Abstract
PURPOSE
To determine the magnitudes of binocular summation for low- and high-contrast letter acuity in a multiple sclerosis (MS) cohort, and to characterize the roles that MS disease, age, interocular difference in acuity, and a history of optic neuritis have on binocular summation. The relation between binocular summation and monocular acuities and vision-specific quality of life (QoL) was also examined.
DESIGN
Cross-sectional observational study.
METHODS
Low-contrast acuity (2.5% and 1.25% contrast) and high-contrast visual acuity (VA) were assessed binocularly and monocularly in patients and disease-free controls at 3 academic centers. Binocular summation was calculated as the difference between the binocular and better eye scores. QoL was measured using the 25-item National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25) and the 10-item neuro-ophthalmic supplement. The relation of the degree of binocular summation to monocular acuity, clinical history of acute optic neuritis, age, interocular acuity difference, and QoL was determined.
RESULTS
Binocular summation was demonstrated at all contrast levels, and was greatest at the lowest level (1.25%). Increasing age (P < .0001), greater interocular differences in acuity (P < .0001), and prior history of optic neuritis (P = .015) were associated with lower magnitudes of binocular summation; binocular inhibition was seen in some of these patients. Higher magnitudes of summation for 2.5% low-contrast acuity were associated with better scores for the NEI VFQ-25 (P = .02) and neuro-ophthalmic supplement (P = .03).
CONCLUSION
Binocular summation of acuity occurs in MS but is reduced by optic neuritis, which may lead to binocular inhibition. Binocular summation and inhibition are important factors in the QoL and visual experience of MS patients, and may explain why some prefer to patch or close 1 eye in the absence of diplopia or ocular misalignment.
Visual function, particularly as measured by low-contrast letter acuity, has been identified as an important component in the profile of patients with multiple sclerosis (MS). Low-contrast acuity is an easily administered quantitative measure that correlates well with overall visual dysfunction,1 quality of life (QoL),2 magnetic resonance imaging (MRI) lesion burden,3 and response to treatment4 in MS. In assessing contrast sensitivity in healthy patient cohorts, investigators have noted the presence of “binocular summation,” or improved vision under binocular viewing conditions, when compared to scores for either eye individually.5 However, the magnitude of binocular summation is not as great in patients with cataracts, advanced age, and amblyopia.6,7 Furthermore, in patients with large interocular differences in contrast sensitivity, there appears to be “binocular inhibition,” by which the contrast sensitivity score with both eyes together is worse than that of the better eye alone.7 The phenomena of binocular summation and inhibition are not well understood, but appear to be related to neural interactions of input from both eyes within the post-geniculate visual pathway.5,6
Binocular low-contrast acuity has been used frequently as a visual outcome in MS trials. At the same time, monocular measurements have been introduced into MS trials with the inclusion of optical coherence tomography (OCT) scanning as a structural correlate to vision in MS. Since binocular and monocular acuities are likely complementary, it will be critical to know how these 2 types of measurements relate to each other, and to determine whether patients with MS have the capacity for binocular summation. For understanding visual function following optic neuritis, it would also be useful to know whether those patients with unilaterally poor vision demonstrate the phenomenon of binocular inhibition. In a study of 15 patients with MS and a history of unilateral optic neuritis, the majority showed evidence of binocular summation.8
The purpose of our study was to determine the magnitude of binocular summation of acuity in patients with MS compared to that of disease-free control subjects, and to determine the impact that age, interocular difference in acuity, and a history of acute optic neuritis have on the magnitude of binocular summation. We also sought to examine how the magnitude of binocular summation relates to vision-specific quality of life and to individual eye acuities in patients with MS.
METHODS
PATIENTS AND DISEASE-FREE CONTROL SUBJECTS WERE enrolled as part of an ongoing prospective study of visual outcome measures in MS at the University of Pennsylvania, Johns Hopkins University, and the University of Texas Southwestern Medical Center at Dallas. MS was diagnosed by standard clinical and neuroimaging criteria.9
A history of 1 or more episodes of acute optic neuritis was determined for eyes of patients with MS by self-report and physician diagnosis, and confirmed by medical record review. The diagnosis of a prior history of clinical optic neuritis was usually made in the presence of clinical symptoms consistent with optic neuritis (acute change in visual acuity with pain on eye movements, loss of color vision, afferent pupillary defect, or decreased contrast sensitivity) with a normal ophthalmology examination, or optic nerve edema. An MRI scan demonstrating optic nerve enhancement was useful but not essential for confirming the diagnosis. Patients were excluded if they had any comorbid ocular conditions that impacted visual acuity (ascertained by a detailed history and examination), or if they had acute optic neuritis within the previous 1 to 3 months. Patients were excluded if their Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity (VA) equivalent on the ETDRS chart was worse than 20/200 in either eye, because such poor vision precludes testing of low-contrast acuity. Disease-free control participants were recruited from staff and family members of patients, and were required to have had no history of ocular or neurologic disease. Controls were excluded if their VA was worse than 20/20 in either eye.
VISUAL FUNCTION TESTING
After a distance refraction was performed, low-contrast acuity was tested with Sloan letter charts at 1.25% and 2.5% contrast levels positioned at 2 meters on a retroilluminated cabinet (Precision Vision, LaSalle, Illinois, USA) using the appropriate refractive correction. Sloan charts have a format similar to the ETDRS VA charts (5 letters per line), and each Sloan chart corresponds to a different contrast level. The low-contrast acuity score is the number of letters identified correctly, with a maximum score of 70 (14 lines). High-contrast visual acuity (VA) was tested using the ETDRS charts at 3.2 meters (Lighthouse Low-Vision Products, Long Island City, New York, USA) with the appropriate refractive correction. The VA score is also the number of letters identified correctly, with a maximum score of 70 (Snellen equivalent of 20/12.5). Each of the 3 acuity measures (2.5%, 1.25%, VA) were tested with both eyes together (binocular testing), and then for each eye individually (monocular testing). All testing was performed by trained technicians experienced in examination of patients for research studies. Technicians adhered to detailed standard protocols, including written scripts and instructions for testing.
For each participant and each contrast level, the difference between the binocular letter score and the monocular score for the better eye was calculated. This value was called the “magnitude of binocular summation” (Table 1). If this value was less than 0, then this was defined as binocular inhibition.
TABLE 1.
Definitions of Terms Used to Characterize Visual Function Tests in Patients With Multiple Sclerosis and Disease-Free Controls
| Acuity Score Term | Definition and Calculation |
|---|---|
| Monocular acuity score |
Number of letters identified correctly using either eye separately |
| Better eye | Eye with greater score for high- contrast visual acuity (VA) based on number of letters identified correctly |
| Binocular acuity score | Number of letters identified correctly using both eyes together |
| Magnitude of binocular summation |
Extent to which binocular acuity score is greater than monocular acuity score for better eye = binocular acuity score minus better eye acuity score |
| Binocular inhibition | Binocular acuity score is less than monocular acuity score for better eye |
QUALITY-OF-LIFE ASSESSMENT
The 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25)10 was self-administered in all participants to assess self-reported, vision-specific QoL. The NEI VFQ-25, the standard QoL measure for ophthalmology clinical trials, consists of 12 sub-scales (general health, general vision, ocular pain, near activities, distance activities, social functioning, mental health, role difficulties, dependency, driving, color vision, and peripheral vision); the composite score is generated as the unweighted average of all items, excluding the single item for general health. A 10-item neuro-ophthalmic supplement to the NEI VFQ-25 was also administered.11
STATISTICAL METHODS
All data analyses were performed using Stata statistical software (version 11.0; StataCorp, College Station, Texas, USA). Groups were compared with respect to mean values for acuity scores and magnitudes of binocular summation using 2-sample t tests. To determine whether the magnitudes of binocular summation were significantly different from zero (and thus likely to represent true improvements of vision for binocular compared to monocular scores), a t test was also used comparing each group mean to the null value of 0; a 2-sided test was used as some patients showed binocular inhibition (magnitude of binocular summation <0). A type I error level α = 0.05 was used for statistical significance.
Evaluation of factors associated with the magnitude of binocular summation
Linear regression analyses were performed to assess the relation between the magnitude of binocular summation and MS vs control status (or optic neuritis vs non-optic neuritis history within the MS cohort), accounting for age. Similar models were constructed to account for interocular acuity differences and to determine their effects on magnitude of binocular summation.
Relation of binocular low-contrast acuity scores and magnitude of summation to monocular acuities
Also using linear regression models, we examined the relation of binocular low-contrast acuity scores and magnitude of summation to 1) better eye low-contrast acuity scores, 2) worse eye low-contrast acuity scores, and 3) average of low-contrast acuity scores for the 2 eyes.
Impact of interocular difference on binocular summation and inhibition
The proportions of patients demonstrating binocular summation and binocular inhibition were calculated for each patient subgroup. Test-retest variability for low-contrast acuity, defined as 2 standard deviations of the published inter-rater difference, is approximately 7 letters for MS and control subjects.12 For purposes of these analyses of proportions, the 7-letter cutoff was used to categorize patients as having binocular inhibition (magnitude of binocular summation ≤–7) or binocular summation (magnitude of summation ≥7). Subjects whose magnitudes of summation were >–7 and <7 were categorized as having neither binocular summation nor binocular inhibition. Proportions of patients demonstrating binocular summation or inhibition within patient groups were compared using the χ2 test.
Relation of binocular acuity scores and magnitude of summation to quality of life
Linear regression analysis was used to determine whether greater magnitudes of binocular summation are associated with better composite and subscale scores for the NEI VFQ-25, accounting for age, interocular acuity difference, and high-contrast VA.
RESULTS
AMONG 1007 PATIENTS WITH MS AND 324 DISEASE-FREE control participants, the mean age of the 2 groups was similar (43 ± 11 years for MS and 40 ± 11 years for controls, P = .19). Within the MS group, 46% of patients had a prior history of acute optic neuritis.
The mean VA and low-contrast acuity scores for each patient group are summarized in Table 2. All groups demonstrated evidence of binocular summation for each measure of visual function. Magnitudes of binocular summation were significantly different from zero for all groups (P < .001, 1-sample t tests). The mean magnitude of binocular summation was greater at lower levels of contrast than at high contrast (4-7 letters vs 1 letter) in all groups.
TABLE 2.
Mean Scores (Numbers of Letters Correct) for High-Contrast Visual Acuity and Low-Contrast Letter Acuity in Patients With Multiple Sclerosis, With or Without a History of Acute Optic Neuritis, and Disease-Free Controls
| Visual Function Test | MS (n = 1007) |
Disease-Free Controls (n = 324) |
MS With No History of ON (n = 544) |
MS With History of ON (n = 463) |
|---|---|---|---|---|
| Visual acuity (high-contrast) | ||||
| Worse eye score | 55 ± 14 | 61 ±7 | 57 ± 11 | 52 ± 17 |
| Better eye score | 61 ±9 | 64 ±6 | 61 ±8 | 60 ± 10 |
| Binocular score | 62 ±8 | 66 ±5 | 63 ±7 | 61 ± 10 |
| Magnitude of binocular summationa | 1 ±3 | 1±2 | 1±3 | 1± 3 |
| P valueb | <.001 | <.001 | <.001 | <.001 |
| Low-contrast acuity 2.5% | ||||
| Worse eye score | 24 ± 13 | 34 ±8 | 27 ± 11 | 20 ± 14 |
| Better eye score | 32 ± 10 | 37 ±7 | 33 ±9 | 30 ± 12 |
| Binocular score | 36 ± 10 | 43 ±6 | 38 ±9 | 35 ± 11 |
| Magnitude of binocular summationa | 5 ±4 | 5±4 | 5±4 | 4± 4 |
| P valueb | <.001 | <.001 | <.001 | <.001 |
| Low-contrast acuity 1.25% | ||||
| Worse eye score | 11 ± 11 | 23 ± 10 | 14 ± 11 | 9 ± 10 |
| Better eye score | 18 ± 11 | 27 ±9 | 19 ± 11 | 17 ± 11 |
| Binocular score | 24 ± 11 | 34 ±8 | 26 ± 11 | 22 ± 12 |
| Magnitude of binocular summationa | 6 ±6 | 7±5 | 7±6 | 5± 6 |
| P valueb | <.001 | <.001 | <.001 | <.001 |
MS = multiple sclerosis; ON = optic neuritis.
Magnitude of binocular summation calculated as binocular letter score minus monocular letter score for better eye.
P values calculated using a 1-sample t test comparing the mean value to 0 for magnitude of binocular summation.
MAGNITUDES OF BINOCULAR SUMMATION
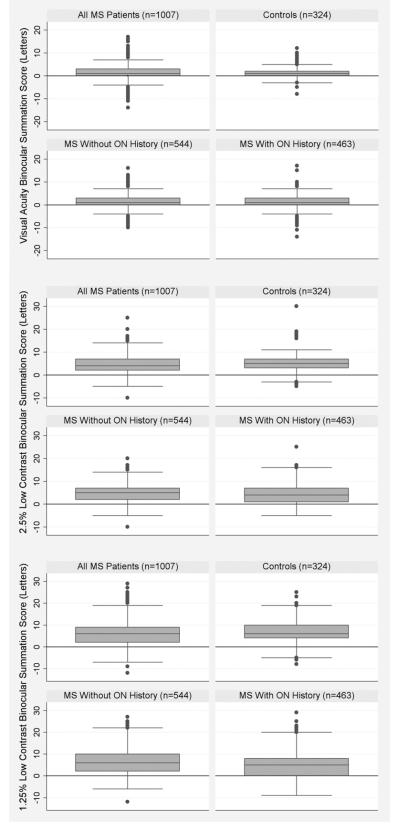
Magnitudes of binocular summation for each patient group are summarized in the Figure. For VA and 2.5% low-contrast acuity, there were no significant differences between the MS cohort and controls. Within the MS cohort, there was no difference between patients with a history of optic neuritis and those with no history of optic neuritis, except at the lowest contrast level. Patients with optic neuritis summated less well than those without optic neuritis for the 1.25% contrast charts (difference of 5.3 ± 6 letters vs 7.0 ± 6 letters, P = .0001, 2-sample t tests).
FIGURE.
Magnitude of binocular summation for low-contrast letter acuity and high-contrast visual acuity (VA) in multiple sclerosis and disease-free control subjects. Binocular summation = binocular letter score minus better eye letter score for all patient subgroups and all contrast levels. (Top) Results for high-contrast VA. (Middle) Results for low-contrast letter acuity at 2.5% contrast. (Bottom) Results for low-contrast letter acuity at 1.25% contrast. Boxes define the median ± interquartile range. Error bars represent 1.5× the interquartile range. Additional points represent outliers. Multiple sclerosis patients without optic neuritis had significantly (P = .015) higher binocular summation scores than those with a history of optic neuritis for 1.25% low-contrast acuity. MS = multiple sclerosis, ON = optic neuritis.
PROPORTIONS OF PATIENTS WITH BINOCULAR SUMMATION AND INHIBITION BEYOND TEST-RETEST VARIABILITY (MAGNITUDE OF SUMMATION ≥7 OR ≤−7 LETTERS)
Less than 2% (14/1331 total study subjects for VA, 2/1331 total study subjects for 2.5% low contrast acuity, and 6/1331 total study subjects for 1.25% low contrast acuity) of patients had binocular inhibition (Table 3). Most of the patients with binocular inhibition had interocular differences between 6 and 20 letters for VA and between 6 and 25 letters for low-contrast acuity. None of the 150 patients who had interocular acuity differences of >25 letters demonstrated binocular inhibition. The percentages of patients with binocular summation (binocular summation score ≥7) for high-contrast VA were less than 5% in all groups (Table 3). The proportions with binocular summation were much higher for 2.5% low-contrast acuity (28%-31%) and 1.25% low-contrast acuity (29%-52%) compared to high-contrast testing. Within MS patients, those with a history of optic neuritis had a lower percentage with binocular summation on 1.25% low-contrast acuity than those without a history of optic neuritis (P < .001).
TABLE 3.
Proportions (95% Confidence Interval) of Multiple Sclerosis and Disease-Free Control Subjects Demonstrating Binocular Summation or Inhibition Beyond Test-Retest Variability (±7 Letters) for High-Contrast Visual Acuity and Low-Contrast Letter Acuity
| All MS (n = 1007) | Controls (n = 324) | MS Without a History of ON (n = 544) |
MS With a History of ON (n = 463) |
|
|---|---|---|---|---|
| VA | ||||
| % binocular summationa | 3.6% (2.5, 4.9) | 3.0% (1.4, 5.6) | 3.1% (1.8, 4.9) | 4.2% (2.4, 6.3) |
| % binocular inhibitionb | 1.3% (0.7, 2.2) | 0.4% (0.01, 1.7) | 1.2% (0.5, 2.6) | 1.4% (0.5, 2.8) |
| 2.5% LCA | ||||
| % binocular summationa | 31% (28, 34) | 31% (26, 36) | 33% (29, 37) | 28% (24, 32) |
| % binocular inhibitionb | 0.2% (0.02, 0.7) | 0 (0, 1.1) | 0.3% (0.04, 1.3) | 0 (0, 8.0) |
| 1.25% LCA | ||||
| % binocular summationa | 44% (41, 47) | 49% (44, 55) | 52% (48, 56) | 29% (25, 33) |
| % binocular inhibitionb | 0.5% (0.16, 1.1) | 0.4% (0.01, 1.7) | 0.2% (0.004, 1.0) | 0.9% (0.2, 2.0) |
LCA = low-contrast letter acuity; MS = multiple sclerosis; ON = optic neuritis; VA = high-contrast visual acuity.
Binocular summation defined as difference between the binocular score and the better eye score >7 letters.
Binocular inhibition defined as difference between the binocular score and the better eye score <–7 letters.
EVALUATING FACTORS ASSOCIATED WITH THE MAGNITUDE OF BINOCULAR SUMMATION
Linear regression models were used to examine the associations between magnitude of binocular summation and MS vs control status (Table 4). Within the MS cohort, optic neuritis vs non–optic neuritis history was also examined for its association with magnitude of binocular summation. MS vs control status was not associated with a decrement in binocular summation at any of the contrast levels, accounting for age (P = .6-.9). However, among patients with MS, a history of optic neuritis was associated with lower magnitudes of binocular summation at the 1.25% contrast level (P < .0001). When interocular difference was also accounted for in the models, both a history of optic neuritis (P = .015) and the degree of interocular difference (P < .0001) were associated with lower magnitudes of binocular summation. For low-contrast acuity, increased age was associated with lower magnitudes of binocular summation at both the 2.5% and 1.25% levels, even accounting for MS vs control or optic neuritis vs non–optic neuritis status (P < .0001, linear regression models).
TABLE 4.
Association of Binocular Summation Score, Disease Status, Age, and Interocular Difference in Multiple Sclerosis Patients for High-Contrast Visual Acuity and Low-Contrast Letter Acuity
| Measure, Variable | Coefficient From Linear Regression Modela |
P Value |
|---|---|---|
| All patients (N = 1331) | ||
| Visual acuity (high-contrast) | ||
| MS vs control | −0.09 | .6 |
| Age, years | −0.01 | .66 |
| Low-contrast, 2.5% | ||
| MS vs control | 0.02 | .9 |
| Age, years | −0.07 | <.0001a |
| Low-contrast, 1.25% | ||
| MS vs control | 0.04 | .9 |
| Age, years | −0.09 | <.0001a |
| MS patients only (N = 1007) | ||
| Visual acuity (high-contrast) | ||
| ON vs no ON | −0.08 | .7 |
| Age, years | −0.01 | .2 |
| Low-contrast, 2.5% | ||
| ON vs no ON | −0.05 | .2 |
| Age, years | −0.07 | <.0001a |
| Low-contrast, 1.25% | ||
| ON vs no ON | −1.72 | <.0001a |
| Age, years | −0.11 | <.0001a |
| MS patients only (N = 1007) | ||
| Visual acuity (high-contrast) | ||
| ON vs no ON | 0.01 | .78 |
| Age, years | −0.01 | .29 |
| Interocular difference, letters | −0.20 | .32 |
| Low-contrast, 2.5% | ||
| ON vs no ON | 0.70 | .8 |
| Age, years | −0.07 | <.0001a |
| Interocular difference, letters | −0.14 | <.0001a |
| Low-contrast, 1.25% | ||
| ON vs no ON | −1.0 | .015a |
| Age, years | −0.12 | <.0001a |
| Interocular difference, letters | −0.25 | <.0001a |
MS = multiple sclerosis; ON = history of optic neuritis.
Linear regression models were used to examine the relation between binocular summation and various patient characteristics.
RELATION OF BINOCULAR ACUITY AND MAGNITUDE OF BINOCULAR SUMMATION TO MONOCULAR VISION
Among patients with MS, the magnitude of binocular summation for the 2 low-contrast tests was weakly correlated with the monocular scores (range of R2 0.03 to 0.10, after accounting for age). The correlation with the magnitude of binocular summation was slightly stronger for the better eye (R2 = 0.1, P < .001 for both 2.5% and 1.25% low-contrast acuity, accounting for age), compared to those for the worse eye (R2 = 0.03, P = .07 for 2.5% low-contrast acuity and R2 = 0.03, P = .7 for 1.25% low-contrast acuity) or an average of acuity scores for the 2 eyes (R2 = 0.06, P < .001 for 2.5% low-contrast acuity and R2 = 0.04, P = .001 for 1.25% low-contrast acuity). Conversely, binocular acuity scores for the 2 low-contrast tests were strongly correlated with the monocular scores (R2 ranging from 0.5 to 0.8). The correlation with the binocular acuity score was slightly stronger for the average score for the 2 eyes (R2 = 0.8, P < .001 for 2.5% low-contrast acuity and R2 = 0.7, P < .001 for 1.25% low-contrast acuity), compared to those for the better eye (R2 = 0.7, P < .001 for 2.5% low-contrast acuity and R2 = 0.6, P < .001 for 1.25% low-contrast acuity) or worse eye (R2 = 0.5, P < .001 for 2.5% low-contrast acuity and R2 = 0.5, P < .001 for 1.25% low-contrast acuity).
BINOCULAR SUMMATION AND QUALITY OF LIFE
Higher magnitudes of summation for low-contrast acuity at 2.5% were associated with higher (better) scores for the NEI VFQ-25 composite (P = .02), distance vision (P = .003), and neuro-ophthalmic supplement scores (P = .03). Using linear regression models accounting for age, a 1-line difference in magnitude of binocular summation for 2.5% low-contrast acuity is associated with a 4-point difference (95% confidence interval: 1, 7) in NEI VFQ-25 composite score. Similarly, a 1-line difference in magnitude of binocular summation for 1.25% low-contrast acuity is associated with a 2-point difference (95% confidence interval: 1, 5). For high-contrast VA, less binocular summation was not associated with worse composite scores (P = .51) after accounting for age and acuity score.
DISCUSSION
BINOCULAR HIGH-CONTRAST VA AND LOW-CONTRAST acuity measures are now frequently used for testing of visual function in MS clinical trials.1–4,13 Although binocular low-contrast acuity has been correlated with vision-related QoL,2 MRI lesion burden,3 retinal nerve fiber layer thickness measures,13,14 and treatment effects4 in MS, it has been unclear whether binocular low-contrast acuity scores reflect those of the better eye, the worse eye, or a value in between the 2 eyes. Binocular low-contrast acuity in young, healthy test subjects reflects some degree of binocular summation, such that the binocular score is greater than the score for the better eye. Since binocular summation most likely occurs at the cortical level,5,6 it follows that patients with MS may not have a “normal” binocular experience, since the pathologic change of MS may interrupt normal cortical signaling. It is not known how prior episodes of optic neuritis, vision loss, or age similarly affect the binocular experience in MS patients. A better understanding of the role that these factors play in an individual’s capacity for binocular summation may provide insight into how these findings negatively impact a patient’s binocular function.
The results of this investigation demonstrate that some binocular summation of low-contrast acuity occurs in MS patients. Furthermore, a history of acute optic neuritis, older age, and greater interocular difference decrease the magnitude of binocular summation in MS patients. This is most evident when patients are tested at the lowest contrast levels. Although the mean magnitude of binocular summation in all patient groups ranged from only 1 to 7 letters, greater magnitudes of summation were associated with a modest improvement in vision-specific QoL. Consistent with other ophthalmologic disorders that affect the anterior visual pathway,6,7,15–19 our study of patients with MS shows that increasing age and greater interocular acuity differences diminish individual patients’ abilities for binocular summation, and ultimately can culminate in binocular inhibition. Of interest is the finding that the magnitude of binocular summation is greatest at lower contrast levels (1.25%), as reported for normal subjects. With respect to metrics that best reflect visual function, the average of the low-contrast acuity scores from the 2 eyes was confirmed to most strongly reflect binocular acuity scores. In contrast, the phenomenon of binocular summation is most strongly associated with the score derived from the better eye. Collectively, these observations are important for the continued implementation of binocular acuity measurements in MS investigations.
Previous large-scale studies of binocular summation have focused on the effects of increasing age and interocular differences in high-contrast VA on the magnitude of summation. Binocular summation has been evaluated for high-contrast VA in 2 large cohorts of patients: The Salisbury Eye Evaluation (SEE)19 and the Los Angeles Latino Eye Study (LALES).15 The SEE group evaluated 2520 elderly (>65 years) Americans, and the LALES evaluated 1831 adults, with ages ranging from 40 to 95 years. The observations from these cohorts differed slightly, with the SEE group demonstrating a very small degree of summation of high-contrast VA and LALES showing less binocular summation (and more binocular inhibition) in the elderly portion of their study group compared to younger participants. Larger interocular differences were also associated with less binocular summation in those population-based studies. The SEE and LALES studies did not evaluate binocular summation at low-contrast acuity.
The effects of aging on binocular summation of contrast sensitivity and spatial frequency discrimination have been recently investigated.6 The mean binocular summation ratio (binocular score / better eye score) in elderly subjects was significantly lower than for younger participants, especially at high spatial frequencies. Further, in the elderly patients, there was evidence of binocular inhibition. Independent of aging, the effect of interocular differences on low-contrast acuity has also been well studied in patients with unilateral cataracts.7 It has been shown that unilateral cataracts, which cause a difference in the monocular low-contrast acuity, can lead to decreased binocular summation, and even binocular inhibition. This occurs once a critical threshold interocular difference is reached.16–18 Identification of such a threshold has been evaluated with varying methods, and has been found to correspond to a 0.7-log decrement between the 2 eyes.18 There has been only 1 study that has evaluated the characteristics of binocular summation specifically in MS patients.8 This study evaluated contrast sensitivity in 15 patients with a history of optic neuritis and 13 healthy controls. This small study did not show differences between patients and controls, yet provided a basis for future investigation.
In our MS cohort, we found that a prior clinical history of optic neuritis was associated with less binocular summation for low-contrast acuity. This finding persisted even when we accounted for interocular differences in acuity, suggesting that other mechanisms may contribute to decreased summation in these patients. This finding could potentially be explained by several hypotheses. First, optic nerves of optic neuritis–affected eyes likely carry visual signals to the lateral geniculate nucleus at a slower rate than the fellow eyes. Perhaps this difference in the speed of signal transduction diminishes binocular summation. This hypothesis could be further tested by characterizing binocular summation in patients with symptoms of the Pulfrich phenomenon, or the perception of elliptical motion for an object that in reality is only moving side-to-side. Neuro-degeneration in the visual pathways is also a likely etiology of less efficient binocular combination in patients with a history of optic neuritis. In addition to white matter axonal loss, it is now recognized that gray matter disease accounts for a substantial amount of disability in patients with MS. Neuronal loss has likewise been demonstrated in the eyes of MS patients, particularly in those with a history of optic neuritis, by reductions in macular volume.20 Future investigation with functional MRI and OCT will further evaluate the contributions of visual pathway axonal and neuronal loss to binocular summation in MS.
In addition to the above findings, we also determined the range of interocular differences in acuity that were associated with binocular inhibition. These differences were 6 to 20 letters for VA and 6 to 25 letters for low-contrast acuity. Patients with greater interocular differences in acuity did not show binocular inhibition. This finding has been demonstrated by other groups18 and may be explained by the fact that at very large interocular differences, the vision in the worse eye is so poor that its signal does not contribute to binocular combination at all (so the binocular vision score approximates that of the better eye). This finding of a threshold level, or “tipping point” at which binocular summation no longer occurs and binocular inhibition appears, is consistent with prior studies of normal subjects using neutral density filters and optical defocus.16,18 These findings add to our understanding of why, in some cases, patients with interocular differences between 2 and 5 lines of low-contrast acuity note difficulty with binocular vision, and may tend to close 1 eye during visual tasks.
The implications of our study are important in understanding the binocular experience of patients who have a history of MS with or without a prior history of optic neuritis. Although MS itself does not appear to impact a patient’s capacity for binocular summation, those patients who have a clinical history of acute optic neuritis are less likely to have binocular summation, and are more likely to experience binocular inhibition, than healthy controls. These findings provide some insight into how visual function in low-contrast settings, such as fog or night-time driving, might be affected in patients with MS.
Acknowledgments
PUBLICATION OF THIS ARTICLE WAS SUPPORTED BY THE NATIONAL MULTIPLE SCLEROSIS SOCIETY, NEW YORK, NEW YORK (grant PP1115, L.J.B.); National Multiple Sclerosis Society Tissue Repair Partnership (grant TR 3760-A-3, L.J.B. and P.A.C.); National Institute of Health/National Eye Institute, Bethesda, Maryland (grant K24 EY 014136, L.J.B. and M.G.M.); DAD’s Foundation, and McNeill Foundation. All authors were involved in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the article (S.L.P., E.E.B., L.S.T., D.J.S., E.M.F., P.A.C., S.L.G., M.G.M., L.J.B.). This study was approved by Institutional Review Boards at the University of Pennsylvania, the Johns Hopkins University, and the University of Texas-Southwestern. The study was HIPAA compliant and adhered to the Declaration of Helsinki and all relevant federal and state laws. All subjects signed informed consent documents.
REFERENCES
- 1.Baier ML, Cutter GR, Rudick RA, et al. Low-contrast letter acuty testing captures visual dysfunction in patients with multiple sclerosis. Neurology. 2005;64(6):992–995. doi: 10.1212/01.WNL.0000154521.40686.63. [DOI] [PubMed] [Google Scholar]
- 2.Mowry EM, Loguidice MJ, Daniels AB, et al. Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J Neurol Neurosug Psychiatry. 2009;80(7):767–772. doi: 10.1136/jnnp.2008.165449. [DOI] [PubMed] [Google Scholar]
- 3.Wu GF, Schwartz ED, Lei T, et al. Relation of vision to global and regional brain MRI in multiple sclerosis. Neurology. 2007;69(23):2128–2135. doi: 10.1212/01.wnl.0000278387.15090.5a. [DOI] [PubMed] [Google Scholar]
- 4.Balcer LJ, Galetta SL, Calabresi PA, et al. Natalizumab reduces visual loss in patients with relapsing multiple sclerosis. Neurology. 2007;68(16):1299–1304. doi: 10.1212/01.wnl.0000259521.14704.a8. [DOI] [PubMed] [Google Scholar]
- 5.Blake R, Sloane M, Fox R. Further developments in binocular summation. Percep Psychophys. 1981;30(3):266–276. doi: 10.3758/bf03214282. [DOI] [PubMed] [Google Scholar]
- 6.Gagnon RWC, Kline KW. Senescent effects on binocular summation for contrast sensitivity and spatial interval acuity. Curr Eye Res. 2003;27(5):315–321. doi: 10.1076/ceyr.27.5.315.17225. [DOI] [PubMed] [Google Scholar]
- 7.Pardhan S, Elliott DB. Clinical measurements of binocular summation and inhibition in patients with cataract. Clin Vision Sci. 1991;6(5):355–359. [Google Scholar]
- 8.Newman NJ, Wolfe JM, Steward MI, Lessell S. Binocular visual function in patients with a history of monocular optic neuritis. Clin Vision Sci. 1991;6(2):95–107. [Google Scholar]
- 9.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50(1):121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 10.Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire (VFQ-25) Arch Ophthalmol. 2001;119(7):1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 11.Raphael BA, Galetta KM, Jacobs DA, et al. Validation and test characteristics of a 10-item neuro-ophthalmic supplement to the NEI-VFQ-25. Am J Ophthalmol. 2006;142(6):1026–1035. doi: 10.1016/j.ajo.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 12.Balcer LJ, Baier ML, Pelak VS, et al. New low-contrast vision charts: reliability and test characteristics in patients with multiple sclerosis. Multiple Sclerosis. 2000;6(3):163–171. doi: 10.1177/135245850000600305. [DOI] [PubMed] [Google Scholar]
- 13.Fisher JB, Jacobs DA, Markowitz CE, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113(2):324–332. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 14.Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749–760. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azen SP, Varma R, Preston-Martin S, Ying-Lai M, Globe D, Hahn S. Binocular visual acuity summation and inhibition in the ocular epidemiological study: the Los Angeles Latino eye study. Invest Ophthalmol Vis Sci. 2002;43(6):1742–1748. [PubMed] [Google Scholar]
- 16.Pardhan S, Gilchrist J. The effect of monocular defocus on binocular contrast sensitivity. Ophthal Physiol Opt. 1990;10(1):33–36. [PubMed] [Google Scholar]
- 17.Pardhan S, Gilchrist J. Binocular contrast sensitivity with monocular glare disability. Ophthal Physiol Opt. 1990;10(1):37–39. [PubMed] [Google Scholar]
- 18.Pardhan S, Gilchrist J, Douthwaite W, Yap M. Binocular inhibition: psychophysical and electrophysiological evidence. Optom Vis Sci. 1990;67(9):688–691. doi: 10.1097/00006324-199009000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Rubin GS, Munoz B, Bandeen-Roche K, et al. Monocular versus binocular visual acuity as measures of vision impairment and predictors of visual disability. Invest Ophthalmol Vis Sci. 2000;41(11):3327–3334. [PubMed] [Google Scholar]