 treated subgroups (high TSH, low FT4, high TSH and low FT4) showed any significant difference in offspring IQ. The results of the study may be affected by the fact that levothyroxine treatment was initiated beyond the critical time point needed for exogenous thyroid hormone replacement (before the 12th week of gestation [before the fetal thyroid produces levothyroxine]). Pregnant women were indeed recruited up to the 16th week of gestation, and the median time of recruitment was 12.6 weeks.
CONCLUSION
Progress has been made in our knowledge of isolated hypothyroxinemia during pregnancy, but serious gaps remain. The optimal clinical test, which is accurate, inexpensive, and widely available, does not exist yet. Similarly, trimester-specific reference ranges in iodine-sufficient and thyroperoxidase antibody–negative populations are yet to be determined. The problem of iodine deficiency still affects many areas around the world. Iodine deficiency may have a negative influence on FT4 values and contribute to the incidence and severity of hypothyroxinemia during pregnancy. However, several studies have evaluated and confirmed the pivotal role exerted by levothyroxine in the development of the fetal brain. Furthermore, 2 intervention studies have demonstrated a beneficial effect of iodine supplementation on neurodevelopment in progeny.
The results of 2 prospective randomized control trials should yield important data on resolving the impact, or lack thereof, of levothyroxine therapy in women with isolated hypothyroxinemia during pregnancy. Preliminary data from the CATS study have been presented at the International Thyroid Congress, and the definitive data analysis should be forthcoming within the year. The National Institutes of Health Maternal Fetal Medicine Unit has an ongoing trial that screened 120 000 women during pregnancy. Pregnancy screening was initiated in the National Institutes of Health trial in 2006, and recruitment has been completed. Women were divided into 2 groups: 1 group treated with levothyroxine to normalize TSH or FT4 values and 1 group left untreated. Each group had a cohort of women with isolated hypothyroxinemia of pregnancy and a cohort of women with subclinical hypothyroidism. The primary outcome of the study is intellectual function of children at 5 years of age as measured by the WPPSI-III. The WPPSI-III scores of progeny of treated women will be compared with the scores of children of untreated women. Secondary outcomes of the study include assessment of fetal growth, rate of preterm delivery, preeclampsia, abruption, stillbirth, and development of postpartum thyroid dysfunction. Results of both studies—complete analysis of the CATS trial and the results of the National Institutes of Health study (which should be known by 2015)—will have a profound impact on furthering our understanding of isolated hypothyroxinemia of pregnancy.
At present, difficulties in FT4 testing are still a common problem and trimester-specific ranges derived from iodine-sufficient areas are lacking. Although studies demonstrating that levothyroxine therapy in pregnant women with isolated hypothyroxinemia has a positive impact on the intellectual development of the offspring are intriguing, they are still inconclusive; thus, levothyroxine treatment of this entity is not recommended. Data suggest that American women of childbearing age may be receiving inadequate amounts of iodine during pregnancy. Accordingly, the American Thyroid Association recommends that all pregnant and breastfeeding women receive daily iodine supplementation, in the form of 150 mcg of potassium iodide. Recent recommendations to decrease the salt intake of all persons to lower the prevalence of myocardial infarction and stroke is laudatory (48), but may have the unintended consequence of worsening the degree of iodine deficiency experienced during pregnancy and lactation (49). It therefore becomes even more critical that supplemental iodine be included in all prenatal vitamins.
Abbreviations
- CATS
Controlled Antenatal Thyroid Study
- FT3
free triiodothyronine
- FT4
free thyroxine
- IQ
intelligence quotient
- LC/MS/MS
liquid chromatography/tandem mass spectrometry
- PDI
Bayley Psychomotor Developmental Index
- T3
triiodothyronine
- T4
thyroxine
- TSH
thyrotropin
- WPPSI-III
Wechsler Preschool and Primary Scale of Intelligence
Footnotes
DISCLOSURE The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Glinoer D, De Nayer P, Delange F, et al. A randomized trial for the treatment of mild iodine deficiency during pregnancy: Maternal and neonatal effects. J Clin Endocrinol Metab. 1995;80:258–269. doi: 10.1210/jcem.80.1.7829623. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen KM, Laurberg P, Iversen E, et al. Amelioration of some pregnancy-associated variations in thyroid function by iodine supplementation. J Clin Endocrinol Metab. 1993;77:1078–1083. doi: 10.1210/jcem.77.4.8408456. [DOI] [PubMed] [Google Scholar]
- 3.Romano R, Jannini EA, Pepe M, et al. The effects of iodoprophylaxis on thyroid size during pregnancy. Am J Obstet Gynecol. 1991;164:482–485. doi: 10.1016/s0002-9378(11)80004-9. [DOI] [PubMed] [Google Scholar]
- 4.Liesenkötter KP, Göpel W, Bogner U, Stach B, Grüters A. Earliest prevention of endemic goiter by iodine supplementation during pregnancy. Eur J Endocrinol. 1996;134:443–448. doi: 10.1530/eje.0.1340443. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell KL, Miller GA, Wang RY, Jain RB, Jones RL. Iodine status of the U.S. population, National Health and Nutrition Examination Survey 2003-2004. Thyroid. 2008;18:1207–1214. doi: 10.1089/thy.2008.0161. [DOI] [PubMed] [Google Scholar]
- 6.WHO Secretariat. Andersson M, de Benoist B, Delange F, Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: Conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10:1606–1611. doi: 10.1017/S1368980007361004. [DOI] [PubMed] [Google Scholar]
- 7.Caron P, Hoff M, Bazzi S, et al. Urinary iodine excretion during normal pregnancy in healthy women living in the southwest of France: Correlation with maternal thyroid parameters. Thyroid. 1997;7:749–754. doi: 10.1089/thy.1997.7.749. [DOI] [PubMed] [Google Scholar]
- 8.Pearce EN, Lazarus JH, Smyth PP, et al. Perchlorate and thiocyanate exposure and thyroid function in first-trimester pregnant women. J Clin Endocrinol Metab. 2010;95:3207–3215. doi: 10.1210/jc.2010-0014. [DOI] [PubMed] [Google Scholar]
- 9.Elnagar B, Eltom A, Wide L, Gebre-Medhin M, Karlsson FA. Iodine status, thyroid function and pregnancy: Study of Swedish and Sudanese women. Eur J Clin Nutr. 1998;52:351–355. doi: 10.1038/sj.ejcn.1600563. [DOI] [PubMed] [Google Scholar]
- 10.Sack J, Goldstein A, Charpak N, et al. Postpartum maternal hyperthyrotropinemia in an area in which iodine supplementation is required. Thyroid. 2003;13:959–964. doi: 10.1089/105072503322511364. [DOI] [PubMed] [Google Scholar]
- 11.Moleti M, Lo Presti VP, Mattina F, et al. Gestational thyroid function abnormalities in conditions of mild iodine deficiency: Early screening versus continuous monitoring of maternal thyroid status. Eur J Endocrinol. 2009;160:611–617. doi: 10.1530/EJE-08-0709. [DOI] [PubMed] [Google Scholar]
- 12.Moleti M, Lo Presti VP, Campolo MC, et al. Iodine prophylaxis using iodized salt and risk of maternal thyroid failure in conditions of mild iodine deficiency. J Clin Endocrinol Metab. 2008;93:2616–2621. doi: 10.1210/jc.2008-0352. [DOI] [PubMed] [Google Scholar]
- 13.Fritz KS, Wilcox RB, Nelson JC. Quantifying spurious free T4 results attributable to thyroxine-binding proteins in serum dialysates and ultrafiltrates. Clin Chem. 2007;53:985–988. doi: 10.1373/clinchem.2007.085316. [DOI] [PubMed] [Google Scholar]
- 14.d’Herbomez M, Forzy G, Gasser F, Massart C, Beaudonnet A, Sapin R. Clinical evaluation of nine free thyroxine assays: Persistent problems in particular populations. Clin Chem Lab Med. 2003;41:942–947. doi: 10.1515/CCLM.2003.143. [DOI] [PubMed] [Google Scholar]
- 15.Lee RH, Spencer CA, Mestman JH, et al. Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol. 2009;200:260.e1–e6. doi: 10.1016/j.ajog.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 16.Sapin R, D’Herbomez M, Schlienger JL. Free thyroxine measured with equilibrium dialysis and nine immunoassays decreases in late pregnancy. Clin Lab. 2004;50:581–584. [PubMed] [Google Scholar]
- 17.Sapin R, d’Herbomez M. Free thyroxine measured by equilibrium dialysis and nine immunoassays in sera with various serum thyroxine-binding capacities. Clin Chem. 2003;49:1531–1535. doi: 10.1373/49.9.1531. [DOI] [PubMed] [Google Scholar]
- 18.Roti E, Gardini E, Minelli R, Bianconi L, Flisi M. Thyroid function evaluation by different commercially available free thyroid hormone measurement kits in term pregnant women and their newborns. J Endocrinol Invest. 1991;14:1–9. doi: 10.1007/BF03350244. [DOI] [PubMed] [Google Scholar]
- 19.Klee GG, Post G. Effect of counting errors on immunoassay precision. Clin Chem. 1989;35:1362–1366. [PubMed] [Google Scholar]
- 20.Levinson SS. Antibody multispecificity in immunoassay interference. Clin Biochem. 1992;25:77–87. doi: 10.1016/0009-9120(92)80048-l. [DOI] [PubMed] [Google Scholar]
- 21.Ward G, McKinnon L, Badrick T, Hickman PE. Heterophilic antibodies remain a problem for the immunoassay laboratory. Am J Clin Pathol. 1997;108:417–421. doi: 10.1093/ajcp/108.4.417. [DOI] [PubMed] [Google Scholar]
- 22.Toft AD, Beckett GJ. Measuring serum thyrotropin and thyroid hormone and assessing thyroid hormone transport. In: Braverman LE, Utiger RD, editors. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text. Lippincott Williams and Wilkins; Philadelphia, PA: 2005. pp. 329–344. [Google Scholar]
- 23.Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: Effects on obstetrical complications. J Clin Endocrinol Metab. 2006;91:2587–2591. doi: 10.1210/jc.2005-1603. [DOI] [PubMed] [Google Scholar]
- 24.Jonklaas J, Kahric-Janicic N, Soldin OP, Soldin SJ. Correlations of free thyroid hormones measured by tandem mass spectrometry and immunoassay with thyroid-stimulating hormone across 4 patient populations. Clin Chem. 2009;55:1380–1388. doi: 10.1373/clinchem.2008.118752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahric-Janicic N, Soldin SJ, Soldin OP, West T, Gu J, Jonklaas J. Tandem mass spectrometry improves the accuracy of free thyroxine measurements during pregnancy. Thyroid. 2007;17:303–311. doi: 10.1089/thy.2006.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thienpont LM, Van Uytfanghe K, Beastall G, et al. IFCC Working Group on Standardization of Thyroid Function Tests Report of the IFCC Working Group for Standardization of Thyroid Function Tests, part 2: Free thyroxine and free triiodothyronine. Clin Chem. 2010;56:912–920. doi: 10.1373/clinchem.2009.140194. [DOI] [PubMed] [Google Scholar]
- 27.Yue B, Rockwood AL, Sandrock T, La’ulu SL, Kushnir MM, Meikle AW. Free thyroid hormones in serum by direct equilibrium dialysis and online solid-phase extraction--liquid chromatography/tandem mass spectrometry. Clin Chem. 2008;54:642–651. doi: 10.1373/clinchem.2007.098293. [DOI] [PubMed] [Google Scholar]
- 28.Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 29.Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:147–155. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 30.Pop VJ, Brouwers EP, Vader HL Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinemia during early pregnancy and subsequent child development: A 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–288. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 31.Vermiglio F, Lo Presti VP, Moleti M, et al. Attention deficit and hyperactivity disorders in the offspring of mothers exposed to mild-moderate iodine deficiency: A possible novel iodine deficiency disorder in developed countries. J Clin Endocrinol Metab. 2004;89:6054–6060. doi: 10.1210/jc.2004-0571. [DOI] [PubMed] [Google Scholar]
- 32.Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161–167. doi: 10.1542/peds.2005-0227. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol (Oxf) 2010;72:825–829. doi: 10.1111/j.1365-2265.2009.03743.x. [DOI] [PubMed] [Google Scholar]
- 34.Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: The Generation R study. J Clin Endocrinol Metab. 2010;95:4227–4234. doi: 10.1210/jc.2010-0415. [DOI] [PubMed] [Google Scholar]
- 35.Contempré B, Jauniaux E, Calvo R, Jurkovic D, Campbell S, de Escobar GM. Detection of thyroid hormones in human embryonic cavities during the first trimester of pregnancy. J Clin Endocrinol Metab. 1993;77:1719–1722. doi: 10.1210/jcem.77.6.8263162. [DOI] [PubMed] [Google Scholar]
- 36.Calvo RM, Jauniaux E, Gulbis B, et al. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab. 2002;87:1768–1777. doi: 10.1210/jcem.87.4.8434. [DOI] [PubMed] [Google Scholar]
- 37.Calvo RM, Obregón MJ, Ruiz de Oña C, Escobar del Rey F, Morreale de Escobar G. Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3′-triiodothyronine in the protection of the fetal brain. J Clin Invest. 1990;86:889–899. doi: 10.1172/JCI114790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obregon MJ, Escobar del Rey F, Morreale de Escobar G. The effects of iodine deficiency on thyroid hormone deiodination. Thyroid. 2005;15:917–929. doi: 10.1089/thy.2005.15.917. [DOI] [PubMed] [Google Scholar]
- 39.de Escobar GM, Obregón MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18:225–248. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Kester MH, Martinez de Mena R, Obregon MJ, et al. Iodothyronine levels in the human developing brain: Major regulatory roles of iodothyronine deiodinases in different areas. J Clin Endocrinol Metab. 2004;89:3117–3128. doi: 10.1210/jc.2003-031832. [DOI] [PubMed] [Google Scholar]
- 41.Lavado-Autric R, Ausó E, Garcia-Velasco JV, et al. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest. 2003;111:1073–1082. doi: 10.1172/JCI16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ausó E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145:4037–4047. doi: 10.1210/en.2004-0274. [DOI] [PubMed] [Google Scholar]
- 43.de Escobar GM, Obregón MJ, del Rey FE. Iodine deficiency and brain development in the first half of pregnancy. Public Health Nutr. 2007;10:1554–1570. doi: 10.1017/S1368980007360928. [DOI] [PubMed] [Google Scholar]
- 44.Morreale de Escobar G, Obregón MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J Clin Endocrinol Metab. 2000;85:3975–3987. doi: 10.1210/jcem.85.11.6961. [DOI] [PubMed] [Google Scholar]
- 45.Berbel P, Obregón MJ, Bernal J, Escobar del Rey F, Morreale de Escobar G. Iodine supplementation during pregnancy: A public health challenge. Trends Endocrinol Metab. 2007;18:338–343. doi: 10.1016/j.tem.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Berbel P, Mestre JL, Santamaría A, et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: The importance of early iodine supplementation. Thyroid. 2009;19:511–519. doi: 10.1089/thy.2008.0341. [DOI] [PubMed] [Google Scholar]
- 47.Velasco I, Carreira M, Santiago P, et al. Effect of iodine prophylaxis during pregnancy on neurocognitive development of children during the first two years of life. J Clin Endocrinol Metab. 2009;94:3234–3241. doi: 10.1210/jc.2008-2652. [DOI] [PubMed] [Google Scholar]
- 48.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Eng J Med. 2010;362:590–599. doi: 10.1056/NEJMoa0907355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Soldin OP, Pearce EN, Stagnaro-Green A. Dietary salt reductions and cardiovascular disease. N Engl J Med. 2010;362:2224. doi: 10.1056/NEJMc1003765. [DOI] [PubMed] [Google Scholar]