Abstract
Relapse of disease remains a major cause of mortality following allogeneic hematopoietic cell therapy (HCT). Over the past decade there has been a tremendous increase in our understanding of the biology underlying the graft-versus-tumor/leukemia (GVT) effect. However, there are several other factors which affect the occurrence and outcome of relapse including conditioning regimen, allograft, and the histology, state and chemotherapy-sensitivity of the disease being treated. The mainstay of relapse treatment is donor leukocyte infusion (DLI), but the efficacy of DLI is quite variable depending on disease histology and state. As such, there is a significant need for novel therapies and strategies for relapse following allogeneic HCT, particularly in patients for which DLI is not an option. The National Cancer Institute is sponsoring an international workshop to address issues and research questions relative to the biology, natural history, prevention and treatment of relapse following allogeneic HCT.
Keywords: allogeneic, relapse, graft-versus-tumor, DLI
INTRODUCTION
In his 1975 review of bone marrow transplantation in the New England Journal of Medicine, E. Donnall Thomas noted that the major barriers to the successful application of this modality were the availability of suitable donors, treatment-related toxicities, and relapse of disease (1). In the past 30 years there has been tremendous progress in addressing the need for donors for allogeneic hematopoietic cell transplantation (HCT) though the utilization of HLA-matched volunteer unrelated donors, haplo-identical related donors, and cord blood units (2). There has been significant improvement in supportive care measure with better agents to treat mucositis and marked increase and efficacy of antibiotics to treat bacterial, viral and fungal infections. There has been also been the introduction of nonmyeloablative and reduced-intensity conditioning regimens which have been associated with as much as 50% reduction in treatment-related mortality rates, as compared to myeloablative conditioning, when utilized in similar patient populations (3,4).
However, there has been very little progress in the reduction of in the incidence and subsequent outcomes of patients who experience relapse following allogeneic HCT, as it remains one of the most common causes of death following allogeneic HCT (Figure 1). This limited improvement has occurred despite a greater understanding of the biology underlying the graft-versus-tumor/leukemia (GVT) effect (5) and, more importantly, the introduction of donor lymphocyte infusion (DLI) as a therapeutic option for patients who experience disease progression or relapse after allogeneic HCT (6). Relative to the biology underlying the GVT effect, there is an awareness of major interactions between various lymphocytes (e.g. T regulatory cells, natural killer cells), antigen (e.g. WT1) and receptor (killer cell immunoglobulin receptors) expression, cytokines (e.g. interleukin [IL]-2, IL-7, IL-15, transforming growth factor β-1), and the tumor environment play in mediating the GVT effect (7). Despite this greater understanding we have not yet been able to translate these findings into significant improvements in outcomes except for a minority of patients (8). In regard to DLI, the disease for which it most effective, chronic myeloid leukemia (CML), utilizes allogeneic HCT only in the minority of patients who are resistant to tyrosine kinase inhibitors. In the vast majority of malignant diseases for which allogeneic HCT is utilized DLI are either ineffective or can provide long-term disease-free survival for only a minority of patients (9). These results are even more disappointing when placed in the context that the relapse risk is significantly higher in individuals who undergo allogeneic HCT following nonmyeloablative and reduced-intensity conditioning.
Figure 1.
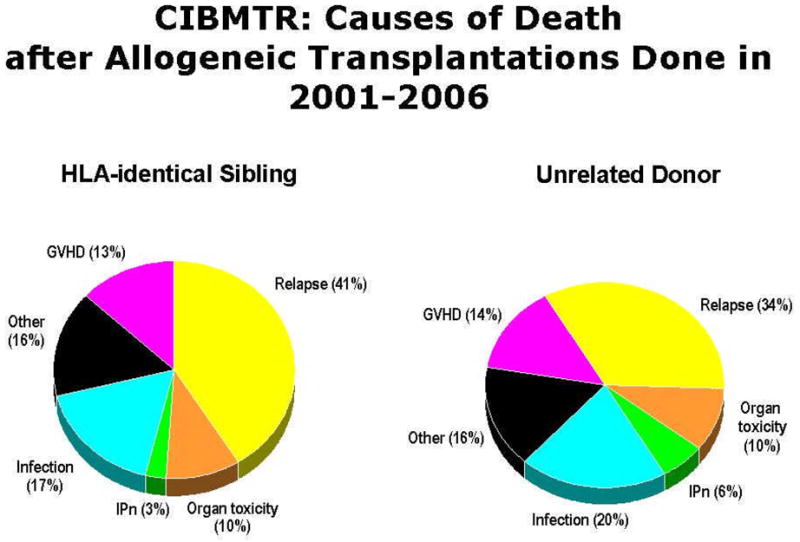
Causes of death following allogeneic hematopoietic cell transplantation as reported to the Center for International Blood and Marrow Transplant Research
The majority of the available medical literature on relapse following allogeneic HCT focuses on treatment, particularly on immunotherapeutic approaches such as withdrawal of immune suppression and DLI. However, an important clinical question is what to do with patients with active graft-versus-host disease (GVHD) for whom DLI is relatively contraindicated. There are relatively few reports on non-immunological treatments despite the fact chemotherapy-sensitivity is commonly cited as the most important prognostic factor associated with relapse. Other important factors impacting relapse include disease, disease state, stem cell source, graft manipulation, immunosuppression, and, as previously mentioned, conditioning regimen. There is a paucity of data on the epidemiology, prevention, and monitoring for relapse of various diseases following allogeneic HCT. Unfortunately, the disease, for which there are the most data, is CML. However, this can serve as a model to study relapses in other diseases. This brief reviews attempt provide an overview on our current understanding of the GVT effect, current treatments approaches, and an attempt to organize the transplant community to address the major laboratory and clinical questions related to relapse following allogeneic HCT.
T CELLS IN THE GRAFT-VERSUS-TUMOR RESPONSE
Although the conditioning regimen remains an important contributor to the anti-tumor potential of allogeneic HCT, the emphasis in the last decades has been on the therapeutic effect of GVT activity. Extensive analysis both in human and animal studies has shown that GVT activity is primarily mediated by T and NK cells, although other cells can contribute through direct or indirect mechanisms. In some cases donor T cells can recognize a tumor-specific (e.g. Bcr/Abl in CML) or a minor histocompatibility antigen, such as a polymorphic antigen with restricted expression on hematopoietic cells (e.g. HA-1/2). However, the major contributors to the GVT activity of donor T cells are likely alloreactive T cells recognizing allo-antigens on tumor cells and normal tissue cells in the recipient. Clinical studies support this notion and have demonstrated an inverse correlation between GVHD, especially chronic GVHD, and post-transplant relapse risk. Therefore, strategies to enhance GVT activity could result in worsening of GVHD, and novel approaches to improve GVT must be evaluated for this potential complication. Here, we briefly review selected approaches for enhancing GVT without exacerbating GVHD. Recent reviews on strategies to promote GVT without exacerbating GVHD are shown in Table 1 (10–15).
Table 1.
T cell approaches to enhance GVT without exacerbating GVHD
Post-transplant environment
Recent studies have resulted in a new appreciation of the post-transplant setting as a unique environment that seems to be conducive to T cell reactivity against tumor cells as well as vaccines aimed at enhanced T cell responses. Recently, investigators studying optimal conditions for cancer immunotherapy “reinvented” myeloablative conditioning followed by autologous HCT as an ideal setting for adoptive T cell therapies (16). Post-transplant infusion of T cells can produce robust T-cell expansion and vaccines and immune-modulating antibodies, also appear to have augmented efficacy in the setting of decreased lymphocytes. Possible mechanisms include increased access to antigen-presenting cells (and MHC/antigen), increased access to cytokines (IL-2, IL-7, IL-15, IL-21), decreased suppressor cell populations, lymphopenia-induced homeostatic proliferation of naïve T cells develop and radiation-induced upregulation of trafficking/adhesion molecules, costimulatory molecules, and activation of dendritic cells (17,18).
T cell reconstitution
Allogeneic HCT patients experience prolonged post-transplant deficiencies in T cell numbers and function, which is associated with increased risks for malignant relapse, development of secondary malignancies, and suboptimal responses to immunotherapeutic strategies such as anti-tumor vaccination. Currently, the most promising approaches to enhance post-transplant T cell reconstitution include cytokines and growth factors, including growth hormone, IGF-I, ghrelin, sex steroid ablation with leuprolide, keratinocyte growth factors, IL-7, IL-12 and IL-15. All these agents have shown promise in animal models and most of them are currently in early clinical trials (19).
T Cell Cytolysis
Cytotoxic T cells execute their function via use of the perforin/granzyme system and death receptor ligands (FasL, tumor necrosis factor (TNF)-related apoptosis-inducing ligand [TRAIL], TNF-like weak inducer of apoptosis [TWEAK]), which trigger the target cell’s own apoptotic pathways (11). Multiple murine models have demonstrated differential use of these cytolytic pathways during GVT and target organ GVHD. For example, FasL is important for liver GVHD, whereas TNF has a critical role in intestinal GVHD. Depending on the tumor model used, each of these pathways can be involved in GVT activity, although GVT activity by TWEAK has not been studied to date. Overexpression of TRAIL in T cells seems to be able to enhance GVT activity against certain malignancies, although TRAIL has been recently implicated in thymic GVHD (MRM van den Brink: unpublished observations).
T Cell Trafficking
Studies in mouse models have demonstrated roles for individual selectins, integrins, and chemokines/chemokine receptors in the pathogenesis of GVHD (20). For example, donor T cells deficient for CCR2 or β7 integrin have decreased capability to home to the liver and GI tract resulting in decreased GVHD, but intact GVT responses. Natalizumab is a humanized antibody to the α4 subunit of certain integrin heterodimers, including α4β7, which is associated with homing to the intestines. Natalizumab has been tested for use in inflammatory bowel disease and multiple sclerosis and could potentially useful for inhibition of migration to GVHD target tissues while still permitting activation of GVT effectors in lymphoid tissue. However, further studies with this drug are being complicated by controversy regarding the increased risk of progressive multifocal leukoencephalopathy observed in patients treated with natalizumab.
Regulatory T cells
A number of investigators have demonstrated in mouse models that regulatory T cells (Tregs) of donor or host origin can inhibit GVHD. CD4+CD25+ Tregs suppress the early expansion of alloreactive donor T cells, and have been reported to limit their expression of interleukin-2-receptor (IL-2R) alpha-chain and their capacity to induce GVHD without abrogating GVT effector function, mediated primarily by the perforin lysis pathway. Thus, at least in mouse models, donor Tregs can separate GVHD from GVT activity. Several clinical studies with Tregs administered to patients receiving an allogeneic HCT are currently underway.
Effector Memory T cells
Upon encounter of their cognate antigen in the context of appropriate costimulatory signals naïve CD8+ T cells will become activated and can develop into effector (TEM) and central memory (TCM) CD8+ T cells. Similar differentiation patterns have been proposed for CD4+ T cells. TCM cells are long-lived and express CD62L and CCR7 in contrast to CD8+ TEM cells. Studies in mouse models have shown that selected CD4+ or CD8+ donor TEM (as opposed to naive) T cells cause less GVHD, but can still exert GVT activity. Several investigators are currently planning clinical trials in allogeneic HCT recipients with selected donor TEM cells.
Adoptive cell therapy and vaccines
Beginning with DLI, the potential of adoptive T cell therapy has been widely recognized as a way to enhance GVT and prevent or treat malignant relapse. Many strategies have been developed that have focused on the ex vivo expansion of donor T cells which can recognize one or more antigens on tumor cells. These cells can be modified with suicide genes (to halt the development of GVHD), specific T cell receptors (TCR) resulting in T cells with dual TCRs, chimeric antigen receptors (which use the antigen binding portion of an antibody in combination with the TCRζ chain for activation), undergo ex vivo polarization towards Th1 or Th17, and a variety of other strategies. Several of these approaches are currently in clinical trials as upfront or delayed adoptive T cell therapy in allogeneic HCT patients.
This brief review could only touch on a few of the many exciting strategies that are being developed to enhance T cell-mediated GVT. Many problems still remain to be solved ranging from feasibility to financial costs to scientific issues. For example, the Achilles heel of T cells is their requirement of antigen recognition when many tumor cells through genetic instability and a variety of other mechanisms can downregulate many potential antigens and avoid elimination by T cells. Therefore, an important question regarding any potential tumor target antigen is whether its expression is indispensable for the survival of the tumor cells. Alternatively, GVT activity of T cells could be directed against non-cancer cells in the tumor stroma, such as the tumor vasculature myeloid-derived suppressor cells. However, as our understanding of T cell biology continues to grow it is expected that new approaches will emerge to optimize GVT by T cells, which remain the most important mediators of GVT.
STRATEGIES AND OPTIONS FOR RECURRENT DISEASE FOLLOWING ALLOGENEIC STEM CELL TRANSPLANTATION
Treatment options for most patients who relapse after allogeneic HCT are limited and prognosis is generally poor, with the exception of CML. In general, the greatest potential for successful treatment of relapse is manipulation or enhancement of donor cells as GVT induction. Hence the most common intervention for relapse is DLI. In some cases, supportive and palliative care may be the most appropriate option. Disease specific chemotherapy or radiation can be considered in some settings but with poor long term survival (21). In some cases, cytokines to activate T, NK or dendritic cells, have resulted in sustained remissions after relapse. Second HCT may be curative, but is associated with extensive morbidity and mortality. Ultimately, newer strategies are needed to maximize efficacy and limit toxicity for treatment of relapse after allogeneic HCT.
Withdrawal of Immunosuppression
To maximize the GVT activity, withdrawal of immunosuppression is often the first intervention for relapse. While there are numerous anecdotal successes, this approach, by itself, is rarely effective in patients with diseases other than CML.
Second Allogeneic SCT
Historically, the role for second allogeneic HCT has been limited by unacceptable relapse rates and high mortality. Treatment-related mortality (TRM) is between 25–45% after myeloablative second transplant and varies between 0–30% after non-myeloablative second transplant, depending on prior therapies, age and time from first transplant. Relapse rates are disease dependent but few studies report relapse rates less than 40%. Interestingly, despite this high risk, survival rates after second myeloablative transplant for acute leukemia are between 25 to 40%, though clearly this represents highly selected patients. Available data do not support a benefit with a second donor, and generally show improved outcomes for younger patients and a longer time (>6–12 months) from transplant to relapse. Data (Figure 2) from the Center for International Blood and Marrow Transplant Research (CIBMTR) showed that the survival for patients less than 20 years old who relapse more than 6 months from transplant was 51% at 5 years and only 3% for older patients who relapsed within 6 months (22). The European Group for Blood and Marrow Transplantation (EBMT) reported the best outcomes for patients with late relapse (>292 days) in remission at time of second transplant with a 53% survival at 3 years (23).
Figure 2. Second Allogeneic Hematopoietic Cell Transplantation as reported to the Center for International Blood and Marrow Transplant Research (22).
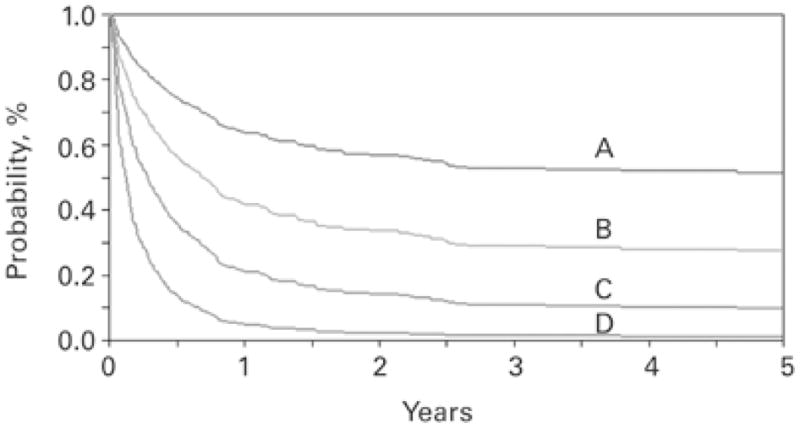
Probability of overall survival after second transplantation. (A) Age ≤20 years, duration of remission >6 months; (B) age >20 years, duration of remission >6 months; (C) age ≤20 years, duration of remission ≤6 months; and (D) age >20 years, duration of remission ≤6 months 2.
Reduced intensity conditioning (RIC) for the second allogeneic transplant is expected to minimize TRM but relapse rates are high. Nevertheless, long-term survival rates between 20 and 60% are reported. Outcomes are dependent on many factors including the intensity of the first and second conditioning regimen, time to relapse, underlying disease, and disease status at transplant. Whether a RIC regimen improves outcome compared to conventional conditioning for a second allogeneic transplant is unknown.
Disease-specific Treatments
Relapsed CML
DLI for relapsed CML is dramatically effective and induces complete molecular remissions in up to 80% of patients who relapse in chronic phase (24). These remissions are sustained in the majority of patients though late relapses raise concern that GVT effects might have a limited life span or that the primitive leukemic stem cell is not eradicated.
Imatinib may be an effective alternative to DLI for relapsed CML without the risk of GVHD. Limited data suggest up to 70% of patients achieve a complete molecular remission. It appears that continued therapy is necessary to prevent progression (25). While combined therapy has not been prospectively studied, the combination of imatinib or other tyrosine kinase inhibitors (TKI) with DLI has the advantage of rapid reduction in leukemia burden and disease control until an effective immune response can develop. Moreover, it is possible that lower T cell doses would be necessary, thus reducing the risk of GVHD. Unfortunately, DLI is less effective for patients with accelerated and blast phase CML. Only 12–28% of these patients achieve remission, and many responses are transient.
When DLI and TKI therapy are not options or are ineffective, interferon-a may be a useful alternative (26). Vaccine strategies also hold particular promise for relapse of indolent diseases like CML (27).
Acute Leukemias
For relapsed acute leukemias, both conventional chemotherapy and newer biological agents result in significant remission rates but poor long term survival. The use of novel agents (e.g. dasatinib or newer TKIs) in patients with Ph+ acute lymphoid leukemia (ALL) or 5-azacytidine for relapsed acute myeloid leukemia (AML) may have particular benefit. For patients with relapsed ALL, outcomes are particularly poor after DLI with response rates between 0–20% and overall survival rates less than 15% (9). Outcomes after DLI for relapsed AML are more variable. Response rates occur in 15–30% of patients but remission duration is generally short and long term survival is only approximately 20%. The EBMT studied outcomes in almost 400 patients with relapsed AML (28). For the 171 recipients of DLI, outcomes were improved if DLI was given to patients who achieved remission by other means. In a good risk population of patients in remission with a favorable karyotype, the 2 year overall survival (OS) was 56%; this is in contrast to patients who received DLI with active disease or during aplasia who had an OS of 9–20% (overall 15%) depending on other risk factors. Nevertheless, patients treated with DLI appear to have better outcomes than patients who never receive DLI with overall survival of 21 versus 9% at 2 years. Furthermore, patients who relapse later after allogeneic HCT and receive DLI have improved outcomes compared to patients who relapse early (29).
Immunotherapy often fails because rapid leukemia cell growth may outpace the cytotoxicity of donor leukocytes. When patients with acute leukemia are given chemotherapy prior to DLI (c-DLI), complete remissions (CRs) are more common. In one study, CRs were reported in 47% of patients (29). Although overall survival at 2 years was 19%, patients who recovered from c-DLI in CR had 1- and 2-year survival rates of 51% and 41%, respectively, compared to a 1-year survival of 5% in non-responders. Survival at 1 year for patients with relapse occurring less than 6 months after transplant was 10% compared to 44% for patients who relapsed more than 6 months after transplant (p<0.001).
DLI for Myeloma, Hodgkin’s Disease, and Non-Hodgkin’s Lymphoma
Response rates and remission duration following DLI in other diseases are less well defined. It is clear that a “graft-versus-myeloma” effect from DLI can induce remissions in some patients who relapse after allogeneic HCT, but relapse rates are high and long term outcomes poor (30). The use of newer biological therapies for myeloma will expand treatment options for relapsed disease. There is relatively limited data on outcomes after DLI for relapsed non-Hodgkin’s lymphoma (NHL) and Hodgkin’s disease (HD); however, experience demonstrates that a meaningful graft-versus-lymphoma reaction can be generated after DLI for NHL and HD (9,31,32).
Relapse after Reduced Intensity Transplantation
As previously described, RIC is associated with higher relapse rates, as compared to myeloablative conditioning prior to allogeneic HCT. While mortality is quite high in patients who relapse after RIC transplant, patients given therapy seem to have better outcomes than patients who receive no intervention (33). Response rates to DLI after RIC allogeneic HCT seem similar to those after conventional SCT.
New Approaches to Relapse Treatment
Despite the achievements with DLI, high response rates are largely limited to CML, and are tempered by significant GVHD and other toxicity. Innovative and novel immunotherapeutic approaches are being studied (Table 2). Among other approaches, non-specific ex-vivo activation and expansion through co-stimulation of donor T cells have been used safely with intriguing GVT responses (34). It may also be possible to generate leukemia specific cytotoxic T cells to use for adoptive immunotherapy (35). Vaccine strategies with tumor specific antigens or modified tumor cells are other promising approaches to generate tumor-specific immunity (27, 36). These strategies are likely to be most effective in the setting of minimal disease. Combining DLI with antibody therapy that may direct effector cells directly to tumor cells may overcome possible resistance mechanisms to GVT induction without excessive toxicity.
Table 2.
Newer Approaches to Cellular Therapies to Treat Relapse
|
Since DLI seems to be most effective for patients with minimal disease, the role of prophylactic DLI for patients in remission needs to be better defined. If donor T cells become tolerant or possibly rapidly senescent after HCT as a mechanism leading to relapse, then the use of repetitive DLI, once patients achieve remission, may be useful (37). In addition, the role of other cell populations (such as NK and dendritic cells) in GVT induction for relapse needs to be explored in further detail. Ultimately, understanding the biology of relapse and mechanisms involved in GVT induction will permit more effective and patient specific approaches for relapsed disease.
FIRST INTERNATIONAL WORKSHOP ON THE BIOLOGY, PREVENTION, AND TREATMENT OF RELAPSE AFTER ALLOGENEIC HEMATOPOIETIC STEM CELL TRANSPLANTATION
To address the problem of relapse following allogeneic HCT, the National Cancer Institute convened a workshop on November 2 and 3, 2009 in Bethesda, Maryland, USA. The planning of this event began in 2008 with the primary objectives of: 1) reviewing the current “state-of-the-science” relative to the biology, natural history, prevention and treatment of relapse following allogeneic HCT, 2) identifying the most important questions and problems that need to be answered relative to the biology, prevention and treatment of relapse following allogeneic HCT over the next 5 years, 3) provide specific recommendations as to what studies and resources are needed in order to answer these questions and provide for the deficits relative to the research related to relapse following allogeneic HCT, and 4) provide a forum for interested researchers to interact and form networks of interest. An international group of more than 60 basic and clinical researchers was assembled and assigned to specific committees addressing the biology, strategies and therapies for prevention, disease-specific methods and strategies for monitoring, and disease-specific treatment of relapse following allogeneic HCT. Each committee generated a list of research priorities, and a summary of their recommendations were presented for open discussion at the workshop, and the final recommendations will be published sequentially in the Biology of Blood and Marrow Transplantation. At the end of the two day workshop a summary report of all the working committees as an executive summary were developed for subsequent publication. A summary of the workshop recommendations will be presented during the 2010 Tandem Transplant Meetings Educational Sessions.
Acknowledgments
This work was supported in part by the Center for Cancer Research, National Cancer Institute, Intramural Research Program.
References
- 1.Thomas ED, Storb R, Clift RA, et al. Bone-marrow transplantation. N Engl J Med. 1975;292:832–843. 292, 895–902. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 2.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 3.Diaconescu R, Flowers CR, Storer B, et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104:1550–1558. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 4.Aoudjhane M, Labopin M, Gorin NC, et al. Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19:2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 5.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 6.Porter DL, Roth MS, McGarigle C, Ferrara JL, Antin JH. Induction of graft-versus-host disease as immunotherapy for relapsed chronic myeloid leukemia. N Engl J Med. 1994;330:100–106. doi: 10.1056/NEJM199401133300204. [DOI] [PubMed] [Google Scholar]
- 7.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 8.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 9.Tomblyn M, Lazarus HM. Donor lymphocyte infusions: the long and winding road: how should it be traveled? Bone Marrow Transplant. 2008;42:569–579. doi: 10.1038/bmt.2008.259. [DOI] [PubMed] [Google Scholar]
- 10.van den Brink MR, Alpdogan O, Boyd RL. Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev Immunol. 2004;4:856–867. doi: 10.1038/nri1484. [DOI] [PubMed] [Google Scholar]
- 11.van den Brink MRM, Burakoff SJ. Cytolytic pathways in haematopoietic stem-cell transplantation. Nature Reviews in Immunology. 2002;2:273–281. doi: 10.1038/nri775. [DOI] [PubMed] [Google Scholar]
- 12.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson BE, Zheng H, Taylor PA, et al. Memory T cells in GVHD and GVL. Biol Blood Marrow Transplant. 2008;14:19–20. [PubMed] [Google Scholar]
- 15.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudley ME, Yang JC, Sherry R, et al. Adoptive Cell Therapy for Patients With Metastatic Melanoma: Evaluation of Intensive Myeloablative Chemoradiation Preparative Regimens. J Clin Oncol. 2008;26:5233–5339. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eyrich M, Burger G, Marquardt K, et al. Sequential expression of adhesion and costimulatory molecules in graft-versus-host disease target organs after murine bone marrow transplantation across minor histocompatibility antigen barriers. Biol Blood Marrow Transplant. 2005;11:371–382. doi: 10.1016/j.bbmt.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Louboutin JP, Zhu J, Rivera AJ, Emerson SG. Preterminal host dendritic cells in irradiated mice prime CD8+ T cell-mediated acute graft-versus-host disease. J Clin Invest. 2002;109:1335–1344. doi: 10.1172/JCI14989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland AM, van den Brink MR. Rejuvenation of the aging T cell compartment. Curr Opin Immunol. 2009;21:454–459. doi: 10.1016/j.coi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw BE, Russell NH. Treatment options for the management of acute leukaemia relapsing following an allogeneic transplant. Bone Marrow Transplant. 2008;41:495–503. doi: 10.1038/sj.bmt.1705888. [DOI] [PubMed] [Google Scholar]
- 22.Eapen M, Giralt SA, Horowitz MM, et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant. 2004;34:721–727. doi: 10.1038/sj.bmt.1704645. [DOI] [PubMed] [Google Scholar]
- 23.Bosi A, Laszlo D, Labopin M, et al. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2001;19:3675–3684. doi: 10.1200/JCO.2001.19.16.3675. [DOI] [PubMed] [Google Scholar]
- 24.Collins R, Shpilberg O, Drobyski W, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 25.Hess G, Bunjes D, Siegert W, et al. Sustained complete molecular remissions after treatment with imatinib-mesylate in patients with failure after allogeneic stem cell transplantation for chronic myelogenous leukemia: results of a prospective phase II open-label multicenter study. J Clin Oncol. 2005;23:7583–7593. doi: 10.1200/JCO.2005.01.3110. [DOI] [PubMed] [Google Scholar]
- 26.Higano CS, Chielens D, Raskind W, et al. Use of alpha-2a-interferon to treat cytogenetic relapse of chronic myeloid leukemia after marrow transplantation. Blood. 1997;90:2549–2554. [PubMed] [Google Scholar]
- 27.Rezvani K, Yong AS, Mielke S, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid C, Labopin M, Nagler A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25:4938–4945. doi: 10.1200/JCO.2007.11.6053. [DOI] [PubMed] [Google Scholar]
- 29.Levine J, Braun T, Penza S, et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem cell transplantation. J Clin Oncol. 2002;20:405–412. doi: 10.1200/JCO.2002.20.2.405. [DOI] [PubMed] [Google Scholar]
- 30.Tricot G, Vesole D, Jagannath S, et al. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87:1196–1198. [PubMed] [Google Scholar]
- 31.Peggs KS, Hunter A, Chopra R, et al. Clinical evidence of a graft-versus-Hodgkin’s-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365:1934–1941. doi: 10.1016/S0140-6736(05)66659-7. [DOI] [PubMed] [Google Scholar]
- 32.Bloor AJ, Thomson K, Chowdhry N, et al. High response rate to donor lymphocyte infusion after allogeneic stem cell transplantation for indolent non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14:50–58. doi: 10.1016/j.bbmt.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Bethge WA, Storer BE, Maris MB, et al. Relapse or progression after hematopoietic cell transplantation using nonmyeloablative conditioning: effect of interventions on outcome. Exp Hematol. 2003;31:974–980. doi: 10.1016/s0301-472x(03)00225-x. [DOI] [PubMed] [Google Scholar]
- 34.Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107:1325–1331. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 35.Falkenburg JH, Wafelman AR, Joosten P, et al. Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood. 1999;94:1201–1208. [PubMed] [Google Scholar]
- 36.Ho V, Vanneman M, Kim H, et al. Biologic Activity of Irradiated, Autologous, GM-CSF Secreting Leukemia Cell Vaccines Early After Allogeneic Stem Cell Transplantation. Proc Natl Acad Sci U S A. 2009 Aug 26; doi: 10.1073/pnas.0908358106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beatty G, Smith J, Reshef R, et al. Functional unresponsiveness and replicative senescence of myeloid leukemia antigen-specific CD8+ T cells after allogeneic stem cell transplantation. Clin Cancer Res. 2009;15:4944–4953. doi: 10.1158/1078-0432.CCR-08-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]


