Abstract
Aims
Hypoxia, acidosis and programmed cell death are each hallmarks of acute myocardial infarction (AMI). We previously described a death pathway of cardiac myocytes mediated by hypoxia-acidosis that was characterized by activation of the Bcl2-family protein Bnip3 and programmed necrosis. The pathway included extensive DNA fragmentation that was sensitive to inhibition of the mitochondrial permeability transition pore (mPTP) and calpain inhibitors, but not caspase inhibitors. We did not identify the DNases responsible for DNA cleavage.
Main methods
Neonatal rat cardiomyocytes were subjected to hypoxia with and without concurrent acidosis and the cellular localization of apoptosis-inducing factor (AIF), DNase II and caspase-dependent DNase (CAD) were determined.
Key findings
Here we report the occurrence of biphasic pH-dependent translocations of AIF and DNase II but no change in CAD or its inhibitor ICAD. AIF co-localized with the mitochondria under aerobic and hypoxia-neutral conditions but translocated to the nucleus at pH ~6.7 coincident with a decrease of the mitochondrial membrane potential. DNase II co-localized with lysozomes under normoxia and hypoxia-neutral conditions and translocated to the nucleus at pH ~6.1 coincident with the appearance of single strand DNA cuts. Inhibition of the mPTP pore with BH4-TAT peptide, calpain inhibition with PD150606 or knockdown (KD) of Bnip3 failed to prevent nuclear translocation of these DNase although Bnip3 KD blocked mitochondrial fission.
Significance
The results suggest that caspase-independent DNA fragmentation is precisely regulated and occurs in parallel but independently from programmed necrosis mediated by hypoxia-acidosis.
Keywords: hypoxia, DNase II, cardiac myocyte, ischemia, apoptosis inducing factor, mitochondrial fission, bnip3, calpains
Introduction
Classical caspase-dependent programmed cell death (apoptosis) and caspase-independent programmed necrosis are both associated with acute myocardial infarction (AMI) (reviewed in Buja, 2005). Apoptosis is characterized by opening of mitochondrial Bax channels, cytochrome c release, nuclear condensation and caspase activation. Programmed necrosis is initiated by mitochondrial swelling, opening of the permeability transition pore (mPTP), and caspase-independent cell death. Both death pathways may include DNA fragmentation. Caspase-activated DNase (CAD) is associated with the classical intrinsic apoptosis pathway and is activated when the CAD inhibitor (ICAD) is cleaved by caspase 3 allowing for the nuclear translocation of CAD. Caspase-independent DNases include apoptosis inducing factor (AIF) and endonuclease G (EndoG). Both are normally sequestered in the mitochondrial intermembrane space and translocate to the nucleus following mitochondrial dysfunction. DNase I is an endonuclease normally bound to actin and is released in association with caspase activation. DNase II is an acid-dependent nuclease associated with lysosomes.
Bnip3 is a member of the BH-3 only subfamily of pro-apoptotic proteins that localizes to the mitochondria and can induce mitochondrial dysfunction and cell death. Bnip3 has been implicated in promoting apoptosis, necrosis and autophagy (Vande Velde et al., 2000; Hamacher-Brady et al., 2006; Kubasiak et al., 2002). Bnip3 is induced by hypoxia but our laboratory reported that acidosis is required to fully activate Bnip3-cell death (Kubasiak et al., 2002). The hypoxia-acidosis death pathway is associated with extensive DNA fragmentation that was insensitive to caspase inhibitors but sensitive to inhibitors of the mPTP. Here we investigated the DNases responsible for DNA cleavage during hypoxia-acidosis cell death. We found no evidence for CAD activation but we report a biphasic pH-regulated cytoplasm-nuclear translocations of AIF and DNase II which occurred in parallel with but independent of hypoxia-acidosis induced programmed cell death. Importantly we found that translocation of these DNases was not blocked by inhibitors of calpains or mPTP opening suggesting independence from the main programmed death pathway. Mitochondrial fission, a marker of the hypoxia-acidosis pathway was prevented by siRNA knockdown of Bnip3 but this also did not inhibit DNase translocation.
Materials and Methods
Reagents
Reagents were obtained from Sigma unless other wise noted.
Cell Culture and Hypoxia
Our methods for preparation of primary cultures of neonatal rat cardiomyocytes and exposure to hypoxia with and without acidosis are described in detail elsewhere (Webster et al., 1999; Kubasiak et al., 2002; Frazier et al., 2006). To maintain a neutral extracellular pH, culture media was replaced with fresh hypoxic media every 12hrs. For hypoxia with acidosis, the media was not changed allowing for the buildup of acid. All cultures were maintained in high glucose DMEM and as described previously (Webster et al,, 1999), Glucose and ATP were monitored and although extracellular glucose fell more rapidly during hypoxia the level remained above 2 mM at all times under both conditions, and ATP remained high (data not shown; see Webster et al,, 1999). To ensure that myocyte death during these conditions was due primarily to acidosis rather than the accumulation of other metabolites and/or energy failure, parallel cultures were exposed to hypoxia without media change and the pH was neutralized every 12h by infusing pre-determined aliquots of HEPES buffer and NaOH (described in Webster et al., 1999). In agreement with our previous report, myocytes exposed to 72 h of hypoxia without medium change but with pH neutralization remained contractile and fully intact with minimal signs of apoptosis, nuclear condensation or DNA fragmentation (data not shown; see Webster et al., 1999). Therefore we can conclude that the effects of spent medium on programmed death, DNA fragmentation and associated pathways in this model are due primarily to the effects of acidosis.
Western Blot analysis
Detailed methods for cell harvesting, lysis and western blot analyses are described elsewhere (Kubasiak et al., 2002). For subcellular fractionation, nuclei were separated by centrifugation at 10,000g for 1min, mitochondria 10,000g for 30min and the remaining supernatant is the cytoplasm. Western blots were probed with antibodies against CAD, ICAD (Santa Cruz Biotechnology), AIF (Oncogene), cytochrome c (Pharmingen), VDAC (Biovision), β-actin, and α-tubulin (Calbiochem).
siRNA
Rat specific Bnip3 siRNA and random control siRNA were obtained from Dharmacon. Cells were transfected with 10nM oligonucleotide overnight using DharmaFECT transfection reagents as previously described (Graham et al., 2007). Bnip3 protein knock-down (KD) was >95% (not shown).
Immunocytochemistry and Organelle labeling
Detailed methods for immunostaining and visualizing cardiac myocytes are presented in (Kubasiak et al., 2002). Lysosomes were labeled with Lysotracker red (0.1μM; Molecular Probes) for 30 min at 37°C followed by incubation with Hoechst 33342 for 10min. Changes in mitochondrial membrane potential (ΔΨM ) was monitored by incubating cells in TMRE (1nM) for 10min at 37 °C. ΔΨM was analyzed using Image J software from NIH. Mitochondria morphology was determined by labeling mitochondrial with MitoTracker Green (Molecular Probes). For immunocytochemistry cells plated on coverslips were fixed in 4% paraformaldehyde and blocked in 10% BSA and 0.6% triton X-100 for 1hr at room temperature. Cells were exposed to antibodies against AIF or DNase II (Santa Cruz Biotechnology) and visualized with FITC conjugated secondary antibody. Nuclei were labeled with Hoechst 33342. Micrograph exposures were maintained between samples.
2-D gel electrophoresis
DNA was isolated as previously described (Graham et al. 2007). Samples (7μg DNA) were subjected to electrophoresis in 2% agarose gel in TAE buffer (neutral) followed by turning the gel 90° and soaking in denaturing buffer (50mM NaOH, 1mM EDTA). The denatured gel was run for a second dimension of alkaline electrophoresis.
Results
CAD remains in the cytoplasm during hypoxia-acidosis
We have previously shown that apoptosis and DNA degradation during hypoxia-acidosis is caspase-independent (Kubasiak et al., 2002). Nuclear translocation of CAD from the cytoplasm following caspase dependent degradation of ICAD is an indicator of caspase dependent genomic degradation. To investigate the role of CAD during hypoxia-acidosis, the cytoplasmic levels of CAD and ICAD were determined. Exposing cells to hypoxia or hypoxia with concurrent acidosis did not affect the cytoplasmic levels of CAD or its inhibitor ICAD when compared to air control plates (Figure 1). In contrast there was a significant reduction in cytoplasmic CAD and ICAD concentrations when cells were exposed to staurosporine, a potent inducer of caspase-dependent apoptotic genomic degradation.
Figure 1. Cytoplasmic levels of CAD and ICAD are unaffected by hypoxia-acidosis.

Cytoplasmic CAD and ICAD levels in cells exposed to air, hypoxia-neutral (Hx-Neut) or hypoxia with progressive acidosis (HA). As a positive control, caspase dependent cell death was induced by treating normoxic cells with staurosporine (St, 1uM for 6hrs). n =3.
Reduction in mitochondrial membrane potential (ΔΨM) occurs early in response to hypoxia acidosis
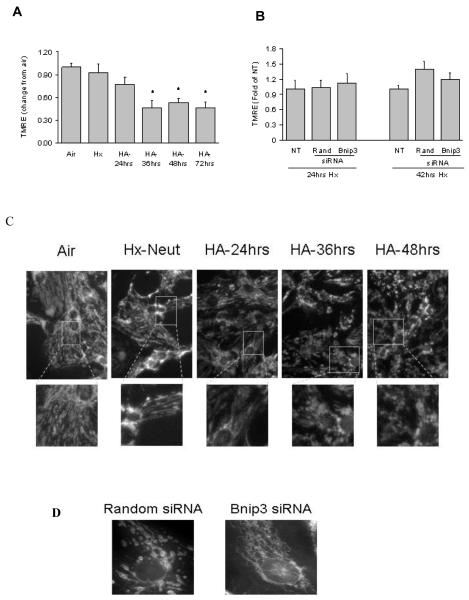
Loss of ΔΨM is associated with the opening of the mPTP and release of pro-apoptotic proteins. To examine the affect of hypoxia-acidosis on mitochondrial membrane potential, cells were incubated with TMRE, a ΔΨM–sensitive fluorescent dye. Exposing cells to hypoxia-neutral or hypoxia with mild extracellular acidosis (24hrs Hx, [pH]o >6.8) did not significantly reduce TMRE staining compared with aerobic cultures (Figure 2a). When extracellular pH decreased to ~6.6 (36h hypoxia), TMRE staining decreased to <50% of normoxic values coinciding with a marked increase of mitochondrial fission (Figure 2a and c). Knockdown of Bnip3 with siRNA did not prevent the decline in TMRE staining (Figure 2b) but significantly blocked mitochondrial fission compared to random sequence siRNA controls (Figure 2d)
Figure 2. Hypoxia-acidosis alters mitochondrial morphology and membrane potential.
Mitochondrial membrane potential was determined by TMRE staining in cells exposed to air, hypoxia-neutral (Hx-Neut) or hypoxia-acidosis (HA) (A). In (B) the effects of Bnip3 specific or random sequence siRNA on mitochondrial membrane potential during hypoxia-acidosis. In (C), mitochondrial morphology in myocytes exposed to air, hypoxia-neutral or hypoxia-acidosis. Lower panel represents magnified views of indicated areas. The effects of Bnip3 or random sequence siRNA on hypoxia-acidosis induced mitochondrial fission is shown in (D). Results are mean ± S.E.M., (* p<0.05 compared to aerobic controls), n = 3.
Early nuclear translocation of AIF
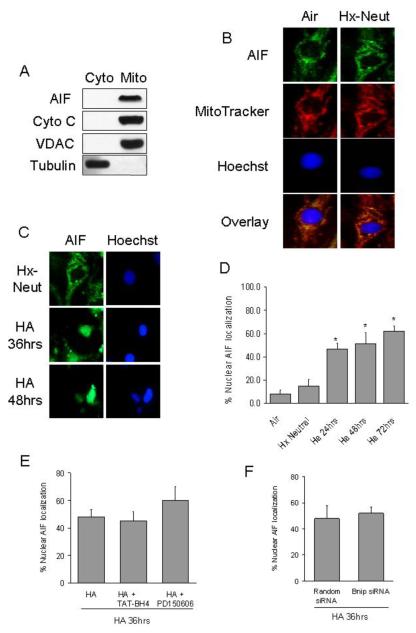
Under non-apoptotic conditions AIF co-localized with cytochrome c and MitoTracker red, a fluorescent mitochondrial marker (Figure 3a and b). However, during hypoxia with progressive acidosis when the [pH]o decreased to ~6.8 AIF was released from the mitochondria, which correlated with increase nuclear localization of AIF indicating translocation (Figure 3c). After 72h of hypoxia/acidosis 62 ± 4.2% of nuclei stained positive for AIF (Figure 3d). Nuclear localization of AIF was not prevented by pre-treating cells with the mPTP inhibitor, TAT-BH4, the calpain inhibitor, PD150606 or Bnip3 KD with siRNA (Figure 3e and f). In contrast pre-treating cells with TAT-BH4 or PD150606 was sufficient to reduce but not completely eliminate DNA fragmentation during hypoxia-acidosis (data not shown).
Figure 3. AIF is released from the mitochondria and translocates to the nucleus in response to hypoxia-acidosis.
AIF co-localizes with cytochrome c in the mitochondrial fraction of normoxic myocytes (A). (B) Immunocytochemistry of AIF and MitoTracker red, a mitochondrial marker, in cells exposed to air and hypoxia-neutral (Hx-Neut). In (C), immunocytochemistry of AIF in cells exposed to hypoxia-neutral or hypoxia with progressive acidosis. Quantification of nuclear AIF localization is shown in (D) (n = 100 nuclei). The effects of the mPTP inhibitor, TAT-BH4, the calpain inhibitor PD150606 and Bnip3 or random sequence siRNA on nuclear localization of AIF is shown in (E and F). Results are means ± S.E.M., (* p< 0.05 compared to hypoxia-neut). n =3.
DNase II translocates to the nucleus during severe hypoxia-acidosis
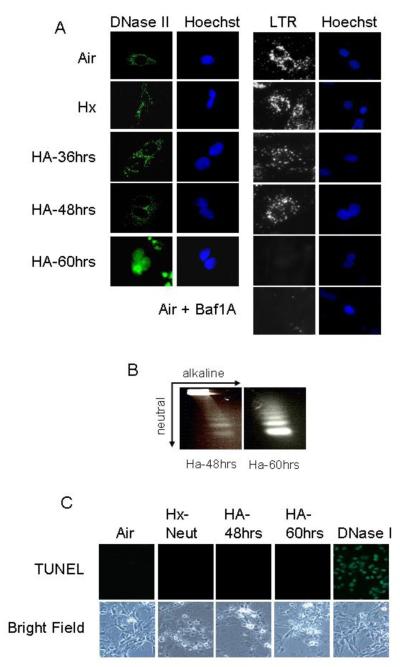
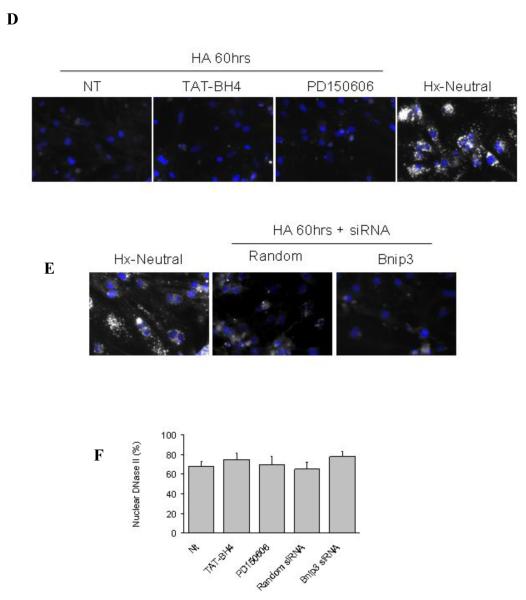
DNase II has been associated with apoptotic cell death (Barry and Eastman, 1993). In cardiac myocytes DNase II was exclusively cytoplasmic during the non-apoptotic conditions of air and hypoxia-neutral (Figure 4a). However during hypoxia with acidosis when the extracellular pH fell below 6.5 there was an abruptly loss of DNase II from the cytoplasm and a subsequent appearance of DNase II in the nuclei (Figures 4a). When the [pH]o decreased to ~6.1; 72% of nuclei stained positive for DNase II. Nuclear DNase II localization correlated with the loss of Lysotracker red staining, suggesting lysosomal rupture (Figure 4a), and the appearance of single-strand DNA breaks (Figure 4b). Cardiac myocytes subjected to late stage death by hypoxia/acidosis were TUNEL-negative consistent with predominant roles for AIF and DNase II in genomic fragmentation, (Figure 4c). Treatment of cells with the mPTP inhibitor, TAT-BH4, calpain inhibitor PD150606 or with Bnip3 siRNA did not prevent the loss of Lysotracker Red staining or nuclear translocation of DNase II (Figure 4d-f).
Figure 4. DNase II translocates to the nucleus in response to hypoxia-acidosis.
Using immunocytochemistry, DNase II and Lysotracker Red (LTR) localization was determined in the non-apoptotic conditions of air and hypoxia-neutral and during hypoxia with progressive acidosis (A). As a positive control, the lysosomes were neutralized by treating a parallel plate with bafilomycin A1 (Baf1A), an inhibitor of the H+-ATPase pump. In (B) single strand DNA cuts were determined by non-denaturing electrophoresis (neutral) followed by a second dimension of denaturing electrophoresis (alkaline). TUNEL DNA labeling is shown in (C). As a positive control, a parallel plate was treated with DNase I to generate TUNEL positive DNA strand breaks. The effects of the mPTP inhibitor, TAT-BH4, the calpain inhibitor PD150606 and Bnip3 or random sequence siRNA on Lysotracker Red staining and DNase II nuclear localization is shown in (D-F). Results are means ± S.E.M., n = 3.
Discussion
We demonstrate that genomic DNA fragmentation associated with programmed cell death of cardiac myocytes subjected to hypoxia/acidosis is accomplished by successive pH-dependent translocation of AIF and DNase II from the mitochondrial and lysosomal fractions respectively to the nuclei. These events were independent of calpains, the mPTP and Bnip3. As expected CAD was not activated and cardiac myocyte nuclei undergoing DNA fragmentation in this pathway were TUNEL-negative. These results are also consistent with previous results from our laboratory demonstrating that broad-spectrum caspase inhibitors were ineffective in preventing DNA fragmentation during hypoxia-acidosis (Kubasiak et al., 2002). These results suggest that the translocation of these DNases occurs in parallel with but independently of the associated death pathway that is driven by hypoxia-acidosis activated Bnip3. Previous studies have demonstrated a combination of programmed death pathways which act in parallel to induce cell death. For example in damaged photoreceptors autophagy, apoptosis and complement-mediated lysis all contributed to cell death (Lohr et al., 2006).
Despite the coincidence of AIF release with decreased ΔΨM and cytochrome c release, our inhibitor studies suggest that AIF translocation was independent of the mPTP and calpains. AIF has been shown to localize to the cytoplasmic side of the mitochondrial outer membrane as well as to the inter-membrane space (IMS) (Yu et al., 2009). Release from the IMS requires mPTP opening and cleavage by calpain I and/or cathepsins (Otera et al., 2005). Conversely release of AIF from the outer membrane pool does not require cleavage or mPTP opening (Yu et al., 2009). In our studies we did not see any change in molecular size of AIF therefore it seems possible that translocated AIF in this model comes exclusively from the outer membrane pool. Calpain- and mPTP-independent release of AIF has been demonstrated previously (Vindis at al., 2005; Ferrand-Drake et al., 2003).
DNase II translocated to the nucleus when acidosis progressed within the range necessary for its activation (Barry and Easman, 1993). Again, the specific pathway of nuclear translocation is unclear. Previous work has shown that calpain I can rupture the lysosomal membrane and release its contents (Yamashima et al., 1996). In our model DNase II translocation was calpain-independent therefore the release mechanism must involve an alternate pathway for disruption of the lysozomes. Lysosomal rupturing and DNase II dependent genomic degradation is associated with programmed death of the hippocampus following global ischemia (Tsukada et al., 2001) and during AMI (Ichihara et al., 1987; Hoffstein et al., 1976). During myocardial ischemia intra-cardiac pH falls rapidly so that 30 minutes of ischemia in the mouse is sufficient to be within the range reported here for AIF and DNase II translocation (Steenbergen et al., 1993).
There have been several reports that AIF and EndoG translocate to the nuclei during neuronal and myocardial ischemia coincident with Bnip3 activation (Hamacher-Brady et al., 2006; Zhang et al., 2011; Zhang et al., 2007). In one of these, Zhang et al., (2011) reported caspase-independent, Bnip3-dependent release of EndoG in cardiac myocytes subjected to simulated ischemia. Our studies suggest that AIF translocation accompanies Bnip3-mediated cell death during hypoxia/acidosis but this translocation is not dependent on Bnip3, mPTP, or calpains. In contrast, we found that mitochondrial fission, a process that has been functionally linked with AIF and EndoG release was Bnip3-dependent (Brooks et al., 2011; Li et al., 2010).
Conclusion
It seems possible that AIF and EndoG release occur by redundant pathways possibly involving other BH3-only proteins. At least 6 BH3-only proteins, including Noxa, PUMA, Bid, Bad, and HGTD-P, may contribute to cell death during myocardial ischemia/reperfusion (Webster et al., 2006).
Acknowledgments
This study was supported by National Institutes of Health grant RO1 HL44578 (to K.A.W), by Predoctoral (J.W.T.) and Postdoctoral (R.M.G.) awards from the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement The authors declare that there are no conflicts of interest
References
- Barry MA, Eastman A. Identification of deoxyribonuclease II as an endonuclease involved in apoptosis. Arch Biochem Biophys. 1993;300(1):440–50. doi: 10.1006/abbi.1993.1060. [DOI] [PubMed] [Google Scholar]
- Brooks C, Cho G, Wang CY, Yang T, Dong Z. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol. 2011;300(3):C447–55. doi: 10.1152/ajpcell.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buja LM. Myocardial ischemia and reperfusion injury. Cardiovas Pathol. 2005;14(4):170–5. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Ferrand-Drake M, Zhu C, Gidö G, Hansen AJ, Karlsson JO, Bahr BA, Zamzami N, Kroemer G, Chan PH, Wieloch T, Blomgren K. Cyclosporin A prevents calpain activation despite increased intracellular calcium concentrations, as well as translocation of apoptosis-inducing factor, cytochrome c and caspase-3 activation in neurons exposed to transient hypoglycemia. J Neurochem. 2003;85(6):1431–42. doi: 10.1046/j.1471-4159.2003.01794.x. [DOI] [PubMed] [Google Scholar]
- Frazier DP, Wilson A, Graham RM, Thompson JW, Bishopric NH, Webster KA. Acidosis regulates the stability, hydrophobicity and activity of the BH3-only protein Bnip3. Antioxid Redox Signal. 2006;8(9-10):1625–34. doi: 10.1089/ars.2006.8.1625. [DOI] [PubMed] [Google Scholar]
- Graham RM, Thompson JW, Wei J, Bishopric NH, Webster KA. Regulation of Bnip3 death pathways by calcium, phosphorylation, and hypoxia-reoxygenation. AntioxidRedox Signal. 2007;9(9):1309–15. doi: 10.1089/ars.2007.1726. [DOI] [PubMed] [Google Scholar]
- Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2006;14(1):146–57. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- Hoffstein S, Weissmann G, Fox AC. Lysosomes in myocardial infarction: studies by means of cytochemistry and subcellular fractionation, with observations on the effects of methylprednisolone. Circulation. 1976;53(3 Suppl):I34–40. [PubMed] [Google Scholar]
- Ichihara K, Haneda T, Onodera S, Abiko Y. Inhibition of ischemia-induced subcellular redistribution of lysosomal enzymes in the perfused rat heart by the calcium entry blocker, diltiazem. J Pharmacol Exp Ther. 1987;242(3):1109–13. [PubMed] [Google Scholar]
- Kubasiak LA, Hernandez OM, Bishopric NH, Webster KA. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A. 2002;99(20):12825–30. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhou J, Li Y, Qin D, Li P. Mitochondrial fission controls DNA fragmentation by regulating endonuclease G. Free Radic Biol Med. 2010;49(4):622–31. doi: 10.1016/j.freeradbiomed.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Lohr HR, Kuntchithapautham K, Sharma AK, Roher B. Multiple, parallel cellular suicide mechanisms participate in photoreceptor cell death. Exp Eye Res. 2006;83(2):380–9. doi: 10.1016/j.exer.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Otera H, Ohsakaya S, Nagaura Z, Ishihara N, Mihara K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005;24(7):1375–86. doi: 10.1038/sj.emboj.7600614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenbergen C, Perlman ME, London RE, Murphy E. Mechanism of preconditioning. Ionic alterations. Circ Res. 1993;72(1):112–25. doi: 10.1161/01.res.72.1.112. [DOI] [PubMed] [Google Scholar]
- Tsukada T, Watanabe M, Yamashima T. Implications of CAD and DNase II in ischemic neuronal necrosis specific for the primate hippocampus. J Neurochem. 2001;79(6):1196–206. doi: 10.1046/j.1471-4159.2001.00679.x. [DOI] [PubMed] [Google Scholar]
- Vande Velde C, Cizeau J, Dubik D, Alimonti J, Brown T, Israels S, Hakem R, Greenberg AH. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol Cell Biol. 2000;20(15):5454–68. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindis C, Elbaz M, Escargueil-Blanc I, Augé N, Heniquez A, Thiers JC, Nègre-Salvayre A, Salvayre R. Two distinct calcium-dependent mitochondrial pathways are involved in oxidized LDL-induced apoptosis. Arterioscler Thromb Vasc Biol. 2005 Mar;25(3):639–45. doi: 10.1161/01.ATV.0000154359.60886.33. [DOI] [PubMed] [Google Scholar]
- Webster KA, Discher DJ, Kaiser S, Hernandez O, Sato B, Bishopric NH. Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. J Clin Investigation. 1999;104(3):239–52. doi: 10.1172/JCI5871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster KA, Graham RM, Thompson JW, Spiga MG, Frazier DP, Wilson A, Bishopric NH. Redox stress and the contributions of BH3-only proteins to infarction. Antioxid Redox Signal. 2006;(9-10):1667–76. doi: 10.1089/ars.2006.8.1667. [DOI] [PubMed] [Google Scholar]
- Yamashima T, Saido TC, Takita M, Miyazawa A, Yamano J, Miyakawa A, Nishijyo H, Yamashita J, Kawashima S, Ono T, Yoshioka T. Transient brain ischemia provokes Ca2+, PIP2, and calpain responses prior to delayed neuronal death in monkeys. Eur. J. Neurosci. 1996;8:1932–44. doi: 10.1111/j.1460-9568.1996.tb01337.x. [DOI] [PubMed] [Google Scholar]
- Yu SW, Wang Y, Frydenlund DS, Ottersen OP, Dawson VL, Dawson TM. Outer mitochondrial membrane localization of apoptosis-inducing factor: mechanistic implications for release. ASN Neuro. 2009;1(5):pii, e00021. doi: 10.1042/AN20090046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ye J, Altafaj A, Cardona M, Bahi N, Llovera M, Cañas X, Cook SA, Comella JX, Sanchis D. EndoG links Bnip3-induced mitochondrial damage and caspase-independent DNA fragmentation in ischemic cardiomyocytes. PLoS One. 2011;6(3):e17998. doi: 10.1371/journal.pone.0017998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yang X, Zhang S, Ma X, Kong J. BNIP3 upregulation and EndoG translocation in delayed neuronal death in stroke and in hypoxia. Stroke. 2007;38(5):1606–13. doi: 10.1161/STROKEAHA.106.475129. [DOI] [PubMed] [Google Scholar]