Abstract
Mycobacterium tuberculosis is highly immunogenic yet appears to have evolved to preserve its antigenicity. The retention of antigenicity is important to the maintenance of a robust immune response that contributes greatly to the late-stage tissue damage required for transmission and completion of the pathogen’s life cycle. Bacterial persistence is achieved through the remodeling of the tissue at site of infection and maintaining the lymphocytes distant to the infected macrophages in the granuloma core. The tissue metabolism within the granuloma leads to lipid sequestration that supports bacterial growth. However, growth on host lipids places metabolic stresses on Mtb, which has evolved to incorporate potentially harmful metabolic intermediates into the very cell wall lipids that induce the remodeling of the host tissue response.
Introduction
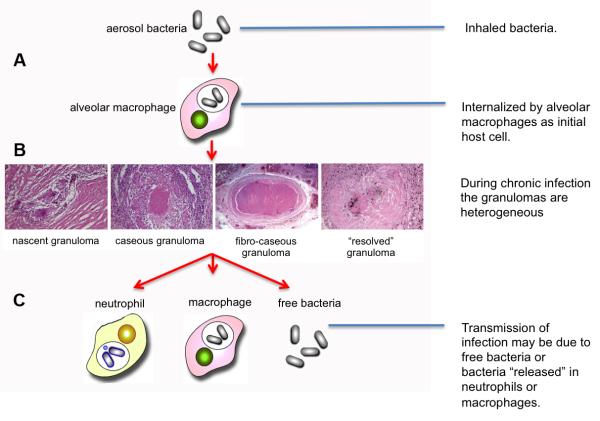
Mycobacterium tuberculosis (Mtb) is a human-specific pathogen with an impressive penetrance of its host population. In the modern era, infection with Mtb leads to active disease in approximately 5-10% of those individuals during the course of their lifetime. This ability to infect many yet cause active disease in only a few at any given instance has likely contributed to the pathogen’s success through co-evolution with its host. The life cycle of the pathogen is shown in Figure 1, which highlights the key points discussed in this article.
Figure 1. The infection cycle of Mycobacterium tuberculosis.
(A) Infection is initiated by inhalation of infectious bacilli that are likely internalized by alveolar macrophages that patrol the airway surfaces of the lung. Mtb induces a proinflammatory reaction through the activation of TLR and NOD pathways and initiates the formation of a macrophage-centric granuloma. (B) During the chronic or latent phase of infection in humans one can observe a wide variety of granulomas in lung tissue that vary from the productive to the controlled. The phenotype(s) of the productive, infectous granulomas have yet to be formally determined. (C) While transmission is conventionally regarded as cavitation of an infected granuloma(s), there is an increasing body of data arguing that migration of infected neutrophis or macrophages to the airways plays a critical role in the release of bacilli and induction of transmission .
Co-evolution of Mtb and mankind
Many publications, even recent ones, describe Mtb as a zoonosis evolving from the bovine pathogen Mycobacterium bovis (Mb) during the development of agriculture around 12,000 years ago [1-3]. However sequencing of both genomes over 10 years ago revealed that the Mtb chromosome was clearly larger than the Mb chromosome, and the size difference was due predominantly to deletions [4-6]. More recent whole genome analysis of Mtb strains representing the sequence diversity in the Mtb complex across the globe generates a fascinating picture of the co-evolution of this pathogen with its human host [7]. The proto-Mtb strain likely existed across Central Africa and infected mankind as they emerged from Africa around 50,000 years ago. Mankind carried the pathogen as they radiated out across the planet and the bacteria evolved independently in different geographic locations until the waves of colonialism lead to the neo-colonization of South American and Southern Africa from the 1500’s onwards. This period of human migration introduced European and Asian strains back into Africa so that the current strain collection on that continent is a genetic mosaic that documents the human migration from its emergence from Africa to its recent re-introduction.
Had Mtb been derived from Mb in the Fertile Crescent 12,000 years ago then much of mankind would have evolved absent selection pressure from Mtb. Were this to be true, one would expect considerable heterogeneity in human susceptibility to this pathogen. But while differential susceptibility to tuberculosis is present, it is comparatively subtle [8-11], except for those catastrophic phenotypes that would be non-sustainable genetic traits absent modern medicine [12]. Such extreme deficiencies have questionable evolutionary significance. The marginal susceptibilities observed are easier to rationalize if, as is now accepted, all of mankind ran the gauntlet of Mtb infection during their emergence from Africa.
Mtb proteins exhibit minimal evidence for antigenic diversity
But what of the evolutionary pressures that shape the Mtb genome? The bacterium requires the human host in order to replicate so clearly must have evolved under consistent selective pressure. However, this selective pressure lacks the environmental diversity experienced by free-living organisms and, like many pathogenic organisms, Mtb has experienced genomic down-sizing [13]. As with any pathogenic organism immune avoidance would constitute a major evolutionary pressure. However, when Hershberg and colleagues extended their analyses to examine the ratio of the rates of non-synonymous and synonymous changes (dN/dS) in 89 genes representing housekeeping genes, virulence-associated genes, and genes encoding proteins that were surface-exposed or secreted they found, surprisingly, that all 3 categories of genes demonstrated comparable ratios of dN/dS mutations [7]. The authors concluded that these genes were selection-neutral with respect to the immune interface with the host.
Mtb contains 2 large gene families that encode the PE and PPE proteins, which have been postulated to represent antigenic variation in Mtb [14]. The genes are thought to encode secreted or cell wall-associated proteins that would interface closely with the host. Both these families of genes show a high degree of genetic variation between family members from different isolates, and the mechanisms of variation, SNPs, intels and deletions also vary considerably. However, analysis of SNPs across an extensive subset of pe and ppe genes revealed a dN/dS ratio close to 1 suggesting that there was no selective advantage for productive mutations [15]. These data indicate that, absent the highly conserved PE region of the protein thought to be involved in association with the cell wall, there is little selective or purifying pressure fixing structural variants. This result adds context to an earlier study where Comas and colleagues found that antigenic epitopes in other genes were highly conserved, which actually implies strong selective pressure against sequence diversity in immunogenic regions [16]. The suggestion that there is no negative selection against Mtb being immunogenic is contrary to most accepted notions of how a pathogen should evolve. Moreover, Mtb is a rich source of agonists for both the TLR and NOD pattern receptors, the activation of which is known to augment the strength of any developing adaptive immune response [17-21].
Why would Mtb not care if it is immunogenic?
At the level of the infected macrophage this seems a bad idea. The macrophage is an antigen-presenting cell capable of informing both CD4+ and CD8+ cells of its infection status, and if activated by interferon-γ (IFN-γ) capable of either killing Mtb or rendering it non-replicative [22]. In the majority of animal models infection by Mtb is marked by a period of rapid bacterial replication preceding the development of the adaptive immune response. Several investigators have noted that this immune response is delayed in comparison to other infections. Wolf and colleagues reported that in murine infections the immune response is driven by the antigen burden in lymph nodes and not in the lung, the major site of bacterial expansion [23]. More recently, temporal analysis of the progression of infection in the rabbit model, which generates a more human-like granuloma, confirmed the delayed nature of the immune response [24]. Transcriptional profiling revealed a delay in peak expression of genes involved in macrophage activation and anti-microbial responses in the lung to 8-12 weeks post-infection. Both these observations suggest either that the lung is a privileged site for Mtb infection, or that the bacterium is able to modulate the immune response at site of infection. This delay is likely critical to the success of the infection allowing bacterial replication and preliminary remodeling of the infection site prior to the development of an adaptive immune response.
Once an adaptive immune response develops, the bacterial load in most immune-competent animal models plateaus. Whether this sub-clinical, or “latent” infection is the product of non-replicating or slowly-replicating bacteria is unclear. Ford and colleagues studied the rate of mutation or SNP acquisition in Mtb throughout the course of disease in Cynomolgous macaques and found that the rates were constant during latency and reactivation, and were the same as those in a logarithmically-growing culture [25]. These data are broadly consistent with those of Gill and coworkers who used Mtb transformed with a replication or “clock” plasmid to measure bacterial replication rates in a murine infection model [26]. They observed sustained loss of the plasmid throughout the course of the infection, although the rate of loss was enhanced by immune suppression of the mouse with dexamethasone indicating that rates did vary as a consequence of immune pressure. These data indicate sustained replication, and therefore a sustained capacity for mutation.
Tempering the impact of immunity through the granuloma
The key to appreciating how Mtb manages host immune pressure comes with the realization that Mtb actually requires the adaptive immune response to complete its life cycle. The late stage tissue damage that culminates in transmission is driven by the host immune response therefore the bacterium has to find other means of modulating host immune function without impairing the robustness of the systemic immune response.
A human TB granuloma in possesses several conserved characteristics, however, within an infected individual you can find granulomas in many different forms, Figure 1B. These are usually discussed as different stages in a continuum [27] but, while this is a useful vehicle, we have little evidence which characteristics are indicative of progression to active infection [28]. The classic granuloma has a caseous center surrounded by a macrophage-rich zone in which you can observe multinucleated giant cells, and foamy macrophages. The bulk of the bacilli are in this region. Outside this are epithelioid macrophages and the collagen-rich fibrotic capsule. Lymphocytes are present in low number within this capsule but both are abundant around its periphery. Transmission is postulated to occur when the necrotic center of the granuloma collapses into the airways release viable, infectious bacteria, however recent reports from both human infections and primate models indicate that neutrophils may carry bacteria to the airways during late stage inflammation without the necessity for cavitation of the granuloma [28,29], Figure 1C.
Although host-derived, the granuloma likely represents the efforts by Mtb to shape the immune response and blunten its edge locally. Mycobacterial lipids released by intracellular Mtb traffic from infected to uninfected cells. When inoculated into mice these lipids can induce many of the characteristics of the Mtb granuloma [30-32]. Trehalose dimycolate, TDM, appears the most bioactive of the bacterial effectors [19]. Although the environment within the granuloma does not represent the extremes experienced by free-living bacteria, it does present a range of conditions that are potentially limiting to bacterial growth [27,28]. The manner in which the bacterium senses and responds to these pressures is key to its success. Although extracellular bacteria can be observed in granulomas, the ability of Mtb to sustain its infection in the macrophage is crucial. Clearly the bacterium’s ability to assess its environment and respond accordingly would be an important pressure shaping its genome.
The intracellular environment shapes bacterial metabolism
Mtb is able to arrest the normal maturation of its phagosome and resides in a vacuole that retains many of the characteristics of a sorting endosome [33]. It has a pH of 6.4, and it remains accessible to early endosomal contents. However, upon activation of the macrophage this blockage is overcome and the bacterium is exposed to a lower pH, more hydrolytically-competent environment. The physiological gradients in the endosome-lysosome continuum provide useful cues to Mtb. pH is well studied and Mtb is known to respond robustly to the acidification of its environment [34,35]. Interestingly, the response to pH can be divided into two types; the physiological response, linked to the maintenance of cytosolic homeostasis [36,37], and an adaptive response that is not obviously linked to pH homeostasis but appears connected to metabolic shifts required for growth in the endosomal continuum [38].
Mtb senses pH through the two-component sensor PhoPR and mutants deficient in phoPR are avirulent [39,40]. We have studied the transcriptional response on Mtb during invasion of the macrophage and the reduction in pH appears responsible for approximately 50% of the genes up-regulated early in infection [34]. Amongst the genes up-regulated is a 3 gene operon, aprABC, which appears to have been acquired by horizontal gene transfer and is unique to the Mtb complex [38]. Deletion of the operon leads to impaired survival in macrophages and a loss of production of polyketide-containing cell wall lipids, such as the virulence-associated phthiocerol dimycocerosates, PDIMs [41].
Recent literature indicates that intracellular Mtb relies of host cholesterol and fatty acids as major carbon sources [42-44], however the utilization of cholesterol can come at a cost to Mtb. Degradation of the side chain of cholesterol gives rise to propionyl-CoA [45-47]. Mtb is exquisitely sensitive to increases in the propionyl-CoA pool and the bacterium has three different means of metabolizing this precursor of potentially-toxic metabolite(s) [48-50]. ICL1 catalyzes the last reaction of the methylcitrate cycle (MCC) that converts methylisocitrate to succinate and pyruvate that feeds into the TCA cycle [43,48]. Alternatively, propionyl-CoA carboxylase can generate methylmalonyl-CoA that will enter the methylmalonyl pathway (MMP) leading to the production of succinyl-CoA [49]. Finally, and of considerable significance to infection, these 3-carbon intermediates in the form of methylmalonyl-CoA may be used as building blocks for the bacterium’s cell wall lipids [51-53]. These bioactive lipids includes phthiocerol dimycocerosates (PDIM), sulfolipid-1 (SL-1), diacyltrehalose (DAT), triacyltrehalose (TAT), and polyacyltrehalose (PAT) [54], which provide an effective “sink” for excess propionyl-CoA as well as a means of manipulation of the host tissue response [19,30-32,55,56].
Subversion of host tissue metabolism to support infection
Transcriptional profiling of caseous human TB granulomas [56,57] revealed dysregulation of lipid metabolism as a pathogen-induced pathology that may drive tissue breakdown. Host proteins key to lipid overload and sequestration localized to cells subtending the caseum by immuno-histology. Furthermore, the caseous core of the TB granuloma contained triacylglycerides, cholesterol and cholesterol ester, together with sphingomyelin and lactosylceramide. The presence of cholesterol ester indicated that foamy macrophages were the most likely source of the lipids in the caseum. Foamy macrophage formation is induced by Mtb infection of macrophages in culture, and most significantly, by trehalose dimycolate (TDM)-coated beads inoculated sub-cutaneously into mice [56,58]. Co-incidentally, TDM is an excellent sink for propionyl-CoA. The data imply that the dysregulation of lipid metabolism is a pathogen-induced phenomenon that plays a key role in the pathology associated with progression to disease [56,57], and may also expand the nutrient pool accessible to the bacilli [59].
Concluding Remarks
Mycobacterium spp. are highly immunogenic and were used previously as constituents in Freund’s Complete Adjuvant. While it is not unusual for pathogens to induce an inflammatory response to promote transmission, such as Vibrio cholera and Salmonella, such a trait is atypical of a chronic infection. Instead of diminishing its immunogenicity Mtb has evolved to model its site of infection to support its persistence despite a strong immune response. This remodeling of the granuloma leads to the exclusion of lymphocytes and the dysregulation of host lipid homeostasis, both of which appear to favor bacterial survival. In a pathogen that relies so heavily on lipid metabolism both for nutrition and for the synthesis of effectors to regulate host behavior it is unsurprising that it has evolved to devote a significant portion of its genome [4] and the majority of its core intracellular transcriptome [60] to these activities. The fact that Mtb has evolved to sustain an infection in the face of a robust systemic immune response remains a considerable problem that has yet to be addressed effectively by any vaccine development program.
Highlights.
Mtb has evolved with mankind for 50,000 years after human migration from Africa.
Mtb shows minimal genetic mutations linked to the avoidance of antigenicity.
Mtb is immunogenic, which is critical to the tissue damage required for transmission.
Mtb avoids the systemic immune response by local modulation of the infection site.
The strategies employed by Mtb have serious consequences for vaccine development.
Acknowledgements
DGR is supported by US Public Health Services grants AI067027, AI095519, and HL055936 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Diamond J. Guns, Germs and Steel: The Fates of Human Societies. W. W. Norton and Company; London: 1997. [Google Scholar]
- 2.Stead WW. The origin and erratic global spread of tuberculosis. How the past explains the present and is the key to the future. Clin Chest Med. 1997;18:65–77. doi: 10.1016/s0272-5231(05)70356-7. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe ND, Dunavan CP, Diamond J. Origins of major human infectious diseases. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, 3rd, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 5.Garnier T, Eiglmeier K, Camus JC, Medina N, Mansoor H, Pryor M, Duthoy S, Grondin S, Lacroix C, Monsempe C, et al. The complete genome sequence of Mycobacterium bovis. Proc Natl Acad Sci U S A. 2003;100:7877–7882. doi: 10.1073/pnas.1130426100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mostowy S, Inwald J, Gordon S, Martin C, Warren R, Kremer K, Cousins D, Behr MA. Revisiting the evolution of Mycobacterium bovis. J Bacteriol. 2005;187:6386–6395. doi: 10.1128/JB.187.18.6386-6395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hershberg R, Lipatov M, Small PM, Sheffer H, Niemann S, Homolka S, Roach JC, Kremer K, Petrov DA, Feldman MW, et al. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 2008;6:e311. doi: 10.1371/journal.pbio.0060311. ** Yes, this is more than 2 years old but it it is a fascinating account of the co-evolution of humans and Mtb, and details the genetic mosaic of Mtb strains in modern Africa. It is a must read article.
- 8.Kleinnijenhuis J, Oosting M, Joosten LA, Netea MG, Van Crevel R. Innate immune recognition of Mycobacterium tuberculosis. Clin Dev Immunol. 2011;2011:405310. doi: 10.1155/2011/405310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moller M, de Wit E, Hoal EG. Past, present and future directions in human genetic susceptibility to tuberculosis. FEMS Immunol Med Microbiol. 2010;58:3–26. doi: 10.1111/j.1574-695X.2009.00600.x. [DOI] [PubMed] [Google Scholar]
- 10.Schurr E. The contribution of host genetics to tuberculosis pathogenesis. Kekkaku. 2011;86:17–28. [PubMed] [Google Scholar]
- 11.Yim JJ, Selvaraj P. Genetic susceptibility in tuberculosis. Respirology. 2010;15:241–256. doi: 10.1111/j.1440-1843.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- 12.Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondaneche MC, Tuerlinckx D, Blanche S, Emile JF, Gaillard JL, Schreiber R, Levin M, et al. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. J Clin Invest. 1997;100:2658–2664. doi: 10.1172/JCI119810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith NH, Hewinson RG, Kremer K, Brosch R, Gordon SV. Myths and misconceptions: the origin and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol. 2009;7:537–544. doi: 10.1038/nrmicro2165. [DOI] [PubMed] [Google Scholar]
- 14.Karboul A, Mazza A, Gey van Pittius NC, Ho JL, Brousseau R, Mardassi H. Frequent homologous recombination events in Mycobacterium tuberculosis PE/PPE multigene families: potential role in antigenic variability. J Bacteriol. 2008;190:7838–7846. doi: 10.1128/JB.00827-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEvoy CR, Cloete R, Muller B, Schurch AC, van Helden PD, Gagneux S, Warren RM, Gey van Pittius NC. Comparative analysis of Mycobacterium tuberculosis pe and ppe genes reveals high sequence variation and an apparent absence of selective constraints. PLoS One. 2012;7:e30593. doi: 10.1371/journal.pone.0030593. * This family of proteins is frequently cited as evidence for antigenic variation in Mtb. This study demonstrates that there is no selection for productive mutations, which argues against their evolution being subject to immune selection.
- 16.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, Ernst JD, Gagneux S. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowdish DM, Sakamoto K, Kim MJ, Kroos M, Mukhopadhyay S, Leifer CA, Tryggvason K, Gordon S, Russell DG. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000474. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ, Langenberg DM, van Crevel R, Adema GJ, Ottenhoff TH, Van der Meer JW, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:279–285. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geisel RE, Sakamoto K, Russell DG, Rhoades ER. In vivo activity of released cell wall lipids of Mycobacterium bovis bacillus Calmette-Guerin is due principally to trehalose mycolates. J Immunol. 2005;174:5007–5015. doi: 10.4049/jimmunol.174.8.5007. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J Biol Chem. 2007;282:36223–36229. doi: 10.1074/jbc.M703079200. [DOI] [PubMed] [Google Scholar]
- 22.Russell DG. Mycobacterium tuberculosis: here today, and here tomorrow. Nat Rev Mol Cell Biol. 2001;2:569–577. doi: 10.1038/35085034. [DOI] [PubMed] [Google Scholar]
- 23.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subbian S, Tsenova L, Yang G, O’Brien P, Parsons S, Peixoto B, Taylor L, Fallows D, Kaplan G. Chronic pulmonary cavitary tuberculosis in rabbits: a failed host immune response. Open Biol. 2011;1:110016. doi: 10.1098/rsob.110016. ** This paper provides an extensive, temporal analysis of the adaptive immune response in the rabbit model of tuberculosis. Intrigingly, the adaptive immune response is delayed extensively and only reaches its full capacity 8-12 weeks post infection.
- 25.Ford CB, Lin PL, Chase MR, Shah RR, Iartchouk O, Galagan J, Mohaideen N, Ioerger TR, Sacchettini JC, Lipsitch M, et al. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat Genet. 2011;43:482–486. doi: 10.1038/ng.811. * There is much debate about the replication status of Mtb during the course of infection. This publication reports that the mutation rates of Mtb in an animal infection is comparable to that in liquid culture, implying that replication of sustained throughout this infection model.
- 26.Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. A replication clock for Mycobacterium tuberculosis. Nat Med. 2009;15:211–214. doi: 10.1038/nm.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russell DG. Who puts the tubercle in tuberculosis? Nat Rev Microbiol. 2007;5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 28.Gideon HP, Flynn JL. Latent tuberculosis: what the host “sees”? Immunol Res. 2011;50:202–212. doi: 10.1007/s12026-011-8229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eum SY, Kong JH, Hong MS, Lee YJ, Kim JH, Hwang SH, Cho SN, Via LE, Barry CE., 3rd Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beatty WL, Rhoades ER, Ullrich HJ, Chatterjee D, Heuser JE, Russell DG. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 2000;1:235–247. doi: 10.1034/j.1600-0854.2000.010306.x. [DOI] [PubMed] [Google Scholar]
- 31.Rhoades E, Hsu F, Torrelles JB, Turk J, Chatterjee D, Russell DG. Identification and macrophage-activating activity of glycolipids released from intracellular Mycobacterium bovis BCG. Mol Microbiol. 2003;48:875–888. doi: 10.1046/j.1365-2958.2003.03473.x. [DOI] [PubMed] [Google Scholar]
- 32.Rhoades ER, Geisel RE, Butcher BA, McDonough S, Russell DG. Cell wall lipids from Mycobacterium bovis BCG are inflammatory when inoculated within a gel matrix: characterization of a new model of the granulomatous response to mycobacterial components. Tuberculosis (Edinb) 2005;85:159–176. doi: 10.1016/j.tube.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev. 2011;240:252–268. doi: 10.1111/j.1600-065X.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rohde KH, Abramovitch RB, Russell DG. Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues. Cell Host Microbe. 2007;2:352–364. doi: 10.1016/j.chom.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Walters SB, Dubnau E, Kolesnikova I, Laval F, Daffe M, Smith I. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol Microbiol. 2006;60:312–330. doi: 10.1111/j.1365-2958.2006.05102.x. [DOI] [PubMed] [Google Scholar]
- 36.Vandal OH, Pierini LM, Schnappinger D, Nathan CF, Ehrt S. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat Med. 2008;14:849–854. doi: 10.1038/nmXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandal OH, Roberts JA, Odaira T, Schnappinger D, Nathan CF, Ehrt S. Acid-susceptible mutants of Mycobacterium tuberculosis share hypersusceptibility to cell wall and oxidative stress and to the host environment. J Bacteriol. 2009;191:625–631. doi: 10.1128/JB.00932-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abramovitch RB, Rohde KH, Hsu FF, Russell DG. aprABC: a Mycobacterium tuberculosis complex-specific locus that modulates pH-driven adaptation to the macrophage phagosome. Mol Microbiol. 2011;80:678–694. doi: 10.1111/j.1365-2958.2011.07601.x. * This report details a novel Mtb operon acquired by horizontal gene transfer that plays a key role in regulating Mtb’s response to an acidic environment
- 39.Martin C, Williams A, Hernandez-Pando R, Cardona PJ, Gormley E, Bordat Y, Soto CY, Clark SO, Hatch GJ, Aguilar D, et al. The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine. 2006;24:3408–3419. doi: 10.1016/j.vaccine.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 40.Perez E, Samper S, Bordas Y, Guilhot C, Gicquel B, Martin C. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol Microbiol. 2001;41:179–187. doi: 10.1046/j.1365-2958.2001.02500.x. [DOI] [PubMed] [Google Scholar]
- 41.Cox JS, Chen B, McNeil M, Jacobs WR., Jr. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 1999;402:79–83. doi: 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- 42.Daniel J, Maamar H, Deb C, Sirakova TD, Kolattukudy PE. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. 2011;7:e1002093. doi: 10.1371/journal.ppat.1002093. * This paper provides formal proof that Mtb accesses and utilizes lipid stores in its host macrophage. The authors use fluorescent lipids that can be visualized in intracelluar Mtb.
- 43.McKinney JD, Honer zu Bentrup K, Munoz-Elias EJ, Miczak A, Chen B, Chan WT, Swenson D, Sacchettini JC, Jacobs WR, Jr., Russell DG. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–738. doi: 10.1038/35021074. [DOI] [PubMed] [Google Scholar]
- 44.Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A. 2008;105:4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griffin JE, Pandey AK, Gilmore SA, Mizrahi V, McKinney JD, Bertozzi CR, Sassetti CM. Cholesterol catabolism by Mycobacterium tuberculosis requires transcriptional and metabolic adaptations. Chem Biol. 2012;19:218–227. doi: 10.1016/j.chembiol.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang X, Nesbitt NM, Dubnau E, Smith I, Sampson NS. Cholesterol metabolism increases the metabolic pool of propionate in Mycobacterium tuberculosis. Biochemistry. 2009;48:3819–3821. doi: 10.1021/bi9005418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas ST, VanderVen BC, Sherman DR, Russell DG, Sampson NS. Pathway profiling in Mycobacterium tuberculosis: elucidation of cholesterol-derived catabolite and enzymes that catalyze its metabolism. J Biol Chem. 2011;286:43668–43678. doi: 10.1074/jbc.M111.313643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munoz-Elias EJ, Upton AM, Cherian J, McKinney JD. Role of the methylcitrate cycle in Mycobacterium tuberculosis metabolism, intracellular growth, and virulence. Mol Microbiol. 2006;60:1109–1122. doi: 10.1111/j.1365-2958.2006.05155.x. [DOI] [PubMed] [Google Scholar]
- 49.Savvi S, Warner DF, Kana BD, McKinney JD, Mizrahi V, Dawes SS. Functional characterization of a vitamin B12-dependent methylmalonyl pathway in Mycobacterium tuberculosis: implications for propionate metabolism during growth on fatty acids. J Bacteriol. 2008;190:3886–3895. doi: 10.1128/JB.01767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Upton AM, McKinney JD. Role of the methylcitrate cycle in propionate metabolism and detoxification in Mycobacterium smegmatis. Microbiology. 2007;153:3973–3982. doi: 10.1099/mic.0.2007/011726-0. [DOI] [PubMed] [Google Scholar]
- 51.Jain M, Petzold CJ, Schelle MW, Leavell MD, Mougous JD, Bertozzi CR, Leary JA, Cox JS. Lipidomics reveals control of Mycobacterium tuberculosis virulence lipids via metabolic coupling. Proc Natl Acad Sci U S A. 2007;104:5133–5138. doi: 10.1073/pnas.0610634104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell DG, VanderVen BC, Lee W, Abramovitch RB, Kim MJ, Homolka S, Niemann S, Rohde KH. Mycobacterium tuberculosis wears what it eats. Cell Host Microbe. 2010;8:68–76. doi: 10.1016/j.chom.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rainwater DL, Kolattukudy PE. Isolation and characterization of acyl coenzyme A carboxylases from Mycobacterium tuberculosis and Mycobacterium bovis, which produce multiple methyl-branched mycocerosic acids. J Bacteriol. 1982;151:905–911. doi: 10.1128/jb.151.2.905-911.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaur D, Guerin ME, Skovierova H, Brennan PJ, Jackson M. Chapter 2: Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. Adv Appl Microbiol. 2009;69:23–78. doi: 10.1016/S0065-2164(09)69002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beatty WL, Ullrich HJ, Russell DG. Mycobacterial surface moieties are released from infected macrophages by a constitutive exocytic event. Eur J Cell Biol. 2001;80:31–40. doi: 10.1078/0171-9335-00131. [DOI] [PubMed] [Google Scholar]
- 56.Kim MJ, Wainwright HC, Locketz M, Bekker LG, Walther GB, Dittrich C, Visser A, Wang W, Hsu FF, Wiehart U, et al. Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol Med. 2010;2:258–274. doi: 10.1002/emmm.201000079. ** This paper provides the first transcriptional profile of isolated human TB granulomas in their caseous state. The authors go on to generate the first characterization and identification of the lipids present in the caseous center of the granuloma. The data demonstrated the dysregulation of host lipid metabolism in tuberculosis.
- 57.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C, Bardou F, Daffe M, Emile JF, Marchou B, Cardona PJ, et al. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4:e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garton NJ, Waddell SJ, Sherratt AL, Lee SM, Smith RJ, Senner C, Hinds J, Rajakumar K, Adegbola RA, Besra GS, et al. Cytological and transcript analyses reveal fat and lazy persister-like bacilli in tuberculous sputum. PLoS Med. 2008;5:e75. doi: 10.1371/journal.pmed.0050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Homolka S, Niemann S, Russell DG, Rohde KH. Functional genetic diversity among Mycobacterium tuberculosis complex clinical isolates: delineation of conserved core and lineage-specific transcriptomes during intracellular survival. PLoS Pathog. 2010;6:e1000988. doi: 10.1371/journal.ppat.1000988. [DOI] [PMC free article] [PubMed] [Google Scholar]