Abstract
Solid tumors often contain regions with insufficient oxygen delivery, a condition called hypoxia. Tumor hypoxia is an independent prognostic factor significantly correlated with advanced stages of malignancy, increased resistance to conventional therapy, and reduced disease-free survival. Hypoxic tumor cells exhibit poorly differentiated phenotypes resembling stem or progenitor cells. Studies have shown that hypoxia can inhibit tumor cell differentiation and promote maintenance of cancer stem cells. In addition, hypoxia also blocks differentiation of mesenchymal stem/progenitor cells, a potential source of tumor-associated stromal cells. Therefore, hypoxia may play a critical role during the evolution of the tumor stromal microenvironment and formation of the putative cancer stem cell niches. Conceptually, hypoxia may help create a microenvironment enriched both in poorly differentiated tumor cells and in undifferentiated stromal cells. Such an undifferentiated hypoxic microenvironment may provide essential cellular interactions and environmental signals for the preferential maintenance of cancer stem cells. This review will discuss the hypoxia-regulated stem cell pathways and their roles in the maintenance of cancer stem cell functions.
Keywords: Cancer stem cells, differentiation, hypoxia, niche, oxygen, tumor microenvironment
Introduction
The transformation from a benign to a malignant state is a rather slow process, often taking years to complete.1, 2 During their malignant progression, tumor cells accumulate multiple mutations in oncogenes, tumor suppressor genes, as well as other epigenetic changes.3, 4 The key to tumor progression is that these mutational events need to take place longitudinally in a linear fashion in a single tumor cell created by the initial oncogenic transformation. In order to complete this protracted journey, this tumor cell must be able to faithfully reproduce itself in order for previously acquired mutations to be inherited and be combined with newly acquired mutations in the daughter cell stages. In other words, this initially formed tumor cell must be capable of self-renewal and must remain in a stem cell-like state along the process toward increased malignancy (Figure 1).
Figure 1. Cancer Cell Stemness is Essential for Tumor Progression.
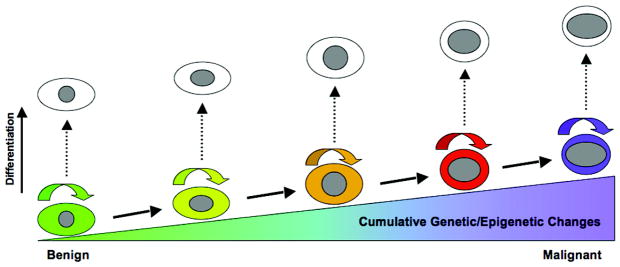
Tumor progression is accompanied by multiple genetic mutations and epigenetic changes that occur over a long period of time. Since these nuclear mutations and changes must be inherited in the same tumor cell or its identical clones, the capacity of self-renewal is absolutely required for malignant progression. Differentiation of a clonal population at any stage of this linear progression will effectively block the tumor progression.
Since being first identified from acute myeloid leukemia (AML) patients in 1997,5 cancer stem cells or tumor-initiating cells have also been found in many commonly diagnosed solid tumors including breast cancers,6 colon cancers,7–9 cancers of the central nervous system,10–12 head-and-neck cancers,13 lung cancers, 14, 15 and pancreatic cancers.16 It is worth noting that cancer stem cells are more or less defined by their biological functions, especially the ability for self-renewal or clonogenic growth and the potential to initiate a tumor upon transplantation in a new host. However, there is a lack of consensus on the cell surface markers that define the cancer stem cell population from a tumor.17, 18 It is highly likely that a tumor may contain a heterogeneous population of cancer stem cells arising from differential clonal evolution or different developmental stages.
Studies using the euploid teratocarcinoma model strongly suggest that cancer could result from mis-regulated differentiation.19 Therefore, cancer stem cells may share functional similarities with normal stem or progenitor cells. Gene expression profiling of gliomas has revealed that high-grade gliomas express neural stem cell markers, whereas low-grade gliomas possess lineage-specific markers of neuronal differentiation.20 Furthermore, cancer stem cells can use many of the same mechanisms that regulate self-renewal of normal stem cells, such as Bmi-1, Notch, Wnt and Sonic Hedgehog.18, 21–23.
Generally speaking, the microenvironment in normal tissues is ideal for differentiated cells to perform their biological functions and is permissive for lineage-specific differentiation of stem and progenitor cells. Therefore, uncommitted stem cells are often found in a unique microenvironment or niche where they can undergo self-renewal without premature or misguided differentiation.24–27 By the same token, cancer stem cells may also rely on a specific microenvironment to protect their stem cell characteristics. Although cancer stem cell niches are still poorly understood, components of the tumor microenvironment have been shown to regulate stem cell functionalities. This review will discuss the potential role of tumor hypoxia in the regulation of cancer cell differentiation and formation of a niche environment that may facilitate cancer stem cell maintenance.
Hypoxia and Tumor Progression
Decreased oxygenation or hypoxia is a common feature of all solid tumors and is an independent prognostic factor for advanced disease progression and poor clinical outcome.28–32 Hypoxia induces a wide range of biological changes that may contribute to the evolution of malignant tumor cells, such as decreased cell proliferation,33 increased expression of drug-resistance gene,34, 35 selection of apoptosis-resistant clones,36 facilitation of tumor invasion and metastasis,37, 38 reduced expression of DNA repair genes,39–42 and increased genomic instability.43, 44 During the last decade, a number of studies have shown that hypoxia has the potential to regulate cell differentiation, which has led to the emergence of a new paradigm that tumor hypoxia may facilitate the maintenance of cancer stem cell characteristics and thus allow a tumor cell with self-renewal potential to accumulate a multitude of genetic and epigenetic changes over a long period of time in order to become increasingly malignant.
Hypoxia-induced signaling is primarily mediated by the ubiquitous hypoxia-inducible factor-1 (HIF-1), a master regulator of O2 homeostasis that consists of O2-regulated HIF-1α and O2-insensitive HIF-1β.45 Genes induced by HIF-1 are involved in a wide range of cellular functions such as cell growth, survival, motility, angiogenesis, energy metabolism, and cellular differentiation.45, 46 The second important member of the HIF family is HIF-2α, which is structurally similar to HIF-1α but appears to have non-overlapping functions.47 In most cases, increased HIF activity is associated with tumor development. Elevated levels of HIF-1α protein are often detected by immunochemical staining in solid tumors, in contrast to normal non-ischemic tissues.45, 48, 49 Clinical studies have shown that elevated levels of HIF-1α protein50, 51 or HIF-2α protein52 show significant statistical correlation with poor patient survival. Furthermore, nuclear accumulation of HIF-1α protein is correlated with poor tumor differentiation in primary pancreatic cancers.53 However, not all tumors benefit from increased HIF expression or activity. Tumors derived from HIF-1α−/− mouse embryonic stem (ES) cells exhibit accelerated growth, due in part to decreased hypoxia-induced apoptosis.54 Similarly, elevated levels of HIF-2α have been shown to decrease growth of rat glioma tumors in vivo.55 These contradicting observations may reflect the differences in the interaction between specific tumor cells and their microenvironment.
Nonetheless, recent studies have shown that increased expression of HIF-1α and/or HIF-2α has been found in the stem cell-like populations of neuroblastomas56, 57 and gliomas.58 Knocking down either HIF-1α or HIF-2α results in reduced tumor sphere growth and survival of glioma stem cells.58 Compared to HIF-1α, HIF-2α appears to have a higher normoxic level of expression in glioma stem cells,58 which might explain why HIF-2α exhibits stronger effects than HIF-1α does. In human neuroblastoma tumors, HIF-2α is preferentially expressed in the immature neural crest-like neuroblastoma cells in vivo and appears to be required for the maintenance of the undifferentiated neuroblastoma cells.56, 57 These studies suggest that activation of the HIF pathway may potentially contribute to the maintenance of undifferentiated cancer stem cell phenotypes and thus facilitate malignant tumor progression.
Hypoxia-Activated Stem Cell Genes and Pathways in Tumor Cells
Several stem cell genes and/or pathways have been shown to be upregulated by hypoxia in tumor cells. However, we are still in the very early stages of understanding the mechanisms by which hypoxia regulates the maintenance or differentiation of cancer stem cells. Kim et al.59 have recently identified the stem cell gene DLK1, or delta-like 1 homolog (Drosophila), as a new transcription target of the HIF pathway in neuronal tumor cells. DLK1, also known as pref-1 (preadipocyte factor 1) and fetal antigen 1 (FA1) among others, is a type I transmembrane protein with abundant expression in embryonic tissues and immature cells but not in differentiated adult tissues,60 suggesting a role of DLK1 in the regulation of stem cells and progenitor cells. Elevated expression of DLK1 has also been reported in several tumor types.61–66 Kim et al.59 have found that DLK1 is robustly expressed in undifferentiated, but not differentiated, tumor cells. Inhibition of DLK1 enhances spontaneous neuronal differentiation, decreases clonogenicity or the colony-forming potential, and suppresses tumorigenicity in vivo. Overexpression of DLK1, on the other hand, inhibits differentiation, enhances clonogenicity, and increases tumorigenicity. The proximal 5′ promoter/enhancer region of the human DLK1 gene contains three putative hypoxia-responsive elements (HRE) with the conserved motif of 5′-ACGTG-3′67 at −758, −402, and −248, respectively, from the transcription start site. Both HIF-1α and HIF-2α can bind to this promoter region under hypoxia, as demonstrated by chromosome immunoprecipitation.59 Further investigation demonstrates that the DLK1 cytoplasmic domain, especially Tyrosine-339 and Serine-355, is required for maintaining both clonogenicity and tumorigenicity.59 These observations demonstrate that HIF and DLK1 constitute an active signal transduction pathway that facilitates the maintenance or selection of cancer stem cells and increases their tumorigenic potential.
Chemokine receptor CXCR4 and its ligand CXCL12 or SDF-1 (stromal cell-derived factor-1) play a critical role during embryogenesis and regulate the functions of subsets of adult stem/progenitor cells.68–70 Hypoxia, as well as activation of HIF pathways due to loss of pVHL tumor suppressor protein, strongly induces expression of CXCR4 and SDF-1 in both cancer cells and normal stem/progenitor cells.71–76 CXCR4-mediated signal transduction plays an important role in homing of stem cells to hypoxic regions via the CXCL12 gradient.71 Similarly, elevated CXCR4 expression in tumors has been found to be associated with increased metastasis and poor patient survival.77–80 Interestingly, the CXCR4+ subpopulations of the CD133+ human pancreatic cancer stem cells are essential for liver metastasis when orthotopically injected in immune-deficient mice,81 suggesting that the CXCR4+ cancer stem cells are the most likely source of tumor metastasis. However, it remains to be determined whether CXCR4-CXCL12 has the potential to regulate stem cell maintenance or cellular differentiation.
The POU family transcription factor POU5F1 (Oct3/4) has been shown to be one of the four to five critical genes that collectively transform adult somatic cells into pluripotent stem cells.82–84 Elevated levels of POU5F1 has been found in germ cell cancers and several types of somatic cancers including human cervical carcinomas, breast carcinomas and pancreatic cancers.85–88 Using transgenic mice with doxycycline-inducible expression of POU5F1, Hochedlinger et al.89 have shown that increased POU5F1 expression results in inhibition of cellular differentiation and dysplastic growths in epithelial tissues, thus demonstrating a direct role of POU5F1 in tumorigenesis. Interestingly, the genetic HIF-2α “knock-in” study has shown that HIF-2α is directly involved in transcription of POU5F1 in mouse embryonic tissues.90 Loss of POU5F1 results in decreased growth of mouse embryonal stem cell-derived teratomas.90 Similarly, Forristal et al.91 have found that reduced HIF-2α expression results in decreased expression of POU5F1 and other stem cell genes in human ES cells cultured at 5% O2. These observations strongly suggest that HIF-2α is an important regulator of cellular stemness. Nevertheless, it remains to be examined whether hypoxia increases POU5F1 expression in common types of clinical cancers and whether hypoxia-dependent upregulation of POU5F1 expression plays a role in the regulation of cancer stem cell characteristics.
The pentaspan transmembrane glycoprotein prominin-1 (CD133) has been widely used as a marker to isolate perspective cancer stem cells from a variety of tumors18 although its functions remain to be investigated. Recent studies have shown that hypoxia (1% O2) can increase CD133 expression in human glioma cells and can promote the expansion of the CD133+ tumor cell population.92–94 Interestingly, the hypoxia-dependent induction of CD133 expression is attenuated in glioma cells when expression of either HIF-1α94 or HIF-2α93 is knocked down. Contradictorily, Matsumoto et al.95 have shown that CD133 expression in several gastric, colorectal, and lung cancer cell lines with elevated basal CD133 levels is down regulated upon exposure to hypoxic conditions (0.1% O2 or deferoxamine). In addition to cell line differences, an important difference between these two experimental models is the degree of hypoxia, i.e. 1% O2 for glioma cells versus 0.1% O2 for other cell lines. Future investigation of the transcriptional regulation of CD133 expression under hypoxia may shed light on the discrepancy between these two experimental models.
Impact of Hypoxia and Cancer Stem Cell Niches
Genetic studies have shown that differentiation status of niche stromal cells can exert strong impact on stem cell maintenance. In mouse bone marrow (BM), destruction of osteoblasts at the early stage of osteoblastogenesis results in severe decrease of hematopoietic stem cells.96 In contrast, ablation of osteoblasts at later stages of differentiation has little effect on hematopoiesis.97 Furthermore, CD146+ osteoprogenitor cells are able to reconstitute the hematopoietic microenvironment in bone marrow.98 These immature CD146+ cells, but not the differentiated CD146− cells, can produce significant amounts of angiopoietin-1 to support the maintenance of hematopoietic stem cells.98–100 These data suggest that immature stromal cells are preferentially suited for the maintenance of stem cells.27 It can therefore be argued that cancer stem cells might also prefer being surrounded by poorly differentiated stromal cells.
With the initiation and progression of a tumor, the tumor stromal microenvironment also undergoes progressive changes characterized by loss of normal tissue structures and reduced numbers of well-differentiated stromal cells.101–103 For example, breast carcinoma is predominantly rich in fibroblasts, whereas normal mammary tissues have abundant adipocytes and sparse connective tissue. Interactions between tumor cells and their microenvironment exert profound influence upon tumor development and progression toward malignancy.101, 104, 105 As an excellent example, Pine et al. have shown that the decision to undergo symmetric or asymmetric cell division by a small population of human lung cancer cells is partially determined by hypoxia and cell-cell contact, among other environmental factors.106
Tumor-associated stromal cells appear to be developmentally immature. Mouse stromal cells isolated from human breast cancer xenografts have been shown to have the ability to form fibroblastoid colonies (CFU-F) in vitro, a hallmark of undifferentiated mesenchymal stem cells.105, 107 In contrast, stromal cells prepared from control Matrigel plugs or from adjacent normal tissues do not form fibroblastoid colonies,105 suggesting that tumor stroma contains higher populations of immature cells than normal stroma does. Interestingly, weakly metastatic human breast carcinoma cells become highly metastatic when injected subcutaneously as a mixture with human BM-derived mesenchymal stem cells.105 Although it remains to be determined whether mesenchymal stem cells play a role in regulation of cancer cell differentiation, this study clearly demonstrates that immature stromal cells facilitate acquisition of aggressive tumor phenotypes.
It is not yet understood what causes the changes in the stromal microenvironment and what is the cellular origin of tumor-associated fibroblasts, although BM-derived mesenchymal stem cells and hematopoietic stem cells are among the likely sources of tumor stromal cells.105, 108, 109 Also not clear is how the cell fate decision of these tumor-infiltrating mesechymal stem cells is regulated by tumor microenvironment. As discussed below, it is possible that tumor hypoxia regulates the cell fate decisions in these mesenchymal stem cells and thus affects the evolution of the tumor stromal microenvironment.
Mesenchymal stem cells are capable of differentiating into several cell types including adipocytes, chondrocytes, and myocytes in response to specific differentiation cues. Interestingly, it has been demonstrated that adipogenic differentiation by mesenchymal stem or progenitor cells is inhibited at physiologically relevant levels of hypoxia or 1–2% O2.110–113 Ectopic expression of constitutively active HIF-1α mutants is sufficient to inhibit adipogenic differentiation under normoxic conditions.112 On the other hand, knocking down HIF-1α by gene-specific siRNA rescues adipogenic differentiation under hypoxic conditions.112 Mechanistically, hypoxia increases the expression of DEC1/Stra13, a putative transcription repressor with homology to the Hairy and Enhancer-of-Split (HES) family of transcription repressors that represses the expression PPARγ2, a critical differentiation-determination gene for terminal adipogenic differentiation.112, 113 In addition, HIF-independent signaling pathways also appear to be involved in the inhibition of adipogenic differentiation by hypoxia. The Transforming Growth Factor β (TGFβ)-Smad pathway in human BM-derived mesenchymal stem cells is activated under hypoxia as shown by increasing levels of phosphorylated Smad2/3, which leads to inhibition of adipogenic differentiation of human BM-derived mesenchymal stem cells.114 However, whether or how the HIF pathway is involved in activation of the TGFβ-Smad pathway remains to be determined. Nonetheless, it is worth noting that mesenchymal stem/progenitor cells remain undifferentiated and uncommitted under hypoxic conditions and can still undergo lineage-specific differentiation once they return to normoxic conditions.112 Collectively, these data suggest that hypoxia has the ability to maintain mesenchymal stem cells in an undifferentiated state and to prevent them from committing to adipogenic differentiation. These findings could provide a plausible mechanism underlying the stromal changes observed in breast cancers.101
Hypoxia also decreases the potential of mesenchymal progenitor cells to undergo myogenic differentiation.115, 116 Nonetheless, the degree of inhibition is dose dependent on the level of hypoxia with strongest inhibition at nearly anoxic pO2 level.116 Interestingly, expression of the key myogenic transcription factor MyoD is only transiently repressed at 0.5–2% O2 and gradually recovers even when the myogenic precursor C2C12 cells are continuously maintained under hypoxic conditions. This observation suggests that myogenic differentiation, in contrast to adipogenic differentiation, can adapt to persistent or chronic hypoxia.116 The mechanisms underlying hypoxia adaptation remain to be fully understood.
There have been conflicting reports regarding the mechanisms of hypoxic inhibition of myogenesis. Yun et al.116 have shown that ectopic expression of constitutively active HIF-1α does not affect myogenic differentiation under normoxia or hypoxia, suggesting that the HIF pathway does not play a significant role in the regulation of myogenic differentiation. Nonetheless, Gustafsson et al.115 have reported that HIF-1α is involved in inhibition of myogenic differentiation via interaction with the Notch intracellular domain (NICD) and subsequently activation of Notch-regulated genes. In contrast, Yun et al.116 have found that expression of Notch family genes, Notch1, Notch2 and Notch3, is decreased at <0.01% O2, but is insignificantly affected at 0.5–2% O2, compared to the ambient (21% O2) culture condition. Furthermore, pharmacological inhibition of Notch signaling by N-[N-(3,5-difluorophenylacetyl-L-alanyl)]-S-phenylglycine t-butylester (DAPT), a specific γ-secretase, has no significant effect on myogenic differentiation of C2C12 myoblasts under either normoxic or hypoxic conditions.116
A study by Sun et al.117 may have provided a clue about the mechanism of hypoxic regulation of myogenic differentiation. Mice homozygous-null for Stra13 (BHLHB2 or DEC1) have defective muscle regeneration with degenerated myotube formation. Primary Stra13−/− myoblasts show elevated Notch activity, increased proliferation, and defective differentiation.117 Interestingly, Stra13 can be co-immunoprecipitated with NICD, and ectopic expression of Stra13 rescues the Notch-dependent inhibition of myogenesis.117 Because Stra13 is a HIF target gene,113, 118, 119 the genetic study by Sun et al. does not support the HIF-dependent activation of Notch as reported by Gustafsson et al. Since myogenic differentiation is only transiently inhibited by moderate hypoxia,116 it is tempting to hypothesize that the transient inhibition of myogenesis might be mediated by the HIF-Notch interaction; whereas the recovery of myogenic differentiation under prolonged hypoxia may potentially be mediated by the inhibition of Notch signaling by the hypoxia-induced gene Stra13.
As discussed above, hypoxia clearly has a profound impact on differentiation of mesenchymal stem cells. Tumor hypoxia undoubtedly plays a significant role in the composition of tumor-associated stromal cells and the evolution of tumor stroma. It is conceivable that hypoxic tumor stroma is enriched in undifferentiated stromal cells, which may provide a favorable microenvironment for maintaining tumor cells in a stem cell state (Figure 2).
Figure 2. Hypoxia Facilitates the Evolution of Cancer Stem Cell Niche.
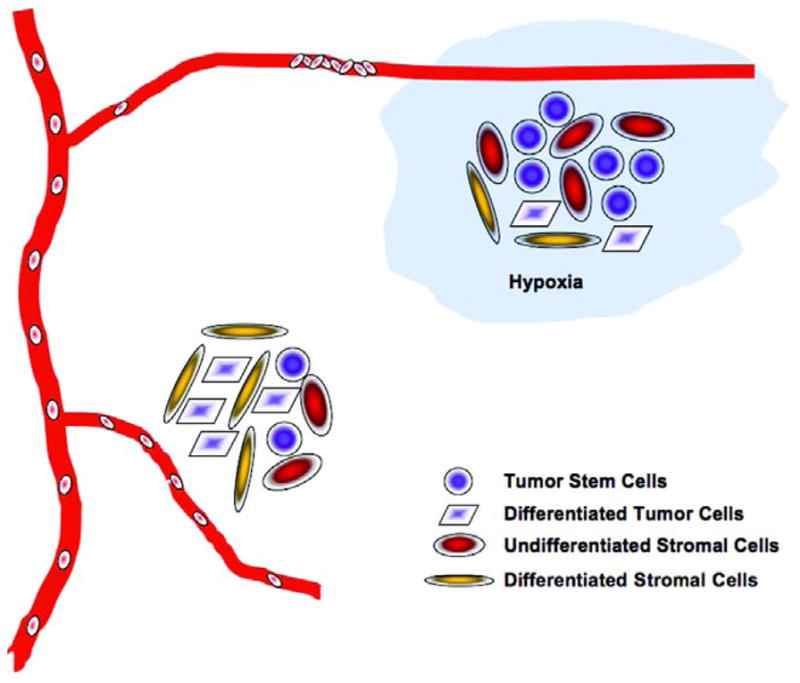
Tumor hypoxia develops mainly due to the abnormal structures and functions of tumor-associated blood vessels. Three potential mechanisms by which hypoxia regulates maintenance and differentiation of cancer stem cells can be envisioned. First, hypoxia directly prevents cancer stem cells from undergoing differentiation by activating intracellular stem cell pathways. Second, hypoxia maintains niche stromal cells in an undifferentiated state to support the self-renewing cancer stem cells. Third, hypoxia may potentially regulate the expression of paracrine factors by endothelial cells for the maintenance of cancer stem cells located in the periendothelial niche.
A recent study has shown that human glioblastoma cells expressing a subset of stem cell genes can be found in the hypoxic perinecrotic regions with positive immunostaining for both HIF-1α and HIF-2α.93 However, brain cancer stem cells can also be identified in perivascular zones.93, 120, 121 Similarly, some immature neural crest-like neuroblastoma cells are reportedly found in a perivascular niche.56 These latter findings seem to argue against the notion that stem cell niches are hypoxic; however, in vitro co-culture assays have shown that endothelial cells promote growth or self-renewal of cancer stem cells, at least in part, via endothelial cell-derived paracrine factors.120 This result suggests that endothelial cells directly regulate cancer stem cells independent of their blood-carrying or other vascular functions. As is widely known, tumor-associated blood vessels are often structurally and functionally abnormal, which results in tumor hypoxia.122, 123 Consistent with this notion, some of the perivascularly located immature neuroblastoma cells express high levels of HIF-2α.56 It will be interesting to determine whether cancer stem cells are preferentially associated with endothelial cells in hypoxic regions.
As suggested by current observations, cancer stem cells may exist in two types of niches: the hypoxic niche located distally from the functional blood vessels and the perivascular niche that may or may not be hypoxic. Nonetheless, there are at least three potential mechanisms by which tumor hypoxia may regulate maintenance and differentiation of cancer stem cells (Figure 2). First, hypoxia directly prevents cancer stem cells from undergoing differentiation. Second, hypoxia inhibits differentiation of niche stromal cells and maintains them in an undifferentiated state. Third, hypoxia may potentially regulate the expression of paracrine factors by endothelial cells for the maintenance of cancer stem cells located in the periendothelial niche.
Implications in Cancer Therapy
Stem cells seem to have superior survival potential. Among different subpopulations of hematopoietic stem cells, the most immature hematopoietic stem cells are more resistant to radiation than partially differentiated stem cells.124 These immature stem cells seem to have robust repair capabilities, which may offer a partial explanation about therapy resistance by stem/progenitor cells. Similarly, as shown recently by Bao et al.125, CD133+ tumor stem cells isolated from both human glioma xenografts and clinical glioblastoma tumors display more robustly activated DNA damage checkpoints in response to ionizing radiation and repair radiation-induced DNA damage more effectively than CD133− tumor cells isolated from the same tumors. These findings indicate that poorly differentiated tumor cells or cancer stem cells are likely to be the source of resistance to conventional therapies such as radiotherapy and chemotherapy. Therefore, targeting cancer stem cells would offer a potentially highly effective approach for cancer therapy.
Despite the close similitude between cancer stem cells and normal stem cells in their gene expression profiles, it remains challenging to identify a marker that is specific to cancer stem cells only. Nonetheless, the distinctive tumor microenvironment, especially hypoxia, may offer unique opportunities for potentially effective targeting of cancer stem cells. As discussed above, HIF-α proteins, especially HIF-2α, are capable of facilitating cancer stem cell maintenance and expansion.57, 58, 93 Inhibitors of the HIF pathway126–128 would have the potential to decrease the self-renewal of cancer stem cells and sensitize them to undergo differentiation. This approach is likely to be useful for targeting both cancer stem cells located in the hypoxic regions and those HIF-α+ cancer stem cells residing around blood vessels.
Targeting the tumor microenvironment would be another promising strategy for effective control of cancer stem cells. Anti-angiogenesis compounds can strongly reduce tumor blood supply if tumor vasculature is severely destroyed, but can also improve tumor blood flow and oxygenation with partial vascular destruction.129, 130 Because at least a subpopulation of cancer stem cells resides in perivascular niches93, 120, 121 and endothelial cell-derived paracrine factors can promote cancer stem cell maintenance,120 ablation of blood vessels would be detrimental to the perivascularly localized cancer stem cells. Furthermore, improved oxygenation after vascular normalization would have the potential to reduce the expression of HIF-α proteins in cancer stem cells located in the hypoxic regions, thus rendering the niche environment permissive for these cancer stem cells to undergo differentiation. Another potential approach for controlling cancer stem cells is to therapeutically target the tumor-associated stromal cells. Altering the composition or differentiation of tumor-associated stromal cells would likely change the cancer stem cell niche from a microenvironment that supports stem cell maintenance to one that facilitates cell differentiation or loss of self-renewal. However, different types of cancer stem cell niches may exist in a typical solid tumor due to the heterogeneity in the tumor microenvironment. Effective control of tumor growth and improvement of disease-free survival will have to rely on a combination of therapeutic approaches that collectively targets different molecular pathways in both cancer stem cells and stromal cells located in different depots of tumor microenvironment.
Acknowledgments
The authors thank the present and past members of the Yun Laboratory for their contribution to the herein referenced work and Lisa Cabral for her excellent editorial assistance. ZY is partly supported by R01CA125021 and R01CA148996 from the National Institutes of Health.
Footnotes
Disclosure: The authors claim no conflicts of interest.
References
- 1.Beerenwinkel N, Antal T, Dingli D, Traulsen A, Kinzler KW, Velculescu VE, et al. Genetic progression and the waiting time to cancer. PLoS Comput Biol. 2007;3:e225. doi: 10.1371/journal.pcbi.0030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones S, Chen WD, Parmigiani G, Diehl F, Beerenwinkel N, Antal T, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A. 2008;105:4283–8. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–99. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 6.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 9.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–5. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 10.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–21. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 11.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. [PubMed] [Google Scholar]
- 12.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 13.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho MM, Ng AV, Lam S, Hung JY. Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res. 2007;67:4827–33. doi: 10.1158/0008-5472.CAN-06-3557. [DOI] [PubMed] [Google Scholar]
- 15.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–14. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 17.Campbell LL, Polyak K. Breast tumor heterogeneity: cancer stem cells or clonal evolution? Cell Cycle. 2007;6:2332–8. doi: 10.4161/cc.6.19.4914. [DOI] [PubMed] [Google Scholar]
- 18.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature reviews. 2008;8:755–68. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 19.Andrews PW. From teratocarcinomas to embryonic stem cells. Philos Trans R Soc Lond B Biol Sci. 2002;357:405–17. doi: 10.1098/rstb.2002.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer cell. 2006;9:157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 22.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nature reviews. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 23.Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer; the polycomb connection. Cell. 2004;118:409–18. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–78. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 25.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–5. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 26.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 27.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 28.Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco-regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol. 2004;43:396–403. doi: 10.1080/02841860410026189. [DOI] [PubMed] [Google Scholar]
- 29.Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, et al. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996;56:941–3. [PubMed] [Google Scholar]
- 30.Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–7. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 31.Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–15. [PubMed] [Google Scholar]
- 32.Young SD, Marshall RS, Hill RP. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A. 1988;85:9533–7. doi: 10.1073/pnas.85.24.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans SM, Hahn SM, Magarelli DP, Koch CJ. Hypoxic heterogeneity in human tumors: EF5 binding, vasculature, necrosis, and proliferation. Am J Clin Oncol. 2001;24:467–72. doi: 10.1097/00000421-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Wartenberg M, Ling FC, Muschen M, Klein F, Acker H, Gassmann M, et al. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J. 2003;17:503–5. doi: 10.1096/fj.02-0358fje. [DOI] [PubMed] [Google Scholar]
- 35.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–94. [PubMed] [Google Scholar]
- 36.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 37.Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol. 2000;76:589–605. doi: 10.1080/095530000138259. [DOI] [PubMed] [Google Scholar]
- 38.Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20:237–50. doi: 10.1023/a:1022939318102. [DOI] [PubMed] [Google Scholar]
- 39.Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P, et al. HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell. 2005;17:793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Mihaylova VT, Bindra RS, Yuan J, Campisi D, Narayanan L, Jensen R, et al. Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol Cell Biol. 2003;23:3265–73. doi: 10.1128/MCB.23.9.3265-3273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, et al. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- 42.Bindra RS, Schaffer PJ, Meng A, Woo J, Maseide K, Roth ME, et al. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24:8504–18. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan J, Narayanan L, Rockwell S, Glazer PM. Diminished DNA repair and elevated mutagenesis in mammalian cells exposed to hypoxia and low pH. Cancer Res. 2000;60:4372–6. [PubMed] [Google Scholar]
- 44.Coquelle A, Toledo F, Stern S, Bieth A, Debatisse M. A new role for hypoxia in tumor progression: induction of fragile site triggering genomic rearrangements and formation of complex DMs and HSRs. Mol Cell. 1998;2:259–65. doi: 10.1016/s1097-2765(00)80137-9. [DOI] [PubMed] [Google Scholar]
- 45.Semenza GL. Targeting HIF-1 for cancer therapy. Nature reviews. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 46.Giaccia AJ, Simon MC, Johnson R. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004;18:2183–94. doi: 10.1101/gad.1243304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol Cell Biol. 2006;26:3514–26. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nature reviews. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 49.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 50.Aebersold DM, Burri P, Beer KT, Laissue J, Djonov V, Greiner RH, et al. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–6. [PubMed] [Google Scholar]
- 51.Burri P, Djonov V, Aebersold DM, Lindel K, Studer U, Altermatt HJ, et al. Significant correlation of hypoxia-inducible factor-1alpha with treatment outcome in cervical cancer treated with radical radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:494–501. doi: 10.1016/s0360-3016(02)04579-0. [DOI] [PubMed] [Google Scholar]
- 52.Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer cell. 2006;10:413–23. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 53.Couvelard A, O’Toole D, Turley H, Leek R, Sauvanet A, Degott C, et al. Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer. 2005;92:94–101. doi: 10.1038/sj.bjc.6602245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 55.Acker T, Diez-Juan A, Aragones J, Tjwa M, Brusselmans K, Moons L, et al. Genetic evidence for a tumor suppressor role of HIF-2alpha. Cancer cell. 2005;8:131–41. doi: 10.1016/j.ccr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 56.Pietras A, Gisselsson D, Ora I, Noguera R, Beckman S, Navarro S, et al. High levels of HIF-2alpha highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche. J Pathol. 2008;214:482–8. doi: 10.1002/path.2304. [DOI] [PubMed] [Google Scholar]
- 57.Pietras A, Hansford LM, Johnsson AS, Bridges E, Sjolund J, Gisselsson D, et al. HIF-2alpha maintains an undifferentiated state in neural crest-like human neuroblastoma tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:16805–10. doi: 10.1073/pnas.0904606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer cell. 2009;15:501–13. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim Y, Lin Q, Zelterman D, Yun Z. Hypoxia-Regulated Delta-like 1 Homologue Enhances Cancer Cell Stemness and Tumorigenicity. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Floridon C, Jensen CH, Thorsen P, Nielsen O, Sunde L, Westergaard JG, et al. Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? A study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation. Differentiation. 2000;66:49–59. doi: 10.1046/j.1432-0436.2000.066001049.x. [DOI] [PubMed] [Google Scholar]
- 61.Jensen CH, Krogh TN, Hojrup P, Clausen PP, Skjodt K, Larsson LI, et al. Protein structure of fetal antigen 1 (FA1). A novel circulating human epidermal-growth-factor-like protein expressed in neuroendocrine tumors and its relation to the gene products of dlk and pG2. Eur J Biochem. 1994;225:83–92. doi: 10.1111/j.1432-1033.1994.00083.x. [DOI] [PubMed] [Google Scholar]
- 62.Tornehave D, Jensen CH, Teisner B, Larsson LI. FA1 immunoreactivity in endocrine tumours and during development of the human fetal pancreas; negative correlation with glucagon expression. Histochem Cell Biol. 1996;106:535–42. doi: 10.1007/BF02473268. [DOI] [PubMed] [Google Scholar]
- 63.Yin D, Xie D, Sakajiri S, Miller CW, Zhu H, Popoviciu ML, et al. DLK1: increased expression in gliomas and associated with oncogenic activities. Oncogene. 2006;25:1852–61. doi: 10.1038/sj.onc.1209219. [DOI] [PubMed] [Google Scholar]
- 64.Sakajiri S, O’Kelly J, Yin D, Miller CW, Hofmann WK, Oshimi K, et al. Dlk1 in normal and abnormal hematopoiesis. Leukemia. 2005;19:1404–10. doi: 10.1038/sj.leu.2403832. [DOI] [PubMed] [Google Scholar]
- 65.Van Limpt VA, Chan AJ, Van Sluis PG, Caron HN, Van Noesel CJ, Versteeg R. High delta-like 1 expression in a subset of neuroblastoma cell lines corresponds to a differentiated chromaffin cell type. Int J Cancer. 2003;105:61–9. doi: 10.1002/ijc.11047. [DOI] [PubMed] [Google Scholar]
- 66.Li L, Forman SJ, Bhatia R. Expression of DLK1 in hematopoietic cells results in inhibition of differentiation and proliferation. Oncogene. 2005;24:4472–6. doi: 10.1038/sj.onc.1208637. [DOI] [PubMed] [Google Scholar]
- 67.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 68.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–8. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 69.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 70.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 71.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 72.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–11. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 73.Zagzag D, Krishnamachary B, Yee H, Okuyama H, Chiriboga L, Ali MA, et al. Stromal cell-derived factor-1alpha and CXCR4 expression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel-Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res. 2005;65:6178–88. doi: 10.1158/0008-5472.CAN-04-4406. [DOI] [PubMed] [Google Scholar]
- 74.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS ONE. 2007;2:e416. doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Richard CL, Blay J. CXCR4 in Cancer and Its Regulation by PPARgamma. PPAR Res. 2008;2008:769413. doi: 10.1155/2008/769413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 78.Salvucci O, Bouchard A, Baccarelli A, Deschenes J, Sauter G, Simon R, et al. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006;97:275–83. doi: 10.1007/s10549-005-9121-8. [DOI] [PubMed] [Google Scholar]
- 79.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–12. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 80.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. The chemokine receptor CXCR4 is required for outgrowth of colon carcinoma micrometastases. Cancer Res. 2003;63:3833–9. [PubMed] [Google Scholar]
- 81.Hermann PC, Huber SL, Herrler T, Alexandra Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell stem cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Meissner A, Wernig M, Jaenisch R. Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol. 2007;25:1177–81. doi: 10.1038/nbt1335. [DOI] [PubMed] [Google Scholar]
- 83.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 84.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 85.Cheng L. Establishing a germ cell origin for metastatic tumors using OCT4 immunohistochemistry. Cancer. 2004;101:2006–10. doi: 10.1002/cncr.20566. [DOI] [PubMed] [Google Scholar]
- 86.Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer cell. 2003;4:361–70. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- 87.Jones TD, Ulbright TM, Eble JN, Cheng L. OCT4: A sensitive and specific biomarker for intratubular germ cell neoplasia of the testis. Clin Cancer Res. 2004;10:8544–7. doi: 10.1158/1078-0432.CCR-04-0688. [DOI] [PubMed] [Google Scholar]
- 88.Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26:495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 89.Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–77. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 90.Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–70. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forristal CE, Wright KL, Hanley NA, Oreffo RO, Houghton FD. Hypoxia inducible factors regulate pluripotency and proliferation in human embryonic stem cells cultured at reduced oxygen tensions. Reproduction. 2010;139:85–97. doi: 10.1530/REP-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Griguer CE, Oliva CR, Gobin E, Marcorelles P, Benos DJ, Lancaster JR, Jr, et al. CD133 is a marker of bioenergetic stress in human glioma. PLoS One. 2008;3:e3655. doi: 10.1371/journal.pone.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seidel S, Garvalov BK, Wirta V, von Stechow L, Schanzer A, Meletis K, et al. A hypoxic niche regulates glioblastoma stem cells through hypoxia inducible factor 2{alpha} Brain. 2010;133:983–95. doi: 10.1093/brain/awq042. [DOI] [PubMed] [Google Scholar]
- 94.Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009 doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- 95.Matsumoto K, Arao T, Tanaka K, Kaneda H, Kudo K, Fujita Y, et al. mTOR signal and hypoxia-inducible factor-1 alpha regulate CD133 expression in cancer cells. Cancer Res. 2009;69:7160–4. doi: 10.1158/0008-5472.CAN-09-1289. [DOI] [PubMed] [Google Scholar]
- 96.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–64. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 97.Corral DA, Amling M, Priemel M, Loyer E, Fuchs S, Ducy P, et al. Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc Natl Acad Sci U S A. 1998;95:13835–40. doi: 10.1073/pnas.95.23.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 99.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 100.Zhang CC, Kaba M, Ge G, Xie K, Tong W, Hug C, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–5. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–7. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 103.Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev. 2001;11:54–9. doi: 10.1016/s0959-437x(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 104.Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 106.Pine SR, Ryan BM, Varticovski L, Robles AI, Harris CC. Microenvironmental modulation of asymmetric cell division in human lung cancer cells. Proc Natl Acad Sci U S A. 2010;107:2195–200. doi: 10.1073/pnas.0909390107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 108.Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M, Marini F. Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol. 2007;86:8–16. doi: 10.1532/IJH97.06230. [DOI] [PubMed] [Google Scholar]
- 109.LaRue AC, Masuya M, Ebihara Y, Fleming PA, Visconti RP, Minamiguchi H, et al. Hematopoietic origins of fibroblasts: I. In vivo studies of fibroblasts associated with solid tumors. Exp Hematol. 2006;34:208–18. doi: 10.1016/j.exphem.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 110.Kim KH, Song MJ, Chung J, Park H, Kim JB. Hypoxia inhibits adipocyte differentiation in a HDAC-independent manner. Biochem Biophys Res Commun. 2005;333:1178–84. doi: 10.1016/j.bbrc.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 111.Sahai A, Patel MS, Zavosh AS, Tannen RL. Chronic hypoxia impairs the differentiation of 3T3-L1 fibroblast in culture: role of sustained protein kinase C activation. J Cell Physiol. 1994;160:107–12. doi: 10.1002/jcp.1041600113. [DOI] [PubMed] [Google Scholar]
- 112.Lin Q, Lee YJ, Yun Z. Differentiation arrest by hypoxia. J Biol Chem. 2006;281:30678–83. doi: 10.1074/jbc.C600120200. [DOI] [PubMed] [Google Scholar]
- 113.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPARg2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–41. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 114.Zhou S, Lechpammer S, Greenberger JS, Glowacki J. Hypoxia inhibition of adipocytogenesis in human bone marrow stromal cells requires transforming growth factor-beta/Smad3 signaling. J Biol Chem. 2005;280:22688–96. doi: 10.1074/jbc.M412953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–28. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 116.Yun Z, Lin Q, Giaccia AJ. Adaptive myogenesis under hypoxia. Mol Cell Biol. 2005;25:3040–55. doi: 10.1128/MCB.25.8.3040-3055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun H, Li L, Vercherat C, Gulbagci NT, Acharjee S, Li J, et al. Stra13 regulates satellite cell activation by antagonizing Notch signaling. J Cell Biol. 2007;177:647–57. doi: 10.1083/jcb.200609007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miyazaki K, Kawamoto T, Tanimoto K, Nishiyama M, Honda H, Kato Y. Identification of functional hypoxia response elements in the promoter region of the DEC1 and DEC2 genes. J Biol Chem. 2002;277:47014–21. doi: 10.1074/jbc.M204938200. [DOI] [PubMed] [Google Scholar]
- 119.Wykoff CC, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Identification of novel hypoxia dependent and independent target genes of the von Hippel-Lindau (VHL) tumour suppressor by mRNA differential expression profiling. Oncogene. 2000;19:6297–305. doi: 10.1038/sj.onc.1204012. [DOI] [PubMed] [Google Scholar]
- 120.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 121.Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22:436–48. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–16. [PubMed] [Google Scholar]
- 123.Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol. 2004;14:198–206. doi: 10.1016/j.semradonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 124.Wagemaker G. Heterogeneity of radiation sensitivity of hemopoietic stem cell subsets. Stem Cells. 1995;13 (Suppl 1):257–60. doi: 10.1002/stem.5530130731. [DOI] [PubMed] [Google Scholar]
- 125.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 126.Onnis B, Rapisarda A, Melillo G. Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med. 2009;13:2780–6. doi: 10.1111/j.1582-4934.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Semenza GL. HIF-1 inhibitors for cancer therapy: from gene expression to drug discovery. Curr Pharm Des. 2009;15:3839–43. doi: 10.2174/138161209789649402. [DOI] [PubMed] [Google Scholar]
- 128.Dimova EY, Michiels C, Kietzmann T. Kinases as upstream regulators of the HIF system: their emerging potential as anti-cancer drug targets. Curr Pharm Des. 2009;15:3867–77. doi: 10.2174/138161209789649358. [DOI] [PubMed] [Google Scholar]
- 129.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009;6:395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 130.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]


