Abstract
Merkel cells are an enigmatic group of rare cells found in the skin of vertebrates. Most make contacts with somatosensory afferents to form Merkel cell-neurite complexes, which are gentle-touch receptors that initiate slowly adapting type I responses. The function of Merkel cells within the complex remains debated despite decades of research. Numerous anatomical studies demonstrate that Merkel cells form synaptic-like contacts with sensory afferent terminals. Moreover, recent molecular analysis reveals that Merkel cells express dozens of presynaptic molecules that are essential for synaptic vesicle release in neurons. Merkel cells also produce a host of neuro-active substances that can act as fast excitatory neurotransmitters or neuromodulators. Here, we review the major neurotransmitters found in Merkel cells and discuss these findings in relation to the potential function of Merkel cells in touch reception.
Keywords: Merkel cell, touch, mechanotransduction, neurotransmitter, neuromodulator, somatosensory
The somatosensory neurons that innervate our skin constantly update our brains about the objects and environmental factors that surround us. A remarkable feature of our skin's nervous system is that it encodes a diversity of chemicals, temperatures and physical forces into membrane potential changes that trigger discrete neural signals. These signals are processed by circuitry within the central nervous system to produce distinct percepts such as touch, pain, warmth, cooling and itch. We rely on this information to navigate our environment and avoid harm. For example, our sense of discriminative touch allows us to perform countless essential behaviors, including feeding and clothing ourselves.
To initiate a range of sensations, cutaneous sensory neurons display an array of anatomical specializations and physiological properties. They can be classified as Aβ, Aδ, or C fibers based on conduction velocity and degree of myelination.1–3 A β afferents are the fastest (~ 35–75 m/s in humans and ~ 10–25 m/s in mice) due to their large diameters and thick myelin sheets. C fibers, which have thin, unmyelinated afferents, are the slowest (~ 0.5–2 m/s in humans and ≤ 1 m/s in mice). Thinly myelinated Aδ fibers, which have fine axonal diameters compared to Aβ afferents, fall between with conduction velocities of ~ 5–30 m/s in humans and ~ 4–10m/s in mice. Sensory neurons can be further designated as mechanoreceptors, thermoreceptors and nociceptors, depending on their modality or the sensory stimuli to which they respond.2–4 Most high threshold mechanoreceptors, thermoreceptors and nociceptors fall into Aδ or C fiber classes. These are thought to terminate in free nerve endings innervating the epidermis, dermis or hair follicles. Most tactile afferents, or low-threshold mechanoreceptors, are classified as Aβ or Aδ afferents. The reader is cautioned that there are numerous exceptions to these general guidelines. For example, a population of unmyelinated low threshold mechanoreceptors (C-tactile, CT), with conduction velocities of ~ 1 m/s in humans and mice, abundantly innervate hairy skin.5,6
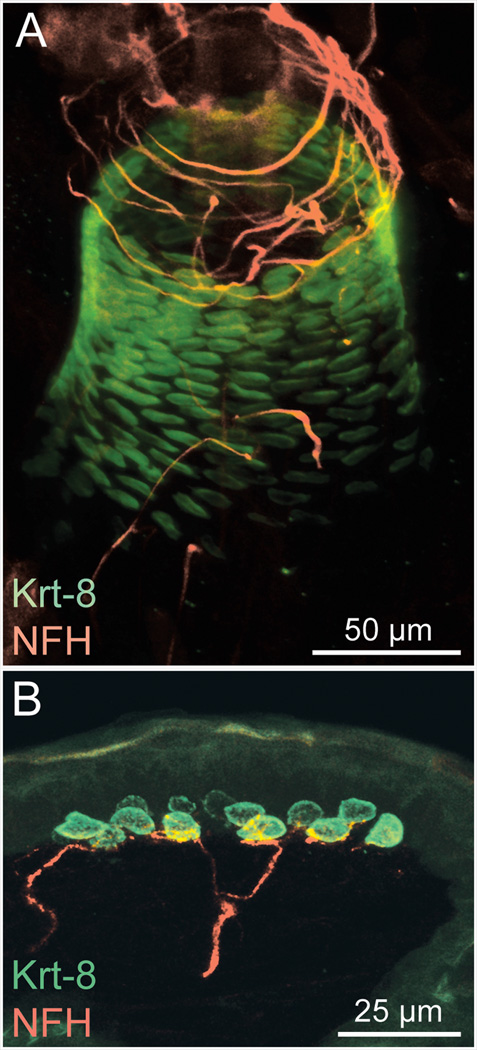
Tactile afferents terminate in morphologically specialized end-organs that govern their mechanosensory responses and allow them to extract distinct features of a complex tactile stimulus.2,3,7 For example, rapidly adapting afferents that encode vibration have encapsulated endings called Meissner’s corpuscles and Pacinian corpuscles. A surprising variety of rapidly adapting afferents also innervate hair follicles to signal hair movements.6 Slowly adapting afferents produce sustained discharges throughout mechanical stimulation. The best characterized of these are slowly adapting type I (SAI) afferents, which are the Aβ afferents that form complexes with epidermal Merkel cells (Fig. 1).
Figure 1.
Merkel cell-neurite complexes in mouse whisker follicles (A) and touch domes (B) from the mouse hairy skin. Merkel cells, marked by keratin 8 (Krt 8; green) are in intimate contact with myelinated sensory afferents, visualized with antibodies against neurofilament heavy chain (NFH; red).
Merkel cell-neurite complexes are required for SAI responses
Based on their distribution and response properties, SAI afferents are thought to encode object features, such as shape, edges and curvature.7 These afferents make contacts with Merkel cells, which cluster in skin regions that are specialized for high tactile acuity. These include fingertips, whisker follicles, (Fig. 1A) and touch domes, which are high-sensitivity areas of hairy skin (Fig. 1B). Importantly, SAI afferents have the highest spatial resolution among mammalian touch receptors and they represent fine spatial details, such as Braille-like characters, with fidelity.7
Though it is well established that Merkel cell-neurite complexes are gentle-touch receptors that mediate SAI responses, the function of Merkel cells in discriminative touch is still a mystery. Based on similarities to inner-ear hair cells, Merkel cells have been proposed to be mechanosensory cells that transduce touch and activate afferent neurons by neurotransmitter release.8,9 Parallels between mechanosensory hair cells and Merkel cells are notable. Merkel cells have elongated microvilli suggestive of the hair cell’s mechanosensitive stereocilia.10 These cell types express the same developmental transcription factors, including mammalian atonal homolog 1 (Atoh1), growth factor independent 1 and Pou4F2.11–15 Moreover, Atoh1 is absolutely essential for Merkel-cell development, as it is for hair cells.16–18
To test whether Merkel cells are required for touch sensation, the Cre-loxP system was used to conditionally delete Atoh1 in the skin of mice. Merkel cells failed to develop in these mice; however touch domes were still innervated by myelinated Aβ afferents.16 A survey of touch-sensitive afferents in ex vivo skin–nerve preparations revealed the selective and complete loss of SAI responses in these mice. Maricich et al. subsequently reported that Merkel-cell knockout mice show a loss of texture preference in behavioral assays.19 This finding is exciting because it provides the first behavioral evidence that animals rely on SAI responses for textural information. Together, these results indicate that epidermal Merkel cells play an integral role in touch-evoked SAI responses;16 however, they do not distinguish between a developmental requirement, a mechanosensory function, or an accessory role.
Studies that disrupted Merkel cell-neurite complexes postnatally have yielded conflicting results.20,21 Removing Merkel cells from the epidermis by photoablation or enzymatic treatment abolished slowly adapting responses in some studies but not in others.22–26 Mice lacking p75 neurotrophin receptor, which lose most Merkel cells postnatally, display slowly adapting responses comparable to those of wild-type mice.27 This disparity from the Atoh1 phenotype may reflect methodological differences between studies, a developmental requirement for Merkel cells, or a postnatal requirement for p75 in SAI afferents. Additional studies are needed to distinguish between these possibilities.
A second model for Merkel-cell function posits that Merkel cells are accessory cells rather than sensory receptor cells.9,28 For example, Merkel cells might release modulatory neurotransmitters that shape the sensitivity of mechanosensitive afferents. Some investigators have argued that the SAI afferent must be the site of mechanotransduction because response latencies at touch onset (~ 200 µs) are too short to include synaptic transmission from Merkel cells.29 A two receptor-site model, postulating that both Merkel cells and afferent terminals contain mechanotransduction channels, reconciles these short latencies with the Merkel cell’s sensory features.30 In this model, the SAI afferent transduces the phasic component of touch, as do rapidly adapting afferents. It has been proposed that Merkel cells mediate the tonic component of the SAI response. This hypothesis has some support from electrophysiological evidence but remains to be thoroughly tested.31–33
The Merkel cell’s synaptic-like contacts
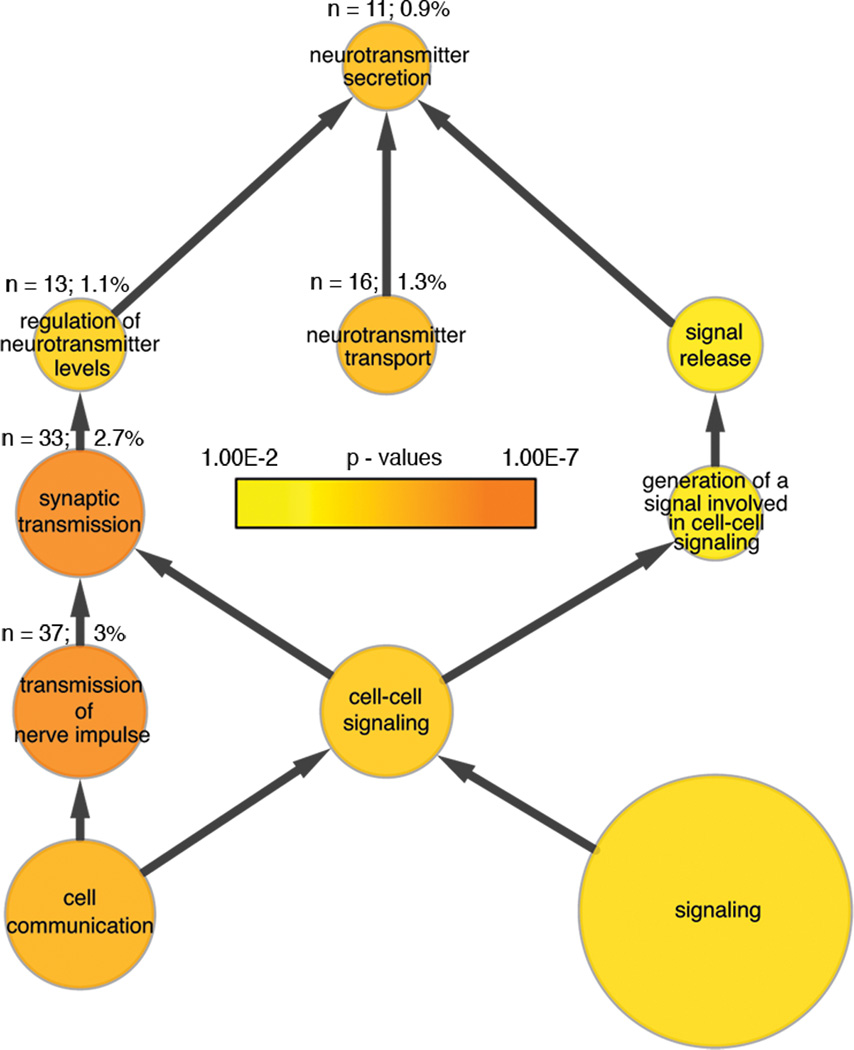
The presence of synaptic-like contacts between Merkel cells and sensory afferents suggests that Merkel cells are presynaptic cells. A substantial body of molecular evidence now supports this hypothesis.13,34,35 Microarray analysis of purified mouse Merkel cells has identified a number of synaptic molecules that are preferentially expressed in Merkel cells.13 These include essential presynaptic components such as active-zone scaffolding proteins, SNARE complex genes, voltage activated calcium channels, calcium sensors such as synaptotagmins 1 and 7, and neurotransmitter transporters. Enrichment at the protein level has been verified for many of the key synaptic components.13,34,35 Furthermore, gene ontology (GO) analysis has revealed that transcripts involved in synaptic transmission are over-represented in Merkel cells (Fig. 2). It is worth noting that a few studies suggest that Merkel cells might form reciprocal synapses with sensory afferents.36
Figure 2.
Gene transcripts involved in synaptic transmission and neurotransmitter release are enriched in Merkel cells. This network is a product of a Gene Ontology (GO) analysis of transcripts enriched in mouse Merkel cells.13 Compared with the mouse genome overall, genes associated with synaptic transmission and neurotransmitter release are significantly overrepresented in Merkel cells. Node size indicates the number of genes associated with a particular GO term (function). For reference, the absolute (n) and relative (%) sizes of nodes associated with synaptic transmission and neurotransmitter release are indicated. Node color indicates the significance level (P value) of enrichment. Arrows show parent-child node relationships.
Although there is no evidence of clear-core vesicles in Merkel cells, they do contain small, dense-core vesicles, which cluster near synaptic-like densities that mark the junctions with the afferent’s membrane.8,36,37 These vesicles show immunoreactivity for numerous neurotransmitters that vary across species, including adenosine triphosphate (ATP), serotonin (5-HT), vasoactive intestinal polypeptide (VIP), calcitonin gene related peptide (CGRP), substance P, met-enkephalin, and cholecystokinin octapeptide (CCK8).9,13,38–47 Glutamate has also been implicated as a potential neurotransmitter at the Merkel cell-neurite complex.13,31,32,34,48
This impressive array of neuro-active molecules poses an obvious question: what is the nature of the signal transmitted at the Merkel cell-neurite complex? If Merkel cells act as sensory receptor cells, sensory afferent terminals should contain ionotropic receptors to specific excitatory neurotransmitters released by Merkel cells. On the other hand, if Merkel cells modulate the SAI afferent’s response properties, sensory terminals might instead express metabotropic receptors. Of the neurotransmitters that have been localized to Merkel cells, three are classical, small-molecule neurotransmitters that have fast ionotropic receptors: glutamate, serotonin, and ATP. Most other neurotransmitters identified in Merkel cells are neuropeptides that, due to their slower mode of action, are more likely to serve neuromodulatory roles than to mediate fast SAI responses.49 Functional studies that have tested the involvement of synaptic transmission in Merkel cell–neurite signaling have provided conflicting evidence regarding sensory or modulatory roles.21,31,32,38,50,51 As a result, the functional significance of the Merkel cell’s synapse is still a mystery.
Classical neurotransmitters
Glutamate is the major excitatory neurotransmitter in vertebrates. The expression of the vesicular glutamate transporter 1 (VGLUT1) or 2 (VGLUT2) is the hallmark of glutamatergic synapses. Expression of VGLUT1, 2, and 3 in Merkel cells has been reported by several groups.13,34,35 Even before the discovery of VGLUTs in Merkel cells, Fagan and Cahusac demonstrated by electrophysiology that kynurenate, a broad-spectrum antagonist of ionotropic glutamate receptors, attenuated SAI responses in rat whisker follicles.31 Interestingly, kynurenate preferentially inhibited the tonic response but had little effect on the phasic response, which lends support to the two receptor-site model. In the search for ionotropic glutamate receptors in the Merkel cell-neurite complex, one was found: the NMDA receptor, which is a heterotetramer comprising multiple NR1 subunits and at least one NR2 subunit.52 In the rat whisker follicle, Cahusac et al. localized NR1 to Merkel cells and sensory afferents.50 Surprisingly, they localized the NR2 subunit to Merkel cells rather than to their associated sensory afferents. Thus, their data suggest that it is the Merkel cells and not the sensory afferents that express functional NMDA receptors.50 Tachibana et al. described an additional glutamate receptor expressed by Merkel cells, metabotropic mGluR5.38 Electrophysiological experiments with metabotropic glutamate receptor antagonists have yielded confusing results, with some potentiating the SAI response and others suppressing its activity.32,53 Though these findings might indicate that Merkel cell glutamate receptors act as auto-receptors that govern Merkel cell output via a feedback mechanism, Cahusac and Mavulati questioned the selectivity of glutamate receptor antagonists used in their studies.32 They argue that these compounds might interfere with SAI signaling, not by binding to glutamate receptors, but by inhibiting mechanotransduction channels (or other targets associated with them). Thus, although several lines of evidence support glutamatergic signaling in Merkel cells, how glutamate shapes the SAI response remains an open question.
5-HT has been considered one of the major candidates for a neurotransmitter at the Merkel cell-neurite complex since the discovery of serotonin-like immunoreactivity in Merkel cells of various organisms.9,40,41,54,55 Like glutamate, 5-HT has both ionotropic and metabotropic receptors. Out of seven 5-HT receptors (5-HT1–5-HT7) only 5-HT3 is ionotropic.56 Using 5-HT2 and 5-HT3 antagonists, He et al. were able to suppress, but not eliminate, rat SAI responses, indicating that 5-HT signaling modulates SAI responses.57 Though they reported that MDL72222, an ionotropic 5-HT3 receptor antagonist, reduced rat SAI responses, 5-HT3 receptors have yet to be located in the Merkel cell-neurite complex. Furthermore, metabotropic 5-HT1 (though not 5-HT2) receptors have been localized on SAI afferent terminals.58 Together with the presence of 5-HT transporters in Merkel cells, these data support the modulatory role of 5-HT in the Merkel cell–neurite complex.58 Combing the same 5-HT3 antagonist, MDL7222, with various levels of displacement, Press et al. were able to completely abolish frog SAI responses, providing the first evidence for a fast excitatory serotonergic transmission in the Merkel cell-neurite complex.33 Thus it is possible that 5-HT can have a dual role in the Merkel cell–neurite complex: as a classical excitatory neurotransmitter, and as a neuromodulator of the SAI response.33
Though glutamate and 5-HT have been discussed separately throughout this review, it is possible that they are co-released from Merkel cells during mechanical stimulation. Merkel cells express VGLUT3, which is also expressed in central serotonergic neurons.59—61 Thus, as proposed by Nunzi et al., VGLUT3-positive Merkel cells might co-release glutamate and serotonin (or any other neurotransmitter for that matter) during tactile stimulation.35 In support of this hypothesis, Press et al. found that in frog Merkel cell–neurite complexes SAI responses are shaped by both 5-HT and glutamate.33
ATP has been a neurotransmitter candidate since Toyoshima and Shimamura confirmed its presence in finch Merkel-cell vesicles with a uranaffin reaction.39 In frogs, large diameter DRG neurons, which include SAI afferents, express metabotropic P2Y1 ATP receptors and application of ATP increases their activity.62 In the rat sinus hair follicle, Tachibana et al. located another metabotropic receptor, P2Y2, on Merkel cells but not on sensory afferents.38 Although a P2Y1 antibody did not show positive reaction, further histological and physiological experiments are needed before conclusions can be made about the role of ATP in Merkel cell-neurite signaling.
Neuropeptides
Merkel cells have been widely reported to produce neuropeptides, though their identities vary from species to species. Even within a single species Merkel cells show heterogeneous patterns of neuropeptide expression.44,63,64 The most common neuropeptides reported in Merkel cells are VIP, CGRP, substance P, met-enkephalin, somatostatin, and CCK8.13,42–47,63 Boulais et al. demonstrated VIP release from cultured Merkel cells.65 Most of these neuropeptides can modulate neuronal activity through metabotropic receptors coupled to heterotrimeric G proteins.66 Tachibana et al. found Go and Gi immunoreactivity in SAI afferent terminals of rat and monkey Merkel cell–neurite complexes.67 Since mostly inhibitory neuropeptide receptors are Gi/Go-coupled, it is possible that some of the neuropeptides released by Merkel cells reduce SAI firing rates, though electrophysiological data is still lacking.51,68 Interestingly, Tachibana and Nawa localized receptors for met-enkephalin, VIP, substance P and CGRP to Merkel cells rather than sensory terminals, suggesting autocrine or paracrine action of these neuropeptides on Merkel cells.51
Some of the neuropeptides found in Merkel cells might serve neuroendocrine rather than sensory functions. Indeed, a subpopulation of non-innervated Merkel cells, which exists in mucosal tissues and hair follicles, have been proposed to be neuroendocrine cells.21,64,69 Merkel cells have been classified as a part of the amine precursor uptake and decarboxylation (APUD) system.21,70,71 As other cells of the APUD system, Merkel cells have dense-core vesicles and are positive for biogenic amines and neuropeptides. Recent reports posit that the neuroendocrine function of Merkel cells might impact skin disorders.65,72–74 It is possible that distinct classes of Merkel cells serve different functions in the skin. Indeed, recent reports propose two secretory pathways in rat Merkel cells: a Ca2+-dependent pathway that serves mechanosensory function and neurotransmitter release, and Ca2+-independent pathway that serves neuroendocrine functions and neuropeptide release.65,72 Functional studies, including the analysis of transgenic mouse models, are needed to test this hypothesis.
Summary and open questions
Merkel cells are one of four conserved cell types in the vertebrate epidermis and yet much about their biological function still remains unclear more than a century after Merkel’s initial description.21,75_ At this point, the role for Merkel cells in touch sensation seems assured – the majority of Merkel cells throughout the vertebrate skin contact sensory terminals, and they localize to highly touch-sensitive areas. Moreover, Merkel-cell knockout mice lack SAI responses and display impaired texture-driven behaviors. Electrophysiological data supports the two receptor-site hypothesis where initial, phasic responses are mediated by SAI afferents, and slower, tonic responses by Merkel cells.32,33 In the last decade glutamate has emerged as the most likely neurotransmitter in the Merkel cell–neurite complex, but confirming this will have to wait until additional experiments are performed. Further complicating this problem is accumulating evidence suggesting that Merkel cells co-secrete multiple neuro-active substances that could be involved not only in mechanoreception (transduction and modulation), but also in neuroendocrine roles unrelated to mechanoreception.33,65,69,72
The morphology of Merkel cell-neurite contacts, which contains features of both fast synaptic transmission and peptide-based neuromodulation, provides many intriguing questions. Physiological and genetic experiments are needed to provide concrete answers. Since both Merkel cells and SAI afferents are VGLUT2-positive, cell-type specific ablation of glutamate signaling is needed to resolve the role of this neurotransmitter in touch-evoked responses.13 To determine whether Merkel cells are required developmentally or postnatally for SAI responses, rapid and selective Merkel-cell ablation is needed. For example, targeted expression of diphtheria toxin receptor in adult Merkel cells could be achieved with Cre-loxP technology and an appropriate Cre driver. Optogenetic tools provide an opportunity to selectively excite and/or inhibit Merkel cells while recording SAI activity. If SAI activity can be provoked by exciting only Merkel cells with light, then Merkel cells indeed release excitatory neurotransmitters. If Merkel cell inhibition with light completely abolishes touch-evoked SAI responses, then Merkel cells not only release excitatory neurotransmitters but also transduce mechanical stimuli. Given the recent explosion of genetic tools for selectively manipulating the excitability of cell populations, we can expect to have a better understanding of the intriguing Merkel cell and its functions in the near future.
Acknowledgements
The work was supported by NIAMS R21 AR062307 (to EAL), NINDS R01 NS073119 (to EAL and Gregory J. Gerling) and NHLB T32HL087745 (SM). We thank Dr. David Ginty, Dr. Michael Rutlin and Ms. Kara Marshall for sharing whole-mount skin staining protocols prior to publication. Confocal microscopy was performed in the Columbia University Skin Disease Research Center Advanced Imaging Core (NIAMS P30AR044535).
Footnotes
The authors declare no conflicts of interest.
References
- 1.Brown AG, Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967;193:707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner EP, Martin JH, Jessell TM. In: Principles of Neuroscience. Kandel ER, Schwartz JH, Jessell TM, editors. Oxford University Press; 2000. pp. 430–449. [Google Scholar]
- 3.Rice FL, Albrecht PJ. In: The Senses: A Comprehensive Reference. Basbaum Allan I, et al., editors. Academic Press; 2008. pp. 1–31. [Google Scholar]
- 4.Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 5.Olausson H, Wessberg J, Morrison I, McGlone F, Vallbo A. The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev. 2010;34:185–191. doi: 10.1016/j.neubiorev.2008.09.011. Epub 2008 Oct 2008. [DOI] [PubMed] [Google Scholar]
- 6.Li L, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol. 2001;11:455–461. doi: 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- 8.Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachibana T, Nawa T. Recent progress in studies on Merkel cell biology. Anat Sci Int. 2002;77:26–33. doi: 10.1046/j.0022-7722.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 10.Toyoshima K, Seta Y, Takeda S, Harada H. Identification of Merkel cells by an antibody to villin. J Histochem Cytochem. 1998;46:1329–1334. doi: 10.1177/002215549804601113. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Arie N, et al. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 2000;127:1039–1048. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- 12.Wallis D, et al. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. 2003;130:221–232. doi: 10.1242/dev.00190. [DOI] [PubMed] [Google Scholar]
- 13.Haeberle H, et al. Molecular profiling reveals synaptic release machinery in Merkel cells. Proc Natl Acad Sci U S A. 2004;101:14503–14508. doi: 10.1073/pnas.0406308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leonard JH, et al. Proneural and proneuroendocrine transcription factor expression in cutaneous mechanoreceptor (Merkel) cells and Merkel cell carcinoma. Int J Cancer. 2002;101:103–110. doi: 10.1002/ijc.10554. [DOI] [PubMed] [Google Scholar]
- 15.Xiang M, et al. Role of the Brn-3 family of POU-domain genes in the development of the auditory/vestibular, somatosensory, and visual systems. Cold Spring Harb Symp Quant Biol. 1997;62:325–336. [PubMed] [Google Scholar]
- 16.Maricich SM, et al. Merkel cells are essential for light-touch responses. Science. 2009;324:1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Keymeulen A, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187:91–100. doi: 10.1083/jcb.200907080. Epub 2009 Sep 2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bermingham NA, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 19.Maricich SM, Morrison KM, Mathes EL, Brewer BM. Rodents rely on Merkel cells for texture discrimination tasks. J Neurosci. 2012;32:3296–3300. doi: 10.1523/JNEUROSCI.5307-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa H. The Merkel cell as a possible mechanoreceptor cell. Prog Neurobiol. 1996;49:317–334. doi: 10.1016/0301-0082(96)00018-4. [DOI] [PubMed] [Google Scholar]
- 21.Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his "Merkel cell", morphology, development, and physiology: review and new results. Anat Rec A Discov Mol Cell Evol Biol. 2003;271:225–239. doi: 10.1002/ar.a.10029. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda I, Yamashita Y, Ono T, Ogawa H. Selective phototoxic destruction of rat Merkel cells abolishes responses of slowly adapting type I mechanoreceptor units. The Journal of physiology. 1994;479(Pt 2):247–256. doi: 10.1113/jphysiol.1994.sp020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senok SS, Baumann KI, Halata Z. Selective phototoxic destruction of quinacrine-loaded Merkel cells is neither selective nor complete. Exp Brain Res. 1996;110:325–334. doi: 10.1007/BF00229133. [DOI] [PubMed] [Google Scholar]
- 24.Senok SS, Halata Z, Baumann KI. Chloroquine specifically impairs Merkel cell mechanoreceptor function in isolated rat sinus hairs. Neuroscience letters. 1996;214:167–170. doi: 10.1016/0304-3940(96)12906-2. [DOI] [PubMed] [Google Scholar]
- 25.Diamond J, Mills LR, Mearow KM. Evidence that the Merkel cell is not the transducer in the mechanosensory Merkel cell-neurite complex. Prog Brain Res. 1988;74:51–56. doi: 10.1016/s0079-6123(08)62997-0. [DOI] [PubMed] [Google Scholar]
- 26.Mills LR, Diamond J. Merkel cells are not the mechanosensory transducers in the touch dome of the rat. J Neurocytol. 1995;24:117–134. doi: 10.1007/BF01181555. [DOI] [PubMed] [Google Scholar]
- 27.Kinkelin I, Stucky CL, Koltzenburg M. Postnatal loss of Merkel cells, but not of slowly adapting mechanoreceptors in mice lacking the neurotrophin receptor p75. Eur J Neurosci. 1999;11:3963–3969. doi: 10.1046/j.1460-9568.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 28.Pasche F, Merot Y, Carraux P, Saurat JH. Relationship between Merkel cells and nerve endings during embryogenesis in the mouse epidermis. J Invest Dermatol. 1990;95:247–251. doi: 10.1111/1523-1747.ep12484847. [DOI] [PubMed] [Google Scholar]
- 29.Gottschaldt KM, Vahle-Hinz C. Merkel cell receptors: structure and transducer function. Science. 1981;214:183–186. doi: 10.1126/science.7280690. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita Y, Ogawa H. Slowly adapting cutaneous mechanoreceptor afferent units associated with Merkel cells in frogs and effects of direct currents. Somatosensory & motor research. 1991;8:87–95. doi: 10.3109/08990229109144732. [DOI] [PubMed] [Google Scholar]
- 31.Fagan BM, Cahusac PM. Evidence for glutamate receptor mediated transmission at mechanoreceptors in the skin. Neuroreport. 2001;12:341–347. doi: 10.1097/00001756-200102120-00032. [DOI] [PubMed] [Google Scholar]
- 32.Cahusac PM, Mavulati SC. Non-competitive metabotropic glutamate 1 receptor antagonists block activity of slowly adapting type I mechanoreceptor units in the rat sinus hair follicle. Neuroscience. 2009;163:933–941. doi: 10.1016/j.neuroscience.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Press D, Mutlu S, Guclu B. Evidence of fast serotonin transmission in frog slowly adapting type 1 responses. Somatosens Mot Res. 2010;27:174–185. doi: 10.3109/08990220.2010.516670. [DOI] [PubMed] [Google Scholar]
- 34.Hitchcock IS, Genever PG, Cahusac PM. Essential components for a glutamatergic synapse between Merkel cell and nerve terminal in rats. Neurosci Lett. 2004;362:196–199. doi: 10.1016/j.neulet.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 35.Nunzi MG, Pisarek A, Mugnaini E. Merkel cells, corpuscular nerve endings and free nerve endings in the mouse palatine mucosa express three subtypes of vesicular glutamate transporters. J Neurocytol. 2004;33:359–376. doi: 10.1023/B:NEUR.0000044196.45602.92. [DOI] [PubMed] [Google Scholar]
- 36.Mihara M, Hashimoto K, Ueda K, Kumakiri M. The specialized junctions between Merkel cell and neurite: an electron microscopic study. J Invest Dermatol. 1979;73:325–334. doi: 10.1111/1523-1747.ep12550322. [DOI] [PubMed] [Google Scholar]
- 37.Hartschuh W, Weihe E. Fine structural analysis of the synaptic junction of Merkel cell-axon-complexes. The Journal of investigative dermatology. 1980;75:159–165. doi: 10.1111/1523-1747.ep12522555. [DOI] [PubMed] [Google Scholar]
- 38.Tachibana T, Endoh M, Kumakami R, Nawa T. Immunohistochemical expressions of mGluR5, P2Y2 receptor, PLC-beta1, and IP3R-I and -II in Merkel cells in rat sinus hair follicles. Histochem Cell Biol. 2003;120:13–21. doi: 10.1007/s00418-003-0540-5. [DOI] [PubMed] [Google Scholar]
- 39.Toyoshima K, Shimamura A. Uranaffin reaction of Merkel corpuscles in the lingual mucosa of the finch, Lonchula striata var. domestica. Journal of anatomy. 1991;179:197–201. [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Caballero T, et al. Localization of serotonin-like immunoreactivity in the Merkel cells of pig snout skin. The Anatomical record. 1989;225:267–271. doi: 10.1002/ar.1092250402. [DOI] [PubMed] [Google Scholar]
- 41.English KB, et al. Serotonin-like immunoreactivity in Merkel cells and their afferent neurons in touch domes from the hairy skin of rats. Anat Rec. 1992;232:112–120. doi: 10.1002/ar.1092320112. [DOI] [PubMed] [Google Scholar]
- 42.Alvarez FJ, et al. Presence of calcitonin gene-related peptide (CGRP) and substance P (SP) immunoreactivity in intraepidermal free nerve endings of cat skin. Brain Res. 1988;442:391–395. doi: 10.1016/0006-8993(88)91532-6. [DOI] [PubMed] [Google Scholar]
- 43.Hartschuh W, Weihe E, Yanaihara N, Reinecke M. Immunohistochemical localization of vasoactive intestinal polypeptide (VIP) in Merkel cells of various mammals: evidence for a neuromodulator function of the Merkel cell. The Journal of investigative dermatology. 1983;81:361–364. doi: 10.1111/1523-1747.ep12519966. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Caballero T, Gallego R, Roson E, Fraga M, Beiras A. Calcitonin gene-related peptide (CGRP) immunoreactivity in the neuroendocrine Merkel cells and nerve fibres of pig and human skin. Histochemistry. 1989;92:127–132. doi: 10.1007/BF00490231. [DOI] [PubMed] [Google Scholar]
- 45.Gauweiler B, Weihe E, Hartschuh W, Yanaihara N. Presence and coexistence of chromogranin A and multiple neuropeptides in Merkel cells of mammalian oral mucosa. Neurosci Lett. 1988;89:121–126. doi: 10.1016/0304-3940(88)90367-9. [DOI] [PubMed] [Google Scholar]
- 46.Hartschuh W, Weihe E. Multiple messenger candidates and marker substance in the mammalian Merkel cell-axon complex: a light and electron microscopic immunohistochemical study. Prog Brain Res. 1988;74:181–187. doi: 10.1016/s0079-6123(08)63012-5. [DOI] [PubMed] [Google Scholar]
- 47.Hartschuh W, et al. Met enkephalin-like immunoreactivity in Merkel cells. Cell Tissue Res. 1979;201:343–348. doi: 10.1007/BF00236994. [DOI] [PubMed] [Google Scholar]
- 48.Morimoto R, et al. Co-expression of vesicular glutamate transporters (VGLUT1 and VGLUT2) and their association with synaptic-like microvesicles in rat pinealocytes. J Neurochem. 2003;84:382–391. doi: 10.1046/j.1471-4159.2003.01532.x. [DOI] [PubMed] [Google Scholar]
- 49.Wellnitz SA, Lesniak DR, Gerling GJ, Lumpkin EA. The regularity of sustained firing reveals two populations of slowly adapting touch receptors in mouse hairy skin. J Neurophysiol. 2010;103:3378–3388. doi: 10.1152/jn.00810.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cahusac PM, Senok SS, Hitchcock IS, Genever PG, Baumann KI. Are unconventional NMDA receptors involved in slowly adapting type I mechanoreceptor responses? Neuroscience. 2005;133:763–773. doi: 10.1016/j.neuroscience.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 51.Tachibana T, Nawa T. Immunohistochemical reactions of receptors to met-enkephalin VIP, substance P, and CGRP located on Merkel cells in the rat sinus hair follicle. Arch Histol Cytol. 2005;68:383–391. doi: 10.1679/aohc.68.383. [DOI] [PubMed] [Google Scholar]
- 52.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 53.Cahusac PM, Senok SS. Metabotropic glutamate receptor antagonists selectively enhance responses of slowly adapting type I mechanoreceptors. Synapse. 2006;59:235–242. doi: 10.1002/syn.20236. [DOI] [PubMed] [Google Scholar]
- 54.Zaccone G. Neuron-specific enolase and serotonin in the Merkel cells of conger-eel (Conger conger) epidermis. An immunohistochemical study. Histochemistry. 1986;85:29–34. doi: 10.1007/BF00508650. [DOI] [PubMed] [Google Scholar]
- 55.Toyoshima K, Shimamura A. Monoamine-containing basal cells in the taste buds of the newt Triturus pyrrhogaster. Arch Oral Biol. 1987;32:619–621. doi: 10.1016/0003-9969(87)90034-3. [DOI] [PubMed] [Google Scholar]
- 56.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 57.He L, Tuckett RP, English KB. 5-HT2 and 3 receptor antagonists suppress the response of rat type I slowly adapting mechanoreceptor: an in vitro study. Brain Res. 2003;969:230–236. doi: 10.1016/s0006-8993(03)02335-7. [DOI] [PubMed] [Google Scholar]
- 58.Tachibana T, Endoh M, Fujiwara N, Nawa T. Receptors and transporter for serotonin in Merkel cell-nerve endings in the rat sinus hair follicle. An immunohistochemical study. Arch Histol Cytol. 2005;68:19–28. doi: 10.1679/aohc.68.19. [DOI] [PubMed] [Google Scholar]
- 59.Fremeau RT, Jr, et al. The identification of vesicular glutamate transporter 3 suggests novel modes of signaling by glutamate. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:14488–14493. doi: 10.1073/pnas.222546799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schafer MK, Varoqui H, Defamie N, Weihe E, Erickson JD. Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J Biol Chem. 2002;277:50734–50748. doi: 10.1074/jbc.M206738200. [DOI] [PubMed] [Google Scholar]
- 61.Gras C, et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22:5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fantini F, Johansson O. Neurochemical markers in human cutaneous Merkel cells. An immunohistochemical investigation. Exp Dermatol. 1995;4:365–371. doi: 10.1111/j.1600-0625.1995.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 64.Moll I, et al. Human Merkel cells--aspects of cell biology, distribution and functions. Eur J Cell Biol. 2005;84:259–271. doi: 10.1016/j.ejcb.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 65.Boulais N, et al. Merkel cells as putative regulatory cells in skin disorders: an in vitro study. PLoS One. 2009;4:e6528. doi: 10.1371/journal.pone.0006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merighi A, Salio C, Ferrini F, Lossi L. Neuromodulatory function of neuropeptides in the normal CNS. J Chem Neuroanat. 2011;42:276–287. doi: 10.1016/j.jchemneu.2011.02.001. Epub 2011 Mar 2016. [DOI] [PubMed] [Google Scholar]
- 67.Tachibana T, Endoh M, Nawa T. Immunohistochemical expression of G protein alpha-subunit isoforms in rat and monkey Merkel cell-neurite complexes. Histochemistry and cell biology. 2001;116:205–213. doi: 10.1007/s004180100318. [DOI] [PubMed] [Google Scholar]
- 68.Tallent MK. Presynaptic inhibition of glutamate release by neuropeptides: use-dependent synaptic modification. Results Probl Cell Differ. 2008;44:177–200. doi: 10.1007/400_2007_037. [DOI] [PubMed] [Google Scholar]
- 69.Boulais N, Misery L. Merkel cells. J Am Acad Dermatol. 2007;57:147–165. doi: 10.1016/j.jaad.2007.02.009. Epub 2007 Apr 2006. [DOI] [PubMed] [Google Scholar]
- 70.Pearse AG. Common cytochemical and ultrastructural characteristics of cells producing polypeptide hormones (the APUD series) and their relevance to thyroid and ultimobranchial C cells and calcitonin. Proc R Soc Lond B Biol Sci. 1968;170:71–80. doi: 10.1098/rspb.1968.0025. [DOI] [PubMed] [Google Scholar]
- 71.Winkelmann RK. The Merkel cell system and a comparison between it and the neurosecretory or APUD cell system. J Invest Dermatol. 1977;69:41–46. doi: 10.1111/1523-1747.ep12497864. [DOI] [PubMed] [Google Scholar]
- 72.Boulais N, et al. Rat Merkel cells are mechanoreceptors and osmoreceptors. PLoS One. 2009;4:e7759. doi: 10.1371/journal.pone.0007759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu T, et al. Neuroendocrine regulatory role of Merkel cells in the pathogenesis of psoriasis. Scientific Research and Essays. 2011;6:4526–4531. [Google Scholar]
- 74.Righi A, et al. Merkel cells in the oral mucosa. Int J Surg Pathol. 2006;14:206–211. doi: 10.1177/1066896906290053. [DOI] [PubMed] [Google Scholar]
- 75.Merkel F. Tastzellen und Tastkörperchen bei den Haustehieren und beim Menschen. Arch Mikrosk Anat. 1985;11:636–652. [Google Scholar]