Abstract
Rationale/Objectives
This study examined the effects of propranolol vs. placebo, administered immediately after a 'retrieval' session of cocaine cue exposure (CCE), on craving and physiological responses occurring 24 hr. later during a subsequent 'test' session of CCE. It was hypothesized that compared to placebo-treated cocaine-dependent (CD) individuals, propranolol-treated CD individuals would evidence attenuated craving and physiological reactivity during the test session. Secondarily, it was expected that group differences identified in the test session would be evident at a 1-week follow-up CCE session. Exploratory analyses of treatment effects on cocaine use were also performed at follow-up.
Methods
CD participants received either 40 mg propranolol or placebo immediately following a 'retrieval' CCE session. The next day, participants received a 'test' session of CCE that was identical to the 'retrieval' session except no medication was administered. Participants underwent a ‘follow-up’ CCE session 1-week later. Craving and other reactivity measures were obtained at multiple time points during the CCE sessions.
Results
Propranolol- vs. placebo-treated participants evidenced significantly greater attenuation of craving and cardiovascular reactivity during the test session. Analysis of the follow-up CCE session data did not reveal any group differences. Although there was no evidence of treatment effects on cocaine use during follow-up, this study was insufficiently powered to rigorously evaluate differential cocaine use.
Conclusions
This double-blind, placebo-controlled laboratory study provides the first evidence that propranolol administration following CCE may modulate memories for learning processes that subserve cocaine craving/cue reactivity in CD humans. Alternative interpretations of the findings were considered and implications of the results for treatment were noted.
Keywords: Reconsolidation, Retrieval, Craving, Cocaine Dependence, Cue Exposure, Human Subjects
Introduction
Memory consolidation refers to a post-learning process (or processes) whereby new information, initially persisting in a relatively fragile state, gradually consolidates or becomes more stable over time (Dudai 2004; McGaugh 2000; Nader and Einarsson 2010; Nader and Hardt 2009). The concept of consolidation is over 100 years old (Lechner et al. 1999) and it has been the impetus for multidisciplinary efforts to elucidate neural and cellular processes that affect lasting memory (Dudai, 2004). Reconsolidation denotes a process (or processes) during which retrieved memories can either be strengthened or otherwise altered by updating or integrating new information into long-term storage (Hardt et al. 2010; Nadel and Hardt 2011; Sara 2000). Generally, the memory retrieval process that defines reconsolidation is initiated by the presentation of cues that putatively elicit targeted memories (cf., Alberini 2005; Hernandez and Kelley 2005).
Some of the earliest basic neuroscience research on reconsolidation showed that the noradrenergic system, likely via action in the basolateral amygdala and related limbic structures, is involved in memory reconsolidation and that administration of an adrenergic receptor antagonist such as propranolol can attenuate or disrupt reconsolidation of memory for learned behaviors (Debiec and Ledoux 2004; Przybyslawski et al. 1999; Sara 2000). In the prototypical experiment (Debiec and Ledoux, 2004), some form of basic associative learning is established in animal subjects, say discrete cue fear conditioning of a conditioned stimulus (CS); later, subjects are exposed to the CS to elicit retrieval/reactivation of the memory for the learned association. Approximately, 24–48 hours later the animals are tested for their response (e.g., freezing) to the fear CS. Evidence of disrupted reconsolidation usually takes the form of a diminished or absent fear response. Importantly, these deficits in responding are not seen in subjects that receive only vehicle at the time of memory retrieval or in subjects that receive propranolol but not retrieval exposure to the CS. This latter observation is important because it shows that disruption of reconsolidation (DoR) is inextricably tied to reactivation of the fear memory (Przybyslawski et al. 1999).
More recent studies have demonstrated DoR in human laboratory studies using propranolol as the disrupting agent to target emotionally aversive memories (Kindt et al. 2009; Schwabe et al. 2012; Sevenster et al. 2012; Soeter and Kindt 2010; 2011; 2012a; b). As the apparent robustness of the DoR phenomenon has become more established, basic science investigators have begun encouraging the clinical science community to test the utility of safe DoR agents in the treatment of human fear-based anxiety disorders (cf., Debiec and Altemus 2006; Debiec and LeDoux 2006; Dudai 2006). PTSD was considered a good candidate disorder given the strong conditioning element to its etiology and given that recurrent, intrusive and emotionally aversive memories are dominant clinical features. To date, two published reports by Brunet and colleagues (Brunet et al. 2008; Brunet et al. 2011b) have provided initial evidence that post-retrieval propranolol (i.e., administered following reactivation of trauma memories) may attenuate fear-based behaviors and PTSD symptoms. Collectively, infrahuman and human laboratory research on DoR in aversively motivated behavior and emerging clinical research on DoR in PTSD begs two questions: (a) does propranolol-induced DoR occur in appetitively-based behavior with infrahumans? and (b) does propranolol-induced DoR occur in appetitively-based learning in humans, perhaps in the form of translational studies targeting the treatment of appetitively-motivated psychopathology such as addictive behavior?
The answer to the first question is that there is a substantial and growing body of infrahuman research showing parallel effects with appetitive conditioning. Przybyslawski, Roullet, & Sara (1999) demonstrated that propranolol-induced performance impairments in a food reinforced, radial arm maze (rat subjects) were most pronounced when subjects received the propranolol injection 5-minutes, as compared to 2-hours, after the maze run. This finding suggests that administering propranolol as soon as possible after the retrieval cue presentation could maximize deficits in memory reconsolidation. Diergaarde, Schoffelmeer, & De Vries (2006) studied the effects of propranolol (in rats) after a retrieval presentation of a context in which a sucrose-reinforced instrumental behavior (nose-pokes) had been learned three weeks earlier. The duration of context re-exposure (i.e., retrieval cue) was varied and the longest exposure demonstrated the most robust effect. A third study (Milton et al. 2008; Experiment 1) showed that propranolol-induced DoR could impair the ability of a sucrose-reinforced cue to serve as a reinforcer for new instrumental behavior. Collectively, these studies suggest that propranolol can impair performance of appetitively-motivated behavior and that DoR is maximized when (a) propranolol is administered immediately after retrieval cue presentation, and (b) the retrieval cue exposure is of relatively long duration.
A logical extension of the appetitive learning/memory studies noted above is to investigate impairment of memory reconsolidation for behaviors that are reinforced with drugs of abuse. There is now a considerable volume of basic neuroscience literature showing that propranolol can disrupt reconsolidation of learning reinforced with cocaine (Bernardi et al. 2006; Fricks-Gleason and Marshall 2008; Milton et al. 2008; Experiment 2) and morphine (Robinson et al. 2011a; Robinson and Franklin 2007; 2010; Robinson et al. 2011b) and alcohol (Font and Cunningham 2012). While there have been some failures to observe propranolol induced DoR in studies that targeted morphine (Robinson et al. 2011a; Experiments 2 & 3) and alcohol (Wouda et al. 2010) reinforced learning, all four of the cocaine studies produced evidence of DoR. Of these four studies, three used a conditioned place preference (CPP) paradigm in which animals learn to prefer a cocaine-paired chamber. The collective findings of these CPP studies indicated that DoR (a) has a long lasting effect on cocaine reinforced behavior that may persist even after re-exposure to cocaine, (b) can be achieved with as few as one medicated retrieval but may be more robust if more than one medicated retrieval is administered, and (c) is likely a consequence of propranolol action in the brain (not peripherally). The fourth study (Milton et al. 2008; Experiment 2) showed that propranolol-induced DoR could impair the ability of a cocaine-reinforced cue to serve as a reinforcer for a new instrumental behavior. In sum, there appears to be a considerable body of evidence that drug-seeking behavior, which is dependent on memory for stimulus-drug associations, can be attenuated/disrupted by propranolol.
Given the substantial and compelling nature of the findings on DoR of memory for appetitively-based learning in the basic neuroscience literature, one might expect that the answer to the second question above might also be in the affirmative. This is not the case. There appears to be neither any laboratory-based investigations in humans that parallel those noted above (e.g., Kindt and colleagues) nor any developing translational work in the addictions treatment literature that corresponds to the efforts of Brunet and colleagues. However, there is one recent study that did examine propranolol induced DoR of drug associated memory in heroin addicts (Zhao et al. 2011). Specifically, Zhao and colleagues recruited 70 male treatment-seeking heroin addicts to participate in a 3-day, placebo controlled study. On the first day, participants memorized a list of 20 heroin-related words (10 positive & 10 negative) and 10 neutral words; the next day, propranolol or placebo was administered prior to half the participants completing a memory retrieval task that involved writing the words from the previous day. On the test day, participants performed free recall of the word list. The results indicated that heroin, but not neutral, word recall was impaired in only the group that received both propranolol and the retrieval task, a finding that was construed as evidence of DoR. The absence of impaired recall in the group that did not receive retrieval suggests that the DoR effect depends on retrieval. Although these findings provide some initial support for the viability of DoR in human addictive behavior, they are only remotely clinically relevant since the learning targeted in the study bears little resemblance or relation to the learning processes that support addictive behavior.
Several neuroscience researchers (e.g., Besnard et al. 2012; Milton and Everitt 2010; Torregrossa et al. 2011; Torregrossa and Taylor 2012; Zhao et al. 2011) have suggested a more fruitful and clinically relevant path of investigation might be pursued by introducing propranolol-induced DoR into existing cue exposure therapy for drug addiction, with the prospect of developing a novel integrated pharmaco-behavior therapy. It has been amply shown that previously neutral cues/stimuli can acquire conditioned incentive-motivational properties following repeated pairing with drug ingestion (Childress et al., 1988; Drobes, Saladin & Tiffany, 2001; Drummond, Tiffany, Glautier, & Remington, 1995). By means of these associative learning processes, drug-associated cues acquire the ability to elicit drug craving and reactivity, which in turn, contribute significantly to the maintenance of drug use and are associated with relapse in persons attempting to abstain (Epstein et al. 2010; Epstein et al. 2009; Hartz et al. 2001; Paliwal et al. 2008; Preston et al. 2009; Sinha et al. 2006; Strong et al. 2011). The cue reactivity paradigm (cf., Drummond et al., 1995; Drobes et al., 2001), which uses controlled, laboratory-based methodologies to measure subjective (e.g., craving), behavioral and physiological responses to drug-related cues, would be an optimal strategy for assessing whether or not propranolol administration following retrieval exposures to cocaine-related cues could result in reduced reactivity to those cues during a subsequent test session of cue exposure. Accordingly, the present study was designed to provide this proof-of-concept.
Study Design
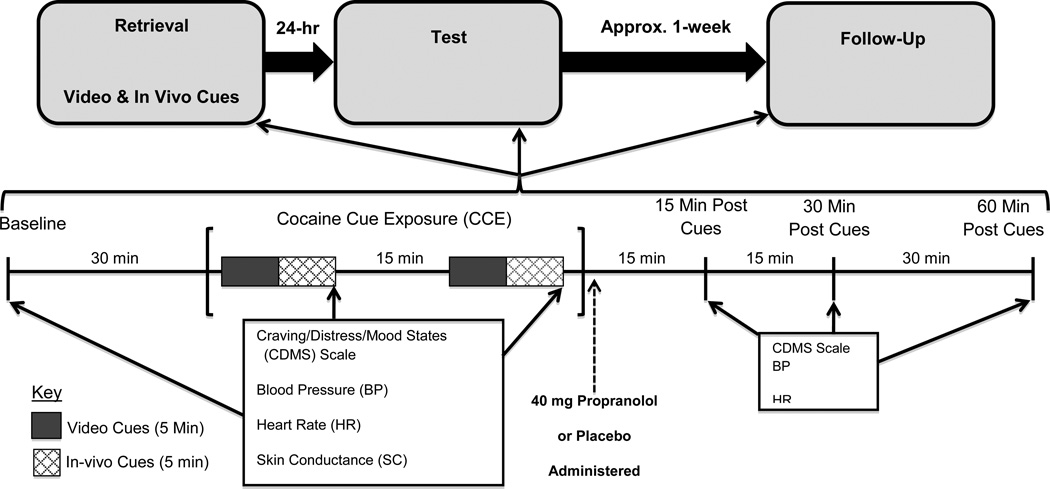
Figure 1 is a diagrammatic summary of the study design and procedures. This study employed cocaine-dependent individuals (N=50) to investigate the acute effects of propranolol (n=26) vs. placebo (n=24), administered immediately after a session of cocaine cue exposure (CCE), on the subjective and physiological responses occurring during a subsequent session of CCE. Participants were randomly assigned to receive 40 mg propranolol (immediate release) or placebo immediately after the first of two CCE sessions occurring on consecutive days of an inpatient stay at the Medical University of South Carolina’s (MUSC) Clinical and Translational Research Center (CTRC). The first CCE session, designated retrieval, putatively served to elicit retrieval and reconsolidation of memories for associations previously established between cues and cocaine administration; the second CCE session, designated test, served to examine the potential modulatory role of propranolol on the reconsolidated memories presumed to be elicited during retrieval. Propranolol and matching placebo were administered in a double-blind fashion and only in the retrieval session. Subjective (e.g., craving) and physiological (e.g., heart rate, skin conductance, blood pressure) measures were obtained at baseline and at regular intervals during and after both cue exposures. Approximately 7 days following the test session, participants returned to MUSC’s CTRC to undergo a 1-week follow-up CCE (follow-up) session that was identical to the test session (i.e., no medication was administered).
Fig. 1.
Study Design and Procedure: The upper portion of the figure shows the timing of the laboratory sessions (3) that comprised the study protocol. The lower portion of the figure shows the timing and order of the procedural elements that occurred in each laboratory session. The boxes list the primary outcome measures and the arrows indicate both the frequency and timing of the measures. It should be noted that 50 sec of continuous HR and SC data were collected at the start of each video and in vivo cue exposure (rather than at the end of each cue type).
The primary hypothesis was that compared to placebo-treated individuals, propranolol-treated participants would evidence lower craving & physiological reactivity during the test CCE session. A secondary hypothesis was that the propranolol group would demonstrate sustained treatment effects, as indicated by lower craving & physiological reactivity, during the 1-week follow-up CCE session. Additionally, we performed exploratory analyses that involved assessment of group differences in cocaine use during the follow-up period (i.e., between the test and follow-up CCE session).
Methods
Participants
Individuals who met DSM-IV criteria for current cocaine dependence (within the past month) but who were not dependent on any other substance, with the exception of nicotine, served as participants. The primary method of recruitment was media advertisement (radio, local papers). Exclusion criteria included severe psychiatric comorbidity (e.g., psychotic disorder, bipolar disorder, severe major depressive disorder with suicidal ideation) or medical illness (e.g., hematological, endocrine, cardiovascular disease) or use of medications that might interact with propranolol (e.g., albuterol, insulin, inhibitors of CYP2D6). The Institutional Review Board at MUSC approved all aspects of the study protocol, including recruitment procedures.
Using the recruitment strategy described above, we screened 121 prospective participants, 54 of which did not meet eligibility criteria (e.g., did not meet dependence criteria, etc.). The remaining 67 individuals were randomized, with 35 and 32 assigned to the propranolol vs. placebo medication condition, respectively. Seventeen participants were excluded after random assignment for one or more of the following reasons: (a) failure to return after completing the screening and diagnostic assessment, (b) positive urine drug screen at time of retrieval CCE session, (c) heart rate too low at time of retrieval CCE session, (d) refusal to continue study participation following completion of the retrieval CCE session. Consequently, 26 propranolol-treated individuals and 24 placebo-treated individuals completed the retrieval and test CCE sessions. The number of individuals completing the 1-week follow-up CCE session was 23 and 20 in the propranolol vs. placebo groups, respectively. Overall, the study completion rate was 86% (43/50).
Screening and Diagnostic Assessment
Trained masters-level research staff administered the Substance Use Disorders section of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I; First et al. 1996) to assess DSM-IV criteria for cocaine dependence, as well as to screen for other substance dependencies. Additional information pertaining to substance use was obtained using a modified version of the Timeline Follow-Back (TLFB; Sobell and Sobell 1992). The TLFB is a calendar-based instrument that uses specific probes to ascertain detailed information about the quantity and frequency of substance use, including frequency and dollar amount of cocaine/other drug use during the three months prior to study involvement and during the week intervening between the test session and the follow-up assessment. The Mini-International Neuropsychiatric Interview or MINI (Sheehan et al. 2003; Sheehan et al. 1998) was used at the screening visit to assess psychiatric status/functioning as it pertained to the exclusion criteria. Lastly, a study physician/physician assistant performed a history/physical exam on all study participants; it included electrocardiogram and blood chemistries.
Randomization
Urn randomization was used to assign participants to the propranolol vs. placebo medication conditions (Stout et al. 1994) while balancing treatment assignment on two variables. To maximize the power for a comparison of effect across gender and minimize confounding, gender was balanced during the randomization. Given that dollar amount of cocaine use at baseline (pre-study involvement) was a likely predictor of one or more of the primary outcome measures, it also served as an urn variable during randomization. Specifically, the total dollar amount of cocaine use in the 3 months prior to study involvement was determined (using the Timeline Follow-Back) and then was used to categorize participants on the following binary variable: (a) participants with ≤ $1000.00 cocaine use pre-study involvement vs. (b) participants with > $1000.00 cocaine use pre-study involvement.
Medication Administration
Medications were compounded, packaged and dispensed by the MUSC Investigational Drug Service, which also administered the randomization procedures and recorded treatment assignment. Propranolol (40 mg, immediate-release) or a matching placebo was administered immediately (within five minutes) after the retrieval session of CCE (see figure 1). The decision to employ a 40 mg dose of propranolol was based on the following evidence and clinical reasoning: (1) a recent study (Zhao et al. 2011; discussed above) employed a 40 mg dose of propranolol and demonstrated disrupted reconsolidation of a drug-related memory in heroin addicts, (2) numerous studies have shown a 40 mg dose of propranolol to be an effective modulator of human emotional memory (De Martino et al. 2008; Kindt et al. 2009; Kroes et al. 2010; Soeter and Kindt 2010; 2012b), and (3) concerns for participant safety (higher doses can increase risk of hypotension, loss of consciousness). The decision to administer the medication after, rather than before, the “retrieval session” of CCE ruled out the possibility that any group differences identified during the test session could be attributed to propranolol’s effects on retrieval rather than reconsolidation processes. The decision was also based on infrahuman studies involving appetitive learning paradigms (cocaine and other appetitively-based learning) in which propranolol was administered after the presentation of retrieval cues (Bernardi et al., 2006; Diergaarde et al., 2006; Przybyslawski et al., 1999), as well as clinical evidence from the PTSD literature (Brunet et al. 2008) in which propranolol administration followed each participant’s description of a traumatic event (i.e., retrieval cue).
CCE Session Measures
The timing and frequency of the measures described here is presented in figure 1. Subjective craving assessment occurred in the context of a larger 13-item scale, the Craving/Distress/Mood States Scale (CDMS Scale; cf., Saladin et al. 2006), which was based on the Within Session Rating Scale (Childress et al. 1986; Childress et al. 1999). This self-report assessment employed 100 mm visual analogue scales, with each being anchored by the adjectival modifiers “not at all” (left side of scale) and “extremely” (right side of the scale). The craving item asked participants to rate the desire to use cocaine “right now”. The remaining 12 items assessed other drug-related subjective states (i.e., withdrawal, anxiety, frustration, anger, depressed mood, etc.). Lastly, the state anxiety scale (i.e., acute, situation specific) of the State-Trait Anxiety Inventory (Spielberger 1983), which is a 20-item self-report scale that measures acute, situation specific anxiety, was administered at baseline and at the last assessment time point (60 min. post-cues). Ten items of this inventory measured stress and anxiety and ten measured feelings of relaxation; each item employed a Likert-scale format with four response options per item (1=not at all, 2=somewhat, 3=moderately so, and 4=very much so).
In addition to the self-report measures, heart rate (HR), blood pressure, and skin conductance (SC) level were measured as indices of physiological arousal during each CCE session. Heart rate was collected via two electrodes, with one affixed to the right shoulder and the other on the bottom left side of the participant’s ribcage. Blood pressure (BP) was measured using an intermittently inflatable cuff. Skin conductance was recorded using Ag/AgCl electrodes attached to the skin over the metacarpal bone of the fifth digit, non-dominant hand. Since physiological arousal/reactivity was likely to be greatest in the earliest portions of a cue exposure sequence, HR and SC data was continuously collected during the initial 50-second epoch of each video and in-vivo cue presentation. The HR and SC signals were amplified using the ECG 100c and GSR 100c Modules of the Biopac MP100 data acquisition system; the Biopac system was interfaced with an Apple (MacBook Pro) laptop for data storage and subsequent reduction.
Cocaine Stimuli
As indicated in figure 1, cocaine cues were presented in two formats. Video cocaine cues consisted of a 5-min. video depicting cocaine use (powder and crack) in a variety of settings. In vivo cues consisted of a small bag of simulated crack cocaine, the participant’s preferred style of crack pipe, a lighter, and money ($20 bill) for those who use crack cocaine. For powder or IV users, simulated powder cocaine, mirror, razor blade, straw and money were used. Video cues always preceded the in vivo cues and this cue combination was presented twice in each CCE session. The approximate duration of the video and in vivo cue exposure was 5-min each. These cues closely resemble those we have employed to effectively elicit cocaine craving and cue reactivity in our previous laboratory studies (cf., Back et al. 2010).
Procedure
Upon arrival at MUSC’s CTRC (approximately 9:00 am), participants provided breath and urine samples to assess abstinence for alcohol and other substances (only 3 participants had to be rescheduled because of positive breath or urine assessment). If the assessment was negative, they remained in the CTRC lounge until 9:30, at which time study personnel escorted them to the cue reactivity laboratory. Nicotine patches were provided to smokers, thereby reducing the risk of nicotine withdrawal during the cue exposure procedure. During the next half hour, a blood pressure cuff was placed on the participant’s arm and leads/sensors were affixed, as described above, for the purpose of collecting HR and SC data. Since these tasks were accomplished in 5–10 min, the participants remained seated quietly for the remaining 20 min period. This waiting period served to acclimate the participant to the laboratory setting (our research group has found acclimation to produce more stable baseline measures in several laboratory studies). At approximately 10:00 am, baseline subjective and physiologic measures were collected. Thirty minutes later, participants received the first sequence of CCE. As noted above (see also figure 1), a sequence of CCE began with a 5-min video cocaine cue presentation and was followed almost immediately by a 5 min in vivo cue exposure. It should be noted that HR and SC data was continuously collected during the first 50 sec of each cue type (video × 2 and in vivo × 2). The CDMS Scale and BP were obtained at the end of the in vivo cues. After the latter measures were obtained, participants remained comfortably seated until the second, identical sequence of CCE commenced (approximately 12–15 min later). At the end of the dual-sequence CCE, participants received their medication. As indicated in figure 1, all dependent measures were obtained at 15 min, 30 min and 60 min time points following termination of CCE. At the end of the retrieval session, research staff assisted the participant in removing the HR/SC leads and BP cuff and then escorted the participant to a research dedicated hospital room where they remained overnight.
The next day, participants were administered the test session at the same time as the first (i.e., 24 hours later). This session was identical to the first, with the except that no medication was administered. Prior to discharge from MUSC’s CTRC, research staff scheduled the follow-up session to occur approximately 1-week after test session (due to scheduling conflicts, the mean number of days between the test and follow-up sessions was 9 days). Approximately half way through recruitment, it was decided that the effectiveness of the participant blind should be assessed. Accordingly, the last 21 participants were queried, just before discharge from the CTRC, as to whether they thought they received propranolol or placebo.
The goal of the follow-up session was to determine the sustainability of any between-group differences identified one week earlier in the test session. The CCE procedures described above were repeated in the follow-up session (no medication was administered). Participants met research staff at the outpatient service of MUSC’s CTRC at approximately (9:00 a.m.). At this time, a UDS and breathalyzer assessments were performed to confirm abstinence. Next, research staff performed a Timeline Follow-Back in order to ascertain the level of drug/alcohol use during the follow-up period. At approximately 10:30 a.m., the CCE procedures described above commenced. Upon completion of the laboratory procedures, the HR/SC leads and BP cuff were removed and participants were compensated for their participation. Appropriate measures were taken to ensure that participants were not discharged while experiencing unmanageable levels of cocaine craving (e.g., brief training in craving management). Research staff offered referrals for treatment.
Data Management
Common summary values (i.e., means, percentages) were computed from demographic and clinical measures obtained during the screening and diagnostic assessment. As indicated in figure 1, all subjective and physiological outcome measures were obtained repeatedly within each laboratory session. Therefore, data reduction necessary for the evaluation of the main hypotheses consisted of computing means from the multiple outcome measures. For the CDMS scale (including the craving item) and BP measure, a mean was derived from two measures obtained during the CCE. As already noted, HR and SC data were collected continuously for the first 50 sec of each of four stimulus presentations (2 video and 2 in vivo) during the CCE; means were first computed from 10 sec bins of HR and SC data and then the resulting five values were used to compute a 50 sec mean; lastly, an overall CCE HR and SC mean was derived from the four, 50 sec values. Baseline values on each outcome measure were derived from a single measure taken at the baseline time point (except the HR and SC measures, which involved the reduction steps noted above). While post-CCE data was collected at three time points (15, 30 and 60 minutes post-CCE), this data was not relevant to hypothesis evaluation and therefore, not substantially analyzed. However, in an effort to demonstrate that propranolol was bioactive in the treated group (i.e., potent independent variable), the post-CCE HR data from the retrieval session was used to show that 40 mg propranolol produced an expected cardiovascular effect.
Data Analysis
The a priori sample size was determined to provide 80% power, at an alpha level of .05, to detect an effect size of .8. The normality assumption was visually assessed via quantile plot; in those cases where the assumption of normality was violated, complementary non-parametric tests were performed. Since parametric and nonparametric methods yielded parallel findings, only parametric results are reported in order to preserve consistency in the reporting of findings. Levene’s tests for equality of variances were performed on all outcomes measures; results failed to identify any significant variance differences.
Group differences on demographic and clinical variables were assessed using either t-tests (two-tailed) or chi square tests of independence. The primary data analytic strategy employed for hypothesis testing was ANCOVA. This approach was adopted because it was important to control for participant differences in initial (retrieval) response to the cocaine cues. Thus, each participant’s response to the cocaine cues presented in the retrieval session (i.e., prior to medication administration) served as a covariate. Finally, although the present study was not powered to detect treatment effects on cocaine use, exploratory analyses were performed using the (self-report) Timeline Follow-Back data collected at the 1-week follow-up CCE session (and contrasted with an equivalent duration of pre-study cocaine use). A type I error protection level of α < .05 was applied to all tests used to evaluate hypotheses involving the primary outcomes (craving item on the CDMS Scale, BP, HR and SC). By contrast, a type I error protection level of α ≤ 0.01 was applied to secondary outcomes and to any collateral (a posteriori) analyses, in an effort to nominally control inflation of the type I error rate associated with tests performed on multiple secondary outcomes (e.g., 12 items on the CDMS Scale). Given the exploratory nature of this study, tests yielding probabilities between .1 and .05 (primary outcome) or .01 (secondary outcome) were considered marginal.
Results
Sample Demographics and Clinical Characteristics
Table 1 contains the demographic and clinical characteristics of the full sample (N=50) and of each group. The mean age of the participants in each group was approximately forty, with the majority being male, African American, high school educated (or less), and unemployed with an income less than (or equal) to $20,000. Less than 10% of the sample was married. Participants began using cocaine in their early twenties and the mean number of years of cocaine use was approximately 15. Most participants were crack cocaine users and most had used greater than $1000 of cocaine during (approximately) 40 of the 90 days preceding study participation. Additionally, the majority of each group was nicotine dependent (10 cigs per day) smokers who evidenced one or more Axis I disorder and reported a modest level of anxiety at the time of testing (STAI min = 20; max = 80).
Table 1.
Demographic and clinical characteristics of overall sample and of groups.
| Overall (N=50) |
Propranolol (n=26) |
Placebo (n=24) |
Statistic | ||
|---|---|---|---|---|---|
| Age† | 39.9(1.3) | 39.1(1.6) | 40.8(2.0) | t = 0.65, p = 0.52 | |
| Genderf | Male | 66.0(33) | 65.4(17) | 66.7(16) | χ2 = 0.01, p = 0.92 |
| Female | 34.0(17) | 34.6(9) | 33.3(8) | ||
| Racef | Black | 66.0(33) | 69.2(18) | 62.5(15) | χ2 = 0.25, p = 0.62 |
| White and other | 34.0(17) | 30.8(8) | 37.5(9) | ||
| Educationf | No HS completion | 30.0(15) | 34.6(9) | 25.0(6) | χ2 = 1.94, p = 0.38 |
| HS grad or equivalent | 36.0(18) | 26.9(7) | 45.8(11) | ||
| Some college or college grad | 34.0(17) | 38.5(10) | 29.2(7) | ||
| Employedf | Yes | 36.0(18) | 38.5(10) | 33.3(8) | χ2 = 0.14, p = 0.71 |
| No | 64.0(32) | 61.5(16) | 66.7(16) | ||
| Annual incomef | ≤ $20,000 | 72.0(36) | 65.4(17) | 79.2(19) | χ2 = 1.18, p = 0.28 |
| > $20,000 | 28.0(14) | 34.6(9) | 20.8(5) | ||
| Marital statusf | Married | 6.0(3) | 7.7(2) | 4.2(1) | χ2 = 0.28, p = 0.60 |
| Other | 94.0(47) | 92.3(24) | 95.8(23) | ||
| Age at first cocaine use† | 22.5(1.2) | 21.8(1.0) | 23.2(2.3) | t = 0.58, p = 0.58 | |
| Total years of cocaine use† | 15.0(1.0) | 15.5(1.5) | 14.4(1.4) | t = 0.53, p = 0.60 | |
| Route of Cocaine Administrationf | Smoked | 66.0(33) | 73.1(19) | 58.3(14) | χ2 = 1.21, p = 0.27 |
| Nasal | 34.0(17) | 26.9(7) | 41.7(10) | ||
| Cocaine use 90 days before screenf | <$1000 | 18.0(9) | 19.2(5) | 16.7(4) | χ2 = 0.06, p = 0.81 |
| >$1000 | 82.0(41) | 80.8(21) | 83.3(20) | ||
| Number days used 90 days before screen† | 41.0(3.1) | 42.3(4.2) | 39.6(4.5) | t = 0.44, p = 0.67 | |
| Cigarettes per day (of smokers)† | 12.8(1.5) | 12.4(1.6) | 13.4(2.9) | t = 0.32, p = 0.75 | |
| Current Cigarette smokerf | Yes | 78.0(39) | 88.5(23) | 66.7(16) | χ2 = 3.46, p = 0.06 |
| No | 22.0(11) | 11.5(3) | 33.3(8) | ||
| STAI - State Anxiety Index score† | 39.1(1.6) | 41.7(2.4) | 36.3(2.3) | t = 1.64, p = 0.11 | |
| Number of MINI diagnosesf | None | 38.0(19) | 26.9(7) | 50.0(12) | χ2 = 2.84, p = 0.24 |
| One | 32.0(16) | 38.5(10) | 25.0(6) | ||
| Two or more | 30.0(15) | 34.6(9) | 25.0(6) | ||
Notes:
indicates mean (SE);
indicates % (number); For cigarettes per day N = 35.
The marital status category “other” contains persons never married, separated, divorced, or widowed. Group differences were assessed via t-tests (continuous) or χ2 (categorical). For most t-tests, df = 48 and for χ2, df = 1; exceptions were df = 33 for cigarettes per day and df = 2 for education, marital status and number of MINI diagnoses.
STAI – State subscale of the State Trait Anxiety Inventory was used to assess acute, situation specific anxiety; MINI - Mini-International Neuropsychiatric Interview was used to assess DSM-IV Axis I disorders and antisocial personality disorder. All p values are from two-tailed tests, with α < 0.05.
The far right column of the table lists the probabilities associated with the statistical test (t-tests for means and chi square tests of independence for categorical measures). The outcomes of these tests indicate that the groups were essentially equivalent on all demographic, cocaine use and other clinical variables with the exception that the propranolol treated group had a marginally greater number of cigarette smokers. To determine whether or not smoking status should serve as a covariate, we ran all the primary analyses with and without smoking status as a covariate. The outcomes indicated that smoking status was not significantly associated with any of the primary outcomes (all F’s < 1.0) and did not appreciably change the outcome of any analysis (i.e., did not moderate the relationship between group membership and outcome). Accordingly, it was not retained as a covariate in the analyses.
It is notable that the urn randomization appears to have successfully balanced the groups on gender and dollar amount of cocaine use in the 90 days prior to study screening. Also, the groups appear to be equivalent with respect to baseline anxiety level (as measured via the STAI) and number of psychiatric comorbidities. Lastly, none of the participants reported daily psychotropic medications use (not in table), although one participant (propranolol group) used a benzodiazepine as needed for anxiety. Other medication use was also infrequent; one participant in each group was using hydrochlorothiazide (diuretic) and one participant in the propranolol group was using pregabalin (for neuropathic pain management). Overall, the high level of similarity between the groups increases confidence that any differences identified following treatment are unlikely to be attributable to group differences on important demographic and clinical variables.
Comparison of groups on outcomes obtained during the retrieval session
Although responses to cocaine cues in the retrieval session (i.e., immediately before the medication) were used as covariates in the primary analyses, we assessed whether there were statistically significant differences between groups on these responses. With regard to the primary outcomes (craving item on the CDMS Scale, systolic/diastolic BP, HR and SC), there were no significant or marginal group differences on any measure (all t’s < 1.0, all p’s > .4). Group comparisons on the secondary outcomes (12 items of the CDMS Scale) suggested a few relatively marginal differences including trends towards a lower mean rating on item 5 (feel less depressed if using; t = 1.76, p = .09) and higher mean rating on items 9 (feeling good; t = 2.43, p = .02) and 13 (feeling relaxed; t = 1.72, p = .09) in the placebo- vs. propranolol-treated group. Thus, the groups were similar on all outcome measures prior to medication administration.
Participant Blind Assessment
As noted above, 21 of 50 participants were asked, before they left the test session (i.e., were discharged from the CTRC), if they thought they received propranolol or placebo in the retrieval session. Once the medication condition was unblinded, it was possible to contrast their responses to this query with the actual medication received. Examination of these data indicated that, of the 13 participants that received propranolol, seven thought they had received placebo while six thought they received propranolol; of the 8 that received placebo, five thought they received placebo and 3 thought they received propranolol. A chi square test of independence applied to these frequencies confirmed that participants were not able to correctly predict the medication they actually received (χ2 < 1.0, p = .70) which suggests that blinding was effective.
Biopotency of Propranolol
In the interest of demonstrating that propranolol was biologically active in the treated group, heart rate data obtained after medication administration (retrieval session) was used to evaluate whether propranolol had a reductive effect on heart rate. Specifically, a difference score was computed such that each participant’s final heart rate measure (obtained 60 min after medication administration; see figure 1) was subtracted from the measure obtained approximately 10–15 minutes after medication administration. Negative values would indicate a heart rate decrease following medication administration whereas positive values would indicate an increase. The mean difference score for the propranolol and placebo groups were −3.9 bpm and +.5 bpm, respectively, and one-sample t-tests applied to these means verified that the propranolol group mean (t = 3.41, p < .01) but not placebo group mean (t < 1.0) differed from 0.
Test Session Outcomes
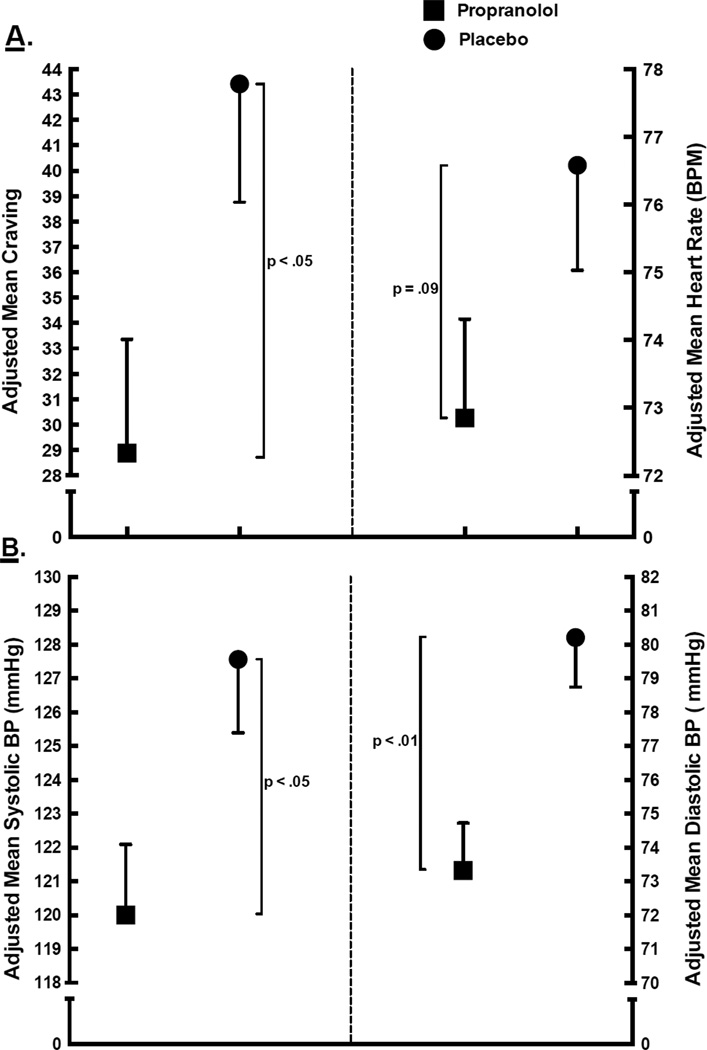
Figure 2 depicts the adjusted mean CDMS craving (A, left), heart rate (A, right), systolic BP (B, left), and diastolic BP (B, right) outcome measures for propranolol-treated (squares) and placebo-treated (circles) groups. The ANCOVA probabilities are presented along side the brackets indicating the means that were compared. The figure indicates that the propranolol group evidenced significantly lower mean craving than the placebo group (F1,47 = 4.98, p = .03). The mean craving reduction, from retrieval to test, was 25.0 (SE = 4.7) for the propranolol group and 10.5 (SE = 4.5) for the placebo group, which suggests that propranolol treatment yielded a craving reduction 1.5 times larger than would be achieved in its absence. The adjusted mean HR evidenced by the propranolol group was marginally lower than that evidenced by the placebo group (F1,44 = 3.06, p = .09). Panel B shows that mean systolic and diastolic BP was lower in the propranolol group relative to the placebo group (F1,47 = 6.28, p = .02 and F1,47 = 11.50, p < .01, respectively). Lastly, there were no group differences in skin conductance responding (F1,43 < 1.0; not shown in figure).
Fig. 2.
Mean craving and heart rate (panel A) and mean systolic/diastolic blood pressure (panel B) by medication group. All means are adjusted for initial response to cocaine cues (i.e., response during the retrieval session).
Table 2 shows the group means for each item of the CDMS Scale. The table shows that the propranolol group evidenced trends towards lower mean ratings of feeling “a cocaine like high” and feeling they “would feel less depressed if using” relative to the placebo group. Propranolol treated individuals did report a significantly lower mean rating of feeling they would “want a rush or a high”. These observations are generally consistent with the lower craving exhibited by the propranolol group.
Table 2.
Test Session Cue Reactivity Data; Craving/Distress/Mood States Scale Mean Scores by Group.
| Craving/Distress/Mood States Scale | Group | Statistic | |
|---|---|---|---|
| Rating Item: Right now, how much do you feel… | Propranolol | Placebo | F =, p = |
| 1. A cocaine-like high | 11.9(2.7) | 18.6(2.8) | 2.97, 0.09 |
| 2. Able to resist using | 51.6(5.4) | 51.2(5.6) | 0.00, 0.96 |
| 3. You could control things better if using | 18.6(3.6) | 23.3(3.7) | 0.85, 0.36 |
| 4. You would feel less depressed if using | 20.7(3.7) | 31.4(3.9) | 3.89, 0.06 |
| 5. You want a rush or a high | 23.8(4.3) | 45.4(4.5) | 11.90, 0.00** |
| 6. Like getting rid of withdrawal or anger | 25.0(5.1) | 36.3(5.3) | 2.39, 0.13 |
| 7. A cocaine withdrawal/crash | 17.2(3.9) | 25.1(4.1) | 1.94, 0.17 |
| 8. Good | 67.0(3.1) | 63.2(3.2) | 0.68, 0.41 |
| 9. Worried/anxious | 22.2(3.0) | 22.4(3.1) | 0.00, 0.96 |
| 10. Angry/hostile | 9.5(1.7) | 10.1(1.8) | 0.06, 0.80 |
| 11. Frustrated | 17.9(3.5) | 24.3(3.7) | 1.56, 0.22 |
| 12. Relaxed | 58.9(3.4) | 58.8(3.6) | 0.00, 0.97 |
Notes: Mean(SE);
p<.01;
For all measures, df = 1, 47. All tabled means are adjusted for initial response to cocaine cues (i.e., response during the retrieval session).
Follow-up Session Outcomes
ANCOVAs performed on the follow-up primary and secondary outcomes failed to identify any significant group differences, with all but two analyses having yielded F’s less than 1 (Table 3 contains the 1 week follow-up results for those measures where significant group differences were identified in the test session). Parenthetically, the mean cocaine craving reduction, from retrieval to follow-up, for the propranolol and placebo groups were 30.6 and 22.9, respectively, indicating that craving was not significantly different between groups (F1,40 = 1.21, p = .28) but was attenuated relative to retrieval levels in both groups (F1,41 = 50.77, p < .01). In sum, it appears that the group differences observed in the test session performed 24 hours after retrieval were not sustained over the follow-up period.
Table 3.
Follow-up outcomes: Group means (SE) for those measures where significant group differences were identified in the test session.
| Group | Statistic | ||
|---|---|---|---|
| Primary Measures | Propranolol | Placebo | F =, p = |
| Subjective Craving | 27.4(4.7) | 35.1(5.1) | 1.21, 0.28 |
| Blood Pressure | |||
| Systolic | 127.2(2.2) | 127.1(2.4) | 0.00, 0.98 |
| Diastolic | 80.5(1.4) | 80.2(1.5) | 0.02, 0.89 |
| Heart Rate (BPM) | 69.3(1.6) | 68.6(1.7) | 0.08, 0.78 |
| Craving/Distress/Mood States Scale | |||
| Item 5. Right now, how much do you feel you want a rush or a high | 26.5(4.9) | 29.7(5.3) | 0.19, 0.66 |
Notes: Mean(SE);
p<.01;
For most measures, df = 1, 47; exception was df=1, 37 for heart rate. All tabled means are adjusted for initial response to cocaine cues (i.e., response during the retrieval session).
Although not powered to detect between group differences in cocaine use during the interim period between the test and follow-up CCE session, preliminary/exploratory analyses using self-report cocaine use data (obtained via TLFB) were performed. The TLFB data indicated that 43% (10/23) of propranolol participants and 35% (7/20) placebo participants reported using cocaine during the follow up period and a chi square test of independence applied to these proportions failed to detect a group difference (χ2(1) < 1.0). An analysis was performed to assess whether there were any group differences in dollar amount of cocaine used during the interim/follow-up period. Specifically, we computed a difference score consisting of the participants mean dollar amount of cocaine used during the follow-up period minus their mean dollar amount of cocaine used during an equivalent number of days prior to study participation. Negative difference scores would indicate an overall reduction in mean dollar amount of cocaine used during follow-up relative to pre-study involvement whereas positive values would indicate an increase. It was observed that there was a mean reduction, from pre-study levels, in dollar amount of cocaine used by the propranolol and placebo groups of −$337 and −$228, respectively. A t-test applied to these means failed to identify a group difference (t < 1.0).
Collateral (A Posteriori) Findings
Since one third of our study sample was female (balanced between groups) we re-computed the analyses described above with sex as a covariate to determine if sex was associated with any of the primary outcomes and whether its presence in the models would change the findings noted above. The results of the analyses indicated that sex was not a significant covariate (all F’s < 1.0) and that the findings did not differ from the original analyses performed without a sex covariate.
Having identified significant and trend level between group differences in responses to cocaine cues during test CCE, it seemed possible that propranolol may have impacted basal or non-cue-elicited responses on those same measures (i.e., craving, HR, SBP, DBP and CDMS items 1, 4 & 5 from table 2). Accordingly, we performed similar group comparisons on test session means derived from the baseline measures obtained 30 min prior to CCE in the test sessions (controlling for baseline response during the retrieval session). Test outcomes with p ≤ .01 were considered significant whereas test outcomes were considered marginal/trends if: .01 < p ≤ .05. With respect to the primary measures, a trend was present for baseline heart rate such that the propranolol group (m = 72.2, se = 1.8) trended towards a lower heart rate than the placebo group (m = 77.4, se = 1.9; F1,43 = 4.00, p = .05). Analyses involving the other primary measures failed to identify any significant or trend level group differences, even though the propranolol group evidenced lower mean baseline craving, SBP and DBP than the placebo group (all p’s > .06). Likewise, for CDMS items 1 (feel a cocaine-like high), 4 (feel less depressed if using) and 5 (want a rush or a high), none of the group differences passed significance or trend level criteria (all p’s > .08).
Given the findings from the test session (figure 2), analyses were performed to assess what level of association existed between craving and physiological (HR, BP) measures of cue reactivity. In the interest of eschewing unnecessary tests, we restricted post hoc correlational analyses to mean craving, HR and BP (systolic & diastolic). Additionally, in an attempt to control for the potential moderating role of anxiety in this analysis, a partial correlation approach was adopted in which the state anxiety score from the STAI obtained at the outset of the test session served as a control variable. Results indicated that HR and BP measures were not significantly associated with mean craving, regardless of whether the analyses were performed on the full sample (n = 46; all r’s < .04, all p’s > .84) or with the propranolol treated group data only (n = 24; HR r = −.15, p = .51; SBP r = .15, p = .49; and DBP r = .37, p = .08).
Since anxiety can influence craving and cue reactivity, the two groups were compared on baseline STAI score obtained the day of the test session (table 1 shows that the groups did not differ on baseline STAI obtained in the retrieval session). The mean STAI scores of the propranolol and placebo groups were 34.5 and 32.9, respectively, and a t-test failed to identify a group difference (t < 1.0). Thus, it is evident that baseline STAI scores did not vary between groups, at either the retrieval or test session.
Discussion
In this double blind, placebo-controlled study, two groups of cocaine dependent individuals were exposed to video and in vivo cocaine cues to elicit retrieval of memories for associative learning that was established over an extensive drug use history that likely consisted of hundreds, if not thousands, of pairings between the cues and cocaine reinforcement. Immediately after the retrieval session of CCE, one group received propranolol (40 mg of immediate release), a putative disruptor/modulator of emotional memory reconsolidation, and the other group received matching placebo. Both 24-hours (test) and approximately 7-days later (follow-up), the groups received a session of CCE to evaluate the impact of the medication on responses to the cocaine cues.
The primary hypothesis of the study was that the propranolol treated group would evidence significantly lower cue-elicited cocaine craving and reactivity relative to the placebo treated group. The observed group differences in responses to the cocaine cues were consistent with this expectation. The propranolol group evidenced lower mean craving, with the craving reduction being one and half times greater than observed in the placebo group. There were also some significant and trend level group differences on other items from the CDMS scale. The propranolol treated group reported significantly lower feelings of wanting a rush or a high and they also reported trend level lower feelings of a cocaine-like high and in feeling less depressed if using. In the case of the physiological responses, both mean systolic and diastolic BP were significantly lower in the propranolol group and there also was a trend towards lower heart rate. In contrast, there were no group differences in skin conductance. Since most of the participants were provided nicotine patches for the test session (61% and 54% of the propranolol and placebo group, respectively) and it is known that nicotine can alter skin conductance (Hori et al. 1994; Lyvers and Miyata 1993; Reid et al. 1998), it is possible that our efforts to minimize nicotine withdrawal may have compromised our ability to detect skin conductance differences (i.e., possibly by inflating measure variability).
Collectively, these findings are suggestive of a general profile of dampened responsiveness to cocaine cues by participants who receive post-retrieval propranolol relative to those who receive placebo. This dampened responding is consistent with the reconsolidation hypothesis, which posits that memory retrieval initiates a period of instability during which memories can be updated, altered or disrupted prior to being reconsolidated (cf., Dudai 2006; Kiefer and Dinter 2013; Milton and Everitt 2012). In the present study, the disrupting agent propranolol was administered immediately after a session of CCE, which served to retrieve memories for learning that underlies cue-elicited cocaine craving and cue reactivity. The diminished responding seen in test CCE session constitutes tentative evidence of propranolol’s disrupting effects on the reconsolidation of memories for learning that develops over the course of habitual drug use. The test session results are also consistent with the established infrahuman research demonstrating propranolol’s disruptive effects on cocaine reinforced learning (Bernardi et al. 2006; Fricks-Gleason and Marshall 2008; Milton et al. 2008) and with the one human study showing that propranolol can disrupt memory of a previously learned drug-related word list in heroin addicts (Zhao et al. 2011).
The secondary hypothesis of this study was that some or all of the acute effects observed at test would be sustained and evident at the 1-week follow-up CCE session. Overall, the findings were not consistent with expectation; craving was lower in the propranolol group but this difference did not exceed threshold for significance/trend. There were no significant/trend level effects on any other subjective or physiological measure. Exploratory analyses of self-report cocaine use during the interim period between the test and follow-up sessions failed to identify group differences. Specifically, the reduction in dollar amount of cocaine used from pre-study levels was greater, albeit non-significantly, in the propranolol group vs. placebo group. While it must be emphasized that the follow-up findings are only suggestive of a trend towards reduced cocaine use, they do hint at the possibility that future efforts directed at amplifying the DoR effect of propranolol may yield more definitive evidence of prolonged disruption of cue responsiveness and/or delayed return to cocaine use.
There were a number of ancillary findings of note. First, it appears that propranolol may have had some modest impact on basal or non-cue elicited responses. For the primary measures, there was a trend towards a lower test session HR in the propranolol vs. placebo group. The findings on baseline craving and BP measures, although in the expected direction, did not exceed significance/trend criteria. No significant or trend level effects were identified on the secondary CDMS scale outcomes (e.g., “feeling a cocaine-like high”, “feel less depressed if using”, and “feel you want a rush or a high”). While these observations suggest the possibility of residual cardiovascular-related medication effects and/or possible generalization of the impact of DoR to basal HR responses, the effects on subjective responses appear to be primarily restricted to cue-elicited responses. Second, there was no association between changes in the physiological measures and changes in craving. This observation is consistent with previous research suggesting dissociation between cue-elicited craving and physiological reactivity measures (cf., Carter and Tiffany 1999; Tiffany 1990; Tiffany and Conklin 2000). Third, the groups did not differ on a standard measure of state anxiety (STAI), either at the beginning of the retrieval or test session. This mitigates the possibility that between-group variation in subjective or physiological measures was due to group differences in anxiousness. Fourth and finally, because no adverse events or side effects were reported by any of the participants at anytime during study participation, it appears that 40 mg propranolol (immediate release) can be used safely with chronic, heavy cocaine users. This observation is consistent with the outcomes of large clinical trials that have documented the safety of propranolol when administered daily by cocaine users (Kampman et al. 1999; Kampman et al. 2001).
As already noted the present findings are generally consistent with the notion that propranolol can disrupt reconsolidation of appetitive drug-related memories. However, there are at least three competing explanations for the present findings. One alternative explanation is that the observed results occurred because propranolol was biologically active at the time of the test session. If this were true, then it could be argued that the HR and BP findings were a consequence of propranolol’s known effects on these measures and that its anxiolytic properties might explain the group differences on the subjective measures, including craving. This explanation seems unlikely for several reasons. First, if decreased anxiety/arousal explains the lower craving, then one would expect the propranolol group would have evidenced a lower level of state anxiety score (as measured with the STAI) on the day of the test session; as already noted above, the groups were equivalent on state anxiety on the day of both the retrieval and test CCE sessions. Second, because immediate release propranolol reaches peak plasma concentration in approximately 90 minutes (Paterson et al. 1970; Shand et al. 1970) and has a half-life of 3–4 hours (Duchateau et al. 1986; George et al. 1972; Riopel and Walle 1980; Shand and Rangno 1972), it seems unlikely that appreciable levels of propranolol would have been present 24 hours post-administration when the test CCE session was performed. Furthermore, a recent study (Zaky et al. 2011) employing a sample of patients with liver cirrhosis reported that an 80 mg dose of propranolol had almost completely cleared (mean plasma propranolol concentration < 10 ng mL) when measured 24 hours post-administration. Since the participants in this study had normal liver function and received a 40 mg dose, it seems very likely that propranolol had completely cleared in the 24 hours preceding the test session. Although it cannot be argued with certainty that this interpretation of the findings is invalid, there appears to be grounds to question its veracity.
Two other related alternative interpretations of the present findings are that (a) propranolol facilitated consolidation of memory for extinction learning in the retrieval session or (b) in the unlikely event that propranolol was still biologically active during the test session, it may have facilitated extinction learning. However, neither of these interpretations appears consistent with the existing research literature on propranolol’s effects. In the case of memory consolidation, propranolol has generally been found to have either no effect (Decker et al. 1990; McGaugh 1989) or an impairing effect (Cahill et al. 2000) on memory consolidation. Additionally, propranolol has been shown to impair consolidation of declarative memory in humans (McGaugh 2000) and to block bicuculline-induced enhancement of extinction consolidation in animals (Berlau and McGaugh 2006). In the case of extinction learning, a number of recent infrahuman studies suggest that propranolol retards extinction of both fear-based context conditioning (Do-Monte et al. 2010) and appetitive sand maze learning (Cohen and Gotthard 2011). The former of these two studies is of special relevance insofar as it indicates that a single administration of propranolol does not affect the rate of extinction, a finding that is in agreement with three previous studies (Cain et al. 2004; Rodriguez-Romaguera et al. 2009; Terry et al. 1990). Moreover, in a recent study by Kindt and colleagues (Bos et al. 2012) propranolol administration was found to have no effect on extinction as measured physiologically (i.e., startle reflex and skin conductance) whereas extinction at the cognitive level (i.e., participant expectancy of shock) was impaired. Taken together, the extant literature on the effects of propranolol on memory consolidation and extinction learning is not consistent with a facilitation of either process.
The present study had a number of design strengths that aided the interpretation of the findings. For example, it was demonstrated, as a precondition of evaluating group differences, that the groups were equivalent on demographic and relevant clinical variables prior to study initiation. Equivalence on dollar amount of cocaine use and gender also verifies the effectiveness of the urn randomization procedure, which was another design strength. Additionally, assessment of the participant blind indicated that participants could not discern with any level of accuracy whether or not they had received propranolol. An effective blind reduces the likelihood that self-report results could be explained by accurate participant knowledge of the medication they received. The heart rate data obtained after the medication administration provided the opportunity to assess the biopotency of the medication manipulation. The observation that only the propranolol group evidenced the expected heart rate decrement provides a measure of confidence that the medication was biologically active. The administration of the propranolol after, rather than before, the retrieval CCE session argues for interpretation of the results as a DoR effect as opposed to a retrieval impairment effect (cf., Kroes et al. 2010).
As well as having the aforementioned strengths, the present study also has a number of weaknesses. Chief among these weaknesses was a lack of a “no retrieval” control group. Many extant infrahuman and human laboratory studies of DoR have included a control group that receives the putative DoR agent in the absence of the retrieval manipulation in order to show that retrieval is necessary for the expression of the DoR effect. The present study had no such control, thereby making unclear whether or not the retrieval CCE session was a necessary precondition for the observed test CCE session results. Unfortunately, this is a compromise that is sometimes made in the interest of conducting “sound translational research in patients” (Brunet et al. 2011a). Another weakness was the lack of determination of plasma propranolol levels following the medicated retrieval session. This assay would have permitted the assessment of the association between plasma concentrations of propranolol and the effects observed in the test CCE session and, to a lesser extent, the follow-up CCE session. Ideally, future studies should include this design feature so that the causal connection between propranolol and its effects can be more clearly discerned. Lastly, while the sample size was sufficient for detecting the more acute effects of propranolol on craving and cue reactivity, it was not sufficient to assess the more distal effects at 1-week follow-up, including cocaine use outcomes.
Human laboratory studies to date have substantially advanced knowledge about DoR (e.g., Soeter and Kindt 2011). The findings of the present study represent an important next step in translational science by evaluating the clinical relevance of this phenomenon. The emphasis in this study was on determining if memories for naturalistic learning processes that develop during cocaine addiction are amenable to modulation by a pharmacological agent with known DOR properties (i.e., propranolol). This study suggests that there may well be reason for optimism about the future development of DoR-based clinical interventions that capitalize on the temporary vulnerability of retrieved drug-related memories. Although there are grounds for optimism, it seems clear that enhancing the effects observed in this study will be an important next step. In fact, both Schiller & Phelps (2011) and Schwabe et al, (2012) have suggested that one way to potentially increase the DoR signal of propranolol would be to increase the dosage of propranolol being routinely used in laboratory studies of DoR. We concur and would add that this same recommendation could be gainfully extended to studies that target more clinically relevant drug-related memories. On a related note, one basic science study has suggested that increasing the number of medicated retrievals might also boost the DoR signal of propranolol (Fricks-Gleason and Marshall 2008). Importantly, this study found that multiple medicated retrievals not only eliminated the previously established cocaine conditioned place preference (CCP) but also prevented reinstatement of the CCP following a priming dose of cocaine. Thus, the augmentation of a propranolol-induced DoR effect may very well be achieved by not only increasing the dosage of propranolol but also by increasing the number of medicated retrievals.
The present study was undertaken to provide “proof of concept” that post-retrieval propranolol can attenuate clinically relevant memories for learning that subserves cocaine addiction. Having achieved this initial step, it seems fitting to consider how this phenomenon might be harnessed to improve clinical outcomes. One avenue of clinical investigation might involve incorporating propranolol, as a pharmacological adjuvant, into existing cue exposure-based treatments for addiction. These treatments, which have traditionally targeted extinction of drug-related learned associations, have been found to be of modest efficacy (Conklin and Tiffany 2002). However, their efficacy might be enhanced if the way they were conducted targeted important memory mechanisms of addiction. Such a hybrid cue exposure-based treatment would conceivably employ cue exposure methodology to retrieve memories of addiction-related learning and then strategically target the disruption of those memories with propranolol. In this pharmaco-behavior therapy, propranolol would serve as a pharmacological constituent of an existing behavioral treatment modality, the primary goal of which is to undermine the learning and memory processes that catalyze addictive behavior. This approach may have some obvious and significant benefits. From a cost effectiveness perspective, the hybrid reconsolidation-focused cue exposure intervention would likely require fewer (one or two) treatment sessions and would not involve the chronic daily dosing associated with most pharmacotherapies for drug addiction. Fewer treatment sessions together with minimal medication administration would almost certainly reduce treatment costs, promote treatment seeking and foster compliance/retention once treatment is initiated.
Acknowledgments
This research was supported by NIDA grant R21DA025155 (Saladin, PI), USPHS grant M01 RR01070 (CTRC, MUSC) and by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, through NIH Grant Numbers UL1 RR029882 and UL1 TR000062.
Footnotes
The authors report no conflicts of interest pertaining to this study.
References
- Alberini CM. Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends in Neuroscience. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin ME, McRae-Clark AL, Price KL, Moran-Santa Maria MM, Baker NL, Spratt E, Kreek MJ, Brady KT. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Bernardi RE, Lattal KM, Berger SP. Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport. 2006;17:1443–1447. doi: 10.1097/01.wnr.0000233098.20655.26. [DOI] [PubMed] [Google Scholar]
- Besnard A, Caboche J, Laroche S. Reconsolidation of memory: A decade of debate. Prog Neurobiol. 2012;99:61–80. doi: 10.1016/j.pneurobio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Bos MG, Beckers T, Kindt M. The effects of noradrenergic blockade on extinction in humans. Biol Psychol. 2012;89:598–605. doi: 10.1016/j.biopsycho.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Brunet A, Ashbaugh AR, Saumier D, Nelson M, Pitman RK, Tremblay J, Roullet P, Birmes P. Does reconsolidation occur in humans: A reply. Front Behav Neurosci. 2011a;5:74. doi: 10.3389/fnbeh.2011.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res. 2008;42:503–506. doi: 10.1016/j.jpsychires.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Brunet A, Poundja J, Tremblay J, Bui E, Thomas E, Orr SP, Azzoug A, Birmes P, Pitman RK. Trauma reactivation under the influence of propranolol decreases posttraumatic stress symptoms and disorder: 3 open-label trials. J Clin Psychopharmacol. 2011b;31:547–550. doi: 10.1097/JCP.0b013e318222f360. [DOI] [PubMed] [Google Scholar]
- Cahill L, Pham CA, Setlow B. Impaired memory consolidation in rats produced with beta -adrenergic blockade. Neurobiology of Learning and Memory. 2000;74:259–266. doi: 10.1006/nlme.1999.3950. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O'Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr. 1988;84:25–43. [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O'Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. British Journal of Addiction. 1986;81:655–660. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Gotthard GH. Extinction of appetitive learning is disrupted by cycloheximide and propranolol in the sand maze in rats. Neurobiol Learn Mem. 2011;95:484–490. doi: 10.1016/j.nlm.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- De Martino B, Strange BA, Dolan RJ. Noradrenergic neuromodulation of human attention for emotional and neutral stimuli. Psychopharmacology (Berl) 2008;197:127–136. doi: 10.1007/s00213-007-1015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, Altemus M. Toward a new treatment for traumatic memories. Cerebrum. 2006:2–11. [PubMed] [Google Scholar]
- Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE. Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann N Y Acad Sci. 2006;1071:521–524. doi: 10.1196/annals.1364.056. [DOI] [PubMed] [Google Scholar]
- Decker MW, Gill TM, McGaugh JL. Concurrent muscarinic and beta-adrenergic blockade in rats impairs place-learning in a water maze and retention of inhibitory avoidance. Brain Res. 1990;513:81–85. doi: 10.1016/0006-8993(90)91091-t. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Schoffelmeer AN, De Vries TJ. Beta-adrenoceptor mediated inhibition of long-term reward-related memory reconsolidation. Behav Brain Res. 2006;170:333–336. doi: 10.1016/j.bbr.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Do-Monte FH, Kincheski GC, Pavesi E, Sordi R, Assreuy J, Carobrez AP. Role of beta-adrenergic receptors in the ventromedial prefrontal cortex during contextual fear extinction in rats. Neurobiol Learn Mem. 2010;94:318–328. doi: 10.1016/j.nlm.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Saladin ME, Tiffany ST. Classical conditioning mechanisms in alcohol dependence. In: Heather N, Peters TJ, Stockwell T, editors. International handbook of alcohol dependence and problems. Chichester: John Wiley & Sons, Ltd.; 2001. pp. 281–297. [Google Scholar]
- Drummond DC, Tiffany ST, Glautier S, Remington B. Addictive Behavior: Cue Exposure Theory and Practice. Chichester: John Wiley & Sons Ltd.; 1995. [Google Scholar]
- Duchateau GSMJE, Zuidema J, Merkus FWHM. Bioavailability of Propranolol After Oral, Sublingual, and Intranasal Administration. Pharmaceutical Research. 1986;3:108–111. doi: 10.1023/A:1016345504153. [DOI] [PubMed] [Google Scholar]
- Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: Craving and use during daily life. Addict Behav. 2010;35:318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I disorders-Patient Edition (SCID-I/P, Version 2.0) New York: Biomectrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Font L, Cunningham CL. Post-retrieval propranolol treatment does not modulate reconsolidation or extinction of ethanol-induced conditioned place preference. Pharmacol Biochem Behav. 2012;101:222–230. doi: 10.1016/j.pbb.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Marshall JF. Post-retrieval beta-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learning and Memory. 2008;15:643–648. doi: 10.1101/lm.1054608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CF, Fenyvesi T, Conolly ME, Dollery CT. Pharmacokinetics of dextro-, laevo- and racemic propranolol in man. Eur J Clin Pharmacol. 1972;4:74–76. doi: 10.1007/BF00562500. [DOI] [PubMed] [Google Scholar]
- Hardt O, Einarsson EO, Nader K. A bridge over troubled water: reconsolidation as a link between cognitive and neuroscientific memory research traditions. Annu Rev Psychol. 2010;61:141–167. doi: 10.1146/annurev.psych.093008.100455. [DOI] [PubMed] [Google Scholar]
- Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63:269–276. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- Hernandez PJ, Kelley AE. Cracking addiction the second time around: reconsolidation of drug-related memories. Neuron. 2005;47:772–775. doi: 10.1016/j.neuron.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Hori T, Hayashi M, Oka M, Agari I, Kawabe K, Takagi M. Re-examination of arousing and de-arousing effects of cigarette smoking. Perceptual and motor skills. 1994;78:787–800. doi: 10.1177/003151259407800321. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Rukstalis M, Ehrman R, McGinnis DE, Gariti P, Volpicelli JR, Pettinati H, O'Brien CP. Open trials as a method of prioritizing medications for inclusion in controlled trials for cocaine dependence. Addict Behav. 1999;24:287–291. doi: 10.1016/s0306-4603(98)00040-9. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, Cnaan A, Poole S, Muller E, Acosta T, Luce D, O'Brien C. Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend. 2001;63:69–78. doi: 10.1016/s0376-8716(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Dinter C. New approaches to addiction treatment based on learning and memory. Curr Top Behav Neurosci. 2013;13:671–684. doi: 10.1007/7854_2011_147. [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Kroes MC, Strange BA, Dolan RJ. Beta-adrenergic blockade during memory retrieval in humans evokes a sustained reduction of declarative emotional memory enhancement. J Neurosci. 2010;30:3959–3963. doi: 10.1523/JNEUROSCI.5469-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner HA, Squire LR, Byrne JH. 100 years of consolidation--remembering Muller and Pilzecker. Learning and Memory. 1999;6:77–87. [PubMed] [Google Scholar]
- Lyvers M, Miyata Y. Effects of cigarette smoking on electrodermal orienting reflexes to stimulus change and stimulus significance. Psychophysiology. 1993;30:231–236. doi: 10.1111/j.1469-8986.1993.tb03348.x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Involvement of hormonal and neuromodulatory systems in the regulation of memory storage. Annu Rev Neurosci. 1989;12:255–287. doi: 10.1146/annurev.ne.12.030189.001351. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. The European journal of neuroscience. 2010;31:2308–2319. doi: 10.1111/j.1460-9568.2010.07249.x. [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The persistence of maladaptive memory: addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–1139. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Everitt BJ. Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on {beta}-adrenergic receptors. Learning & Memory. 2008;15:88–92. doi: 10.1101/lm.825008. [DOI] [PubMed] [Google Scholar]
- Nadel L, Hardt O. Update on memory systems and processes. Neuropsychopharmacology. 2011;36:251–273. doi: 10.1038/npp.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Einarsson EO. Memory reconsolidation: an update. Ann N Y Acad Sci. 2010;1191:27–41. doi: 10.1111/j.1749-6632.2010.05443.x. [DOI] [PubMed] [Google Scholar]
- Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- Paliwal P, Hyman SM, Sinha R. Craving predicts time to cocaine relapse: further validation of the Now and Brief versions of the cocaine craving questionnaire. Drug Alcohol Depend. 2008;93:252–259. doi: 10.1016/j.drugalcdep.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson JW, Conolly ME, Dollery CT, Hayes A, Cooper RG. The pharmacodynamics and metabolism of propranolol in man. European Journal of Clinical Pharmacology. 1970;2:127–133. [Google Scholar]
- Preston KL, Vahabzadeh M, Schmittner J, Lin JL, Gorelick DA, Epstein DH. Cocaine craving and use during daily life. Psychopharmacology (Berl) 2009;207:291–301. doi: 10.1007/s00213-009-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyslawski J, Roullet P, Sara SJ. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Riopel DA, Walle T. Kinetic and clinical observations in cyanotic children on propranolol therapy. Clinical pharmacology and therapeutics. 1980;28:743–750. doi: 10.1038/clpt.1980.230. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Armson M, Franklin KB. The effect of propranolol and midazolam on the reconsolidation of a morphine place preference in chronically treated rats. Front Behav Neurosci. 2011a;5:42. doi: 10.3389/fnbeh.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Franklin KB. Central but not peripheral beta-adrenergic antagonism blocks reconsolidation for a morphine place preference. Behav Brain Res. 2007;182:129–134. doi: 10.1016/j.bbr.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Franklin KB. Reconsolidation of a morphine place preference: impact of the strength and age of memory on disruption by propranolol and midazolam. Behav Brain Res. 2010;213:201–207. doi: 10.1016/j.bbr.2010.04.056. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Ross EC, Franklin KB. The effect of propranolol dose and novelty of the reactivation procedure on the reconsolidation of a morphine place preference. Behav Brain Res. 2011b;216:281–284. doi: 10.1016/j.bbr.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Sotres-Bayon F, Mueller D, Quirk GJ. Systemic propranolol acts centrally to reduce conditioned fear in rats without impairing extinction. Biol Psychiatry. 2009;65:887–892. doi: 10.1016/j.biopsych.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin ME, Brady KT, Graap K, Rothbaum BO. A preliminary report on the use of virtual reality technology to elicit craving and cue reactivity in cocaine dependent individuals. Addict Behav. 2006;31:1881–1894. doi: 10.1016/j.addbeh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learning and Memory. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- Schiller D, Phelps EA. Does reconsolidation occur in humans? Front Behav Neurosci. 2011;5:24. doi: 10.3389/fnbeh.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Nader K, Wolf OT, Beaudry T, Pruessner JC. Neural signature of reconsolidation impairments by propranolol in humans. Biol Psychiatry. 2012;71:380–386. doi: 10.1016/j.biopsych.2011.10.028. [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Retrieval per se is not sufficient to trigger reconsolidation of human fear memory. Neurobiol Learn Mem. 2012;97:338–345. doi: 10.1016/j.nlm.2012.01.009. [DOI] [PubMed] [Google Scholar]
- Shand DG, Nuckolls EM, Oates JA. Plasma propranolol levels in adults with observations in four children. Clinical pharmacology and therapeutics. 1970;11:112–120. doi: 10.1002/cpt1970111112. [DOI] [PubMed] [Google Scholar]
- Shand DG, Rangno RE. The disposition of propranolol. I. Elimination during oral absorption in man. Pharmacology. 1972;7:159–168. doi: 10.1159/000136285. [DOI] [PubMed] [Google Scholar]
- Sheehan D, Janavs J, Baker R, Harnett-Sheehan K, Knapp E, Sheehan M, Lecrubier Y, Weiller E, Hergueta P, Amorim P, Bonora LI, Lepine JP. MINI: Mini International Neuropsychiatric Interview. Florida & Paris: Authors; 2003. pp. 1–24. [Google Scholar]
- Sheehan D, Lecrubier Y, Hartnett-Sheehan K, Amorim P, Janavas J, Weiller E, Hergueta T, Baker R, Dunbar G. The Mini International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnaostic psychiatric interview. Journal of Clinical Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of General Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: Psychological and Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Soeter M, Kindt M. Dissociating response systems: erasing fear from memory. Neurobiol Learn Mem. 2010;94:30–41. doi: 10.1016/j.nlm.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Disrupting reconsolidation: pharmacological and behavioral manipulations. Learn Mem. 2011;18:357–366. doi: 10.1101/lm.2148511. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Erasing fear for an imagined threat event. Psychoneuroendocrinology. 2012a;37:1769–1779. doi: 10.1016/j.psyneuen.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Soeter M, Kindt M. Stimulation of the Noradrenergic System during Memory Formation Impairs Extinction Learning but not the Disruption of Reconsolidation. Neuropsychopharmacology. 2012b;37:1204–1215. doi: 10.1038/npp.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Menlo Park, CA: Mind Garden; 1983. [Google Scholar]
- Stout RL, Wirtz PW, Carbonari JP, DelBoca FK. Ensuring balanced distribution of prognostic factors in treatment outcome research. Journal of Studies on Alcohol. 1994;12:70–75. doi: 10.15288/jsas.1994.s12.70. [DOI] [PubMed] [Google Scholar]
- Strong DR, Leventhal AM, Evatt DP, Haber S, Greenberg BD, Abrams D, Niaura R. Positive reactions to tobacco predict relapse after cessation. J Abnorm Psychol. 2011;120:999–1005. doi: 10.1037/a0023666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry P, Wray N, Salmon P. Acute and chronic effects of propranolol on extinction of rewarded running in the rat. Pharmacol Biochem Behav. 1990;36:249–253. doi: 10.1016/0091-3057(90)90399-3. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Conklin CA. A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction. 2000;95(Suppl 2):S145–S153. doi: 10.1080/09652140050111717. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol Learn Mem. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Taylor JR. Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouda JA, Diergaarde L, Riga D, van Mourik Y, Schoffelmeer AN, De Vries TJ. Disruption of Long-Term Alcohol-Related Memory Reconsolidation: Role of beta-Adrenoceptors and NMDA Receptors. Front Behav Neurosci. 2010;4:179. doi: 10.3389/fnbeh.2010.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaky S, Fouad EA, Kotb HIM. The effect of rectal ozone on the portal vein oxygenation and pharmacokinetics of propranolol in liver cirrhosis (a preliminary human study) British Journal of Clinical Pharmacology. 2011;71:411–415. doi: 10.1111/j.1365-2125.2010.03851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LY, Sun LL, Shi J, Li P, Zhang Y, Lu L. Effects of beta-adrenergic receptor blockade on drug-related memory reconsolidation in abstinent heroin addicts. Drug Alcohol Depend. 2011;118:224–229. doi: 10.1016/j.drugalcdep.2011.03.025. [DOI] [PubMed] [Google Scholar]