Abstract
PURPOSE
To study the pattern of facilitatory and suppressive binocular interactions in stereo-deficient patients with strabismus and in normal controls.
METHODS
Visual Evoked Potentials were recorded in response to a vernier onset/offset pattern presented to one eye, either monocularly or paired dichoptically with a straight vertical square-wave grating, which when fused with the target in the other eye gave rise to a percept of a series of bands appearing in depth from an otherwise uniform plane or with a grating that contained offsets that produced a standing disparity and the appearance of a constantly segmented image, portions of which moved in depth.
RESULTS
Participants with normal stereopsis showed facilitative and suppressive binocular interactions that depended on which dichoptic target was presented. Patients with long-standing, constant strabismus lacked normal facilitative binocular interactions. The response to a normally facilitative stimulus was reduced below the monocular level when it was presented to the dominant eye of patients without anisometropia, consistent with classical strabismic suppression of the non dominant eye. The dominant eye of strabismic patients without anisometropia retained a suppressive input from crossed but not uncrossed disparity stimuli presented to the non-dominant eye.
CONCLUSIONS
Abnormal disparity processing can be detected with the dichoptic VEP method we describe. Our results suggest that suppression in stereoblind, non-amblyopic observers is determined by a binocular mechanism responsive to disparity. In some cases, the sign of the disparity is important and this suggests a mechanism that can explain diplopia in patients made exotropic after surgery for esotropia.
Introduction
In a natural scene, many features in the visual field will lie at different horizontal, and sometimes vertical, positions in the two retinal half-images. Fusion converts the horizontal differences (disparities) into a stereoscopic depth, assigning each feature a unique three-dimensional position in visual space. Fusional mechanisms also change the visual direction so that the fused percept appears at a horizontal and vertical location midway between the locations in the half-images. It is easy enough to work out the projected location and depth of a feature from the binocular geometry, but how does the binocular system implement this fusion process neurally? Viewed alone, the monocular location of the target is different from the binocular location. What happens to the neural responses to monocular location when the images are fused?
Numerous psychophysical studies have demonstrated that information in the monocular half-images is lost during normal fusion1–4. In a study that is particularly relevant to this paper, McKee and Harrad5 measured monocular Vernier acuity using a standard target that consisted of two vertical lines, presented one above the other. Predictably, thresholds for detecting misalignment were in the hyperacuity range (< 10 arcsec). However, thresholds rose dramatically when this target was paired stereoscopically with a Vernier target in the other eye containing a large fixed offset. Because of the large offset, the upper line appeared at a different depth from the lower line. Provided that the upper line remained fused binocularly (for offsets covering a range of 6 – 60 arcmin of disparity), thresholds were elevated, compared to the monocular Vernier threshold, by as much as a log unit. This “fusional suppression” is not a form of dichoptic masking, because if the Vernier target is fused with a straight line in the other eye (0 offset), thresholds are very low, since the binocular target is now essentially a stereoacuity target.
Fusional suppression is probably part of the neural network that determines a unique stereoscopic match. A monocular target will weakly stimulate all disparity-tuned neurons that receive input from the target’s retinal location. When a matching feature is presented to the other eye that strongly excites neurons tuned to a particular disparity, these neurons may inhibit or suppress the weaker responses from neurons tuned to other disparities and locations. An inhibitory network of this type will enhance the correct match by reducing the competitors.
Previous work on normal observers6, has found electrophysiological evidence for these inhibitory interactions. Using a variant of the McKee-Harrad stimulus, we presented an oscillating Vernier target in one eye and paired it stereoscopically with a static fusable target in the other eye (see Fig. 1). The visual evoked potential (VEP) is particularly sensitive to the misalignment of contours or surfaces7, 8. The static target by itself elicited no VEP, but when fused with the oscillating Vernier target, it modulated the Vernier-driven response. The response was enhanced when paired with straight static lines (middle panel of Fig. 1), but was suppressed by the offset static target (third panel of Fig. 1). As in the psychophysical studies, binocular fusion of a disparate target suppressed the monocular VEP response, and the inhibitory interaction was dependent on the target disparity.
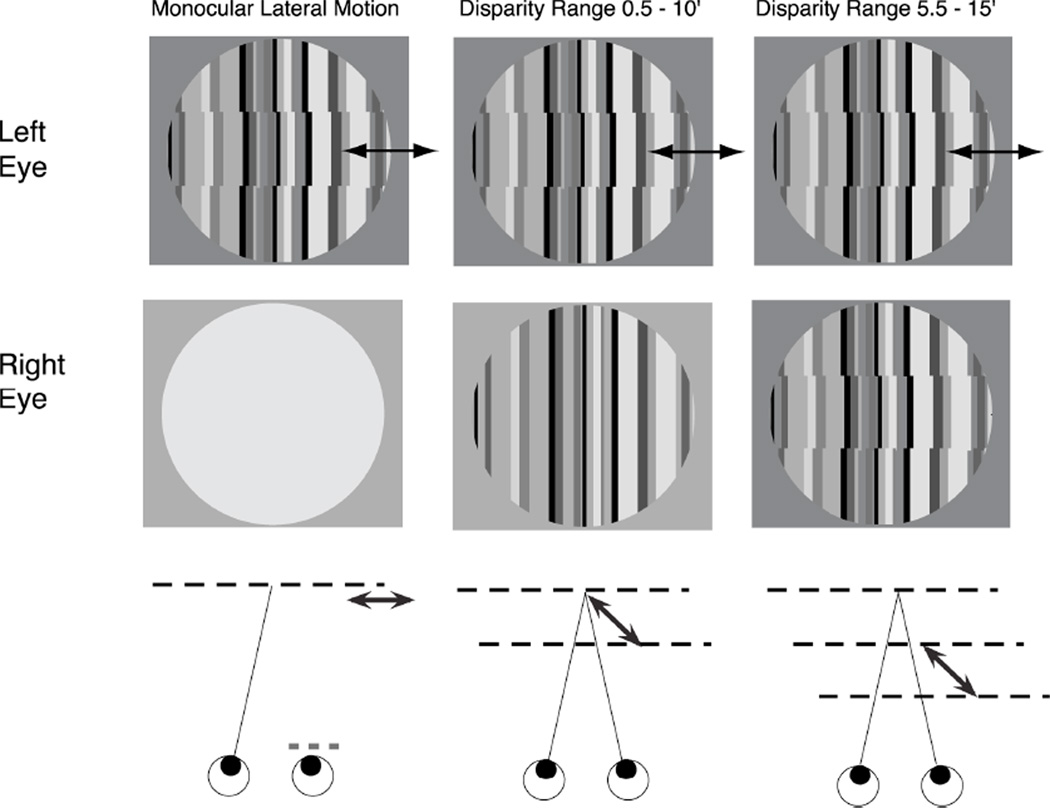
FIG 1.
Stimulus schematic. A vernier onset/offset target was presented to one eye while the other eye viewed either a blank screen (Monocular Lateral Motion), a collinear bar pattern (Disparity Range 0.5–10’) or an offset bar pattern (Disparity Range 5.5–15’). The test stimulus is in the left eye in this illustration, but the tests were presented in both eyes and a range of both crossed and uncrossed disparities was presented in separate conditions. The lower row illustrates the perceived surfaces and motions. In the Monocular lateral motion condition, the dynamic offsets appear to move laterally in the fixation plane over a range of displacements between 0.5 and 10’. In the Disparity Range 0.5–10’ condition, lateral motion and motion in depth are both seen and the stimulus alternates between collinear flat plane and a segmented set of panels, illustrated as two depth planes with arrows between them. In the Disparity Offset 5.5–15’ condition, the offsets in the right eye create a standing crossed disparity and the dynamic panels alternate between two planes that lie in front of the fixation plane.
Due to the misalignment of their eyes during early development, most strabismic observers have no functional stereopsis. Nevertheless, they do have binocular interactions, primarily of a suppressive or inhibitory kind9. Harrad and Hess10 explored whether suppression could be explained as normal dichoptic masking with unequal contrast thresholds. They found that even when the difference in thresholds was taken into account, masking by the dominant (fixing) eye was greater than predicted from normal dichoptic masking functions. Since strabismic suppression is not explained by dichoptic contrast masking, it must involve some other form of binocular inhibition. Given that normal fusion involves suppression, strabismic suppression may be a residual component of the non-functional stereo system11,12. The fact that strabismic suppression is only observed between similar contours13 and that cross-oriented gratings presented dichoptically produce rivalry in strabismics as in normal observers supports this idea11.
In this study, we used the VEP to explore the electrophysiological characteristics of suppression in strabismic patients with and without anisometropia. We find evidence consistent with strabismic suppression being a component of an aberrant stereo network that when operative, prevents diplopia in the absence of stereopsis. We have also found a special case where suppression fails in a disparity-dependent fashion.
Materials and Methods
Observers
Nine healthy adult observers with normal monocular and binocular vision and no previous history of amblyopia, patching or intermittent strabismus consented to participate. Each observer had a corrected LogMar visual acuity of 0 (20/20) or better in each eye and normal stereopsis on testing with TNO plates. We defined normal stereopsis as ≤ 60 arcsec; however, the normal participants all had a stereoacuity of ≤ 30 arcsec. Thirty-two patients with a history of abnormal visual experience during development, either due to strabismus with anisometropia (n=14) or strabismus without anisometropia (n=12). The stereoacuity of the patients ranged from 120 arc sec to unmeasurable. Patients were considered to be anisometropic if their spherical equivalent refractions differed by more than 0.75 diopters between the two eyes. Patients were considered to be amblyopic if their interocular acuity differed by 0.2 LogMAR or more. Local Ethical Committee approval was obtained and each observer gave fully informed consent. The research complied with the principles of the Declaration of Helsinki.
Stimulus generation and apparatus
Details of the apparatus and basic signal acquisition and processing operations are described in detail in a previous publication14 and are described here only briefly. The active VEP display, described schematically in Fig. 1, comprised a circular image of 14 deg diameter. Computer generated nonius lines for alignment in both the horizontal and vertical planes were presented around the aperture and the stimulus was further surrounded by a fusible pattern of small circles that aided accurate superimposition of the images. The observers were asked to physically align the nonius lines by movement of the mirrors and to regularly check their position between stimulus trials.
VEP stimulation protocol
Six stimulus conditions were presented to each eye. Each condition consisted of the same dynamic “test” stimulus presented to one eye, with one of three static images presented to the other eye. The Monocular condition consisted of an oscillating vernier onset/offset stimulus presented to one eye and a mean luminance central field surrounded by the binocular fixation pattern presented to the second eye. In a second condition type (Binocular 0 disparity), the oscillating vernier stimulus was paired with a static, collinear bar pattern. The vernier offset created either crossed or uncrossed disparities. The third condition type (Binocular 5 arcmin) was the same as the second, except that the static bar pattern also contained vernier offsets that created a standing crossed or uncrossed disparity pedestal of 5 arcmin. Crossed disparities will be referred to as positive numbers and uncrossed disparities as negative numbers. When presented alone, the static patterns did not produce a VEP response, but when fused with the temporally modulated pattern, they modified the normal observer’s perception of the stimulus in terms of its position in both the lateral and depth domains.
The dynamic ‘test’ pattern consisted of vertical randomly generated black and green bars of spatial frequency 1± 0.49 c/deg, with 80% contrast. The pattern was divided into 1° horizontal bands. An oscillating vernier pattern was created by laterally shifting alternating bands back and forth, into and out of alignment with the static bands, at a frequency of 2 Hz (square-wave temporal profile). Over a trial period of 10 seconds, the vernier offsets increased in size in 10 equal logarithmic steps from 0.5 arcmin to 10 arcmin.
When the ‘test’ stimulus was combined with a blank mean luminance half-image, the observers perceived purely lateral displacement (see cartoon at bottom of Fig. 1). When combined with the straight static pattern (Figure 1B) the displacement produced a horizontal disparity so that normal observers perceived the oscillating bands appearing and disappearing in depth from a collinear background. The moving panels jumped to an apparent position that was either in front of, or behind the static bands for crossed and uncrossed disparities, respectively. In the other binocular condition type, the static pattern was also divided into bands matching those in the dynamic pattern. These bands were assigned a constant lateral offset of +/− 5 arcmin in the direction opposite to the offset in the dynamic pattern. Thus, as the vernier oscillation swept from 0.5 to 10 arcmin, the disparity of the bands swept from 5.5 to 15.0 arcmin or −5.5 to −15.0 arcmin.
VEP quantification and statistical analysis
The complex numbers representing the amplitudes and phases of the 2Hz first harmonic of the evoked response were coherently averaged over all trials for each stimulus condition for each observer. Coherent averaging uses both amplitude and phase information. Group averages were computed in a similar way, e.g. the average sine and cosine coefficients were calculated across observers before calculating a magnitude. For the plots of response magnitude (Fig. 2), we computed an error measure by pooling the errors on the sine and cosine coefficients in quadrature as has previously been done for single observer averages15. This measure assumes that the sine and cosine coefficients are uncorrelated. However, correlations occur between these measurements in the case of cross-observer averages due to the presence of individual differences in overall response amplitude. For significance testing, we therefore used a method (Multi-variate Analysis of Variance; MANOVA) that takes these correlations into account. MANOVA also correctly models the correlation structure of our repeated measures design. When we plot data in the complex plane, we show two-dimensional standard errors (Fig. 3). These error bounds are often elliptical due to correlations between the real and imaginary values that arise from individual differences in absolute amplitude. Note that these errors are the same as those that would be computed for a between subjects’ design and they therefore do not reflect the within observer errors that are used to assess significance when the MANOVA is used to compare responses across conditions measured within a given group. In all of the statistical evaluations, we used the last bin of the sweep to test for effects of stimulus condition because this bin generally had the largest response across conditions and observer group.
FIG 2.
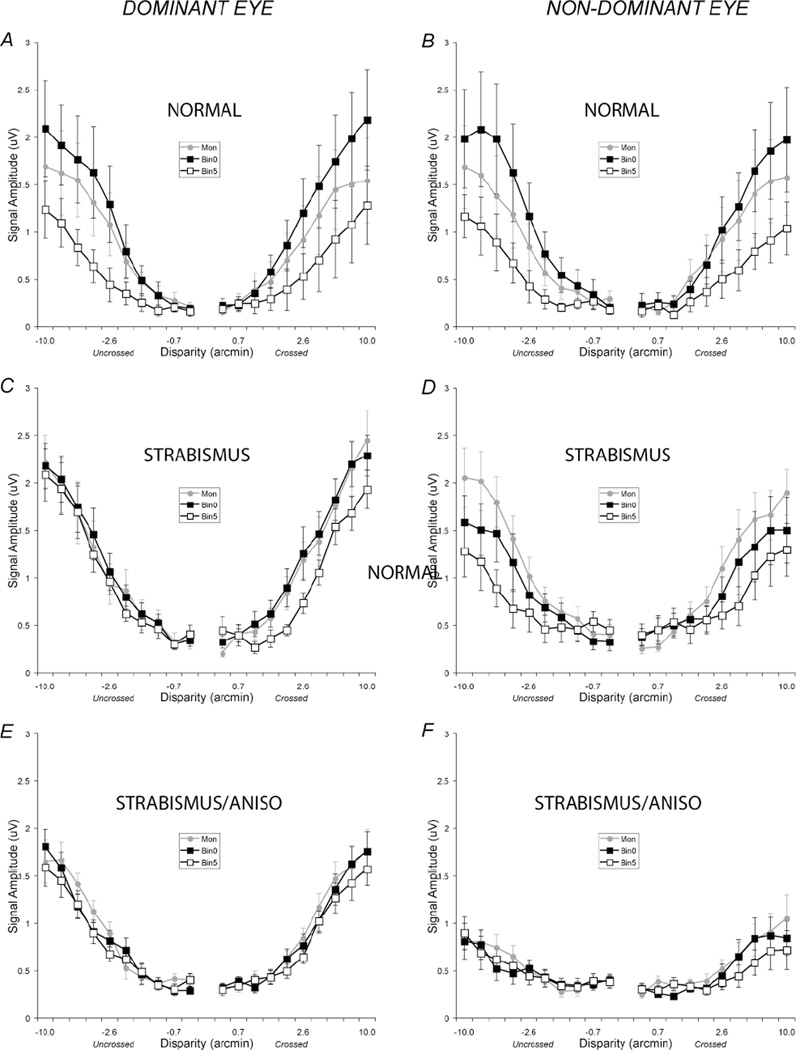
Disparity tuning functions for the first harmonic (1F) components for the normal vision participants (A,B), patients with strabismus without anisometropia (C,D) and strabismus patients with anisometropia (E,F). Data collected when the test was in the dominant eyes are plotted in the first column (Dominant Eye). Data collected when the test was in the non-dominant eye are plotted in the right column (Non-Dominant Eye). Gray filled circles plot the monocular data (Mon), black filled squares plot the 0 disparity pedestal data (Bin0) and black open squares plot the 5 arc min disparity pedestal data (Bin5). In the normal vision observers (A,B), the first harmonic response of the 0 disparity pedestal data lies above the monocular data but the 5 arc min disparity pedestal data lies below the monocular data. In each of the patient groups, the facilitation in the Bin0 condition is reduced. The error bars were calculated by summing the errors on the sine and cosine coefficients computed across observers in quadrature44. See text for details.
FIG 3.
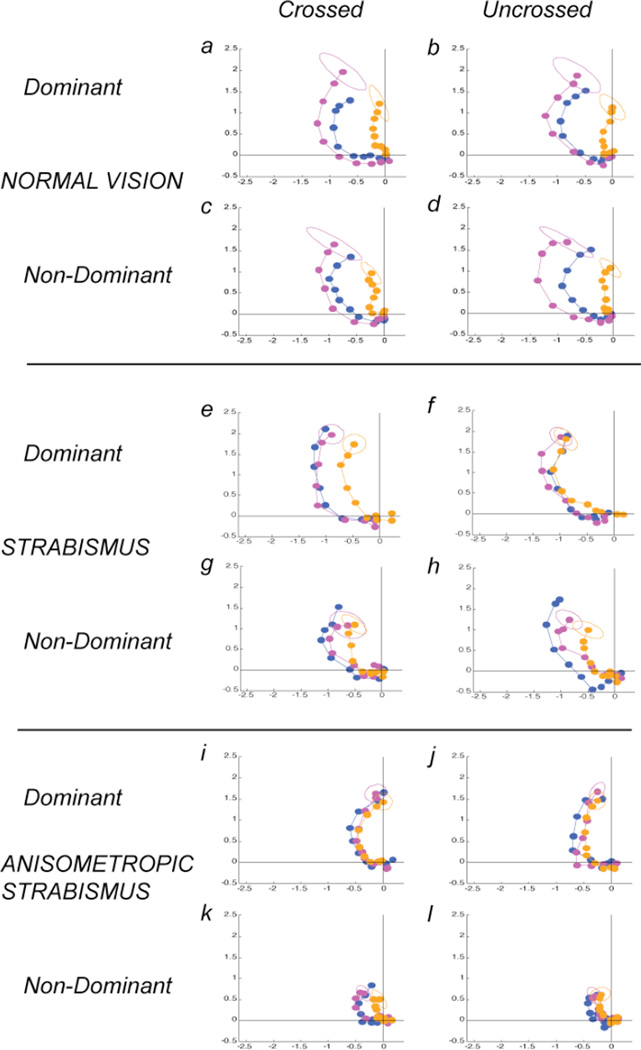
Two-dimensional plots of the complex-values underlying response amplitude and phase. The x-axis plots the real/cosine component and the y-axis the imaginary/sine component in units of microvolts. Data from the dominant eyes are presented in the left two columns separately for uncrossed and crossed disparities and data from the non-dominant eyes are presented in the right two columns. Data from each observer group are presented as rows. Within each panel, the monocular data are plotted in blue, the 0 disparity pedestal data in magenta and the disparate pedestal data in orange. As amplitude increases, the response phase shifts towards the phase origin (in the clockwise direction, especially in the monocular and zero disparity pedestal conditions. The ellipses plot the dispersion at a nominal 1 s.e.m.. See text for details.
RESULTS
Periodic vernier onset/offset stimuli produce both odd and even harmonic responses that increase monotonically with offset size6, 7,14. The current analysis will focus on the first harmonic component of the response because previous work has shown this component to be selective for relative position in the case of lateral motion 7 and to the disparity of dichoptic targets6. The second harmonic responses were also examined in the present study, but they did not show clear disparity effects.
The data will be presented in two formats. First, VEP amplitude will be plotted as a function of the magnitude of the swept parameter (displacement) for ease of visualization of amplitude effects. Secondly, because the spectral analysis yields phase as well as amplitude information, the data will be re-plotted in the complex plane so that both amplitude and phase effects can be visualized. Statistical evaluation will be performed on the complex-values.
The effects of disparate pedestals in normal observers
In normal observers, the addition of a zero-disparity, collinear pedestal leads to a larger first harmonic response than that recorded under monocular viewing conditions (F(2,7)=5.9, p=0.031), replicating previous reports with similar stimuli6,14. This effect can be seen in Fig. 2A and 2B, by comparing the monocular data (gray filled circles; Mon) to the binocular zero disparity pedestal data (black filled squares; Bin0). In this condition, there is a motion-in-depth percept in addition to the perceived alternation between a collinear set of bars and a segmented set that is present in the monocular condition.
The disparate pedestal condition (Fig. 2A and 2B, black open squares; Bin5)) produces a lower amplitude response than does the monocular condition or the zero disparity pedestal condition, again replicating previous results6,14. Although this condition also leads to a percept of motion in depth, it does not result in a perceived change of segmentation, even though the dynamic half-image alternates between collinear and non-collinear states (see cartoon below the targets in Fig. 1). The level of standing disparity thus controls the nature of the response to the dynamic test, leading to either increases or decreases in response amplitude that are well modeled as gain changes14.
Combined amplitude and phase effects
Spectral analysis of the VEP yields both amplitude and phase values for each level of the sweep. Fig. 3 plots the data in the complex plane where distance from the origin corresponds to response amplitude. The time/phase origin for the plots is at the positive x-axis and increasing phase lag/delay is in the counter-clockwise direction. Successive points on the tuning function are connected with lines.
As the size of the displacement increases, the response increases in amplitude In addition, the response phase also depends on the size of the displacement and on the type of pedestal in the other eye. As the magnitude of the monocular test offset increases, response phase progresses towards the phase origin of the plot, consistent with a speeding of the response as the stimulus becomes progressively supra-threshold. The addition of the zero-disparity pedestal preserves the same progression of phases seen in the monocular condition, but with an increased magnitude response at each corresponding displacement (compare magenta to dark blue circles in Fig. 3). In contrast, the disparate pedestal shifts all response phases towards the origin and reduces the amplitude of the response (see orange circles in Fig. 3). The effect of disparity (0 min vs 5 min) on the response amplitude is significant for each combination of eye and disparity sign in normals (See Table 1 for significance values).
Table 1.
Significance values for the comparison between the zero disparity and 5 arc min disparity pedestal conditions.
| Eye | Disparity | F | p | |
|---|---|---|---|---|
| NORMAL | DOM | CROSSED | F(2,7)=25.65 | <0.001 |
| DOM | UNCROSSED | F(2,7)=19.87 | 0.001 | |
| NONDOM | CROSSED | F(2,7)=17.52 | 0.002 | |
| NONDOM | UNCROSSED | F(2,7)=5.53 | 0.036 | |
| STRABISMUS | DOM | CROSSED | F(2,9)=5.68 | 0.025 |
| DOM | UNCROSSED | F(2,9)=0.15 | 0.862 | |
| NONDOM | CROSSED | F(2,9)=0.15 | 0.863 | |
| NONDOM | UNCROSSED | F(2,9)=3.10 | 0.094 | |
| STRABANISO | DOM | CROSSED | F(2,12)=4.35 | 0.038 |
| DOM | UNCROSSED | F(2,12)=1.68 | 0.296 | |
| NONDOM | CROSSED | F(2,12)=2.58 | 0.117 | |
| NONDOM | UNCROSSED | F(2,2)=0.36 | 0.705 |
The effects of disparate pedestals in strabismus patients without anisometropia
In contrast to normal observers, patients with constant strabismus and no anisometropia showed substantial differences in the pattern of responses between their dominant and non-dominant eyes, as well as differences between each of their eyes and either eye of normals. These data are plotted in Fig. 2C and 2D and in Fig. 3e–h. These patients did not, in general, have amblyopia (9 of 11). We eliminated the single patient in this group with demonstrable stereopsis from the analysis for comparability to the group of strabismic-anisometropic patients who had no demonstrable stereopsis.
Monocular condition
The monocular response amplitudes in the non-dominant eyes of this group of patients are approximately equal to those of the dominant eye, consistent with the relative lack of amblyopia in this group.
Zero-disparity pedestal condition
When the dynamic test was in the dominant eye of patients in this group, there is no increase in amplitude in the zero disparity pedestal condition relative to the monocular condition, unlike our normal observers (see Fig. 2C) and Fig. 3e,f. The response amplitudes and phases were, in fact, very similar to those measured in the monocular condition.
When the test was in the non-dominant eye and the zero disparity pedestal was in the dominant eye, the response was lower than in the monocular condition (Fig. 2D and 3g,h), although this effect did not reach significance (F(2,9)=2.24, p=0.162). However, when we compared the pattern of results in the monocular and binocular zero condition between patients and normals, there was a significant interaction effect between stimulus type and patient group (F(2,17)=7.66, p<.001). The different relationship between monocular and zero disparity responses in the two groups is consistent with strabismic suppression replacing normal facilitation when the test was in the non-dominant eye.
The reduced rather than enhanced zero-disparity pedestal response was also present in the smaller group of 9 patients who did not have amblyopia. The reduction of the response to the test in the non-dominant eye when the zero disparity pedestal is presented to the dominant eye is thus due to active suppression of the non-dominant eye by the dominant eye, even when non-dominant eye has normal acuity.
Disparate pedestal condition
The disparate pedestal reduces the non-dominant eye response relative to the monocular response (Fig. 2D). This reduction, considered by itself, is similar to what is seen for normal observers, but it occurs in the absence of the normal binocular enhancement by the zero disparity pedestal. In these strabismic observers, there is no significant difference between the zero-disparity and disparate pedestal conditions, as there is in normal observers (see Table 1 for significance values). We consider it likely that the reduction produced by the 5 arcmin pedestal is also due to strabismic suppression, because the influence of this stimulus is much less when the pedestal is in the non-dominant eye (e.g. Fig. 2C).
When the test is in the dominant eye, the non-dominant eye has a weaker effect (see Fig. 2C). When the pedestal carries an uncrossed disparity, there is no difference between the zero-disparity pedestal and the disparate pedestal conditions (see 2C-D and Fig. 3e–h and Table 1) in three of four comparisons. The non-dominant eye does however exert an effect on the dominant eye, if the disparate pedestal has a crossed disparity (Fig. 2C open squares and Fig. 3f). The effect is a small, but significant reduction in amplitude over a range of supra-threshold disparities (see Table 1). The dominant eye response is thus largely independent of the stimulus presented to the nondominant eye, with the exception of a small, suppressive input from crossed disparities.
The effects of disparate pedestals in strabismus patients with anisometropia
Monocular condition
Almost all of the strabismus patients who were anisometopic also had amblyopia (12 of 14) and all had defective stereopsis. Consequently, there was a large overall difference in response magnitude between the dominant and non-dominant eyes that was not present in the other patient group (compare Fig. 2E to 2F). Monocular sensitivity differences at the first harmonic are correlated with perceptual measures of vernier offset sensitivity and with letter acuity 16.
Zero disparity condition
The normal increase in amplitude in the binocular zero condition relative to the monocular response was absent when the test was in either eye (see Fig. 2E and 2F and 3i–l), as was also seen in the strabismus patients who did not have anisometropia, When the test was in the non-dominant eyes and the zero-disparity pedestal was in the dominant eye, the response did not differ from the monocular condition (F(2,31) = 1.439, p = 0.253).
Disparate pedestal condition
Overall, there were only small differences between the monocular, binocular zero and the disparate pedestal conditions in the patients with strabismus and anisometropia (Fig. 2E and 2F, Fig. 3i–l). In only one condition (test in the dominant eye, crossed disparities; Fig. 3j, see Table 1) was there a significant difference between the disparate and non-disparate pedestal conditions. This input from the non-dominant eye was also present in the strabismus only group (Figs. 2C, 3e). In the non-dominant eye, the response to the disparate pedestal condition was very similar to the monocular condition, unlike in the same eye of the patients without anisometropia who showed lower responses in this condition. Most of these eyes were amblyopic, and the lack of effect of either pedestal when presented in the dominant eye suggests that neither fusional or strabismic suppression is present in this patient group.
Monocular response differences in dominant eyes
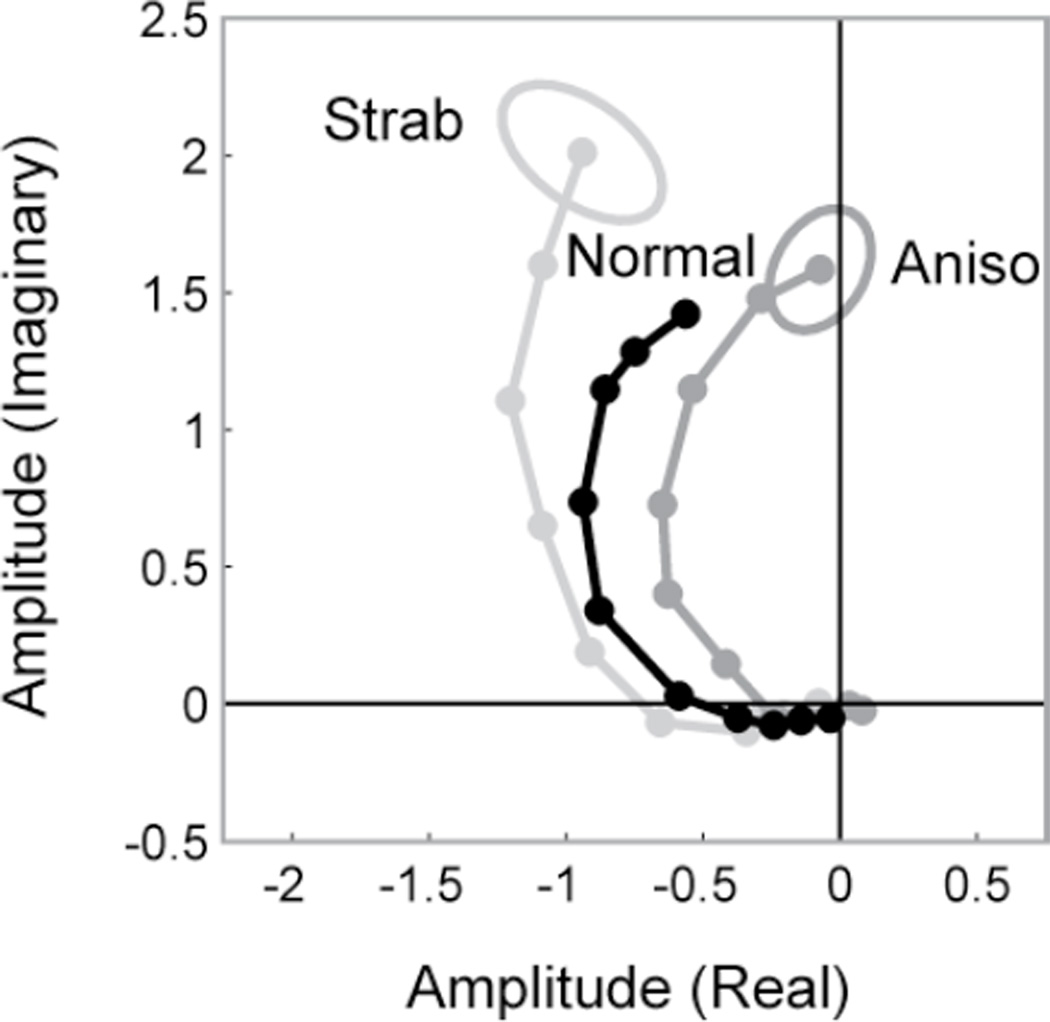
Although the visual acuity of the dominant eyes of both patient groups was comparable, we noted a previously unreported difference in the overall phase of the dominant eye responses of the strabismus patients with and without anisometropia. This is shown for the monocular responses of each group in Fig. 4. The curves of the strabismus-only patients are shifted in the lag direction (counterclockwise) relative to either the anisometropic strabismus patients or the normals and the responses in the strabismus-only group are slightly larger than those patients with anisometropia. The combination of phase shifts and amplitude changes is significant on a between subjects’ analysis F(2,22)=4.49, p=0.023. The 30 deg phase difference between the two strabismic groups corresponds to about 30–40 msec, i.e. the pure strabismics show a delay. Thus the dynamics of the monocular response of the dominant eye depend on the developmental history and status of the non-dominant eye. The major sensory difference between the strabismic groups is the presence of amblyopia in the nondominant eye.
FIG 4.
Response amplitude and phase in the dominant eyes of normal vision participants (black; Normal); patients with strabismus with (dark gray; Aniso) and without (light gray; Strab) anisometropia. Data are from the monocular viewing condition. The phase of the response in the strabismus patients without anisometropia is shifted to increased lags/time delays relative to that of the normal vision observers. The phase of the response in the group with strabismus and anisometropia is shifted towards decreased lag/time delay, relative to that of the normal vision participants.
Discussion
Our results suggest that suppression in stereoblind, non-amblyopic observers is determined by a binocular mechanism responsive to disparity. As in normal observers, the effect of the static stimulus on the oscillating Vernier target depends on the disparity specified by the binocular configuration, although the normal facilitation by the zero disparity pedestal is absent. The differential effect of crossed and uncrossed disparity pedestals on the dominant eye response of the observers with strabismus also argues for a disparity-tuned mechanism.
The disparity dependant effects in the dominant eye of the strabismus patients are relevant to the clinical observation that patients who are esotropic and are made exotropic by surgery complain of double vision. Because of the esotropia, the patient will not have had experience of crossed disparities and may therefore have never developed a mechanism to suppress images that carry them. By contrast, it would be necessary to have developed a means of suppressing the nearly constant presence of large uncrossed disparities in order to prevent diplopia. The haploscope allowed us to present crossed and uncrossed disparities in a controlled fashion and to thus observe a residual “unsuppressable” crossed input from the non-dominant eye that affects the responses of the dominant eye. There have been only three previous reports of the nondominant eye having an effect on the dominant eye17,10{Baker, 2008 #2444}, but this is the first study to show the presence of disparity-tuned suppressive interactions.
In contrast to the preservation of suppressive interactions, we find that patients with long-standing, constant strabismus lack normal facilitative binocular interactions. This interaction is elicited in normal observers in the zero disparity pedestal condition that adds a binocular disparity cue to the monocularly visible cues for discontinuity that are present in the monocular control condition. The same disparity cue that produces enhancement of the monocular response in normals can result in a lower response in the patients, especially when the static pedestal is presented to the dominant eye of strabismus patients who do not have amblyopia (Fig. 2D). The trend for the responses of this normally facilitative stimulus to be reduced below the monocular level is consistent with classical strabismic suppression.
A recent psychophysical study has found evidence for intact facilitative binocular interactions in patients with strabismic amblyopia {Baker, 2007 #2445}. They were able to find these interactions after equating the contrast levels in the two eyes of the patients for distance above detection threshold. When they did this compensation, they found normal levels of binocular summation. Another study found evidence for facilitative binocular interactions in patients with strabismic amblyopia using a dichoptic masking task {Baker, 2008 #2444}, although an earlier study did not.10 While contrast sensitivity differences may have played a role in the loss of facilitation in our patients with amblyopia, they are unlikely to have been present in the group that did not have amblyopia. These patients also lost the facilitation conferred by the zero disparity pedestal. Consistent with this, Lema and Blake 19 found no evidence of binocular summation in stereoblind individuals with equal contrast sensitivity in their two eyes. Our non-amblyopic strabismus patients are probably similar to their stereoblind subjects. The degree to which residual binocular facilitation is present may depend on what function is being tested and the patient’s history of abnormal binocular interaction.
Comparison with previous VEP measures of binocular interaction
The paradigm we have developed is designed to probe the pattern of disparity-dependent binocular interactions. Unlike other VEP paradigms for binocular interaction, such as summation indices18–24 or cyclopean random dot responses25–34, we are able to separately asses the contribution of each eye to the interaction. This is possible because we effectively tag each eye’s input with a different time course, rather than using the same time course in each eye. Because one eye’s image is dynamic and the other eye’s input is static, the time-locked evoked response is labeled for the eye of origin. In this way, we can assay the inputs from the other eye, without directly recording an evoked response from that eye. In a traditional binocular summation paradigm, monocular responses are recorded from each eye separately and the sum of these responses is compared to that measured when both eyes see the same image. This method can detect binocular interaction as an additivity failure, but it cannot recover the separate contributions of each eye during binocular stimulation.
The timing format of our method is similar to other temporal tagging methods that examine binocular interaction under dichoptic conditions with the target in one eye being static and the other dynamic12, 35–43. Other techniques use distinct temporal frequencies in each eye12, 35, 37. With these latter methods, responses of each eye are recorded simultaneously at harmonics of the respective eye-tagging frequencies and definitive evidence for binocular interaction can be obtained by detecting responses at frequencies equal to sums and difference of the eye-tag frequencies. The main difference between our method and previous dichoptic tagging methods is that we focus on binocular interaction in the network of cells responsible for low-level detection of image discontinuities. Our method is thus more focused on spatial relationships than on the contrast processes that have been the focus of previous studies. Our method also provides a measure of monocular vernier acuity that can be used to quantify the degree of amblyopia16.
Timing abnormalities in the dominant eyes
We have observed an alteration in the response timing in the dominant eyes of patients that depends on whether their strabismus is accompanied by anisometropia, or not. The dominant eyes of the strabismus patients all had normal acuity and thus reduced spatial acuity is not a factor. At present we have no explanation for the basis of this effect and simply report its presence. The altered response timing could be the result of several factors: genetic or other constitutional differences between anisometropic and non-anisometropic strabismus patients, differences in the pattern of binocular interaction or differences in the treatment history. Anomalies in response timing may provide additional clues about the pathophysiology of strabismus and amblyopia.
Acknowledgements
Supported by EY 015790 (AMN, MWP), EY 006644 (SPM) and the Smith-Kettlewell Eye Research Institute.
References
- 1.Tyler CW. Stereoscopic depth movement: two eyes less sensitive than one. Science (New York, NY. 1971;174:958–961. doi: 10.1126/science.174.4012.958. [DOI] [PubMed] [Google Scholar]
- 2.Tyler CW, Foley JM. Stereomovement suppression for transient disparity changes. Perception. 1974;3:287–296. doi: 10.1068/p030287. [DOI] [PubMed] [Google Scholar]
- 3.McKee SP, Levi DM, Bowne SF. The imprecision of stereopsis. Vision research. 1990;30:1763–1779. doi: 10.1016/0042-6989(90)90158-h. [DOI] [PubMed] [Google Scholar]
- 4.Harris JM, McKee SP, Watamaniuk SNJ. Visual search for motion-indepth: stereomotion does not ‘pop out’from disparity noise. Nature neuroscience. 1998;1:165–168. doi: 10.1038/418. [DOI] [PubMed] [Google Scholar]
- 5.McKee SP, Harrad RA. Fusional suppression in normal and stereoanomalous observers. Vision research. 1993;33:1645–1658. doi: 10.1016/0042-6989(93)90030-z. [DOI] [PubMed] [Google Scholar]
- 6.Norcia AM, McKee SP, Bonneh Y, Pettet MW. Suppression of monocular visual direction under fused binocular stimulation: evoked potential measurements. Journal of vision [electronic resource] 2005;5:34–44. doi: 10.1167/5.1.4. [DOI] [PubMed] [Google Scholar]
- 7.Norcia AM, Wesemann W, Manny RE. Electrophysiological correlates of vernier and relative motion mechanisms in human visual cortex. Visual neuroscience. 1999;16:1123–1131. doi: 10.1017/s0952523899166124. [DOI] [PubMed] [Google Scholar]
- 8.Levi DM, Manny RE, Klein SA, Steinman SB. Electrophysiological correlates of hyperacuity in the human visual cortex. Nature. 1983;306:468–470. doi: 10.1038/306468a0. [DOI] [PubMed] [Google Scholar]
- 9.Levi DM, Harwerth RS, Smith EL., 3rd Humans deprived of normal binocular vision have binocular interactions tuned to size and orientation. Science (New York, NY. 1979;206:852–854. doi: 10.1126/science.493988. [DOI] [PubMed] [Google Scholar]
- 10.Harrad RA, Hess RF. Binocular integration of contrast information in amblyopia. Vision research. 1992;32:2135–2150. doi: 10.1016/0042-6989(92)90075-t. [DOI] [PubMed] [Google Scholar]
- 11.Schor CM. Visual stimuli for strabismic suppression. Perception. 1977;6:583–593. doi: 10.1068/p060583. [DOI] [PubMed] [Google Scholar]
- 12.Norcia AM, Harrad RA, Brown RJ. Changes in cortical activity during suppression in stereoblindness. Neuroreport. 2000;11:1007–1012. doi: 10.1097/00001756-200004070-00022. [DOI] [PubMed] [Google Scholar]
- 13.Jampolsky A. Characteristics of suppression in strabismus. AMA. 1955;54:683–696. doi: 10.1001/archopht.1955.00930020689010. [DOI] [PubMed] [Google Scholar]
- 14.Hale J, Harrad RA, McKee SP, Pettet MW, Norcia AM. A VEP measure of the binocular fusion of horizontal and vertical disparities. Investigative ophthalmology & visual science. 2005;46:1786–1790. doi: 10.1167/iovs.04-0954. [DOI] [PubMed] [Google Scholar]
- 15.Victor JD, Mast J. A new statistic for steady-state evoked potentials. Electroencephalography and clinical neurophysiology. 1991;78:378–388. doi: 10.1016/0013-4694(91)90099-p. [DOI] [PubMed] [Google Scholar]
- 16.Hou C, Good WV, Norcia AM. Validation study of VEP vernier acuity in normalvision and amblyopic adults. Investigative ophthalmology & visual science. 2007;48:4070–4078. doi: 10.1167/iovs.06-1368. [DOI] [PubMed] [Google Scholar]
- 17.Hood AS, Morrison JD. The dependence of binocular contrast sensitivities on binocular single vision in normal and amblyopic human subjects. The Journal of physiology. 2002;540:607–622. doi: 10.1113/jphysiol.2001.013420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srebro R. The visually evoked response. Binocular facilitation and failure when binocular vision is disturbed. Archives of ophthalmology. 1978;96:839–844. doi: 10.1001/archopht.1978.03910050445009. [DOI] [PubMed] [Google Scholar]
- 19.Apkarian PA, Nakayama K, Tyler CW. Binocularity in the human visual evoked potential: facilitation, summation and suppression. Electroencephalography and clinical neurophysiology. 1981;51:32–48. doi: 10.1016/0013-4694(81)91507-8. [DOI] [PubMed] [Google Scholar]
- 20.Perry NW, Jr, Childers DG, McCoy JG. Binocular addition of the visual evoked response at different cortical locations. Vision research. 1968;8:567–573. doi: 10.1016/0042-6989(68)90097-7. [DOI] [PubMed] [Google Scholar]
- 21.Ciganek L. Binocular addition of the visually evoked response with different stimulus intensities in man. Vision research. 1970;10:479–487. doi: 10.1016/0042-6989(70)90004-0. [DOI] [PubMed] [Google Scholar]
- 22.Lennerstrand G. Binocular interaction studied with visual evoked responses (VER) in humans with normal or impaired binocular vision. Acta Ophthalmol (Copenh) 1978;56:628–637. doi: 10.1111/j.1755-3768.1978.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 23.Campos EC, Chiesi C. Binocularity in comitant strabismus: II. Objective evaluation with visual evoked responses. Documenta ophthalmologica. 1983;55:277–293. doi: 10.1007/BF00161285. [DOI] [PubMed] [Google Scholar]
- 24.Harter MR, Seiple WH, Salmon L. Binocular summation of visually evoked responses to pattern stimuli in humans. Vision research. 1973;13:1433–1446. doi: 10.1016/0042-6989(73)90004-7. [DOI] [PubMed] [Google Scholar]
- 25.Julesz B, Kropfl W, Petrig B. Large evoked potentials to dynamic random-dot correlograms and stereograms permit quick determination of stereopsis. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:2348–2351. doi: 10.1073/pnas.77.4.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodis-Wollner I, Barris MC, Mylin LH, Julesz B, Kropfl W. Binocular stimulation reveals cortical components of the human visual evoked potential. Electroencephalography and clinical neurophysiology. 1981;52:298–305. doi: 10.1016/0013-4694(81)90058-4. [DOI] [PubMed] [Google Scholar]
- 27.Braddick O, Atkinson J, Julesz B, Kropfl W, Bodis-Wollner I, Raab E. Cortical binocularity in infants. Nature. 1980;288:363–365. doi: 10.1038/288363a0. [DOI] [PubMed] [Google Scholar]
- 28.Petrig B, Julesz B, Kropfl W, Baumgartner G, Anliker M. Development of stereopsis and cortical binocularity in human infants: electrophysiological evidence. Science (New York, NY. 1981;213:1402–1405. doi: 10.1126/science.7268443. [DOI] [PubMed] [Google Scholar]
- 29.Norcia AM, Tyler CW. Temporal frequency limits for stereoscopic apparent motion processes. Vision research. 1984;24:395–401. doi: 10.1016/0042-6989(84)90037-3. [DOI] [PubMed] [Google Scholar]
- 30.Lehmann D, Skrandies W, Lindenmaier C. Binocularly evoked potentials: slow, late components to random-dot stereograms and correlograms (dynamic Julesz patterns) Progress in brain research. 1980;54:286–290. doi: 10.1016/S0079-6123(08)61636-2. [DOI] [PubMed] [Google Scholar]
- 31.Eizenman M, Westall CA, Geer I, et al. Electrophysiological evidence of cortical fusion in children with early- onset esotropia. Investigative ophthalmology & visual science. 1999;40:354–362. [PubMed] [Google Scholar]
- 32.Westall CA, Eizenman M, Kraft SP, Panton CM, Chatterjee S, Sigesmund D. Cortical binocularity and monocular optokinetic asymmetry in early- onset esotropia. Investigative ophthalmology & visual science. 1998;39:1352–1360. [PubMed] [Google Scholar]
- 33.Skarf B, Eizenman M, Katz LM, Bachynski B, Klein R. A new VEP system for studying binocular single vision in human infants. Journal of pediatric ophthalmology and strabismus. 1993;30:237–242. doi: 10.3928/0191-3913-19930701-05. [DOI] [PubMed] [Google Scholar]
- 34.Lehmann D, Julesz B. Lateralized cortical potentials evoked in humans by dynamic random-dot stereograms. Vision research. 1978;18:1265–1271. doi: 10.1016/0042-6989(78)90216-x. [DOI] [PubMed] [Google Scholar]
- 35.Baitch LW, Levi DM. Evidence for nonlinear binocular interactions in human visual cortex. Vision research. 1988;28:1139–1143. doi: 10.1016/0042-6989(88)90140-x. [DOI] [PubMed] [Google Scholar]
- 36.Baitch LW, Levi DM. Binocular beats: psychophysical studies of binocular interaction in normal and stereoblind humans. Vision research. 1989;29:27–35. doi: 10.1016/0042-6989(89)90171-5. [DOI] [PubMed] [Google Scholar]
- 37.Suter S, Suter PS, Perrier DT, Parker KL, Fox JA, Roessler JS. Differentiation of VEP intermodulation and second harmonic components by dichoptic, monocular, and binocular stimulation. Visual neuroscience. 1996;13:1157–1166. doi: 10.1017/s0952523800007793. [DOI] [PubMed] [Google Scholar]
- 38.Brown RJ, Candy TR, Norcia AM. Development of rivalry and dichoptic masking in human infants. Investigative ophthalmology& visual science. 1999;40:3324–3333. [PubMed] [Google Scholar]
- 39.Harter MR, Seiple WH, Musso M. Binocular summation and suppression: visually evoked cortical responses to dichoptically presented patterns of different spatial frequencies. Vision research. 1974;14:1169–1180. doi: 10.1016/0042-6989(74)90213-2. [DOI] [PubMed] [Google Scholar]
- 40.Harter MR, Seiple WH, Salmon LE. Evoked cortical responses to dichoptically presented patterned light flashes: interocular interaction. TIT J Life Sci. 1972;2:27–33. [PubMed] [Google Scholar]
- 41.Wright KW, Fox BE, Eriksen KJ. PVEP evidence of true suppression in adult onset strabismus. Journal of pediatric ophthalmology and strabismus. 1990;27:196–201. doi: 10.3928/0191-3913-19900701-07. [DOI] [PubMed] [Google Scholar]
- 42.Lehmann D, Fender DH. Monocularly evoked electroencephalogram potentials: influence of target structure presented to the other eye. Nature. 1967;215:204–205. doi: 10.1038/215204a0. [DOI] [PubMed] [Google Scholar]
- 43.Lehmann D, Fender DH. Component analysis of human averaged evoked potentials: dichoptic stimuli using different target structure. Electroencephalography and clinical neurophysiology. 1968;24:542–553. doi: 10.1016/0013-4694(68)90043-6. [DOI] [PubMed] [Google Scholar]
- 44.Norcia AM, Pei F, Bonneh Y, Hou C, Sampath V, Pettet MW. Development of sensitivity to texture and contour information in the human infant. Journal of cognitive neuroscience. 2005;17:569–579. doi: 10.1162/0898929053467596. [DOI] [PubMed] [Google Scholar]