Abstract
Auditory hair cells transduce sound vibrations into membrane potential changes, ultimately leading to changes in neuronal firing and sound perception. This review provides an overview of the characteristics and repair capabilities of traumatized auditory sensory epithelium in the adult vertebrate ear. Injured mammalian auditory epithelium repairs itself by forming permanent scars but is unable to regenerate replacement hair cells. In contrast, injured non-mammalian vertebrate ear generates replacement hair cells to restore hearing functions. Non-sensory support cells within the auditory epithelium play key roles in the repair processes.
Keywords: Support cell, hair cell regeneration, auditory, cochlea, repair
1. Introduction
A complete survey of the adult cochlear sensory epithelium after trauma would exhaust the space allotted for this review. It has been necessary, therefore, to be selective in the choice of topics. The point of reference is the support cells of adult vertebrate cochleae, primarily those of the organ of Corti, as these cells are potentially critical for repairing damaged human auditory epithelium. The focus is on experimental findings and hypotheses generated within the last 10–15 years.
The auditory sensory epithelia of all vertebrate ears (e.g., amphibian papilla, basilar papilla, organ of Corti) are comprised of hair cells, support cells, and neural endings connecting the hair cells to the brain (Fig. 1). Readers are referred to comprehensive reviews for detail (Smith and Takasaka, 1971; Wever, 1978, 1985; Smith, 1981; Gleich and Manley, 2000; Raphael and Altschuler, 2003; Köppl, 2011; Manley, 2011; Warchol, 2011; Van Dijk et al., 2011). The sensory hair cells typically form a relatively small percentage of the total population of the cells present in vertebrate auditory epithelia. When hair cells die, neighboring support cells close the lesion that is created. In non-mammalian vertebrate auditory epithelium, the support cells also go on to generate replacement hair cells via two mechanisms: 1) support cells re-enter the cell division cycle, divide, and the daughter cells subsequently differentiate into hair and/or support cells (Corwin and Cotanche, 1988; Ryals and Rubel, 1988), and 2) support cells convert into hair cells without an intervening cell division, a process called “direct transdifferentiation” (Adler and Raphael, 1996; Baird et al., 1996; Roberson et al., 1996, 2004). In mammals, there is no hair cell regeneration in the mature organ of Corti. Instead, support cells form permanent scars and repair the epithelium, and/or the sensory epithelium is replaced by an unspecialized epithelium (reviewed in Raphael et al., 2007). A critical question is why robust hair cell regeneration occurs in damaged, non-mammalian, vertebrate, auditory sensory epithelium but not in the mature organ of Corti.
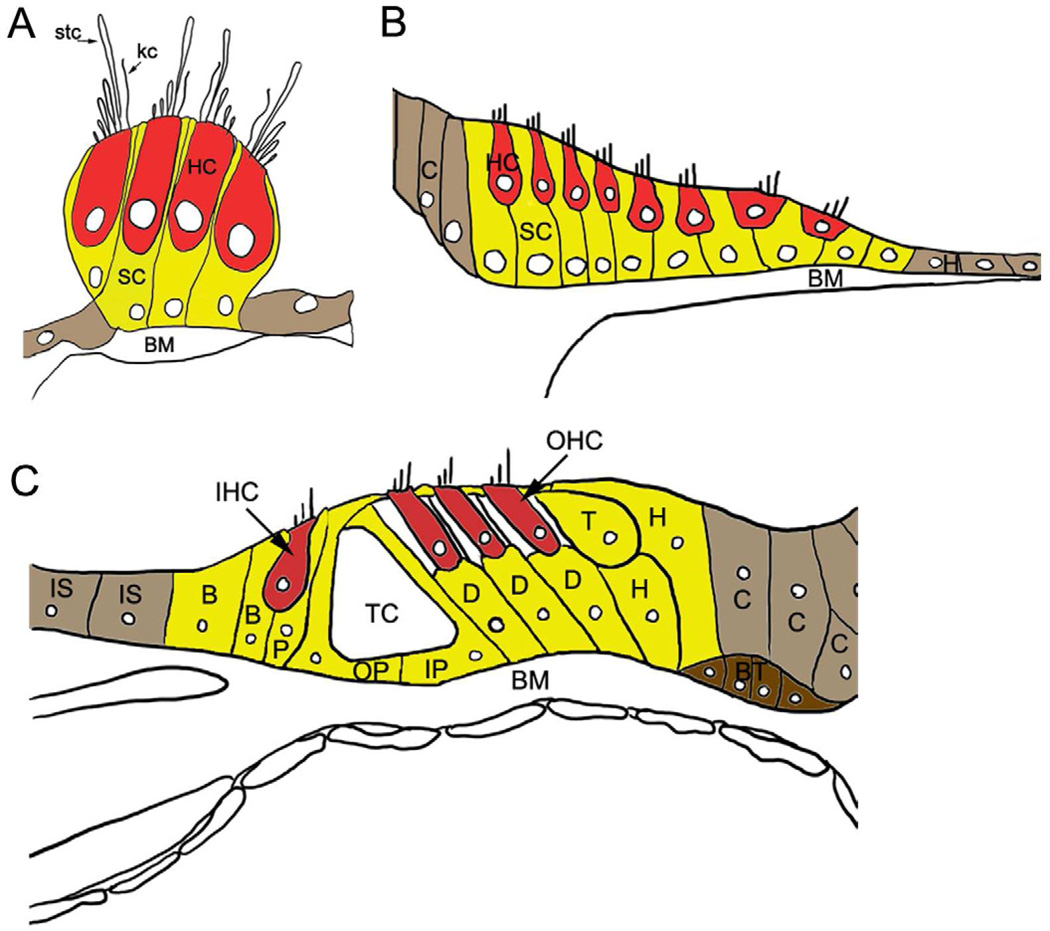
Figure 1. Schematic diagrams of auditory hair cell epithelia in adult vertebrate inner ear.
(A) Reptilian (alligator lizard) basilar papilla. (B) Bird basilar papilla. (C) Mammalian organ of Corti. All vertebrate auditory epithelia contain mechanosensitive sensory receptor cells, hair cells (red, HC), and support cells (yellow, SC). Hair cells are distributed along the lumenal surface of the epithelium; their cell bodies do not make contact with the basilar membrane (BM). A bundle of hair-like stereocilia (stc) protruding from the apical surface of the hair cell extends into the lumen. In the reptilian basilar papilla (A), a single long cilium called the kinocilium (kc) is present in the mature bundle. Support cells (with the exception of the tectal cells in the organ of Corti) extend the entire depth of the epithelium, from the lumen to the basilar membrane, and their nuclei reside primarily within the basal half of the epithelium. (B) The bird basilar papilla containing tall and short hair cells (HC) is flanked by non-sensory clear cells (C) and hyaline cells (H). (C) The mammalian organ of Corti is a highly patterned, complex tissue that contains a single row of inner hair cells (IHC), 3 rows of outer hair cells (OHC), and support cells with a variety of specialized morphologies. In contrast to non-mammalian vertebrates, support cells in the organ of Corti are structurally and functionally diversified and are subtyped as Hensen’s (H), Tectal (T), Deiters’ (D), inner pillar (IP), outer pillar (OP), inner phalangeal (P), and border (B) cells. Non-sensory inner sulcus cells (IS), Boettcher’s cells (BT), and Claudius cells (C) border the sensory epithelium. Inner phalangeal and border cells are closely associated with inner hair cells, and inner pillar and Deiters’ cells are associated with outer hair cells. Pillar and Deiters’ cells provide rigidity and structure to the epithelium. The tunnel of Corti (TC) and support cell specializations may be adaptations necessary for higher frequency hearing (Dallos and Harris, 1978; Hudspeth, 1985). Excellent descriptions of the normal architecture of the organ of Corti, including descriptions of support cell subtypes, can be found in Spicer and Schulte (1994), Slepecky (1996), and Taylor et al. (2012). Nerve fibers and endings are not depicted in the figure.
2. Damaging mature auditory sensory epithelium
Mature vertebrate auditory epithelium is vulnerable to a variety of damaging insults, including intense sounds, exposure to aminoglycosides or certain anti-cancer drugs (e.g., cisplatin), hereditary genetic changes, and aging. These insults can induce varying degrees of destruction to the epithelium that range from the loss of a few hair cells to total destruction, where the normal epithelium is replaced by an unspecialized epithelium (i.e., a single layer of unidentified cuboid epithelial cells) with no patterned organization that has been termed a “monolayer” or “flat” epithelium (Kim and Raphael, 2007; Raphael et al., 2007).
Auditory hair cells are directly vulnerable to the insults listed above, whereas support cells and spiral ganglion cells, bipolar neurons cells that innervate the hair cells and send their axons into the central nervous system (CNS), are thought to have secondary sensitivity to the insults. Support cell and spiral ganglion cell death are thought to occur as a result of primary hair cell loss. However, recent findings in mature vertebrate auditory epithelium suggest support cells and spiral ganglion cells have primary sensitivity in some instances. Fluorescently tagged aminoglycoside uptake studies have shown rapid aminoglycoside uptake in organ of Corti support cells (Dai et al., 2006; Wang and Steyger 2009), corroborating earlier immunohistochemical, ultrastructural, and autoradiographical studies (e.g., von Ilberg et al., 1971; de Groot et al., 1990; Imamura and Adams, 2003). In aminoglycoside-damaged guinea pigs, degeneration to a flat epithelium can occur rapidly, within 1 week (Izumikawa et al., 2008), and it is possible that the rapid degeneration is due to primary support cell damage. Primary spiral ganglion loss (ganglion cell loss in the absence of hair cell loss) has been reported after noise overexposure (Kujawa and Liberman, 2009), aminoglycoside insult (Hinojosa et al., 2001; Teufert et al., 2006), and aging (Makary et al., 2011). Cisplatin is also though to target spiral ganglion cells directly (van Ruijven et al., 2004, 2005). In sum, these findings suggest that overexposure to sound, aminoglycosides, or certain anti-cancer drugs can injure support cells and spiral ganglion cells directly, in addition to hair cells.
3. Repair capacities of traumatized vertebrate auditory epithelium
3.1. Regenerative processes in non-mammalian vertebrate auditory epithelia
Traumatized non-mammalian vertebrate inner ear epithelium quickly repairs itself and the mechanisms of the regenerative process have been covered in numerous recent reviews (e.g., Stone and Cotanche, 2007; Oesterle and Stone, 2008; Collado et al., 2008; Edge and Chen, 2008; Brignull et al., 2009; Cotanche and Kaiser, 2010, Groves, 2010; Warchol 2010; Ronaghi et al., 2012), so only a brief summary is necessary here. Hair cell death triggers regenerative processes in support cells close to the dying hair cell. Prior to the extrusion of dying hair cells from the damaged epithelium, nearby support cells begin to transdifferentiate directly into hair cells in the absence of any cell division (Roberson et al., 2004; Duncan et al., 2006; Cafaro et al., 2007). Some support cells begin to re-enter the cell division cycle 16 hours after hair cell injury (Warchol and Corwin, 1996), and replacement hair cells begin appearing within 3 days of the initial insult (Corwin and Cotanche, 1988; Ryals and Rubel, 1988; Janas et al., 1995; Duncan et al., 2006). It is not clear whether the capacity to divide and differentiate into hair cells is a property shared by all support cells, or whether there is a smaller subpopulation with stem cell-like characteristics or other properties that favor regeneration. It is estimated that only 15% of the support cells in the mature bird auditory epithelium can enter the cell division cycle after damage (Roberson et al., 1996), and fewer than fewer than 5% appear to divide more than once during regeneration (Stone et al., 1999).
Dying hair cells in chicken auditory epithelium are quickly extruded from the lumenal surface of the epithelium (Cotanche, 1987; Hirose et al., 2004: Duncan et al., 2006), in contrast to vertebrate vestibular epithelium where apoptotic vestibular hair cells are generally phagocytosed by adjacent support cells (Forge and Li, 2000; Bird et al., 2010). Recent findings suggest that macrophages are not essential for removal of dying hair cells (Warchol and Hirose, 2012).
In cases of extremely severe damage, where both hair cells and support cells are damaged, the non-mammalian vertebrate auditory epithelium is unable to regenerate replacement hair cells. In chicken auditory epithelium exposed to intense sound, nearby non-sensory hyaline cells divide (Girod et al., 1989) and migrate into the sensory epithelium to permanently cover the surface of the basilar membrane in the damaged region, possibly serving to maintain the epithelial barrier between endolymph and perilymph fluids and the biomechanical components of the basilar membrane complex (Cotanche et al., 1995). Regenerated hair cells are not seen in chicken auditory epithelium exposed to cisplatin, a chemotherapeutic agent with antimitotic properties that is widely used in the treatment of solid tumors. Cisplatin treatment causes both hair cell and support cell death, blocks support cell proliferation, and prevents support cell transdifferentiation into hair cells (Slattery and Warchol, 2010). In sum, these studies show that damaging the support cells decreases their ability to repopulate the epithelium with replacement hair cells, and epithelial cells from areas flanking the sensory epithelium can migrate in and contribute to the maintenance of the epithelial layer.
Hair cell genesis in the repairing non-mammalian ear occurs in an environment where the hair cell precursors coexist with differentiated functioning hair cells, in contrast to developing auditory epithelium. However, signaling regulators (e.g., Notch) and transcription factors (e.g., Atoh1) that were used to regulate the production of hair and support cells during embryonic development appear to be re-employed during hair cell regeneration, and the process of lateral inhibition appears to function in much the same way as it did during inner ear development to generate the correct ratios of hair and support cells during regeneration (reviewed in Cotanche and Kaiser, 2010; Bermingham-McDonogh and Reh, 2011).
3.2. Limited hair cell repair in mammalian auditory epithelium
In contrast to the non-mammalian vertebrate ear, traumatized auditory epithelium in the mature mammalian ear is unable to regenerate new cells. Studies have consistently shown that support cells in the mature organ of Corti have no (e.g., Roberson and Rubel, 1994) or little (e.g., Yamasoba and Kondo, 2006) proliferative ability after extensive hair cell lesions, and spontaneous hair cell replacement fails to occur. The reasons for the lack of regenerative capacity are unknown. Support cells in the mammalian organ of Corti are highly specialized in contrast to support cells in non-mammalian auditory epithelia, and it is possible that as a consequence of their specialization, they have lost the ability to divide or transdifferentiate following hair cell loss. It has been hypothesized that support cell proliferative ability is lost because of the absence of certain mitogenic receptors and signaling pathways, the expression of cell cycle inhibitors such as p27Kip1, the lack of expression of cell cycle-positive regulators such as cyclin D1, the expression of additional inhibitory signaling pathways, and/or changes in the actin cytoskeleton (e.g., White et al., 2006; Burns et al., 2008; Laine et al., 2010; McCullar et al., 2010; Oesterle et al., 2011; Liu et al., 2012c). It has been shown that pluripotent stem cells, present in regenerating organs such as intestine and skin, appear to be absent in the mature cochlea. While stem-like cells have been isolated from the cochlea of neonatal mice, they have not been identified in the cochleae of adult mice (Oshima et al., 2007).
Hair cell stereociliary bundles are susceptible to acoustic trauma, and hair cells in lower vertebrates and mammalian vestibular sensory epithelia can survive bundle loss and spontaneously undergo self-repair of the stereocilia (Baird et al., 1996, 2000; Gale et al., 2002, Zheng et al., 1999). Albeit several older studies suggest damaged mammalian cochlear hair cells might be capable of self-repair and regrowing their stereocilia (Sobkowicz et al., 1992, 1996), a recent study reports the opposite, namely the inability of cochlear hair cells to self-repair lost bundles. Damaged bundleless hair cells in postnatal organ of Corti can survive and continue functional development for several weeks, but they do not spontaneously regenerate their hair bundle (Jia et al., 2009). Auditory hair cells, including mammalian hair cells, can repair broken stereocilia tip links, however (Zhao et al., 1996: Husbands et al., 1999; Jia et al., 2009).
4. Scar formation in damaged organ of Corti
While support cells in the injured organ of Corti do not replenish the epithelium with replacement hair cells, they are actively involved in repair processes. They expand, rearrange, and develop scar formations at the apical surface of the reticular lamina at the sites of hair cell loss (e.g., Hawkins, 1976; Johnsson et al., 1981; Forge, 1985; Raphael and Altschuler, 1991a, b). The scarring prevents further damage to the sensory epithelium, as it prevents mixing of endolymphatic and organ of Corti fluids, thereby maintaining the endolymphatic potential and ensuring that residual hair cells can continue to function (Bohne and Rabbitt, 1983; Forge, 1985). Auditory hair cells operate within a precisely defined extracellular environment that supports mechanotransduction. Stereocilia extending from the hair cell lumenal surface are bathed in endolymph, a unique extracellular fluid with a high potassium concentration, whereas basolateral hair cell surfaces are surrounded by the intercellular fluid within the organ of Corti, interstitial perilymph with a very different low-potassium composition. The support cells form an important epithelial barrier with the hair cells that limits ion flow between endolymph and interstitial perilymph. Tight junctions at the endolymphatic surface of the epithelium are a key component of this barrier. Recent studies have shown that mutations in the tight junction proteins CLDN9, CLDN14, and TRIC enable the ingress of endolymph into the fluid spaces within the organ of Corti and result in hair cell degeneration (Wilcox et al., 2001; Ben-Yosef et al., 2003; Riazuddin et al., 2006; Nakano et al., 2009). Increased potassium levels surrounding the basolateral membranes of the hair cells are thought to be largely responsible for the hair cell death (Raphael et al., 2007).
As hair cells degenerate after insult, a highly regulated and complex mechanism of scar formation is initiated by the specialized columnar support cells that abut each dying hair cell (Forge, 1985, Leonova and Raphael, 1997, Raphael and Altschuler, 1991a, b; Taylor et al., 2012). Epithelial barrier integrity is maintained during hair cell death (McDowell et al., 1989; Bird et al., 2010), minimizing further hair cell death that would otherwise occur by the infusion of endolymph fluids into the fluid bathing the basal domain of hair cells where terminals of the auditory nerve reside (Bohne and Rabbitt, 1983). Recent findings in non-mammalian vertebrates show that support cells repair the epithelial barrier using an actin-based mechanism (Hordichok et al., 2007; Bird et al., 2010) and phagocytose dying hair cells (Bird et al., 2010). These findings remain to be established in the adult mammalian cochlea, where it is possible that support cell activity in the structurally complex organ of Corti could differ from that in non-mammalian vertebrates where the support cells are not as highly differentiated. Mammalian outer hair cell remains are thought to be phagocytosed by neighboring support cells within the epithelium, with the phagocytosis being speculated to occur after the injured hair cell has sent an “eat me” signal to its support cell neighbors before its death (Abrashkin et al., 2006). Macrophages are recruited to the damaged organ of Corti (e.g., Hirose et al., 2005), but they are not thought to play a critical role in repairing the organ following hair cell loss (Taylor et al., 2012). It has been postulated that calpain proteolysis might be involved in the structural changes in Deiters’ phalangeal processes involved in the scarring process that follows outer hair cell loss (Ladrech et al., 2004). Future studies are needed to identify the signals that mediate the elimination of hair cell corpses by the support cells and control scar formation. It is conceivable that minimizing or preventing support cell scar formation might assist regenerative processes in damaged organ of Corti.
5. Support cell phenotype changes after damage
It is not known whether support cells in mature non-mammalian vertebrate ears dedifferentiate in response to the hair cell loss and revert back to a simpler less differentiated stage, with this process then allowing the cell to proliferate before re-differentiating and leading to the replacement of lost hair cells. Dedifferentiation does occur in some tissues before regenerative proliferation (e.g., retinal Müller cells and retinal pigment epithelium (RPE): reviewed in Bermingham-McDonogh and Reh, 2011; Schwann cells: reviewed in Jopling et al., 2011), but it is not necessary for mature cells to proliferate (Jopling et al., 2011).
It had been presumed that loss of inner and outer hair cells in damaged adult organ of Corti would lead to a collapse of the tunnel of Corti and result in generation of a flat epithelium. However, in long-deafened aged animal models where the organ of Corti is severely damaged by aminoglycosides, differentiated support cells can be maintained for long periods of time in remnant organ of Corti in the absence of hair cells (Sugawara et al., 2005; Oesterle et al., 2009). The remnant organ of Corti has also been called the “repaired organ of Corti” or the “repaired columnar epithelium” (Taylor et al., 2012), and the latter term will be used here. Morphological analyses of human temporal bones in patients with severe or profound hearing loss have shown cochlear regions where differentiated support cells remain intact despite total hair cell loss, as well as regions where the organ has been replaced with a flat unspecialized epithelium (Teufert et al., 2006; Hoa et al., 2010). Severe-to-profoundly deaf patients with a viable auditory nerve are potential candidates for restorative therapies once therapies such as hair cell regeneration or stem cell implantation become a reality. Hence, research aimed towards identifying methods to biologically restore the damaged epithelium must focus both on the columnar support cells retained in the repaired columnar epithelium that continue to exhibit some specialized molecular and structural features (Fig. 2B, D–E) and on the short nondescript cuboidal cells in the flat epithelium that lack features of differentiated support cells (Fig. 2C, F–G). Both epithelial types may constitute the substrate for potential future therapy in clinical cases, and understanding the characteristics of the cells that remain after hair cell loss will be crucial to identifying feasible regenerative procedures. The condition of the remaining cells will likely dictate the choice of therapy. Remarkably little is known regarding the characteristics of the remaining cells, and it is summarized below.
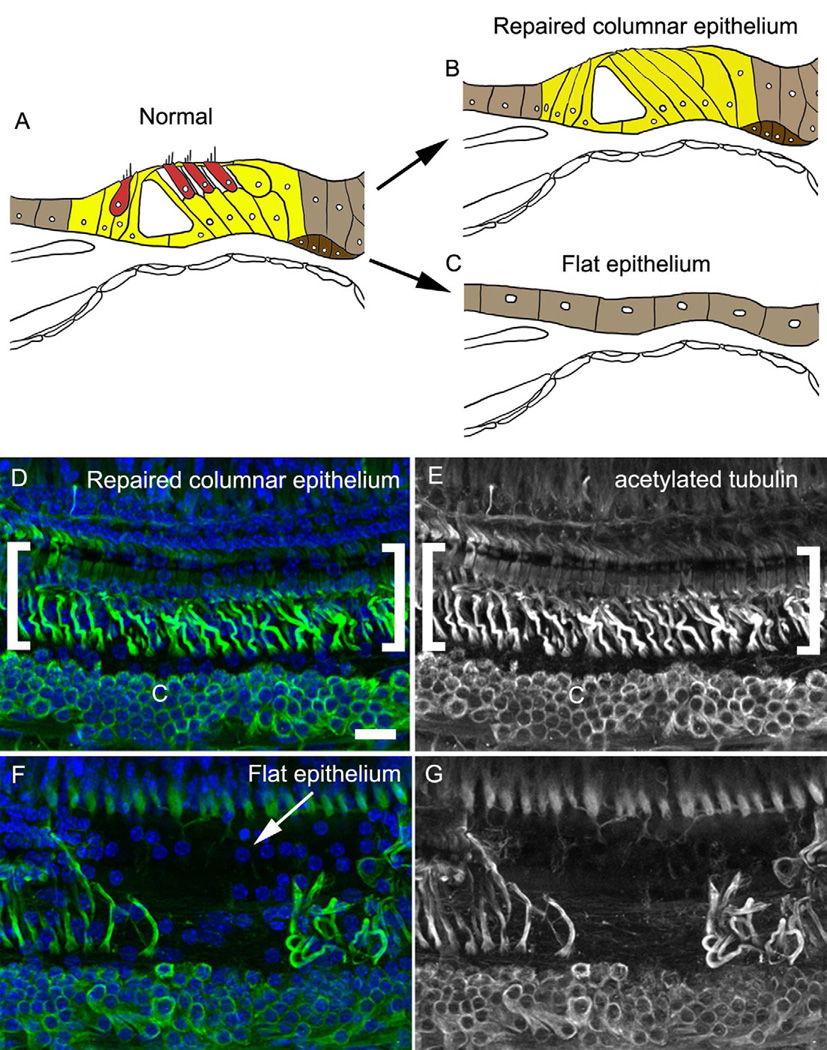
Figure 2. Lesion pathologies in severely damaged organ of Corti.
Schematic diagrams of the normal organ of Corti (A) and two lesion pathologies seen in severely damaged organ of Corti, the repaired columnar epithelium (B) and the flat epithelium (C). Hair cells (red) are absent in both lesion pathologies. Differentiated support cells (yellow) remain in the repaired columnar epithelium, in contrast to the flat epithelium where non-sensory cells flanking the epithelium (light brown) may migrate into the region formerly occupied by the organ of Corti. Deiters’ cells can spread into the tunnel of Corti area in the repaired columnar epithelium (Taylor et al., 2012). Both damaged epithelial types may constitute the substrate for potential future therapy in clinical cases, and understanding the characteristics of the cells that remain after hair cell loss will be crucial to identifying feasible regenerative procedures. (D–G) Adult C57BL/6 mouse organ of Corti damaged by an aminoglycoside-diuretic combination described previously (single high-dose injection of kanamycin coupled with a single injection of furosemide, Oesterle et al., 2008; Oesterle and Campbell, 2009). Tissues were collected two months after the injections, prepared as whole mounts, immunolabeled for acetylated tubulin (green), and nuclei were counterstained with DAPI (blue) as described previously (Oesterle and Campbell, 2009). All images in D–G are taken from whole-mount preparations of the middle turn. Shown are brightest point projections from confocal Z-series spanning the full depth of the sensory epithelium. (D–E) Repaired columnar epithelium. Some support cells retained in the repaired columnar epithelium continue to express acetylated tubulin (region indicated by the white brackets), a component of differentiated pillar and Deiters’ cells that is associated with the intracellular microtubule bundles present within these support cell subtypes. Acetylated tubulin labeling is also present in the Claudius cell region (C). (F–G) A small stretch of flat epithelium (white arrow) is flanked by regions of repaired columnar epithelium. Note the abrupt transition between the regions and the decreased nuclear density and absence of acetylated tubulin labeling in the flat epithelium. Scale bar in D = 20 µm and applies to panels D–F.
Looking first at the repaired columnar epithelium, where differentiated support cells remain despite massive or complete hair cell loss, it has been hypothesized that future reparative approaches may be more successful when differentiated support cells remain in the tissue. A controversial study, that remains to be verified elsewhere, reports that forced Atoh1 expression in damaged, differentiated, adult guinea pig organ of Corti (containing pillar and Deiters’ cells) induces the support cells to convert into hair cells (Izumikawa et al., 2005). In contrast, attempts by the same investigators to force Atoh1 expression in the flat epithelium have not proven successful towards generating new hair cells (Izumikawa et al., 2007). A recent study using CreER/loxP technologies to force Atoh1 expression in aminoglycoside-damaged juvenile mice also failed to induce pillar and Deiters’ cell conversion into hair cells (Liu et al., 2012a).
The preponderance of experimental evidence suggests that support cells in the adult organ of Corti do not dedifferentiate or revert to a less mature state after organ of Corti trauma. Light and electron microscopy studies have shown that the distinctive specializations of pillar and Deiters’ cells, such as the prominent intracellular bundles of closely packed microtubules that are acquired during late states of organ of Corti maturation (Forge et al., 1997; Souter et al., 1997), persist in the repaired organ when columnar support cells remain (Oesterle et al., 2008, Oesterle and Campbell, 2009; Taylor et al., 2012). Further, histochemical studies show that acetylated tubulin, a component of the distinctive intracellular bundles (Tannenbaum and Slepecky, 1997; Saha and Slepecky, 2000) is retained (Oesterle and Campbell, 2009; Taylor et al., 2012). Studies of KCC4, a plasma membrane protein involved in potassium uptake that is upregulated during the latter stages of organ of Corti maturation in inner border and Deiters’ cells (Boettger et al., 2002), indicate KCC4 is retained in repaired organ of Corti when columnar support cells remain despite massive or complete hair cell loss (Taylor et al., 2012). Taylor and colleagues (2012) showed that the glutamate aspartate transporter (GLAST), whose expression increases in inner border, inner phalangeal, and Deiters’ cells during latter stages of organ of Corti development (Jin et al., 2003), is retained, and connexin expression patterns and gap-junctional intercellular connections, including compartmentalization of coupled cells within the organ, are retained in the repaired columnar epithelium (Taylor et al., 2012). Support cells in the repaired epithelium continue to express Sox2, a transcription factor expressed normally in all developing and mature organ of Corti support cell subtypes (Hume et al., 2007; Oesterle et al., 2008, Oesterle and Campbell, 2009). Interestingly, a recent study using tamoxifen-inducible CreER/loxP technologies to conditionally delete Sox2 in normal postnatal pillar and Deiters’ cells suggests that Sox2 may not be necessary to maintain their cell fate (Liu et al., 2012c). In sum, support cells remaining in the repaired columnar epithelium in the mammalian organ of Corti retain differentiated features that include prominent intracellular microtubule bundles. The continuing differentiation of support cells in the mammalian organ of Corti may be an impediment to inducing support cell proliferation or transdifferentiation (Warchol, 2010), and it may not be a conducive environment for nurturing differentiation of hair cell precursors into mature hair cells (Li et al., 2003).
Looking at the flat epithelium, severe lesions to the adult organ of Corti can lead to the loss of hair cells and differentiated support cells. A variety of etiologies may lead to degeneration of the auditory epithelium to the flat state, including severe presbycusis (Bhatt et al., 2001), severe ototoxic injury (Coco et al., 2007; Forge et al., 1998; Kim and Raphael, 2007), hereditary cochlear pathologies (Webster, 1992), or cochlear implant implantation (Nadol et al., 1994). Small patches of flat epithelium can be interspersed with segments of repaired columnar epithelium containing pillar and Deiters’ cells but missing hair cells (Fig. 2F–G). Transitions from repaired columnar epithelium to the flat epithelium are abrupt, with no evidence for intermediate stages between cells with cytoskeletal specializations of differentiated pillar and Deiters’ cells and the simple cuboidal-like cells of the flat epithelium (Taylor et al., 2012, Fig. 2F–G).
The mechanisms inducing transformation of the damaged epithelium to an undifferentiated flat epithelium are not clear. It is not known whether the flat epithelium consists of support cells that have dedifferentiated, or whether cells in the flat epithelium are derived from flanking areas, such as the inner or outer sulcus, with the flanking cells migrating into the damaged region to replace the original support cells that have died. As discussed earlier, support cells are sensitive to certain insults that could conceivably induce primary support cell death. Secondary delayed degeneration may also take place (Forge et al., 1998). At present, there is no direct evidence that support cells dedifferentiate into the less specialized squamous cells of the flat epithelium. Reduced numbers of cells are seen in the flat epithelium relative to the repaired columnar epithelium with differentiated support cells, leading to the speculation that pillar and Deiters’ cell death has occurred (Taylor et al., 2012). Future cell-fate mapping studies, cell isolation and transcriptome analyses studies, and phenotypic characterization of the flat epithelium should help to determine the identity of these cells, and this would facilitate future genetic manipulations of these cells for therapeutic purposes. Proteins that have been detected in the flat epithelium include the tight junction associated protein ZO-1 (Kim and Raphael, 2007) and the gap-junctional proteins connexin 26 and connexin 30 (Taylor et al., 2012), indicating that cells in the flat epithelium are connected to each other with tight junctions and gap junctions. Low levels of the cell cycle inhibitor protein p27Kip1 are seen in the flat epithelium relative to the normal organ of Corti, and cells in the flat epithelium, in contrast to the repaired differentiated epithelium, are not quiescent. The flat epithelium can undergo a robust proliferative phase (Kim and Raphael, 2007).
6. Role of auditory support cells in maintaining spiral ganglion cells after damage
The degeneration of spiral ganglion neurons (SGNs) is a prominent characteristic of the damaged adult mammalian cochlea and an important component of sensori-neural hearing loss. The long-term survival of SGNs is critical for success of cochlear implant prostheses and will also be a requirement for hair cell replacement strategies. Consequently, it is an active area of current research. Recent advancements for maintaining and regenerating nerves in damaged cochlea, with an emphasis on the therapeutic capacity of neurotrophic factors delivered to the inner ear after an insult, are reviewed in Shibata et al. (2011). Auditory sensory epithelial cell types thought to be responsible for maintaining the survival of adult cochlear sensory neurons are examined here. The prevailing view has been that SGN survival depends primarily on trophic support provided by inner hair cells (e.g., Bredberg, 1968; Spoendlin 1973; Ylikoski et al., 1974, reviewed in Shibata et al., 2011). However, support cells, speculated more than 50 years ago to be important for auditory nerve survival (e.g., Schuknecht, 1953; Spoendlin and Gacek, 1963), have recently been validated as playing a critical role in maintaining SGNs. Recent studies in adult organ of Corti indicate inner phalangeal and inner border cells, support cell subtypes flanking the inner hair cells, are critical for auditory nerve survival (Zilberstein et al., 2012). Triggers for SGN loss may originate from these cells rather than the inner hair cells. This idea is based on the following observations: (1) Adult support cells express trophic factors known to promote SGN survival (e.g., brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT3): Sobkowicz et al., 2002; Stankovic et al., 2004; Sugawara et al., 2007; Zuccotti et al., 2012); (2) Transgenic mouse models show that support cell dysfunction causes SGN dysfunction. For example, loss of erbB receptor signaling in cochlear support cells leads to SGN degeneration after the onset of hearing in the absence of hair cell and support cell loss (Stankovic et al., 2004). Studies in conditional BDNF knock-out mice show BDNF (expressed in support cells) is crucial for maintaining inner hair cell transmitter release sites and numbers of afferent fibers (Zuccotti et al., 2012); (3) Studies using a mouse model of inner hair cell loss that doesn’t involve noise or ototoxic drugs have shown that inner hair cells are not required for SGN survival in the adult cochlea. Mice lacking the gene for the high-affinity thiamine (vitamin B1) transporter (Slc19a2) have normal cochlear structure and function when fed a regular (thiamine-rich) diet, and dietary thiamine restriction causes widespread inner hair cell loss with no significant loss of SGNs (Zilberstein et al., 2012); and (4) The correlation between inner hair cell loss and SGN death arises because acoustic trauma and ototoxic drugs typically destroy most support cells in the regions where inner hair cells degenerate (Sugawara et al., 2005), and the insult has direct effects on neurons (Spoendlin, 1971; Liberman and Mulroy, 1982; Robertson, 1983). Support cell survival, when hair cells are lost via aminoglycosides, enhances SGN survival for years post-treatment (Sugawara et al., 2005). SGN survival is critical for prosthetic auditory rehabilitation, as cochlear implant function depends on stimulating the surviving SGNs. Further, support cell maintenance of the SGNs will be critical for biologically restorative therapies, as regenerated hair cells will need to be innervated by the SGN to carry the transduced signals to the brain.
It is unknown whether support cell subtypes abutting the outer hair cells (e.g., Deiters’ cells) play a role in maintaining type II SGNs. Spiral (type II) afferent fibers are frequently enveloped by Deiters’ cells that form an incomplete sheath around several fibers (Ades and Engström, 1974).
7. Conclusions
Several avenues are currently being explored to coax hair cell regeneration in the damaged mammalian ear, an in situ repair approach and a stem cell/graft therapy approach, and they are covered in depth in recent reviews (Raphael et al., 2007; Edge and Chen, 2008; Brigande and Heller, 2009; Wei and Yamoah, 2009; Oshima et al., 2010a; de Felipe et al., 2011). The in situ repair approach entails mobilizing the cells remaining in the damaged ear to effect the repair by inducing these cells to convert into hair cells and/or stimulating their division and deriving hair cells from the daughter cells. The stem cell approach uses cells that will differentiate into new hair cells and incorporates them into the damaged epithelium. The stem cell approach has been used successfully in regenerative medicine, albeit for a limited number of treatments (e.g., hematopoietic stem cell transplantation after cancer therapy, transplantation of in vitro-reconstituted skin to severely burned patients), and exciting advances have been made over the past few years in terms of generating cells with hair cell characteristics from mouse embryonic stem cells and induced pluripotent stem cells (Li et al., 2003; Oshima et al., 2010b; reviewed in Groves, 2010; Oshima et al., 2010a; de Felipe et al., 2011). Both approaches are promising, and understanding more about the cells that remain in the damaged ear is a clear prerequisite to achieving the goal of regenerating replacement hair cells in the damaged human ear.
The complexity of the severely damaged ear, with patches of flat epithelium interspersed with stretches of repaired columnar epithelium within an individual ear, may pose difficulties. Different regenerative therapies are likely to require different cellular substrates on which to work, and lesion variability within an individual cochlea may require application of more than one therapeutic approach (Taylor et al., 2012).
Research in the past few decades has uncovered key intracellular events that can cause hair cell death (reviewed in Cheng et al., 2005) and candidate protectants such as antioxidants, caspase inhibitors, jun kinase inhibitors, and growth factors have been evaluated (Kopke et al., 1997; Liu et al., 1998; Yamasoba et al., 1999; Matsui et al., 2002; Sugahara et al., 2006, reviewed in Shibata and Raphael, 2010). In contrast, little is known regarding intracellular events causing support cell death and potential support cell protectants. Minimizing support cell death may prove important, as future reparative approaches may be more successful when differentiated support cells remain in the tissue (Izumikawa et al., 2008).
Overview of traumatized auditory epithelium in the adult vertebrate ear is provided
Repair capacities of traumatized vertebrate auditory epithelium are discussed
Support cell phenotype changes after damage are examined
Support cell roles in maintaining spiral ganglion cells after damage is discussed
Acknowledgements
Regeneration research in the author’s lab is supported by NIDCD R01 grant DC03944, with additional support for imaging and computing from NIDCD P30 grant DC04661 and NICHHD P30 grant HD002274.
Abbreviations
- SGN
spiral ganglion neuron
- BDNF
brain-derived neurotrophic factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrashkin KA, Izumikawa M, Miyazawa T, Wang CH, Crumling MA, Swiderski DL, Beyer LA, Gong TW, Raphael Y. The fate of outer hair cells after acoustic or ototoxic insults. Hearing Res. 2006;218(1–2):20–29. doi: 10.1016/j.heares.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Ades HW, Engström H. Anatomy of the inner ear. In: Keidel, Neff, editors. Handbook of Sensory Physiology. Vol. V/I. New York: Springer-Verlag; 1974. pp. 125–158. [Google Scholar]
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci. Lett. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann. N.Y. Acad. Sci. 1996;19(781):59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- Baird RA, Burton MD, Lysakowski A, Fashena DS, Naeger RA. Hair cell recovery in mitotically blocked cultures of the bullfrog saccule. Proc. Natl. Acad. Sci. U.S.A. 2000;97(22):11722–11729. doi: 10.1073/pnas.97.22.11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yosef T, Belyantseva IA, Saunders TL, Hughes ED, Kawamoto K, Van Itallie CM, Beyer LA, Halsey K, Gardner DJ, Wilcox ER, Rasmussen J, Anderson JM, Dolan DF, Forge A, Raphael Y, Camper SA, Friedman TB. Claudin 14 knockout mice, a model for autosomal recessive deafness DFNB29, are deaf due to cochlear hair cell degeneration. Hum. Mol. Genet. 2003;12:2049–2061. doi: 10.1093/hmg/ddg210. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Reh TA. Regulated reprogramming in the regeneration of sensory receptor cells. Neuron. 2011;71(3):389–405. doi: 10.1016/j.neuron.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt KA, Liberman MC, Nadol JB., Jr Morphometric analysis of age-related changes in the human basilar membrane. Ann. Otol. Rhinol. Laryngol. 2001;110(12):1147–1153. doi: 10.1177/000348940111001212. [DOI] [PubMed] [Google Scholar]
- Bird JE, Daudet N, Warchol ME, Gale JE. Supporting cells eliminate dying sensory hair cells to maintain epithelial integrity in the avian inner ear. J. Neurosci. 2010;30(37):12545–12556. doi: 10.1523/JNEUROSCI.3042-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger T, Hubner CA, Maier H, Rust MB, Beck FX, et al. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature. 2002;416:874–878. doi: 10.1038/416874a. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Rabbitt KD. Holes in the reticular lamina after noise exposure: Implication for continuing damage in the organ of Corti. Hearing Research. 1983;11:41–53. doi: 10.1016/0378-5955(83)90044-8. [DOI] [PubMed] [Google Scholar]
- Bredberg G. Cellular pattern and nerve supply of the human organ of Corti. Acta Otolaryngol. 1968;(Suppl 236):1. [PubMed] [Google Scholar]
- Brigande JV, Heller S. Quo vadis, hair cell regeneration? Nat. Neurosci. 2009;12(6):679–685. doi: 10.1038/nn.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignull HR, Raible DW, Stone JS. Feathers and fins: non-mammalian models for hair cell regeneration. Brain Res. 2009;1277:12–23. doi: 10.1016/j.brainres.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Christophel JJ, Collado MS, Magnus C, Carfrae M, Corwin JT. Reinforcement of cell junctions correlates with the absence of hair cell regeneration in mammals and its occurrence in birds. J. Comp. Neurol. 2008;511(3):396–414. doi: 10.1002/cne.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev. Dyn. 2007;236:156–170. doi: 10.1002/dvdy.21023. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr. Opin. Otolaryngol. Head Neck Surg. 2005;13:343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- Coco A, Epp SB, Fallon JB, Xu J, Millard RE, Shepherd RK. Does cochlear implantation and electrical stimulation affect residual hair cells and spiral ganglion neurons? Hearing Res. 2007;225(1–2):60–70. doi: 10.1016/j.heares.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado MS, Burns JC, Hu Z, Corwin JT. Recent advances in hair cell regeneration research. Curr. Opin. Otolaryngol. Head Neck Surg. 2008;16(5):465–471. doi: 10.1097/MOO.0b013e32830f4ab5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240(4860):1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hearing Res. 1987;30:181–196. doi: 10.1016/0378-5955(87)90135-3. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Kaiser CL. Hair cell fate decisions in cochlear development and regeneration. Hearing Res. 2010;266(1–2):18–25. doi: 10.1016/j.heares.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotanche DA, Messana EP, Ofsie MS. Migration of hyaline cells into the chick basilar papilla during severe noise damage. Hear Res. 1995;91(1–2):148–159. doi: 10.1016/0378-5955(95)00185-9. [DOI] [PubMed] [Google Scholar]
- Dai CF, Mangiardi D, Cotanche DA, Steyger PS. Uptake of fluorescent gentamicin by vertebrate sensory cells in vivo. Hearing Res. 2006;213(1–2):64–78. doi: 10.1016/j.heares.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J. Neurophysiol. 1978;41(2):365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- de Felipe MM, Feijoo Redondo AF, García-Sancho J, Schimmang T, Alonso MB. Cell- and gene-therapy approaches to inner ear repair. Histol. Histopathol. 2011;26(7):923–940. doi: 10.14670/HH-26.923. [DOI] [PubMed] [Google Scholar]
- de Groot JC, Meeuwsen F, Ruizendaal WE, Veldman JE. Ultrastructural localization of gentamicin in the cochlea. Hearing Res. 1990;50(1–2):35–42. doi: 10.1016/0378-5955(90)90031-j. [DOI] [PubMed] [Google Scholar]
- Duncan LJ, Mangiardi DA, Matsui JI, Anderson JK, McLaughlin-Williamson K, Cotanche DA. Differential expression of unconventional myosins in apoptotic and regenerating chick hair cells confirms two regeneration mechanisms. J. Comp. Neurol. 2006;499:691–701. doi: 10.1002/cne.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge A, Chen Z-Y. Hair cell regeneration. Current Opinion in Neurobiology. 2008;18:377–382. doi: 10.1016/j.conb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A. Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hearing Res. 1985;19(2):171–182. doi: 10.1016/0378-5955(85)90121-2. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hearing Res. 2000;139(1–2):97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Nevill G. Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J. Comp. Neurol. 1998;397(1):69–88. [PubMed] [Google Scholar]
- Forge A, Souter M, Denman-Johnson K. Structural development of sensory cells in the ear. Semin. Cell Dev. Biol. 1997;8:225–237. doi: 10.1006/scdb.1997.0147. [DOI] [PubMed] [Google Scholar]
- Gale JE, Meyers JR, Periasamy A, Corwin JT. Survival of bundleless hair cells and subsequent bundle replacement in the bullfrog's saccule. J. Neurobiol. 2002;50(2):81–92. doi: 10.1002/neu.10002. [DOI] [PubMed] [Google Scholar]
- Girod DA, Duckert LG, Rubel EW. Possible precursors of regenerated hair cells in the avian cochlea following acoustic trauma. Hearing Res. 1989;42(2–3):175–194. doi: 10.1016/0378-5955(89)90143-3. [DOI] [PubMed] [Google Scholar]
- Gleich O, Manley GA. The hearing organ of birds and crocodilia. In: Dooling RJ, Fay RR, Popper AN, editors. Comparative Hearing: Birds and Reptiles. New York: Springer Verlag; 2000. pp. 70–138. [Google Scholar]
- Groves AK. The challenge of hair cell regeneration. Exp. Biol. Med. (Maywood) 2010;235(4):434–446. doi: 10.1258/ebm.2009.009281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JE, Jr, Johnsson LG, Stebbins WC, Moody DB, Coombs SL. Hearing loss and cochlear pathology in monkeys after noise exposure. Acta Otolaryngol. 1976;81(3–4):337–343. doi: 10.3109/00016487609119971. [DOI] [PubMed] [Google Scholar]
- Hinojosa R, Nelson EG, Lerner SA, Redleaf MI, Schramm DR. Aminoglycoside ototoxicity: a human temporal bone study. Laryngoscope. 2001;111(10):1797–1805. doi: 10.1097/00005537-200110000-00025. [DOI] [PubMed] [Google Scholar]
- Hirose K, Discolo CM, Keasler JR, Ransohoff R. Mononuclear phagocytes migrate into the murine cochlea after acoustic trauma. J. Comp. Neurol. 2005;489(2):180–194. doi: 10.1002/cne.20619. [DOI] [PubMed] [Google Scholar]
- Hirose K, Westrum LE, Cunningham DE, Rubel EW. Electron microscopy of degenerative changes in the chick basilar papilla after gentamicin exposure. J. Comp. Neurol. 2004;470(2):164–180. doi: 10.1002/cne.11046. [DOI] [PubMed] [Google Scholar]
- Hoa M, Linthicum FH, Merchant S, Segil N. Supporting cell survival in temporal bones of patients with known hearing loss. Assoc. Res. Otolaryngol. 2010 Abstract 33. [Google Scholar]
- Hordichok AJ, Steyger PS. Closure of supporting cell scar formations requires dynamic actin mechanisms. Hearing Res. 2007;232(1–2):1–19. doi: 10.1016/j.heares.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ. The cellular basis of hearing: the biophysics of hair cells. Science. 1985;230(4727):745–752. doi: 10.1126/science.2414845. [DOI] [PubMed] [Google Scholar]
- Hume CR, Bratt DL, Oesterle EC. Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expression Patterns. 2007;7:798–807. doi: 10.1016/j.modgep.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husbands JM, Steinberg SA, Kurian R, Saunders JC. Tip-link integrity on chick tall hair cell stereocilia following intense sound exposure. Hearing Res. 1999;135(1–2):135–145. doi: 10.1016/s0378-5955(99)00101-x. [DOI] [PubMed] [Google Scholar]
- Imamura S, Adams JC. Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J. Assoc. Res. Otolaryngol. 2003;4(2):176–195. doi: 10.1007/s10162-002-2036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Batts SA, Miyazawa T, Swiderski DL, Raphael Y. Response of the flat cochlear epithelium to forced expression of Atoh1. Hearing Res. 2008;240(1–2):52–56. doi: 10.1016/j.heares.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Janas JD, Cotanche DA, Rubel EW. Avian cochlear hair cell regeneration: stereological analyses of damage and recovery from a single high dose of gentamicin. Hearing Res. 1995;92(1–2):17–29. doi: 10.1016/0378-5955(95)00190-5. [DOI] [PubMed] [Google Scholar]
- Jia S, Yang S, Guo W, He DZ. Fate of mammalian cochlear hair cells and stereocilia after loss of the stereocilia. J. Neurosci. 2009;29(48):15277–15285. doi: 10.1523/JNEUROSCI.3231-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin ZH, Kikuchi T, Tanaka K, Kobayashi T. Expression of glutamate transporter GLAST in the developing mouse cochlea. Tohoku J. Exp. Med. 2003;200:137–144. doi: 10.1620/tjem.200.137. [DOI] [PubMed] [Google Scholar]
- Johnsson LG, Hawkins JE, Jr, Kingsley TC, Black FO, Matz GJ. Aminoglycoside-induced cochlear pathology in man. Acta Otolaryngol. Suppl. 1981;383:1–19. [PubMed] [Google Scholar]
- Jopling C, Boue S, Izpisua Belmonte JC. Dedifferentiation, transdifferentiation and reprogramming: three routes to regeneration. Nat. Rev. Mol. Cell Biol. 2011;12(2):79–89. doi: 10.1038/nrm3043. [DOI] [PubMed] [Google Scholar]
- Kim YH, Raphael Y. Cell division and maintenance of epithelial integrity in the deafened auditory epithelium. Cell Cycle. 2007;6(5):612–619. doi: 10.4161/cc.6.5.3929. [DOI] [PubMed] [Google Scholar]
- Kopke RD, Liu W, Gabaizadeh R, Jacono A, Feghali J, Spray D, et al. Use of organotypic cultures of Corti's organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am. J. Otol. 1997;18:559–571. [PubMed] [Google Scholar]
- Köppl C. Birds--same thing, but different? Convergent evolution in the avian and mammalian auditory systems provides informative comparative models. Hearing Res. 2011;273(1–2):65–71. doi: 10.1016/j.heares.2010.03.095. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J. Neurosci. 2009;29(45):14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladrech S, Guitton M, Saido T, Lenoir M. Calpain activity in the amikacin-damaged rat cochlea. J. Comp. Neurol. 2004;477(2):149–160. doi: 10.1002/cne.20252. [DOI] [PubMed] [Google Scholar]
- Laine H, Sulg M, Kirjavainen A, Pirvola U. Cell cycle regulation in the inner ear sensory epithelia: role of cyclin D1 and cyclin-dependent kinase inhibitors. Dev. Biol. 2010;337(1):134–146. doi: 10.1016/j.ydbio.2009.10.027. [DOI] [PubMed] [Google Scholar]
- Leonova EV, Raphael Y. Organization of cell junctions and cytoskeleton in the reticular lamina in normal and ototoxically damaged organ of Corti. Hearing Res. 1997;113(1–2):14–28. doi: 10.1016/s0378-5955(97)00130-5. [DOI] [PubMed] [Google Scholar]
- Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Mulroy MJ. Acute and chronic effects of acoustic trauma: cochlear pathology and auditory nerve pathophysiology. In: Hamernik RP, Henderson D, Salvi R, editors. New perspectives on noise-induced hearing loss. New York: Raven; 1982. pp. 105–136. [Google Scholar]
- Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, Ayrault O, Zindy F, Gan L, Roussel MF, Zuo J. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters' cells to immature hair cells by Atoh1 ectopic expression. J. Neurosci. 2012a;32(19):6600–6610. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Owen T, Fang J, Zuo J. Overactivation of Notch1 signaling induces ectopic hair cells in the mouse inner ear in an age-dependent manner. PLoS One. 2012b;7(3):34123. doi: 10.1371/journal.pone.0034123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Staecker H, Stupak H, Malgrange B, Lefebvre P, Van De Water TR. Caspase inhibitors prevent cisplatin-induced apoptosis of auditory sensory cells. Neuroreport. 1998;9:2609–2614. doi: 10.1097/00001756-199808030-00034. [DOI] [PubMed] [Google Scholar]
- Liu Z, Walters BJ, Owen T, Brimble MA, Steigelman KA, Zhang L, Mellado Lagarde MM, Valentine MB, Yu Y, Cox BC, Zuo J. Regulation of p27Kip1 by Sox2 maintains quiescence of inner pillar cells in the murine auditory sensory epithelium. J. Neurosci. 2012c;32(31):10530–10540. doi: 10.1523/JNEUROSCI.0686-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, Liberman MC, Merchant SN. Age-related primary cochlear neuronal degeneration in human temporal bones. J. Assoc. Res. Otolaryngol. 2011;12(6):711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. Lizard auditory papillae: an evolutionary kaleidoscope. Hearing Res. 2011;273(1–2):59–64. doi: 10.1016/j.heares.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Cotanche DA. Sensory hair cell death and regeneration: two halves of the same equation. Curr. Opin. Otolaryngol. Head Neck Surg. 2004;12(5):418–425. doi: 10.1097/01.moo.0000136873.56878.56. [DOI] [PubMed] [Google Scholar]
- McCullar JS, Ty S, Campbell S, Oesterle EC. Activin potentiates proliferation in mature avian auditory sensory epithelium. J. Neurosci. 2010;30(2):478–490. doi: 10.1523/JNEUROSCI.5154-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell B, Davies S, Forge A. The effect of gentamicin-induced hair cell loss on the tight junctions of the reticular lamina. Hearing Res. 1989;40(3):221–232. doi: 10.1016/0378-5955(89)90163-9. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Jr, Ketten DR, Burgess BJ. Otopathology in a case of multichannel cochlear implantation. Laryngoscope. 1994;104(3 Pt 1):299–303. doi: 10.1288/00005537-199403000-00010. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Kim SH, Kim HM, Sanneman JD, Zhang Y, Smith RJ, Marcus DC, Wangemann P, Nessler RA, Banfi B. A claudin-9-based ion permeability barrier is essential for hearing. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000610. e1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle E, Campbell S. Supporting cell characteristics in long-deafened aged mouse ears. J. Assoc. Res. Otolaryngol. 2009;10(4):525–544. doi: 10.1007/s10162-009-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle E, Campbell S, Taylor R, Forge A, Hume CR. Sox2 and Jagged1 expression in adult mouse inner ear sensory epithelia. J. Assoc. Res. Otolaryngol. 2008;9(1):65–89. doi: 10.1007/s10162-007-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC, Chien W-M, Campbell S, Nellimarla P, Fero ML. p27Kip1 is required to maintain proliferative quiescence in the adult cochlea and pituitary. Cell Cycle. 2011;10(8):1–12. doi: 10.4161/cc.10.8.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC, Stone JS. Hair cell regeneration: Mechanisms guiding cell proliferation and differentiation. In: Salvi RJ, Popper AN, Fay RR, editors. Hair Cell Regeneration, Repair, and Protection. New York: Springer-Verlag; 2008. pp. 141–197. [Google Scholar]
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Géléoc GS, Edge A, Holt JR, Heller S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J. Assoc. Res. Otolaryngol. 2007;8(1):18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010b;141(4):704–716. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Suchert S, Blevins NH, Heller S. Curing hearing loss: Patient expectations, health care practitioners, and basic science. J. Communication Disorders. 2010a;43:311–318. doi: 10.1016/j.jcomdis.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Scar formation after drug-induced cochlear insult. Hear. Res. 1991a;51(2):173–183. doi: 10.1016/0378-5955(91)90034-7. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil. Cytoskeleton. 1991b;18(3):215–227. doi: 10.1002/cm.970180307. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Structure and innervation of the cochlea. Brain Res. Bull. 2003;60:397–422. doi: 10.1016/s0361-9230(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Kim YH, Osumi Y, Izumikawa M. Non-sensory cells in the deafened organ of Corti: approaches for repair. Int. J. Dev. Biol. 2007;51(6–7):649–654. doi: 10.1387/ijdb.072370yr. [DOI] [PubMed] [Google Scholar]
- Riazuddin S, Ahmed ZM, Fanning AS, Lagziel A, Kitajiri S, Ramzan K, Khan SN, Chattaraj P, Friedman PL, Anderson JM, Belyantseva IA, Forge A, Friedman TB. Tricellulin is a tight-junction protein necessary for hearing. Am. J. Hum. Genet. 2006;79:1040–1051. doi: 10.1086/510022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Aud. Neurosci. 1996;2:195–205. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Rubel EW. Cell division in the gerbil cochlea after acoustic trauma. Am. J. Otol. 1994;15:28–34. [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J. Neurosci. Res. 2004;78:461–471. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Robertson D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hearing Res. 1983;9(3):263–278. doi: 10.1016/0378-5955(83)90031-x. [DOI] [PubMed] [Google Scholar]
- Ronaghi M, Nasr M, Heller S. Concise review: Inner ear stem cells--an oxymoron, but why? Stem Cells. 2012;30(1):69–74. doi: 10.1002/stem.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240(4860):1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Saha S, Slepecky NB. Age-related changes in microtubules in the guinea pig organ of Corti. Tubulin isoform shifts with increasing age suggest changes in micromechanical properties of the sensory epithelium. Cell Tissue Res. 2000;300(1):29–46. doi: 10.1007/s004419900163. [DOI] [PubMed] [Google Scholar]
- Schuknecht H. Lesions of the organ of Corti. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1953;57:366–383. [PubMed] [Google Scholar]
- Shibata SB, Budenz CL, Bowling SA, Pfingst BE, Raphael Y. Nerve maintenance and regeneration in the damaged cochlea. Hearing Res. 2011;281(1–2):56–64. doi: 10.1016/j.heares.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata SB, Raphael Y. Future approaches for inner ear protection and repair. J. Commun. Disord. 2010;43(4):295–310. doi: 10.1016/j.jcomdis.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery EL, Warchol ME. Cisplatin ototoxicity blocks sensory regeneration in the avian inner ear. J. Neurosci. 2010;30(9):3473–3481. doi: 10.1523/JNEUROSCI.4316-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky NB. The structure of the mammalian cochlea. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer-Verlag; 1996. pp. 44–129. [Google Scholar]
- Smith CA. In: Progress in Sensory Physiology 2, Recent Advances in Structural Correlates of Auditory Receptors. Autrum, Ottoson, Schmidt, editors. New York: Springer-Verlag; 1981. [Google Scholar]
- Smith CA, Takasaka T. Auditory receptor organs of reptiles, birds, and mammals. Contrib. Sens. Physiol. 1971;5:129–178. doi: 10.1016/b978-0-12-151805-9.50010-9. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, August BK, Slapnick SM. Epithelial repair following mechanical injury of the developing organ of Corti in culture: an electron microscopic and autoradiographic study. Exp. Neurol. 1992;115(1):44–49. doi: 10.1016/0014-4886(92)90219-g. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, August BK, Slapnick SM. Post-traumatic survival and recovery of the auditory sensory cells in culture. Acta Otolaryngol. 1996;116(2):257–262. doi: 10.3109/00016489609137836. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, August BK, Slapnick SM. Influence of neurotrophins on the synaptogenesis of inner hair cells in the deaf Bronx waltzer (bv) mouse organ of Corti in culture. Int. J. Dev. Neurosci. 2002;20(7):537–554. doi: 10.1016/s0736-5748(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Souter M, Nevill G, Forge A. Postnatal maturation of the organ of Corti in gerbils: morphology and physiological responses. J. Comp. Neurol. 1997;386:635–651. [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Differences along the place-frequency map in the structure of supporting cells in the gerbil cochlea. Hearing Res. 1994;79:161–177. doi: 10.1016/0378-5955(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Degeneration behaviour of the cochlear nerve. Arch. Klin. Exp. Ohren Nasen Kehlkopfheilkd. 1971;200(4):275–291. doi: 10.1007/BF00373310. [DOI] [PubMed] [Google Scholar]
- Spoendlin HH. The innervation of the cochlear receptor. Proceeding of a Symposium on Basic Mechanisms in Hearing; Academic Press; New York. 1973. pp. 185–234. [Google Scholar]
- Spoendlin HH, Gacek RR. Electron microscopic study of the efferent and afferent innervation of the organ of Corti in the cat. Ann. Otol. Rhinol. Laryngol. 1963;72:660–686. doi: 10.1177/000348946307200307. [DOI] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J. Neurosci. 2004;24(40):8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int. J. Dev. Biol. 2007;51:633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Cunningham LL, Rubel EW. Protection of adult vestibular hair cells against aminoglycoside toxicity by inhibition in the JNK signaling pathway. Hearing Res. 2006;221:128–135. doi: 10.1016/j.heares.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara M, Corfas G, Liberman MC. Influence of supporting cells on neuronal degeneration after hair cell loss. J. Assoc. Res. Otolaryngol. 2005;6(2):136–147. doi: 10.1007/s10162-004-5050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara M, Murtie JC, Stankovic KM, Liberman MC, Corfas G. Dynamic patterns of neurotrophin 3 expression in the postnatal mouse inner ear. J. Comp. Neurol. 2007;501(1):30–37. doi: 10.1002/cne.21227. [DOI] [PubMed] [Google Scholar]
- Tannenbaum J, Slepecky NB. Localization of microtubules containing posttranslationally modified tubulin in cochlear epithelial cells during development. Cell Motil. Cytoskeleton. 1997;38(2):146–162. doi: 10.1002/(SICI)1097-0169(1997)38:2<146::AID-CM4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Taylor RR, Jagger DJ, Forge A. Defining the cellular environment in the organ of Corti following extensive hair cell loss: a basis for future sensory cell replacement in the Cochlea. PLoS One. 2012;7(1):e30577. doi: 10.1371/journal.pone.0030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teufert KB, Linthicum FH, Jr, Connell SS. The effect of organ of Corti loss on ganglion cell survival in humans. Otol. Neurotol. 2006;27(8):1146–1151. doi: 10.1097/01.mao.0000232006.16363.44. [DOI] [PubMed] [Google Scholar]
- Van Dijk P, Mason MJ, Schoffelen RL, Narins PM, Meenderink SW. Mechanics of the frog ear. Hearing Res. 2011;273(1–2):46–58. doi: 10.1016/j.heares.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ruijven MW, de Groot JC, Smoorenburg GF. Time sequence of degeneration pattern in the guinea pig cochlea during cisplatin administration. A quantitative histological study. Hear Res. 2004;197(1–2):44–54. doi: 10.1016/j.heares.2004.07.014. [DOI] [PubMed] [Google Scholar]
- van Ruijven MW, de Groot JC, Klis SF, Smoorenburg GF. The cochlear targets of cisplatin: an electrophysiological and morphological time-sequence study. Hear Res. 2005;205(1–2):241–248. doi: 10.1016/j.heares.2005.03.023. [DOI] [PubMed] [Google Scholar]
- von Ilberg C, Spoendlin H, Arnold W. Autoradiographical distribution of locally applied dihydrostreptomycin in the inner ear. Acta Otolaryngol. 1971;71(2):159–165. doi: 10.3109/00016487109125345. [DOI] [PubMed] [Google Scholar]
- Wang Q, Steyger PS. Trafficking of systemic fluorescent gentamicin into the cochlea and hair cells. J. Assoc. Res. Otolaryngol. 2009;10(2):205–219. doi: 10.1007/s10162-009-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME. Cellular mechanisms of aminoglycoside ototoxicity. Curr. Opin. Otolaryngol. Head Neck Surg. 2010;18(5):454–458. doi: 10.1097/MOO.0b013e32833e05ec. [DOI] [PubMed] [Google Scholar]
- Warchol ME. Sensory regeneration in the vertebrate inner ear: differences at the levels of cells and species. Hearing Res. 2011;273(1–2):72–79. doi: 10.1016/j.heares.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Corwin JT. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J. Neurosci. 1996;16(17):5466–5477. doi: 10.1523/JNEUROSCI.16-17-05466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol M, Hirose K. Resident macrophages are not required for normal hair cell regeneration in the avian cochlea. Assoc. Res. Otolaryngol. Abstr. 2012;35:244. [Google Scholar]
- Webster DB. Degeneration followed by partial regeneration of the organ of Corti in deafness (dn/dn) mice. Exp. Neurol. 1992;115(1):27–31. doi: 10.1016/0014-4886(92)90216-d. [DOI] [PubMed] [Google Scholar]
- Wei D, Yamoah EN. Regeneration of the mammalian inner ear sensory epithelium. Curr. Opin. Otolaryngol. Head Neck Surg. 2009;17(5):373–380. doi: 10.1097/MOO.0b013e328330345b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever EG. The Reptile Ear. Princeton N.J: Princeton Univ. Press; 1978. [Google Scholar]
- Wever EG. The Amphibian Ear. Princeton N.J: Princeton Univ. Press; 1985. [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd NA, Morell RJ, Kachar B, Wu DK, Griffith AJ, Friedman TB. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104:165–172. doi: 10.1016/s0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Kondo K. Supporting cell proliferation after hair cell injury in mature guinea pig cochlea in vivo. Cell Tissue Res. 2006;325:23–31. doi: 10.1007/s00441-006-0157-9. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Schacht J, Shoji F, Miller JM. Attenuation of cochlear damage from noise trauma by an iron chelator, a free radical scavenger and glial cell line-derived neurotrophic factor in vivo. Brain Res. 1999;815:317–325. doi: 10.1016/s0006-8993(98)01100-7. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Wersäll J, Björkroth B. Degeneration of neural elements in the cochlea of the guinea-pig after damage to the organ of corti by ototoxic antibiotics. Acta Otolaryngol. Suppl. 1974;326:23–41. doi: 10.3109/00016487409129730. [DOI] [PubMed] [Google Scholar]
- Zhao Y-D, Yamoah EN, Gillespie PG. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc. Natl. Acad. Sci. U.S.A. 1996;94:15469–15474. doi: 10.1073/pnas.93.26.15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Keller G, Gao WQ. Immunocytochemical and morphological evidence for intracellular self-repair as an important contributor to mammalian hair cell recovery. J. Neurosci. 1999;19:2161–2170. doi: 10.1523/JNEUROSCI.19-06-02161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein Y, Liberman MC, Corfas G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J. Neurosci. 2012;32(2):405–410. doi: 10.1523/JNEUROSCI.4678-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccotti A, Kuhn S, Johnson SL, Franz C, Singer W, Hecker D, Geisler HS, Köpschall I, Rohbock K, Gutsche K, Dlugaiczyk J, Schick B, Marcotti W, Rüttiger L, Schimmang T, Knipper M. Lack of brain-derived neurotrophic factor hampers inner hair cell synapse physiology, but protects against noise-induced hearing loss. J. Neurosci. 2012;32(25):8545–8553. doi: 10.1523/JNEUROSCI.1247-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]