Abstract
With the development of effective therapies against human immunodeficiency virus (HIV), hepatitis C virus (HCV) infection has become a major cause of morbidity and mortality among patients with both infections (coinfection). In addition to the high prevalence of chronic HCV, particularly among HIV-infected injection drug users, the rate of incident HIV infections is increasing among HIV-infected men who have sex with men, leading to recommendations for education and screening for HCV in this population. Liver disease is the second leading and, in some cases, a preventable cause of death among coinfected patients. Those at risk for liver disease progression are usually treated with a combination of interferon (IFN) and ribavirin (RBV), which is not highly effective; it has low rates of sustained virologic response (SVR), especially for coinfected patients with HCV genotype 1 and those of African descent. Direct-acting antivirals might overcome factors such as immunodeficiency that can reduce the efficacy of IFN. However, for now it remains challenging to treat coinfected patients due to interactions among drugs, additive drug toxicities, and the continued need for combination therapies that include pegylated IFN. Recently developed HCV protease inhibitors such as telaprevir and boceprevir, given in combination with pegylated IFN and RBV, could increase the rate of SVR with manageable toxicity and drug interactions. We review the latest developments and obstacles to treating coinfected patients.
Keywords: HIV, HCV, Treatment, Liver Disease, Drug Interaction
Chronic infection with hepatitis C virus (HCV) is a global health burden; HCV is estimated to infect approximately 170 million people worldwide.1 Due to shared methods of transmission, coinfection with human immunodeficiency virus (HIV) and HCV is common; on average, 15%–30% of HIV-infected patients are also infected with HCV, although the prevalence of coinfection varies significantly by mode of transmission.2 Highly active combination antiretroviral therapies (ART) for HIV have greatly reduced the incidence of death from acquired immunodeficiency syndrome (AIDS), making liver disease a leading cause of morbidity and mortality among coinfected individuals and a major factor in the health care utilization burden posed by this population.3–5
Although the standard of care regimen, composed of interferon (IFN) formulations in combination with ribavirin (RBV), has been approved for the treatment of chronic HCV in HIV-infected patients, multiple factors have limited their effectiveness in this population. Compared with patients infected with only HCV, coinfected patients have a lower virologic response6,7 and less access to therapy because of provider and patient biases.8 They also frequently have comorbid conditions, such as substance abuse and psychiatric disease, which are contraindications to treatment for many patients. Furthermore, in coinfected patients, antiretroviral drugs could interact with other medications, increasing the risk of complications and the complexity of treatment regimens.
Over time, the treatment of HIV infection has improved and is better tolerated and more effective than earlier regimens, increasing focus on comorbid conditions including HCV-related liver disease. The recent approval by the Food and Drug Administration (FDA) and other global regulatory agencies of the direct-acting antivirals (DAA) for HCV, boceprevir and telaprevir, could increase virologic response rates in coinfected patients. Although the safety and efficacy of these drugs in patients with HIV infection has not been established, coinfected patients are likely to be considered for off-label treatment.9,10 However, there are unique challenges to the use of these first-generation DAAs in the difficult-to-treat, coinfected population that require careful consideration, including the potential for increased drug toxicity, interactions with ART, and unknown efficacy.
Epidemiology and Natural History
HCV is a positive, single-stranded RNA virus that is most efficiently transmitted via blood exposure. HCV causes chronic infection in 70%–80% of exposed individuals and exists as 7 distinct genotypes that vary in prevalence across geographic locations.11 The major clinical consequence of chronic HCV infection is the development of fibrosis and progression to cirrhosis and its potential complications, which include variceal hemorrhage, ascites and spontaneous bacterial peritonitis, and hepatocellular carcinoma.12 Factors consistently associated with progression of fibrosis include fibrosis stage, age at time of infection, duration of infection, consumption of alcohol (most data support >50 g/day), HIV coinfection, CD4+ T-cell counts <200 cells/mL, male sex, and body mass index, diabetes, and steatosis (Figure 1).13
Figure 1.
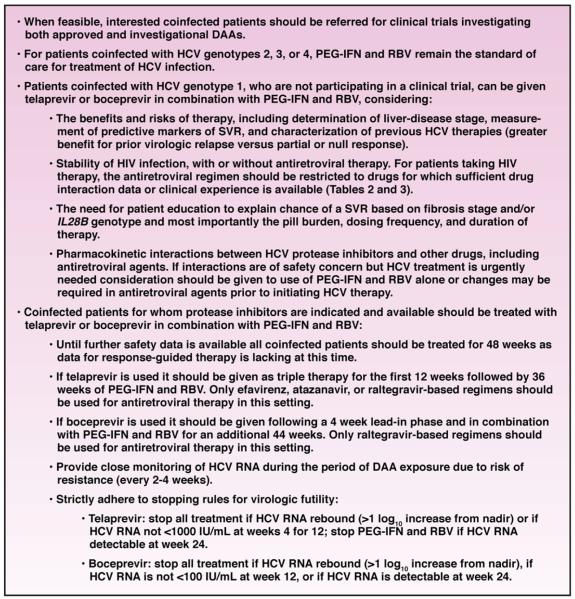
Mechanisms of fibrosis progression in coinfected patients. The mechanism of more advanced fibrosis progression reported in coinfected patients is multifactorial. There are HIV-related, HCV-related, and host-related factors that can lead to fibrosis. The damage to the liver tissue can occur by steatosis, necroinflammation, or stellate cell activation or a combination of any of these mechanisms. Ultimately, ongoing damage via these mechanisms results in fibrosis, which can progress to cirrhosis if the etiology is not identified and managed.
Fibrosis progresses more rapidly in coinfected individuals, which was first reported in the hemophiliac population.14 There have been multiple reports and a meta-analysis showing that coinfected patients have a 2-fold higher relative risk (RR) of developing cirrhosis and a 6-fold higher RR of developing decompensated liver disease than patients infected with only HCV.15 Mechanisms that mediate the more rapid progression of fibrosis in coinfected patients appear to be multifactorial (Figure 1). HIV viremia could increase fibrosis via several possible pathways. HIV infection induces an inflammatory response that can lead to liver injury by activating hepatic stellate cells, which increase production of fibrogenic cytokines, or by reducing the ratio of CD4+ to CD8+ T cells. The external envelope protein of HIV, gp120, induces chemokine receptor-mediated signaling, which also activates hepatic stellate cells. HIV depletes intestinal lymphocytes, increasing microbial translocation, which has been correlated with progression of HCV-related liver disease.16–18 In addition, antiretroviral agents have been associated with liver injury, either directly (dideoxynucleosides lead to microsteatosis from lactic acidosis, protease inhibitors [PIs] can cause hepatitis, non-nucleoside reverse transcriptase inhibitors cause hypersensitivity reactions) or indirectly, promoting development of metabolic syndrome (insulin resistance, dyslipidemia, and increased body mass index).19,20 The prevalence of harmful alcohol ingestion is more common among HIV-infected patients than the general population and is another well-recognized risk factor for fibrosis.21,22
Effective ART has significantly improved outcomes of coinfected patients and been associated with decreased risk of liver disease progression, possibly by reversing HIV-induced immunosuppression, by reducing inflammation, or by decreasing immune system exposure to HIV proteins such as gp120.23 In one large cohort study that followed coinfected patients initiated on ART, a low incidence of liver disease progression was reported, although liver-related disease remained the leading cause of death (43% of deaths).24 The study also associated increases in CD4+ T-cell counts following ART with fewer liver-related outcomes, including death. Although these data support the role of ART in slowing the progression of HCV-related disease in HIV-infected patients, a recent meta-analysis found that, even after accounting for the use of ART, patients with coinfection have an RR of cirrhosis of 1.72, compared with patients infected with only HCV25; the RR for cirrhosis was 2.49 among coinfected patients not on ART. Thus, despite effective anti-HIV therapies, liver disease remains a leading cause of death in coinfected patients.
Transmission
After World War II and through the 1980s, the most common means of transmission of HCV was receipt of contaminated blood or blood products or through sharing of contaminated drug paraphernalia, primarily syringes and needles. In early stages of the HIV epidemic, men with bleeding disorders who had been exposed to blood products derived from multiple donors had high rates of HIV and HCV infection.1 Since the initiation of voluntary donation and routine screening of blood products in the United States, first with antibody and more recently with nucleic acid tests, the incidence of new infections has significantly declined. However, the Centers for Disease Control and Prevention report approximately 18,000 new infections per year.26 Of these new cases, 56% reported recent injection drug use (IDU) and 16% reported recent sex with an HCV-infected partner; 34% reported ≥2 new sexual partners in the past 6 months. IDU remains the leading risk factor for HCV infection in the United States.
Although sexual exposure is considered to be an inefficient means of HCV transmission, the potential for sexual acquisition is supported by the isolation of HCV RNA from semen and cervical smear samples.27,28 Large prospective cohort studies of heterosexual serodiscordant couples reported no increased risk of HCV transmission over a 10-year period.29,30 Similarly, among HIV-uninfected men who have sex with men (MSM), there is little evidence of sexual transmission of HCV, even among those engaging in high-risk sexual behaviors.31 However, persons reporting multiple sexual partners have more than twice the likelihood of acquiring HCV infection (adjusted odds ratio, 2.2–2.9).32,33 Furthermore, concurrent sexually transmitted infections can increase the risk of acquiring HCV by heterosexual exposure.34
HIV-infected individuals are at highest risk for sexual acquisition of HCV infection, regardless of sexual preference. Large cross-sectional studies of HIV-infected heterosexuals reported significant increases in risk of HCV infection, especially among people with high-risk sexual behaviors or multiple sexual partners.35,36 Over the past decade, acute HCV infection among HIV-infected MSM who have not used injection drugs has become a public health concern in many regions of the world. Multiple studies of acute HCV infection in this population suggest that the primary mode of HCV acquisition is mucosal, often in the context of unprotected anal receptive intercourse. In Europe, an international phylogenetic analysis revealed a large network of HCV transmission among HIV-infected MSM.37 A phylogenetic analysis of HCV NS5B from 266 patients in 5 countries found that 84% of the subjects carried a strain of HCV that was most similar to a strain detected in another study participant. This approach identified a large, European, MSM-specific transmission network in which most infections appeared related to permucosal injury. Another MSM-specific transmission network identified in Australia, which included IDUs, had little overlap with the network in Europe. Data from 12 cohorts within the Concerted Action on Sero-Conversion to AIDS and Death in Europe (CASCADE) collaboration revealed that a substantial increase in HCV incidence among HIV-infected MSM began in 2002, although the initial increase started as early as the late 1990s.38 Based on these trends in HCV incidence, the increase in HCV infection correlates with the introduction of effective ART. It is hypothesized that this trend suggests the availability of ART may have changed the perception of risk of sexually transmitted disease and increased risk-taking behaviors.
Since the early reports of HCV outbreaks among HIV-infected MSM, multiple studies have examined the independent relationship of sexual risk behaviors and HCV acquisition. A large cross-sectional study from The Netherlands independently associated HIV infection, IDU, fisting, and noninjecting recreational drug use with HCV infection.39 Similarly, a case-control study completed in the United Kingdom associated permucosal, rather than percutaneous, transmission risk factors with case versus control status.40 Cases reported more sexual partners, engaged in more risky sexual behaviors (unprotected anal intercourse, rimming, fisting, use of sex toys, group sex), and were more likely to use noninjected recreational drugs, including intranasal and anal routes than controls. These data together support a changing epidemiology of HCV transmission among the HIV-infected population of MSM, associating permucosal traumatic sexual practices (particularly in the context of multiple sexual partners), use of recreational drugs, and concomitant ulcerative sexually transmitted infections with acute HCV infection. Similar results were recently reported from a case-control study in New York City.41 A recent review by the Centers for Disease Control and Prevention suggested that in addition to performing HCV tests on all individuals who begin HIV care, annual tests should be given to patients who are not HCV positive but continue to report high-risk behaviors for transmission.42 Multiple methods for screening have been proposed, including annual HCV antibody, use of reflex testing for HCV antibody if screening alanine aminotransferase test result is abnormal, or pooled HCV RNA.41,43 Most current guidelines recommend annual tests for HCV enzyme immunoassay in HIV-infected persons who are HCV negative but continue to engage in risky behavior.
Management and Decision Making
The rapid progression of liver disease and risk of severe liver disease–related outcomes make it important to manage HCV infection in all coinfected patients. Management considerations include assessments of liver disease severity, screening, prevention, education, and eligibility for anti-HCV therapy. Patients should be advised to avoid any consumption of alcohol, which accelerates progression of liver disease.44 If indicated, enrollment in alcohol treatment programs is strongly recommended. Assessments of liver disease should include confirmation of chronic HCV infection by quantitative HCV RNA analysis and exclusion of infection with other viruses that cause hepatitis, such as hepatitis B virus (HBV) and hepatitis A virus. Patients without serologic evidence of immunity to either hepatitis A virus or HBV (based on positive test results for surface antibodies) should be vaccinated.
In coinfected patients, an isolated positive test result for HBV core antibody does not ensure immunity to HBV. Up to 74% of HIV-infected patients with this serologic finding will develop immunity in response to the full vaccine series and thus should be considered.45 HIV-infected patients, particularly those with advanced immunosuppression (CD4+ T-cell counts <200 cells/mm3), might be less likely to develop protective immunity to the standard vaccine series for HBV. Alternative vaccination strategies have therefore been studied. A recent large, open-label, multicenter, randomized trial found that among adults infected with HIV-1, a 4-shot series of a double-dose or intradermal low-dose recombinant HBV vaccine improved serologic response rates compared with the standard HBV vaccine regimen.46 Serologic response rates can also be improved using the standard 3-dose series of double-dose HBV vaccine.47 Some experts recommend using these enhanced vaccination strategies for all HIV-infected persons. Furthermore, there have been recommendations to delay HBV vaccination of persons with advanced immunodeficiency until after CD4+ T-cell counts reach 200 cells/mm3 and/or until HIV suppression is achieved.
Routine assessment of liver disease is recommended in all coinfected persons because knowledge of the stage of liver disease is necessary for providers to make proper management decisions. Inexpensive laboratory tests that provide information on synthetic liver function in HIV-infected persons include assays for prothrombin time (to determine the international normalized ratio), albumin, and total bilirubin (which might be artificially increased by exposure to drugs that inhibit the uridine diphosphate glucuronosyltransferase A1A enzyme system, such as the HIV PI atazanavir).48 Although thrombocytopenia can result from HIV-associated immune thrombocytopenic purpura, thrombocytopenia has also been associated with cirrhosis and portal hypertension among coinfected persons in whom HIV is well controlled with ART.49,50 Since the development of ART, coinfected patients with abnormal levels of synthetic markers or signs of thrombocytopenia should be aggressively evaluated for advanced liver disease. Although liver cancer has been increasingly reported in coinfected patients, the effects of HIV on development of hepatocellular carcinoma (HCC) are not fully understood, and the effects of screening for HCC have not been adequately studied in this population. However, most experts recommend that coinfected patients with advanced fibrosis are screened for HCC by ultrasonography every 6–12 months. The test for α-fetoprotein has relatively poor specificity and sensitivity and should therefore not be the only screening method for HCC. Persons with cirrhosis should be periodically assessed with validated prognostic models, such as the Model for End-Stage Liver Disease (MELD) score, which determines risk of mortality and need for liver transplantation.51
Evaluation of Fibrosis
In patients with normal values from laboratory tests and no clinical signs or sequelae of cirrhosis, liver biopsy analysis is the standard for staging liver disease and also determining prognosis and guiding HCV treatment decisions. Although noninvasive tests are available and can identify patients with minimal fibrosis and those with cirrhosis, they fail to adequately distinguish among intermediate stages of liver disease.52–54 Generally, these noninvasive tests for serum markers are less accurate for coinfected patients than for patients with only HCV infection; thus, results should be interpreted with caution. Transient elastography is another noninvasive test used extensively in many regions of the world but not approved for evaluation of fibrosis in the United States. Using ultrasound technology, it measures liver stiffness, a more accurate determinant of fibrosis than serologic markers.55
This methodology has been studied extensively in coinfected persons and, where available, might be a useful modality for staging liver disease in this population.55 Current HCV treatment guidelines recommend providing therapy for persons at greatest risk for progression of liver disease; thus, staging of fibrosis remains the standard to determine the necessity for immediate treatment. The clinical utility of liver disease staging is directly tied to the availability of safe, tolerable, and effective HCV treatments. The development of the DAAs telaprevir and boceprevir has increased the rate of sustained virologic response (SVR, an undetectable level of HCV RNA 6 months after the end of treatment) from approximately 40% to approximately 70% among patients with HCV genotype 1 infection.56,57 As combination therapies for HCV further increase rates of SVR, and as regimens become more tolerable, fewer patients with minimal levels of liver disease will defer therapy. Therefore, the clinical utility of fibrosis staging in making treatment decisions will wane. However, for the time being, fibrosis staging should remain part of the management strategy for coinfected patients, because there are little data on SVR with DAAs, current DAA regimens are in combination with pegylated interferon (PEG-IFN) and RBV, and the current FDA-approved DAAs have a high pill burden and add to the adverse effect profile of HCV therapy.
Treatment
PEG-IFN and RBV
The goals of HCV therapy include eradication of the infection, which prevents progression of hepatic fibrosis and, among persons with HCV-related cirrhosis, decreases rates of end-stage liver disease, HCC, need for liver transplantation, and death.58,59 Before the approval of boceprevir and telaprevir, the standard of care for HCV treatment was PEG-IFN alfa-2a or −2b in combination with RBV. However, this regimen leads to an SVR in only 14%–29% of patients with HIV and HCV genotype 1 infections (Supplementary Table 1).60–66 In more recent studies, the SVR rate of patients of African descent infected with HIV and HCV genotype 1 was ~15%.62 As in other populations, the IL28B genotype is associated with response of coinfected patients to PEG-IFN and RBV therapy; those with the CC polymorphism have higher rates of SVR than those with the CT or TT genotypes. However, the CT or TT polymorphisms are more common among persons of African and European descent.67
Because of the adverse effects of PEG-IFN and RBV, many coinfected patients have either not been eligible for therapy or not treated because the perceived potential benefit was low. Therefore, in most settings, relatively few coinfected patients have undergone treatment for HCV infection.68 However, the approval of telaprevir and boceprevir, as well as the potential for additional potent oral DAAs, with multiple mechanisms of action (such as NS5B nucleoside and non-nucleoside polymerase inhibitors, NS5A inhibitors, cyclophilin inhibitors) will bring improved SVR. As current investigational agents complete phase 3 study and become clinically available, there will also be improved tolerability of HCV therapy and the potential for interferon sparing, both of which should improve treatment uptake for the coinfected patient and ultimately result in higher viral eradication rates for this difficult-to-treat population.
Protease Inhibitors
Boceprevir and telaprevir significantly increase rates of SVR among patients with HCV genotype 1 infections, commencing the routine use of DAAs in HCV therapy.56,57,69,70 Compared with treatment with only PEG-IFN and RBV, the addition of telaprevir or boceprevir increased rates of SVR among patients with HCV genotype 1 infection who have not been previously treated, as well as those who have previously failed to respond to PEG-IFN and RBV. A benefit of triple therapy compared with PEG-IFN and RBV alone was observed in patients who typically have a poor response to IFN, including those with unfavorable IL28B polymorphisms, African descent, advanced fibrosis or cirrhosis, and high baseline levels of HCV RNA.71–74 However, compared with patients with characteristics that increase their response to IFN, the overall rate of SVR was lower in these poorly responding patients. In addition to improved response rates, potent DAAs provide the opportunity to achieve SVR in a shorter time for a proportion of treatment-naive and prior viral relapse patients. On the other hand, the adverse effect profile has not improved; in fact, both new agents add to the adverse effect profile with issues such as increased rates of anemia, rash, pruritis, and dysgeusia. When considered in the context of the AEs and the increased pill burden of therapy, these DAAs significantly increase the complexity of therapy and therefore continue to limit the number of HCV-infected patients eligible for treatment.
There are limited data on the safety and efficacy of boceprevir and telaprevir in coinfected patients. Importantly, these drugs are not active against HIV infection, and yet their use in coinfected patients raises several unique and complex considerations. It will be important to determine if these drugs interact with antiretroviral agents that are metabolized by, or induce, CYP3A4 and to determine if they have additive or new adverse effects such as anemia and/or neutropenia. Because PEG-IFN and RBV therapy is relatively ineffective in coinfected patients, the overall SVR to DAAs could also be lower than for patients with only HCV infection. To date, drug interaction data from healthy volunteers and interim safety, tolerability, and efficacy data in HIV/HCV treatment-naive coinfected patients have been presented publically for 2 small phase 2 studies of the HCV PIs in combination with PEG-IFN/RBV (Supplementary Table 2 and Table 1).75–81
Table 1.
Phase 2 Studies of HCV Protease Inhibitors in Coinfected Patients
| Telaprevir | Boceprevir | |
|---|---|---|
| HCV treatment population | Naive Genotype 1 |
Naive Genotype 1 |
| HIV treatment population | CD4 ≥500a cells/mm3; CD4 ≥300b cells/mm3 HIV RNA ≤100,000a copies/mL; ≤50b copies/mL |
CD4 ≥200 cells/mm3 HIV RNA ≤50 copies/mL |
| Antiretroviral therapy | EFV + TDF/FTC ATV/r + TDF/FTC |
No NNRTIs All other drugs permitted |
| HCV regimen | TLV 750 mg every 8 hours or 1125 mg every 8 hours (if EFV was coadministered) + PEG-IFN alfa-2a 180 μg/wk + RBV 800 mg/dayc |
BOC 800 mg every 8 hours + PEG-IFN alfa-2b (1.5 μg · kg−1 · day−1) + weight-based RBV (600–1400 mg/day) |
| Lead-in phase | No | Yes |
| Duration of PI treatment (wk) | 12 | 44 |
| Duration of PR treatment (wk) | 48 | 48 |
| Response-guided therapy | No | No |
| Virologic futility rules | Wk 4 or 8 HCV RNA ≥1000 IU/mL Week 12 ≥1000 IU/mLd Week 12 ≤2 log10 declinee Week 24 detectable HCV RNA |
Week 12 ≤2 log10 decline Week 24 detectable HCV RNA |
| HCV PI PK measured | Yes | Yes |
| ART PK measured | Yes | No |
ATV/r, atazanavir boosted with low-dose ritonavir; BOC, boceprevir; EFV, efavirenz; FTC, emtricitabine; NNRTI, non-nucleoside reverse transcriptase inhibitor (ie, EFV); PR, PEG-IFN plus ribavirin; PK, pharmacokinetics; TDF, tenofovir; TLV, telaprevir.
Study arm of patients not requiring antiretrovirals.
Study arm of patients on select antiretrovirals.
Five patients received weight-based ribavirin dosing.
In those patients with week 4 or 8 HCV RNA ≥1000 IU/mL.
In all other patients.
A phase 2a study compared the safety and efficacy of 12 weeks of treatment with a combination of telaprevir, PEG-IFN, and RBV, followed by 36 weeks of PEG-IFN and RBV, with that of 48 weeks of treatment with only PEG-IFN and RBV therapy in coinfected patients.75 An interim analysis reported that rates of rapid virologic response (undetectable HCV RNA at week 4 of therapy), complete early virologic response (undetectable HCV RNA at week 12 of therapy), and sustained virologic response at week 12 (SVR-12, undetectable HCV RNA 12 weeks after the end of therapy) were 60%–75%, 67%–88%, and 69%–80%, respectively (Figure 2). Although this study included a small number of patients, there was no difference in virologic outcomes among patients on no antiretroviral agents, atazanavir boosted with ritonavir (atazanavir/r) plus tenofovir and emtricitabine or lamivudine, or a fixed-dose combination of efavirenz with tenofovir and emtricitabine. Seven patients experienced HCV virologic breakthrough while on telaprevir, 4 on efavirenz-based regimens, and 3 on atazanavir/r-based regimens. Adverse events (AEs) were common among patients given telaprevir, with serious AEs in 7 (18%), although discontinuation of study drugs due to AEs was rare (5% developed jaundice, cholelithiasis, or hemolytic anemia). There was no report of severe rash, and pruritis, headache, nausea, dizziness, pyrexia, and depression were more common among patients given telaprevir.75 The drug interaction data provided significant insight into the potential complications of treating coinfected patients; health care providers should proceed cautiously when choosing to treat coinfected patients outside of a clinical trial.
Figure 2.
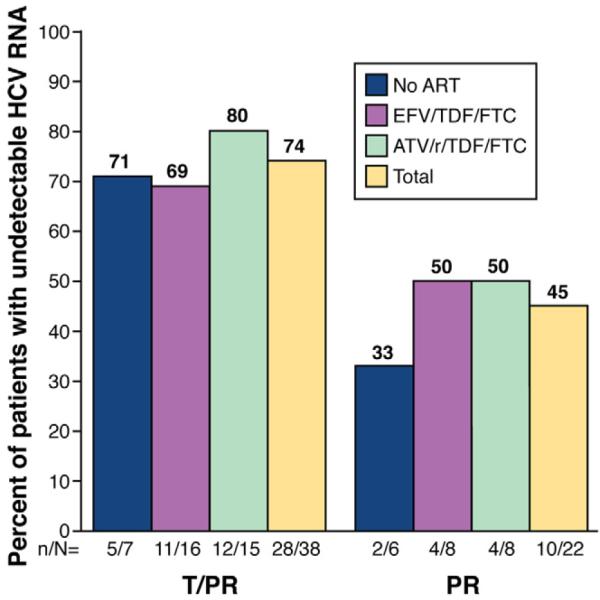
Phase 2 SVR-12 interim analysis: telaprevir in addition to PEG-IFN and RBV in coinfected patients. Study 110 was a randomized phase 2 trial assessing the efficacy and safety of telaprevir in combination with pegylated interferon alfa-2a and ribavirin (T/PR) compared with pegylated interferon alfa-2a and ribavirin alone (PR). All patients enrolled completed 48 weeks of therapy. Those in the study arms received telaprevir at 750 mg every 8 hours or 1125 mg every 8 hours (if on efavirenz [EFV]) for 12 weeks and PR for 48 weeks. Those in the control arm received placebo for 12 weeks and PR for 48 weeks. Part A of the study included patients not on ART. Part B of the study included patients on either EFV in combination with tenofovir (TDF) and emtricitabine (FTC) or atazanavir boosted with ritonavir (ATV/r) in combination with TDF and either FTC or lamivudine (3TC). This SVR-12 interim analysis reports that 74% of patients in the treatment study arm (T/PR) maintained an undetectable HCV RNA (Roche COBAS TaqMan v2.0; lower limit of detection, <10 IU/mL) as compared with 45% of those in the standard of care arm (PR).
Telaprevir is a substrate and selective inhibitor of the CYP3A4 enzyme,77,78 and based on publicly available data, telaprevir has been studied in combination with efavirenz, tenofovir, multiple HIV PIs, and raltegravir (Table 1).77,78 There are several antiretroviral agents that appear to be safe for use in combination with telaprevir, including efavirenz (the dose of telaprevir must be increased to 1125 mg every 8 hours, as was done in the phase 2a study), tenofovir, emtricitabine, atazanavir/r, and raltegravir. Combining telaprevir with other ritonavir-boosted HIV PIs, including lopinavir, darunavir, and fosamprenavir, could reduce exposure of the antiretroviral agents or telaprevir, so they are not recommended for use in combination at this time (Table 1).
A phase 2a study investigating the safety and efficacy of boceprevir in patients infected with HIV and HCV genotype 1 compared a 4-week lead-in phase of PEG-IFN and RBV followed by 44 weeks of triple therapy with boceprevir in combination with PEG-IFN and RBV with 48 weeks of treatment with PEG-IFN and RBV alone.76 In an interim analysis 42% and 73% of patients had undetectable levels of HCV RNA at weeks 8 and 24 of therapy, respectively. Sixty-one percent of patients in the therapy arm achieved SVR-12 as compared to 27% in the control arm (Figure 3). AEs were common in both study arms; 7 of 34 patients (21%) in the control arm and 11 of 64 patients (17%) in the study arm experienced serious AEs. This resulted in discontinuation in 9% and 20% of patients, respectively. Primary AEs included pyrexia, anorexia, headache, dysgeusia, vomiting, and neutropenia. Anemia, which was the most common AE in the phase 3 studies, was not a primary reason for treatment discontinuation in this phase 2a study in coinfected patients.
Figure 3.
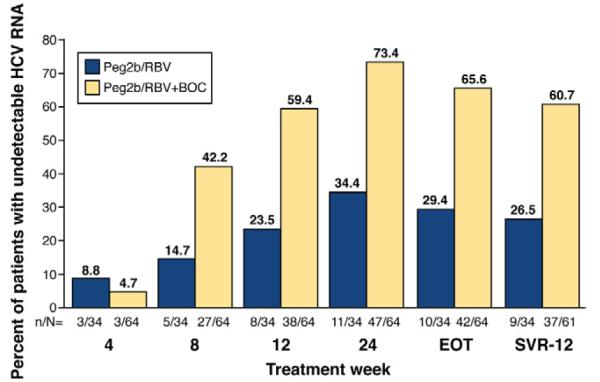
Phase 2 SVR-12 interim analysis: boceprevir in addition to PEG-IFN and RBV in coinfected patients. The P05411 study was a randomized phase 2 trial assessing the efficacy and safety of boceprevir in combination with PEG-IFN alfa-2b and RBV (Peg2b/RBV+BOC) compared with PEG-IFN alfa-2a and RBV alone (Peg2b/RBV). All patients enrolled completed 48 weeks of therapy, starting with a 4-week lead-in phase of Peg2b/RBV. Following the lead-in phase, those in the study arm received boceprevir at 800 mg every 8 hours and Peg2b/RBV for an additional 44 weeks. Those in the control arm received placebo and Peg2b/RBV for an additional 44 weeks. This SVR-12 interim analysis reports that 61% of patients in the study treatment arm maintained an undetectable HCV RNA (Roche TaqMan v2.0; lower limit of detection, 9.3 IU/mL) as compared with 27% of those on the standard of care Peg2b/RBV.
Boceprevir is a primary substrate of aldoketoreductase (1C2 and 1C3) and is a secondary substrate and potent inhibitor of the CYP3A4 enzyme and P-glycoprotein transporter.79 Based on the limited drug interactions studies available at the time of boceprevir and antiretrovirals (Table 1), the concomitant antiretrovirals allowed in the phase 2a study described are different than with telaprevir.79 A total of 84% of patients given boceprevir were on ritonavir-boosted HIV-1 PIs, including lopinavir (25%), atazanavir (31%), and darunavir (19%); 94% were on a nucleoside reverse transcriptase; and 19% were on another regimen (primarily raltegravir).76 The use of non-nucleoside reverse transcriptase inhibitors such as efavirenz was not permitted due to concern for drug interactions.79 More extensive pharmacokinetic studies of the interactions between boceprevir and antiretroviral drugs were conducted in healthy volunteers and identified concerning interactions of boceprevir and ritonavir-boosted protease inhibitors including atazanavir, darunavir, and lopinavir.80 Additional drug interaction studies in coinfected patients will be performed in coinfected subjects by the Adult AIDS Clinical Trials Group (ClinicalTrials.gov identifier: NCT01482767).
In light of these preliminary studies, there remain many important questions about using first-generation HCV PIs to treat coinfected patients. The potential interaction between inhibitors of the HCV NS3/4A protease and antiretroviral agents is complex and incompletely characterized. Data support the use of these agents in coinfected patients with high CD4+ T-cell counts who are not taking antiretroviral agents or those on select antiretroviral regimens for which sufficient safety data have been provided. Data support the use of telaprevir in combination with tenofovir/emtricitabine, atazanavir/r, efavirenz (with a higher dose of telaprevir), and raltegravir. Boceprevir can be safely used in combination with abacavir and lamivudine, tenofovir, and emtricitabine plus raltegravir but not efavirenz or other non-nucleoside reverse transcriptase inhibitors. Recent data suggest boceprevir should not be administered with ritonavir-boosted HIV protease inhibitors due to decreased AUC and Cmin, as well as evidence of HIV virologic breakthroughs. As such, before patients are given either of the approved HCV PIs, all medications, including those used to treat non–HIV-related conditions, should be carefully assessed for potential drug interactions; resources such as the University of Liverpool’s hepatitis tools (www.hep-interactions.org) are available.
Complexity of Therapy
The combination of HCV and HIV treatment requires many patients to take multiple daily doses of medication (a high pill burden), with and without food. Currently, the dosing of both telaprevir and boceprevir is every 7 to 9 hours with the requirement that these drugs be taken with food. To increase absorption, telaprevir must be ingested with at least 20 g of fat (such as a bagel with cream cheese, a half-cup of nuts, or 1 cup of ice cream). The daily pill burden is high for a patient taking boceprevir (12 200-mg capsules daily, in 3 doses) and telaprevir (6 375-mg tablets daily, in 3 doses; 9 tablets when taken with efavirenz). HIV-infected patients who take these HCV PIs, along with RBV, have an even greater and more complicated pill burden. For example, efavirenz should be taken in a fasting state, whereas boceprevir and telaprevir should be taken with food. It is not known how this level of complexity affects treatment of coinfected patients; it might lead to poor compliance, although many coinfected patients are accustomed to taking daily medications and have access to adherence support tools.
Unless a coinfected patient is being treated as part of a clinical trial, the total duration of therapy for either a boceprevir- or telaprevir-based regimen will be 48 weeks. Although response-guided therapy can reduce treatment time and is a major therapeutic advance, it was not part of the phase 2a study design for either drug. Studies are needed to test the appropriateness of response-guided therapy for this population; until that time, there are no data to support the discontinuation of treatment at an earlier time point for coinfected patients with an early virologic response.
The efficacy of telaprevir and boceprevir regimens has not been studied in coinfected patients who failed to respond to prior treatment with PEG-IFN and RBV. Findings from the trials of patients with only HCV infection who did not respond to prior treatment indicate that SVRs are greatest among patients who had experienced virologic relapse and lower among those with prior partial or no virologic response. Patients infected with only HCV who did not respond to previous therapy and had bridging fibrosis or compensated cirrhosis had low rates of SVR (14%–39%), and many developed drug-resistant variants.71 Treatment-experienced coinfected patients will be included in the active phase 3 studies, and those results will be eagerly awaited.
Thus, at this time, the data to support the use of these complicated telaprevir or boceprevir regimens for the treatment of HCV in HIV-infected patients are limited. Despite these challenges, these sparse data indicate that telaprevir or boceprevir in combination with PEG-IFN/RBV can substantially increase the rate of viral suppression with manageable toxicity and drug interactions. Although these FDA-approved medications are not yet indicated for treatment of HIV/HCV-coinfected persons, the benefits of using these medications will outweigh the risks for some individuals. As such, a framework for the management of HCV in HIV-coinfected patients in the era of HCV PIs can be constructed (Figure 4).82,83
Figure 4.
A framework for the management of HCV in HIV-coinfected patients.
Combining DAAs With or Without IFN
In deciding whether to treat coinfected patients with telaprevir- or boceprevir-based regimens, it is important to consider the rapid development of additional DAAs and IFN-sparing regimens. The antiviral effects of IFN vary among coinfected patients, based on effects of HIV infection and/or disease, and many coinfected patients are not eligible for IFN-based therapy due to comorbid conditions, particularly psychiatric diseases. The bone marrow–suppressive effects of IFN are exacerbated in many HIV-infected patients, further complicating treatment. The development of combination regimens of DAAs that are safe for coinfected patients is therefore of high priority.
However, combining multiple antiviral agents is complex. Recommended antiretroviral regimens for the treatment of HIV include the use of at least 3 antiretroviral agents and, in some cases, the use of ritonavir to inhibit CYP3A/4 to boost the HIV-1 PI. Although the interactions among these antiretroviral agents are well understood, the effects of adding HCV DAAs to these regimens are difficult to predict and must be evaluated in careful pharmacokinetic studies and then in larger phase 2 and 3 trials. Although there are many potential interactions between all approved antiretroviral drugs and the HCV DAAs in development, studies of DAA regimens in patients on select antiretroviral regimens will be less complex. For example, HCV NS3A/4 PIs and NS5A inhibitors, which are primarily metabolized by the liver, could be studied in combination with raltegravir and tenofovir/emtricitabine, which are primarily metabolized by the kidneys. The identification of safe antiretrovirals would allow providers to consider temporary modifications in ART because most combination DAA therapies would be limited to 24 weeks or less. This is true even now of telaprevir, which is only required for the first 12 weeks of therapy. In this context, studies of IFN-free DAA-containing regimens for coinfected patients should not be delayed until the completion of studies in patients with only HCV infection.
Liver Transplantation
Despite improvements in anti-HCV therapy, many coinfected patients will develop end-stage liver disease or HCC. HIV-infected patients with end-stage liver disease can receive liver transplants, and these have been successful at multiple institutions worldwide, although outcomes have varied. Initial attempts of liver transplantation in HIV-infected individuals, before the use of highly active ART, had poor results; several case series reported 3-year survival rates of 44%.84 However, following the introduction of highly active ART, survival times increased. A study of 24 HIV-infected subjects reported rates of 3-year survival similar to those of HIV-negative recipients (78%).85 However, in this study, HCV infection was the leading indication for liver transplantation and was independently associated with poorer survival. In the largest prospective study to date, a National Institutes of Allergy and Infectious Diseases–funded HIV-transplant study, coinfected patients (N = 81) had lower rates of graft survival (59% vs 67%) and 3-year survival than patients with only HCV infection (N = 231), although these rates were better than historical reports.86 In the multivariable analysis, factors associated with graft loss in the coinfected patients included treatment for acute rejection, dual liver-kidney transplant, HCV-positive donor, and body mass index <21 kg/m2; HIV-related complications did not appear to contribute to mortality. Raltegravir, a first-in-class integrase inhibitor used to treat HIV infection, has fewer interactions with immunosuppressive drugs and could improve treatment options for HIV-infected patients who require solid organ transplants.
Future Directions
Similar to the approval of the first HIV-1 PI, saquinavir, the advent of the HCV NS3/4A PIs, telaprevir and boceprevir, heralds a new era of more effective HCV treatment. There are currently more than 50 investigational agents in human studies for HCV infection, with 7 different mechanisms of action directed toward the virus and the host.87 This drug development pipeline could lead to once-daily dosing, fewer adverse effects, potency across all HCV genotypes, and reduced selection of resistant viral strains, either through the use of combinations of agents with different mechanisms of action or resistance profiles or the use of agents with high individual barriers to resistance (eg, nucleoside analogue NS5B polymerase inhibitors). Most importantly, these combinations of DAAs could eradicate HCV without the need for PEG-IFN.88–90
On the other hand, the HCV DAAs could interact with antiretrovirals and with other DAAs in treatment regimens. Interactions among drug combinations will need to be carefully assessed before they are tested in coinfected patients. The challenges and limitations of IFN-based therapy are major barriers to controlling HCV-related liver disease in HIV-infected patients, so innovative and more effective approaches are welcomed. Trials of IFN-sparing, DAA combination therapies should be initiated early in coinfected patients, before the regulatory approval of combination regimens, so that access to these highly effective compounds is not limited to those with only HCV infection.
Supplementary Material
Supplementary Table 1. Randomized Controlled Trials of PEG-IFN and RBV in Coinfected Patients
Supplementary Table 2. Interactions Among HCV Protease Inhibitors and Antiretroviral Agents
Funding
Supported by grant DA-16065 from the National Institute on Drug Abuse and grant K23-AI096913-02 for SN, DA-16065 (M.S.).
Abbreviations used in this paper
- AE
adverse event
- ART
antiretroviral therapy
- DAA
direct-acting antiviral
- FDA
Food and Drug Administration
- HIV
human immunodeficiency virus
- IDU
injection drug use
- IFN
interferon
- MSM
men who have sex with men
- PEG-IFN
peginterferon
- PI
protease inhibitor
- RBV
ribavirin
- RR
relative risk
- SVR
sustained virologic response
Footnotes
Supplementary Material Note: The first 50 references associated with this article are available below in print. The remaining references accompanying this article are available online only with the electronic version of this article. To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi:10.1053/j.gastro.2012.02.012.
Conflicts of interest The authors disclose the following: Susanna Naggie receives research support from Vertex, Anadys, Synexis, and Medtronic; and serves on the advisory boards for Vertex and Boehringer Ingelheim. Mark S. Sulkowski receives grant support and consulting fees from Abbott, BMS, BIPI, Gilead, Janssen, Merck, Roche, and Vertex; and receives fees from Pfizer for participation in review activities.
References
- 1.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 2.Sherman KE, Rouster SD, Chung RT, et al. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US Adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–837. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin C, Friis-Møller N, et al. Liver-related deaths in persons infected with the HIV: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 4.Tencer T, Friedman HS, Li-McLeod J, et al. Medical costs and resource utilization for hemophilia patients with and without HIV and HCV infection. J Manage Care Pharm. 2007;13:790–798. doi: 10.18553/jmcp.2007.13.9.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naggie S, Park L, Gellad Z, et al. Healthcare utilization and mortality associated with chronic HIV and HCV: more to the story than chronic infection. Presented at: 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. February 27 to March 2, 2011; Abstract 915. [Google Scholar]
- 6.Chung RT, Anderson J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 8.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33:126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. [Accessed November 6, 2011];Telaprevir [prescribing information] www.incivek.com.
- 10. [Accessed November 6, 2011];Boceprevir [prescribing information] www.victrelis.com.
- 11.Scott JD, Gretch DR. Molecular diagnostics of hepatitis C virus infection: a systematic review. JAMA. 2007;297:724–732. doi: 10.1001/jama.297.7.724. [DOI] [PubMed] [Google Scholar]
- 12.Poynard T, Bedossa P, Opolon P, for the OBSVIRC, METAVIR, CLINIVIR and DOSVIRC Groups Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 13.Massard J, Ratziu V, Thabut D, et al. Natural history and predictors of disease severity in chronic hepatitis C. J Hepatol. 2006;44:S19–S24. doi: 10.1016/j.jhep.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Darby SC, Ewart DW, Giangrande PL, et al. Mortality from liver cancer and liver disease in haemophilic men and boys in UK given blood products contaminated with hepatitis C. UK Haemophilia Centre Directors’ Organization. Lancet. 1997;350:1425–1431. doi: 10.1016/s0140-6736(97)05413-5. [DOI] [PubMed] [Google Scholar]
- 15.Graham C, Baden L, Yu E, et al. Influence of HIV infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 16.Joshi D, O’Grady J, Dieterich D, et al. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377:1198–1209. doi: 10.1016/S0140-6736(10)62001-6. [DOI] [PubMed] [Google Scholar]
- 17.Lin W, Weinberg EM, Tai AW, et al. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology. 2008;134:803–811. doi: 10.1053/j.gastro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soto B, Sanchez-Quijano A, Rodrigo L, et al. Human Immunodeficiency virus infection modifies the natural history of chronic parenterraly-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 20.Tebas P. Insulin resistance and diabetes mellitus associated with antiretroviral use in HIV-infected patients: pathogenesis, prevention, and treatment options. J Acquir Immune Defic Syndr. 2008;49:S86–S92. doi: 10.1097/QAI.0b013e31818651e6. [DOI] [PubMed] [Google Scholar]
- 21.Bonacini M. Alcohol use among patients with HIV infection. Ann Hepatol. 2011;10:502–507. [PubMed] [Google Scholar]
- 22.Chaudhry AA, Sulkowski MS, Chander G, et al. Hazardous drinking is associated with an elevated aspartate aminotransferase to platelet ratio index in an urban HIV-infected clinical cohort. HIV Med. 2009;10:133–142. doi: 10.1111/j.1468-1293.2008.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qurishi N, Kreuzberg C, Lüchters G, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708–1713. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 24.Pineda JA, Garcia-Garcia JA, Aguilar-Guisado M, et al. Clinical progression of hepatitis C virus-related chronic liver disease in human immunodeficiency virus-infected patients undergoing highly active antiretroviral therapy. Hepatology. 2007;46:622–630. doi: 10.1002/hep.21757. [DOI] [PubMed] [Google Scholar]
- 25.Thein H, Yi Q, Dore GJ, et al. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22:1979–1991. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention [Accessed October 5, 2011];Viral hepatitis statistics & surveillance. www.cdc.gov/hepatitis/Statistics/index.htm.
- 27.Leruez-Ville M, Kunstmann JM, De Almeida M, et al. Detection of hepatitis C virus in the semen of infected men. Lancet. 2000;356:42–43. doi: 10.1016/S0140-6736(00)02435-1. [DOI] [PubMed] [Google Scholar]
- 28.Manavir M, Watkins-Riedel T, Kucera E, et al. Evidence of hepatitis C virus in cervical smears. J Infect. 1999;38:60–61. doi: 10.1016/s0163-4453(99)90038-5. [DOI] [PubMed] [Google Scholar]
- 29.Vandelli C, Renzo F, Romano L, et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am J Gastroenterol. 2004;99:855–859. doi: 10.1111/j.1572-0241.2004.04150.x. [DOI] [PubMed] [Google Scholar]
- 30.Marincovich B, Castilla J, del Romero J, et al. Absence of hepatitis C virus transmission in a prospective cohort of heterosexual serodiscordant couples. Sex Transm Infect. 2003;79:160–162. doi: 10.1136/sti.79.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alary M, Joly JR, Vincelette J, et al. Lack of evidence of sexual transmission of hepatitis C virus in a prospective cohort study of men who have sex with men. Am J Public Health. 2005;95:502–505. doi: 10.2105/AJPH.2003.020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salleras L, Bruguera M, Vidal J, et al. Importance of sexual transmission of hepatitis C virus in seropositive pregnant women: a case-control study. J Med Virol. 1997;52:164–167. doi: 10.1002/(sici)1096-9071(199706)52:2<164::aid-jmv8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 33.Feldman JG, Minkoff H, Landesman S, et al. Heterosexual transmission of hepatitis C, hepatitis B, and HIV-1 in a sample of inner-city women. Sex Transm Dis. 2000;27:338–342. doi: 10.1097/00007435-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Thomas DL, Zenilman JM, Alter HJ, et al. Sexual transmission of hepatitis C virus among patients attending sexually transmitted diseases clinics in Baltimore-an analysis of 309 sex partnerships. J Infect Dis. 1995;171:768–775. doi: 10.1093/infdis/171.4.768. [DOI] [PubMed] [Google Scholar]
- 35.Hershow RC, Kalish LA, Sha B, et al. Hepatitis C virus infection in Chicago women with or at risk for HIV infection: evidence for sexual transmission. Sex Transm Dis. 1998;25:527–532. doi: 10.1097/00007435-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Frederick T, Burian P, Terrault N, et al. Factors associated with prevalent hepatitis C infection among HIV-infected women with no reported history of injection drug use: the Women’s Interagency HIV Study (WIHS) AIDS Patient Care STDS. 2009;23:915–923. doi: 10.1089/apc.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van de Laar T, Pybus O, Bruisen S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609–1617. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der helm JJ, Prins M, del Amo J, et al. The hepatitis C epidemic among HIV-positive MSM: incidence estimates from 1990 to 2007. AIDS. 2011;25:1083–1091. doi: 10.1097/QAD.0b013e3283471cce. [DOI] [PubMed] [Google Scholar]
- 39.Urbanus AT, van de Laar TJ, Stolte IG, et al. Hepatitis C virus infections among HIV-infected men who have sex with men: an expanding epidemic. AIDS. 2009;23:F1–F7. doi: 10.1097/QAD.0b013e32832e5631. [DOI] [PubMed] [Google Scholar]
- 40.Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983–991. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- 41.Fierer DS, Factor SH, Uriel AJ, et al. Sexual transmission of hepatitis C virus among HIV-infected men who have sex with men – New York City, 2005-1010. MMWR. 2011;60:945–950. [PubMed] [Google Scholar]
- 42.Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010;52:1497–1505. doi: 10.1002/hep.23808. [DOI] [PubMed] [Google Scholar]
- 43.Taylor LE, DeLong AK, Maynard MA, et al. Acute hepatitis C virus in an HIV clinic: a screening strategy, risk factors, and perception of risk. AIDS Patient Care STDS. 2011;25:571–577. doi: 10.1089/apc.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiley TE, McCarthy M, Breidi L, et al. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805–809. doi: 10.1002/hep.510280330. [DOI] [PubMed] [Google Scholar]
- 45.Chakvetadze C, Bani-Sadr F, Le Pendeven C, et al. Serologic response to hepatitis B vaccination in HIV-infected patients with isolated positivity for antibodies to hepatitis B core antigen. Clin Infect Dis. 2010;50:1184–1186. doi: 10.1086/651422. [DOI] [PubMed] [Google Scholar]
- 46.Launay O, van der Vliet D, Rosenberg AR, et al. Safety and immunogenicity of 4 intramuscular double doses and 4 intradermal low doses vs standard hepatitis B vaccine regimen in adults with HIV-1: a randomized controlled trial. JAMA. 2011;305:1432–1440. doi: 10.1001/jama.2011.351. [DOI] [PubMed] [Google Scholar]
- 47.Cardell K, Akerlind B, Sallberg M, et al. Excellent response rate to a double dose of the combined hepatitis A and B vaccine in previous nonresponders to hepatitis B vaccine. J Infect Dis. 2008;198:299–304. doi: 10.1086/589722. [DOI] [PubMed] [Google Scholar]
- 48.Zucker SD, Qin X, Rouster SD, et al. Mechanism of indinavir-induced hyperbilirubinemia. Proc Natl Acad Sci U S A. 2001;98:12671–12676. doi: 10.1073/pnas.231140698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris L, Distenfeld A, Amorosi E, et al. Autoimmune thrombocytopenic purpura in homosexual men. Ann Intern Med. 1982;96:714–717. doi: 10.7326/0003-4819-96-6-714. [DOI] [PubMed] [Google Scholar]
- 50.Marks KM, Clarke RM, Bussel JB, et al. Risk factors for thrombocytopenia in HIV-infected patients in the era of potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;52:595–599. doi: 10.1097/QAI.0b013e3181b79aff. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Randomized Controlled Trials of PEG-IFN and RBV in Coinfected Patients
Supplementary Table 2. Interactions Among HCV Protease Inhibitors and Antiretroviral Agents