Abstract
Objectives
To determine pediatric reference ranges for zinc protoporphyrin (ZPP).
Design and Methods
The study was conducted in a large pediatric hospital on patient blood specimens (n = 6,478) (0 – 17 y) accrued from January 2001 to June 2002. The data was analyzed employing the Hoffman approach, which was computer adapted.
Results and Conclusions
The 2.5th and 97.5th percentiles for children age 0 to 12 months were 9 to 40 μg/dL (16.6 –73.6 μmol/mol heme) for female subjects and 8.5 to 34.5 μg/dL (15.6 – 63.5 μmol/mol heme) for males. The 97.5th percentiles decreased for the 13 to 24 months age group for females (32 μg/dL) (58.9 μmol/mol heme). There was a significant decrease in the 97.5th percentile for zinc protoporphyrin (ZPP) concentrations for the 5 to 9 yr age group, the 97.5th percentile being 30 μg/dL (55.2 μmol/mol heme) in both genders, which increased to 33.5 μg/dL (61.6 μmol/mol heme) in the 10 to 17 yr female age group but not for the males (31.5 μg/dL) (58.0 μmol/mol heme). The highest medians were 25.5 μg/dL (46.9 μmol/mol heme) for females, and 21.5 μg/dL (39.6 μmol/mol heme) for males in the 0 to 12 months age group.
Keywords: Reference values, Human, Adolescent, Environmental exposure, Environmental monitoring, Iron deficiency anemia, Lead poisoning
1. Introduction
Heme is synthesized in large amounts by the bone marrow where it is incorporated into hemoglobin, and by the liver, where most of it is incorporated into cytochromes. Protoporphyrin is the immediate precursor of the heme molecule requiring iron for the final step of heme synthesis. When plasma iron concentrations decrease, erythrocyte protoporphyrin accumulates and increased ZPP and free erythrocyte protoporphyrin (FEP) concentrations may be measured [1]. Increased blood ZPP and FEP concentrations are not specific; they can occur with iron deficiency, lead poisoning, many erythrocyte disorders, any autosomal recessive porphyria, and sometimes autosomal dominant acute porphyrias.
ZPP and FEP determinations are clinically useful in classifying microcytic red blood cells caused by disorders of heme synthesis or by disorders of globin synthesis [2]. In the assessment of iron status, the ratio of ZPP or FEP and heme are calculated, as it correlates well with values of plasma ferritin, plasma iron, transferrin saturation, hemoglobin and hematocrit, being the more sensitive index of iron status [3–5]. ZPP or FEP are also the differential tests for the three most common porphyrias and erythropoietic protoporphyria, which is due to an enzyme deficiency of the heme biosynthetic pathway. ZPP and FEP tests may be useful in situations in which the diagnosis of beta thalassemia minor is unclear; ZPP and FEP values are within the normal range in patients with the beta thalassemia trait, but are elevated in patients with iron deficiency or lead poisoning [6,7]. Elevated ZPP and FEP concentrations are frequently found in patients with advanced chronic hepatitis C infection [8]. ZPP and FEP can be used in the clinical laboratory as a preliminary, usually out of hours, screening test to evaluate the need for a blood lead measurement and the possibility of lead poisoning in children. [9,10]. ZPP and FEP are not equivalent, the minor nonheme porphyrins in healthy erythrocytes consist of ZPP (approximately 95%) and FEP (approximately 5%). In certain diseases these ratios can be significantly altered [1,11].
We embarked on this study to determine the reference ranges for ZPP and so complement previous studies conducted by our group [12–19]. To have specific reference values for blood ZPP concentrations for both genders in the specific age groups, we calculated the pediatric reference ranges for ZPP for age groups 0 to 12 months, 13 to 24 months, 2 to 5y, 6 to 9y, and 10 to 17 yr old separately for female subjects and for male subjects.
2. Materials and methods
2.1. Patients and sample collection
ZPP levels were determined during routine clinical chemistry testing of whole blood samples. The samples were obtained between January 1, 2001 and June 8, 2002 from a population of outpatient and hospitalized children, which included 3,259 male and 3,219 female patients newborn to 17 yr. (Table 1) 56.6% of females and 59.1% of the males were in the 2 to 5 yr age group. Only 1.9% of females and 1.9% (n = 61 each) of the males were in the 10 to 17 yr age group. Although the numbers in each group are large, this gives more weight to the larger groups and less to the 10 to 17 yr groups.
Table 1.
ZPP demographics for females and males age 0–17 years
| Age Groups Females |
Sample Size | % of Females | % of Total Patients |
|---|---|---|---|
| 0–12 months | 203 | 6.31 | 3.1 |
| 13–24 months | 725 | 22.52 | 11.1 |
| 2–5 years | 1,822 | 56.60 | 28.1 |
| 6–9 years | 408 | 12.67 | 6.2 |
| 10–17 years | 61 | 1.89 | 0.9 |
| Total | 3,219 | 100% | 49.6 |
| Males | Sample Size | % of Males | % of Total Patients |
| 0–12 months | 145 | 4.44 | 2.2 |
| 13–24 months | 605 | 18.56 | 9.3 |
| 2–5 years | 1,926 | 59.09 | 29.7 |
| 6–9 years | 522 | 16.01 | 8.0 |
| 10–17 years | 61 | 1.87 | 0.9 |
| Total | 3,259 | 100% | 50.4 |
The samples were identified only by age and gender. The specimens used for ZPP testing were kept refrigerated for no longer than 96 h at 2° to 4°C. Children known to have conditions affecting ZPP levels were not excluded from the study, as the analytic method for calculating the reference ranges eliminated abnormal or outlier values resulting from such conditions.
2.2. ZPP assay
ZPP concentrations were measured in 50 μL anticoagulated whole blood samples containing either heparin or EDTA using a zinc protoporphyrin hematofluorometer (ZP Hematofluorometer Model 206, AVIV Biomedical Inc., Lakewood, NJ). After calibration of the instrument a drop of blood is placed on the slide. The excitation wavelength is 415 nm and the measurement wavelength is 596 nm. The average of three or more hematofluorometer readings are reported for each specimen, and readings over 50 mg/dL (92 μmol/mol heme) are corrected for the patient’s hematocrit. Appropriate control samples at the low, medium, and high levels were run after every tenth sample. The results were reported as μg/dL and converted to μmol/mol heme by multiplying by a factor of 1.84. This latter conversion makes the assumption that the hemoglobin concentration is 14 g/dL and hence may introduce an error into the results reported. Nevertheless, it is the recommended NCCLS unit.
2.3. Statistical analysis
ZPP values, reported in μg/dL and in μmol/mol heme, and child age and gender were entered into a Microsoft Excel spreadsheet. The data sets were separated by gender, and stratified by age. Abnormal and outlier values were truncated from each individual age category according to the Hoffman method [20]. Generally, the top and bottom 10% of the data was discarded and a line drawn to the central linear portion of the graph. The remaining data, which was of normal Gaussain distribution, was used to calculate the 2.5th and 97.5th percentiles for each of the age groups. We plotted percent cumulative frequency vs. ZPP concentration on percent cumulative frequency paper so that the 2.5th and 97.5th percentiles could be calculated. These were used as the final reported reference ranges.
3. Results
The results in Table 2 display the 2.5th and 97.5th percentile ZPP levels for males ages 0 to 17 yr and for females age 0 to 17 yr.
Table 2.
Pediatric reference ranges of ZPP blood concentrations (2.5th–97.5th percentiles μg/dL) for females and males age 0–17 years
| Age Groups | Blood ZPP Reference Ranges 2.5th–97.5th percentiles (μg/dL)*
|
Blood ZPP Reference Ranges 2.5th–97.5th percentiles (μmol/mol heme)
|
n | |||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| 0–12 months | 8.5–34.5 | 9–40 | 15.6–63.5 | 16.6–73.6 | 145 | 203 |
| 13–24 months | 10–34 | 11–32 | 18.4–62.6 | 20.2–58.9 | 605 | 725 |
| 2–5 years | 5–35 | 10–31 | 9.2–64.4 | 18.4–57.0 | 1,926 | 1,822 |
| 6–9 years | 6–31 | 9–30 | 11.0–57.0 | 16.6–55.2 | 522 | 408 |
| 10–17 years | 5.5–31.5 | 5–33.5 | 10.1–58.0 | 9.2–61.6 | 61 | 61 |
| Total | 3,259 | 3,219 | ||||
Multiply μg/dL by 1.83 to convert to μmol/mol heme.
For male subjects, the ZPP values ranged from 5 to 35 μg/dL, while the ZPP values ranged from 5 to 40 μg/dL in female subjects. A general decrease in the ZPP values with increasing age was evident in both the male and female subjects for the 2.5th, 50th, and 97.5th percentiles as well as the geometric mean ZPP values for each age group.
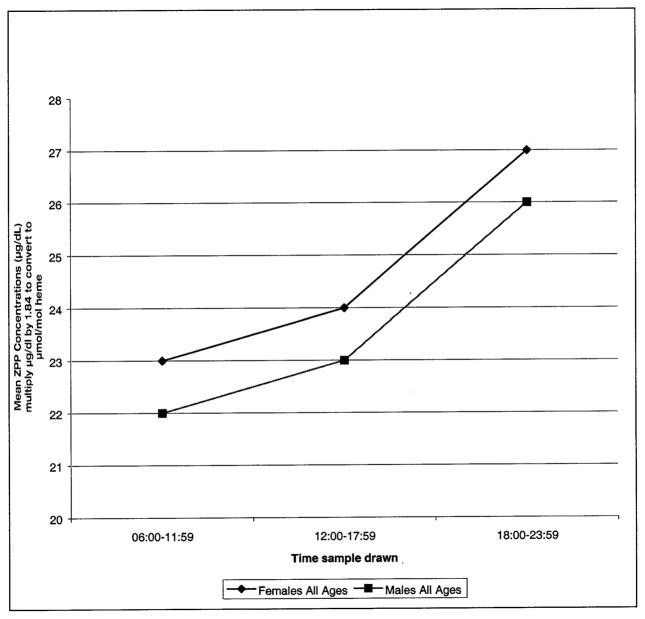
Diurnal variation was evident for both females and males, and for all age groups, ZPP concentrations being higher between 18:00 to 23:59. (Table 4) (Figure 1).
Table 4.
Diurnal effects on ZPP blood concentrations males and females age 0 to 17 years
| Time | ZPP Geometric Means (μg/dL) (μmol/mol heme)*
|
|||||
|---|---|---|---|---|---|---|
| 0–12m | 13–24m | 2–5y | 6–9y | 10–17y | All ages | |
| Females | ||||||
| 06:00–11:59 | 29 (53.4) | 26 (47.8) | 22 (40.5) | 20.5 (37.7) | 28 (51.5) | 23 (42.3) |
| 12:00–17:59 | 27 (49.7) | 26 (47.8) | 24 (44.2) | 23 (42.3) | 21 (38.6) | 24 (44.2) |
| 18:00–23:59 | 30 (55.2) | 27 (49.7) | 27 (49.7) | 25 (46.0) | 22 (40.5) | 27 (49.7) |
| Males | ||||||
| 06:00–11:59 | 25 (46.0) | 25 (46.0) | 23 (42.3) | 20 (36.8) | 20.5 (37.7) | 22 (40.5) |
| 12:00–17:59 | 26 (47.8) | 25 (46.0) | 23 (42.3) | 21 (38.6) | 20 (36.8) | 23 (42.3) |
| 18:00–23:59 | 26 (47.8) | 27 (49.7) | 26 (47.8) | 23 (42.3) | 24 (44.2) | 26 (47.8) |
Multiply μg/dL by 1.83 to convert to μmol/mol heme
Fig. 1.
Diurnal effects on ZPP blood concentrations for females and males of ages 0 to 17 years.
4. Discussion
Low ZPP and FEP values have no known clinical significance. Therefore, the focus of interest should be on the upper limit of the reference interval. Age and sex-related values for ZPP are not available in the scientific literature, although The Second National Health and Nutrition Examination Survey (NHANES II) conducted in the late 1970s does provide FEP ranges for children 1 to 5 yr, and adults 18 to 44 yr [21].
The present study summarizes data on 3,219 female and 3,259 male infants and children 0 to 17 yr screened for zinc protoporphyrin concentrations between January 2000 and June 2002 in the Greater Washington D.C. area and determines reference ranges for zinc protoporphyrin separately for females and for males, stratified in five age groups. These pediatric reference ranges are important in determining the normal ranges for ZPP, for the urgent determination of possible lead poisoning, for the diagnosis of iron deficiency anemia and the diagnosis of other diseases.
ZPPs are heme precursors that accumulate in iron deficiency and in lead poisoning. Lead inhibits iron binding to the heme molecule (by interfering with the activity of the enzymes δ-aminolevulinic dehydratase (ALA) and ferro-chelatase) leading to ZPP accumulation [22,23]. A significant positive correlation was demonstrated between blood-lead concentrations >20 μg/dL (36.8 μmol/mol heme) and ZPP concentrations [24–26]. ZPP is not present as free type protoporphyrin (FPP) but rather is chelated with zinc (ZPP) in lead poisoning and iron deficiency anemia [27]. The ratio of ZPP to FPP in erythrocytes decreases with the progress of lead intoxication, the amount of FPP being greater than that of ZPP in the severe stage of intoxication.
ZPP assay sensitivity decreases at lower blood lead concentrations. This is of concern because even 10 to 20 μg/dL (8.4–36.8 μmol/mol heme) blood lead concentrations can cause significant pediatric neurologic impairments. The ZPP assay is now used as an added evaluation for lead exposure, direct lead testing being the prime measure of lead toxicity. ZPP is also used, together with other tests, to assess longer-term effects of lead exposure and the effectiveness of chelation therapy [28].
The geometric means for ZPP for all females was 24.3 μg/dL (95% Confidence Interval (CI) 23.8 to 24.8) or (44.7 μmol/mol heme; CI 43.8– 45.6 μmol/mol heme), similarly the geometric mean for all males was 24.1 μg/dL (CI 23.8–24.4) or (44.3 μmol/mol heme, CI 43.8– 44.9 μmol/mol heme). (Table 3). ZPP geometric means and medians for all age groups decreased with increasing age, the only exception being the 10 to 17y group where the geometric mean increased. In addition, the geometric means and medians were of similar ZPP concentration for each of the age groups (males and females together), the only exception being the 10 to 17y group where the geometric mean was 23.1 μg/dL (CI 21.0–25.3) (42.5 μmol/mol heme; CI 38.6–46.6 μmol/mol heme) and median 21 μg/dL (38.6 μmol/mol heme). A closer look determines the difference is due to the female 10 to 17 y group (n = 37). This age group is the only one that the female geometric mean (25.4 μg/dL CI 21.3–30.4) (46.7 μmol/mol heme; CI 39.2–55.9 μmol/mol heme) is significantly different from the male geometric mean (21.7 μg/dL CI 19.4–24.2 μg/dL) (39.9 μmol/mol heme; CI 35.7– 44.5 μmol/mol heme) (10–17 age group; n = 57), although the medians for both females and males of 10 to 17 yr group were similar 22 μg/dL (40.5 μmol/mol heme) vs. 21 μg/dL (38.6 μmol/mol heme) respectively. The small sample size relative to the other group sizes may denote an inaccuracy to the calculations. ZPP medians for females and males at 0 to 5y are different and have a different rate of decline. After age 5 they stabilize male median concentrations being consistently lower than those of females.
Table 3.
Geometric means for blood ZPP concentrations (μg/dL)* for all females and males, and for age groups 0–17 years
| Gender | Geometric Mean (95% Confidence Interval) (μg/dL) | Geometric Mean (95% Confidence Interval) (μmol/mol heme) | n |
|---|---|---|---|
| Females | 24.3 (23.9–24.8) | 44.7 (44.0–45.6) | 1,812 |
| Males | 24.1 (23.8–24.4) | 44.3 (43.8–44.9) | 3,096 |
| Age Group | |||
| 0–12 months | 26.0 (24.8–27.2) | 47.8 (45.6–50.0) | 185 |
| 13–24 months | 25.8 (25.1–26.5) | 47.5 (46.2–48.8) | 836 |
| 2–4 years | 24.3 (23.9–24.6) | 44.7 (44.0–45.3) | 3,080 |
| 5–9 years | 21.7 (21.2–22.2) | 39.9 (39.0–40.8) | 713 |
| 10–17 years | 23.1 (21.0–25.3) | 42.5 (38.6–46.5) | 94 |
Multiply μg/dL by 1.83 to convert to μmol/mol heme
There appears to be a difference between the females and males within the same age group, with females showing higher levels, possibly a result of iron-deficiency induced by blood loss due to menstruation and possibly a difference in dietary pattern from males [29]. The median ZPP concentrations were similar for the females and males in each of the age groups and declined with increasing age. Infants tend to have higher ZPP levels than older children, the difference likely reflecting immature heme synthesis and increased erythrocyte volume in newborn babies. Other studies noted newborns cord blood FEP levels were higher than the maternal FEP levels [30].
Diurnal variation was evident for both females and males, and for all age groups, ZPP geometric means being higher between 18:00 to 23:59. (Table 4). The trend was similar when females of all ages and males of all ages were compared (Figure. 1). However, one group, females 10 to 17 yr old did not follow the pattern and distinctly had higher ZPP concentrations in the morning hours and lower concentrations from 12:00 to 23:59 (Table 4).
Acknowledgments
The authors would like to thank Dr. John C. Pezzullo, Clinical Pharmacology, Georgetown University for help with some of the statistical analysis. Ms. Maureen Miller’s study was supported by the Colaco Foundation for students of excellence.
Footnotes
All three authors have no conflict of interest.
References
- 1.Marsh WL, Jr, Nelson DP, Koenig HM. Free erythrocyte protoporphyrin (FEP) I. Normal values for adults and evaluation of the hematofluorometer. Am J Clin Pathol. 1983;79(6):655– 60. doi: 10.1093/ajcp/79.6.655. [DOI] [PubMed] [Google Scholar]
- 2.Marsh WL, Jr, Nelson DP, Koenig HM. Free erythrocyte protoporphyrin (FEP) II. The FEP test is clinically useful in classifying microcytic RBC disorders in adults. Am J Clin Pathol. 1983;79(6):661– 6. doi: 10.1093/ajcp/79.6.661. [DOI] [PubMed] [Google Scholar]
- 3.Kitajima H, Kaneko T, Akatsuka J. The measurement of erythrocyte zinc protoporphyrin/heme ratio in various anemias in childhood. Rinsho Ketsueki. 1992;33(9):1199–203. [PubMed] [Google Scholar]
- 4.Lamola AA, Eisinger J, Blumberg WE. Erythrocyte protoporphyrin/heme ratio by hematofluorometry. Clin Chem. 1980;26(5):677– 8. [PubMed] [Google Scholar]
- 5.Labbe RF, Finch CA, Smith NJ, Doan RN, Sood SK, Madan N. Erythrocyte protoporphyrin/heme ratio in the assessment of iron status. Clin Chem. 1979;25(1):87–92. [PubMed] [Google Scholar]
- 6.Stockman JA, 3rd, Weiner LS, Simon GE, Stuart MJ, Oski FA. The measurement of free erythrocyte porphyrin (FEP) as a simple means of distinguishing iron deficiency from beta-thalassemia trait in subjects with microcytosis. J Lab Clin Med. 1975;85(1):113–9. [PubMed] [Google Scholar]
- 7.Takeshita K. Thalassemia, Beta. eMedicine J. 2002;3(2) [Google Scholar]
- 8.Vogeser M, Jacob K, Zachoval R. Erythrocyte protoporphyrins in hepatitis C viral infection. Clin Biochem. 2000;33(5):387–91. doi: 10.1016/s0009-9120(00)00149-1. [DOI] [PubMed] [Google Scholar]
- 9.Piomelli S, Davidow B, Guinee VF, Young P, Gay G. The FEP (free erythrocyte porphyrins) test: a screening micromethod for lead poisoning. Pediatrics. 1973;51(2):254–9. [PubMed] [Google Scholar]
- 10.Davidow B, Slavin G, Piomelli S. Measurement of free erythrocyte protoporphyrin in blood collected on filter paper as a screening test to detect lead poisoning in children. Ann Clin Lab Sci. 1976;6(3):209–13. [PubMed] [Google Scholar]
- 11.Labbe RF, Vreman HJ, Stevenson DK. Zinc protoporphyrin: a metabolite with a mission. Clin Chem. 1999;45(12):2060–72. [PubMed] [Google Scholar]
- 12.Soldin OP, Hanak B, Soldin SJ. Pedaitric Referene Ranges for Blood Lead. Clinica Chimica Acta. 2002 (In Press) [Google Scholar]
- 13.Soldin SJ, Morales A, Albalos F, Lenherr S, Rifai N. Pediatric reference ranges on the Abbott IMx for FSH, LH, prolactin, TSH, T4, T3, free T4, free T3, T-uptake, IgE, and ferritin. Clin Biochem. 1995;28(6):603– 6. doi: 10.1016/0009-9120(95)00038-5. [DOI] [PubMed] [Google Scholar]
- 14.Soldin SJ, Murthy JN, Agarwalla PK, Ojeifo O, Chea J. Pediatric reference ranges for creatine kinase, CKMB, Troponin I, iron, and cortisol. Clin Biochem. 1999;32(1):77– 80. doi: 10.1016/s0009-9120(98)00084-8. [DOI] [PubMed] [Google Scholar]
- 15.Soldin SJ, Brugnara C, Hicks JM. Pediatric Reference Ranges. 3. Washington DC: AACC; 1999. [Google Scholar]
- 16.Quivers ES, Murthy JN, Soldin SJ. The effect of gestational age, birth weight, and disease on troponin I and creatine kinase MB in the first year of life. Clin Biochem. 1999;32(6):419–21. doi: 10.1016/s0009-9120(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 17.Krafte-Jacobs B, Williams J, Soldin SJ. Plasma erythropoietin reference ranges in children. J Pediatr. 1995;126(4):601–3. doi: 10.1016/s0022-3476(95)70360-8. [DOI] [PubMed] [Google Scholar]
- 18.Murthy JN, Hicks JM, Soldin SJ. Evaluation of the Technicon Immuno I random access immunoassay analyzer and calculation of pediatric reference ranges for endocrine tests, T-uptake, and ferritin. Clin Biochem. 1995;28(2):181–5. doi: 10.1016/0009-9120(94)00080-f. [DOI] [PubMed] [Google Scholar]
- 19.Hicks JM, Cook J, Godwin ID, Soldin SJ. Vitamin B12, and folate. Pediatric reference ranges. Arch Pathol Lab Med. 1993;117(7):704– 6. [PubMed] [Google Scholar]
- 20.Hoffman RG. Statistics in the practice of medicine. JAMA. 1963;185:864–73. doi: 10.1001/jama.1963.03060110068020. [DOI] [PubMed] [Google Scholar]
- 21.Yip R, Johnson C, Dallman PR. Age-related changes in laboratory values used in the diagnosis of anemia and iron deficiency. Am J Clin Nutr. 1984;39(3):427–36. doi: 10.1093/ajcn/39.3.427. [DOI] [PubMed] [Google Scholar]
- 22.Calderon Salinas V, Hernandez-Luna C, Maldonado M, Saenz D. Mechanisms of the toxic effect of lead. I. Free lead in erythrocyte. J Expo Anal Environ Epidemiol. 1993;3(Suppl 1):153– 64. [PubMed] [Google Scholar]
- 23.Antonowicz J, Andrzejak R, Smolik R. Influence of heavy metal mixtures on erythrocyte metabolism. Int Arch Occup Environ Health. 1990;62(3):195– 8. doi: 10.1007/BF00379431. [DOI] [PubMed] [Google Scholar]
- 24.Wada Y, Sato S, Yamaguchi T, Katsumi A, Kobayashi M. Relationship between alpha-aminolevulinic acid dehydratase activity, free erythrocyte protoporphyrin concentration and blood lead in calves from lead contaminated farm. Vet Hum Toxicol. 1993;35(5):393–5. [PubMed] [Google Scholar]
- 25.Sun GL. Dose-effect relationships between blood lead and free erythrocyte protoporphyrin or zinc protoporphyrin. Zhonghua Yu Fang Yi Xue Za Zhi. 1989;23(5):279– 82. [PubMed] [Google Scholar]
- 26.Toriumi H, Kawai M. Free erythrocyte protoporphyrin (FEP) in a general population, workers exposed to low-level lead, and organic-solvent workers. Environ Res. 1981;25(2):310– 6. doi: 10.1016/0013-9351(81)90033-5. [DOI] [PubMed] [Google Scholar]
- 27.Lamola AA, Yamane T. Zinc protoporphyrin in the erythrocytes of patients with lead intoxication and iron deficiency anemia. Science. 1974;186(4167):936– 8. doi: 10.1126/science.186.4167.936. [DOI] [PubMed] [Google Scholar]
- 28.Antonowicz J, Andrzejczak R, Kuliczkowski K, Smolik R. Levels of trace elements in the serum and erythrocytes and some parameters of erythrocyte heme metabolism (FEP, ALA-D, ALA-U) in copper foundry workers. Pol J Occup Med. 1991;4(4):339– 47. [PubMed] [Google Scholar]
- 29.Ohmori S, Harada K, Miura H. Sex difference in free erythrocyte protoporphyrin (FEP) level. I. Sex difference in FEP level in healthy rural residents. Sangyo Igaku. 1992;34(4):342– 8. doi: 10.1539/joh1959.34.342. [DOI] [PubMed] [Google Scholar]
- 30.Koren G, Chang N, Gonen R, et al. Lead exposure among mothers and their newborns in Toronto. CMAJ. 1990;142(11):1241– 4. [PMC free article] [PubMed] [Google Scholar]