Abstract
Objective
Scleroderma is associated with intractable hand pain from vasospasm, digital ischemia, tenosynovitis, and nerve entrapment. This study investigated the effect of hydrodissection of the carpal tunnel followed by corticosteroid injection for the painful scleroderma hand.
Methods
26 consecutive subjects (12 with painful scleroderma hand and 14 with rheumatoid arthritis and carpal tunnel syndrome (RA/CTS) underwent sonographically-observed carpal tunnel hydrodissection with 3 mls of 1% lidocaine administered with a 25 gauge 1 inch needle on a 3 ml RPD mechanical syringe (reciprocating procedure device). After hydrodissection a syringe exchange was performed and 80 mg of triamcinolone acetonide was injected. Baseline pain, procedural pain, pain at outcome, responders, therapeutic duration, and reinjection interval were determined.
Results
Hydrodissection and injection with corticosteroid significantly reduced pain scores by 67% in scleroderma (p<0.001) and by 47% in RA/CT (p <0.001). Scleroderma and RA/CTS were similar in outcome measures: injection pain (p=0.47), pain scores at outcome (p = 0.13), responders (Scleroderma: 83.3%, RA/CTS: 57.1%, p = 0.15), pain at 6 months (p =0.15), and therapeutic duration (p=0.07). Scleroderma patients responded better in time to next injection (Scleroderma: 8.5±3.0 months; RA/CTS: 5.2±3.1 months, p = 0.03). Reduced Raynaud’s attacks and healing of digital ulcers occurred in 83% of subjects. There were no complications.
Conclusion
Hydrodissection with lidocaine followed by injection of triamcinolone reduces pain and vasomotor changes in the scleroderma hand. The mechanism may be a combination of hydrodissection-mediated mechanical freeing of entrapped arteries, nerves, and tendinous structures and corticosteroid-induced reduction of inflammatory vasospasm.
Keywords: Scleroderma, Corticosteroid, Carpal Tunnel, Injection, Ultrasound, Pain
Introduction
Scleroderma (systemic sclerosis) is characterized by severe complications of the hand, including intractable pain, fibrosis, contractures, arthritis, digital ischemia and necrosis, ulcerations, vasomotor changes, Raynaud’s phenomenon, fibrinous tenosynovitis, and digital neuropathic symptoms [1–4]. Therapy for scleroderma hand has included calcium channel blockers, angiotensin converting enzyme inhibitors, prostanoids, endothelin receptor antagonists, and phosphodiesterase type-5 inhibitors as well as sympathetic blockade, sympathetectomy, microsurgical revascularization and other methods primary focused on preventing vasospasm [1–11]. However, vasospasm is not the only etiology for hand symptoms in scleroderma; arthritis, tenosynovitis, and entrapment syndromes are increasingly recognized as important to hand disability [1–3, 11–14]. Further, recently the tendon apparatus has been recognized to be important to the development of contractures in scleroderma, indicating tendinous entrapment [1–3]. Arthritis and tenosynovitis in the scleroderma hand as demonstrated by imaging is extensive, emphasizing that scleroderma and its complications are not purely vascular in nature and may not respond to purely vascular interventions [1–14]. For example, the carpal tunnel can be affected in scleroderma with fibrosis, nerve entrapment, and tenosynovitis, but treatment of the painful scleroderma hand by injection of the carpal tunnel with a long-acting corticosteroid is rarely used probably because of concern regarding potential nerve damage and corticosteroid-induced scleroderma renal crisis [12–18]. Ultrasound can image the median nerve in the carpal tunnel regardless of condition and permits the safe introduction of the needle for corticosteroid injection, but the technique of needle hydrodissection in the scleroderma hand prior to corticosteroid injection has not been described [19–25].
We hypothesized that a component of hand pain and vascular dysfunction in the scleroderma hand is caused by entrapment and compression of tendinous, vascular and neural structures in the carpal tunnel that could be reversed by a combination of hydrodissection to free entrapped structures and corticosteroid injection to atrophy excessive resident connective tissue. The present study investigated the effect of hydrodissection of the carpal tunnel followed by corticosteroid injection for the painful scleroderma hand.
Materials and Methods
Subjects
This project complied with the Helsinki Declaration, was approved by the institutional review board (IRB), was registered at ClinicalTrials.gov (Clinical Trial Identifier NCT00651625), and is a long-term investigation of concerning the safety of injection therapy for musculoskeletal diseases [26]. Patient confidentiality and privacy was protected according to the Health Insurance Portability and Accountability Act (HIPAA). All products used in these procedures were commercially available, FDA-approved, CE-marked and used on-label.
The prospective study design of the present investigation compared hydrodissection of the carpal tunnel follow by corticosteroid injection in 12 consecutive subjects with painful scleroderma hand to a control group of 14 consecutive patients with painful hand due to rheumatoid arthritis with carpal tunnel syndrome (RA/CTS). Scleroderma was classified by the updated 1980 American College of Rheumatology clinical criteria [27,28]. Inclusion criteria for injection of the carpal tunnel in scleroderma patients included: 1) the presence of scleroderma; 2) persistent hand pain; 3) significant pain in the affected hand by 0–10 cm Visual Analogue Pain Sale (VAS) where VAS ≥ 5 cm; 5), 4) failure of oral medications and local measures, and 5) the desire of the patient to have a corticosteroid injection [29–31]. The presence or absence of carpal tunnel syndrome was not used as an enrollment criterion, but 4/12 (33%) had clinical symptoms suggestive of carpal tunnel syndrome as detailed below. Rheumatoid arthritis (RA) was classified using the American College of Rheumatology 1987 revised criteria [32]. Inclusion criteria for RA patients with carpal tunnel syndrome (RA/CTS) patients included: 1) the presence of rheumatoid arthritis, 2) hand numbness and tingling in the distribution of the median nerve; 3) decreased grip strength; 4) persistent hand pain; 5) nocturnal hand pain; 6) significant pain in the affected hand by 0–10 cm Visual Analogue Pain Sale (VAS) where VAS ≥ 5 cm; 7) positive Tinel’s and/or Phalen’s sign, 8) failure of splinting, and 9) the desire of the patient to have a corticosteroid injection [33,34]. Exclusion criteria included 1) thenar atrophy, 2) prior carpal tunnel decompressive surgery, 3) thoracic outlet syndrome, 4) polyneuropathy, 5) hemorrhagic diathesis, 6) use of warfarin or anti-platelet drugs, 7) the presence of infection, or 8) clinical cervical radiculopathy or spinal stenosis, or 9) previous corticosteroid injection in last 4 months.
Injection Technique
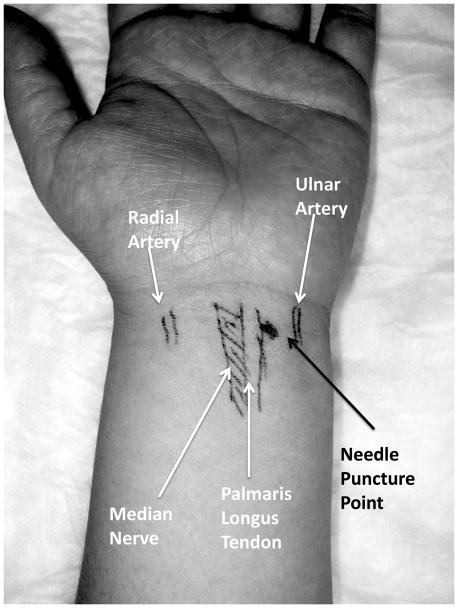
The ulnar border of the palmaris longus tendon was determined by having the patient oppose all the digits together while flexing the wrist and was then marked with a surgical pen on the ulnar side of the palmaris longus tendon at the second skin crease proximal to the palmar-wrist junction. Next the ulnar artery was palpated and marked (Figure 1). The entry point was between these two markings as described by Smith et al for the transverse ulnar (short-axis or axial) approach, or by Grassi et al for the sagittal (long-axis or sagittal) approach [20, 21] (Figure 1). This positioning was then confirmed with sonographic interrogation and identification of the median nerve, palmaris longus tendon, radial artery, and ulnar artery with Doppler imaging (Figure 2). These procedures were performed in a teaching hospital where an experienced proceduralist held the ultrasound probe, and directed the fellow-in-training who performed the syringe procedure. However, this same procedure can be performed by an experienced operator who controls the syringe in one hand and the ultrasound probe in the other.
Figure 1. Injection Point for Carpal Tunnel Injection.
The needle entry point for sagittal injection technique is at the second crease from the palm just ulnar to the palmaris longus tendon, is marked with ink, and is confirmed with both palpation and sonographic imaging, importantly excluding the median nerve, and ulnar nerve and artery. The needle is directed toward the ring finger.
Figure 2. Sonographic Anatomy for Injection of the Carpal Tunnel.
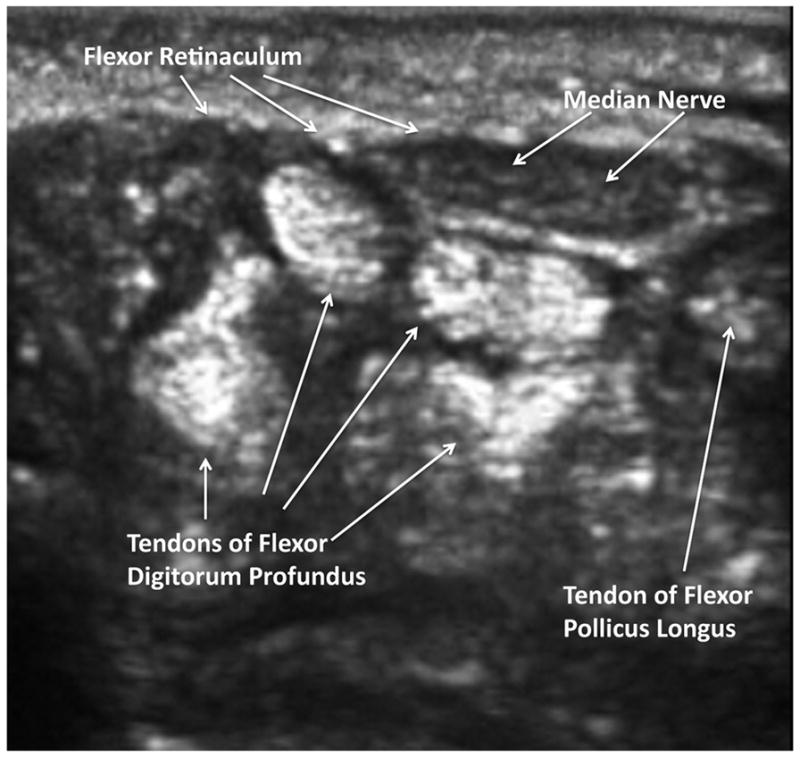
This more distal transverse image confirms that the penetration point to the ulnar side of the median nerve will permit the needle to pierce the flexor retinaculum and dilate the carpal tunnel space by hydrodissection with 1% lidocaine, and avoiding intratendinous, intraarterial, or intraneural injection.
A portable ultrasound unit with a 10–5 MHz 38 mm broadband liner array transducer (Sonosite M-Turbo, SonoSite, Inc. 21919 30th Drive SE, Bothell, WA 98021, website: www.sonosite.com) was used. The one-needle two-syringe technique was used where 1) one needle is used for anesthesia, aspiration to assure non-vascular positioning, dilation of the intra-carpal tunnel space, and carpal tunnel injection; 2) a first syringe is used to anesthetize, aspirate, and then hydrodissect and dilate the carpal tunnel space, and 3) a second syringe is used to inject the corticosteroid therapy [25]. A 25 gauge 1.0 inch (2.5-cm) needle (305783-25 g 1.5” BD Needle, BD, 1 Becton Drive, Franklin Lakes, NJ 07417, website: http://www.bd.com) was mounted on a 3 ml RPD mechanical syringe (Reciprocating Procedure Device procedure syringe, AVANCA Medical Devices, Inc, Albuquerque, New Mexico, USA. website: www.AVANCAMedical.com). The RPD syringe is a mechanical syringe formed around the core of a conventional syringe barrel and plunger, but has a parallel accessory plunger and an accessory barrel to control the motion of the accessory plunger (Figure 3). This device permits easy detection of small amounts of fluid or blood that flash back into the barrel confirming true needle tip positioning, and provides enhanced control [25,26,35,36]. Prior to the procedure the mechanical syringe was filled with 3 ml of 1% lidocaine (Xylocaine® 1%, AstraZeneca Pharmaceuticals LP, 1800 Concord Pike, P.O. Box 15437, Wilmington, DE 19850-5437).
After appropriate sterile preparation of the skin with chlorhexidine, the 25 gauge 1.0 inch BD safety needle on the mechanical procedure syringe safety syringe was inserted at a 30 degree angle between the ulnar artery and ulnar border of the palmaris longus tendon and pointed radially or longitudinally as described above (Figure 3). The needle penetrated the overlying skin and subcutaneous tissues and lidocaine anesthesia was administered to the level of the aponeurosis. The needle was slowly advanced while aspirating to assure a non-vascular position of the needle tip by depressing the aspiration plunger of the mechanical syringe (Figure 3), and the patient was asked if any pain or nerve symptoms were occurring. After reaching a depth of 60 to 70% of the needle shaft, depending on the size of the wrist, the mechanical syringe aspiration plunger was depressed to aspirate to ensure no blood return. The remainder of the 3 ml of lidocaine was then slowing injected, anesthetizing, hydrodissecting, and freeing entrapped structures (Figures 4 and 5). After lidocaine was completely injected and the structures completely hydrodissected as observed by sonography (Figure 5), using one hand to hold the mechanical syringe and the other hand the needle hub, the mechanical syringe was rotated off the needle, and a 3 ml conventional syringe prefilled with 80 mg triamcinolone acetonide suspension (Kenalog® 40, Westwood-Squibb Pharmaceuticals, Inc (Bristol-Myers Squibb), 345 Park Ave, New York, NY 10154-0004, USA) was rotated onto the intracarpal-tunnel needle, aspirated for blood return, and if not present, the treatment was injected slowly again observing for neurological symptoms (Figure 6). We have previously demonstrated that these larger doses are effective and safe [25]. The operator ensured that there was no resistance to injection and continuously monitored the patient that there were no pain or nerve symptoms while directly observing with sonography. After the procedure the needle was then extracted, firm pressure applied to the puncture site, the safety needle was inactivated, and a sterile dressing applied to the puncture site. The patient was not permitted to drive an automobile home for 2 hours after the procedure.
Figure 4. Sonographic Needle Introduction.
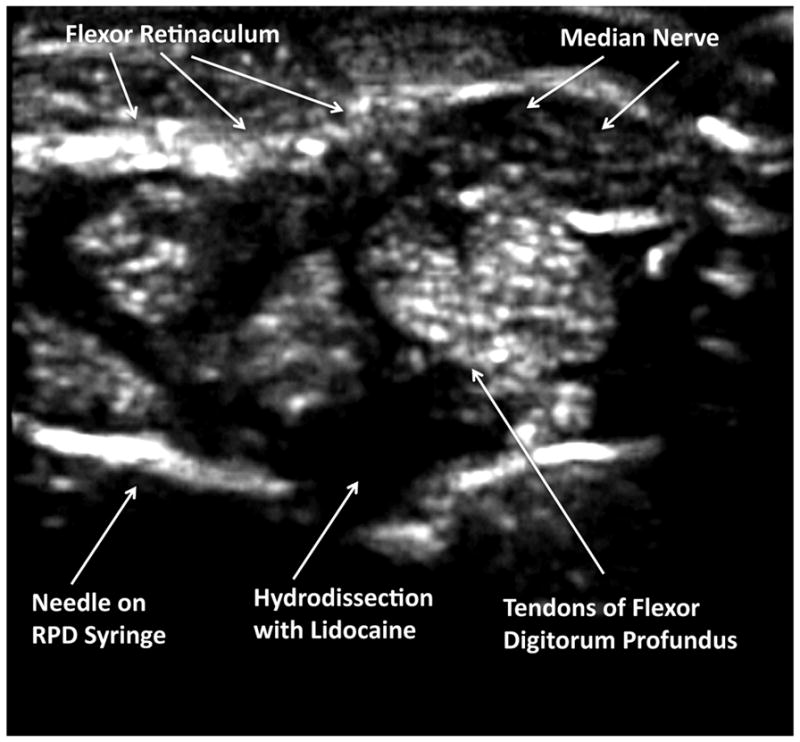
In this transverse sonographic image, using the transverse (short-axis) injection approach, the needle is advanced under direct sonographic guidance until the needle tip has pierced the flexor retinaculum, but has not penetrated the fibers of the flexor digitorum profundis. The mechanical syringe is used to determine aspiration of fluid, and then 1% lidocaine is injected to anesthetize the structures and hydrodissect, creating a space for the corticosteroid and lysing adhesions, and pushing away important structures including the median nerve and tendons.
Figure 5. Sonographic Visualization of Hydrodissection.
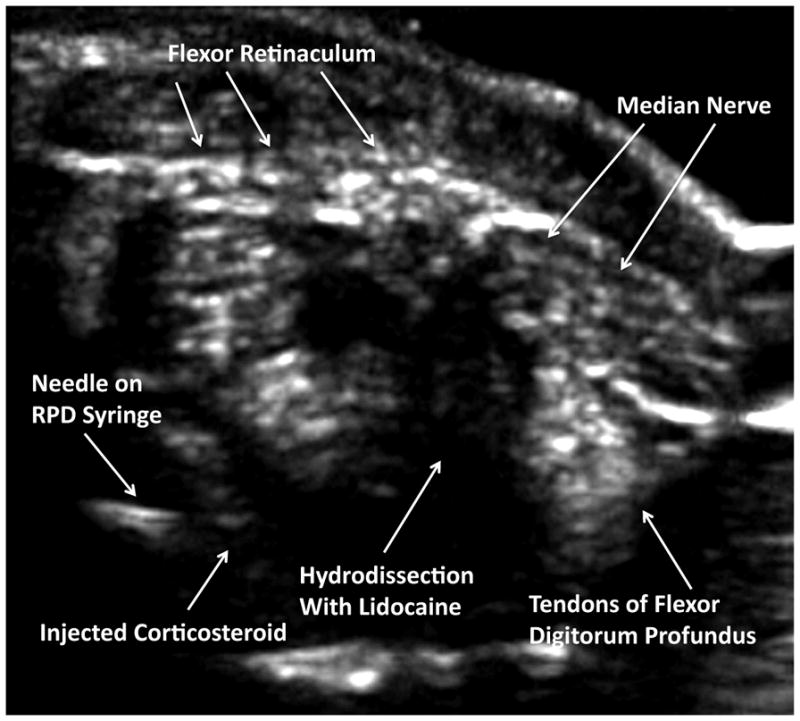
In this transverse image, using the transverse (short-axis) injection approach, after manipulation and rotation of the bevel, the carpal tunnel space is dilated under the flexor retinaculum, avoiding direct injection into the tendon fibers, and specifically avoiding any proximity to the median nerve or ulnar artery.
Figure 6. Corticosteroid Injection.
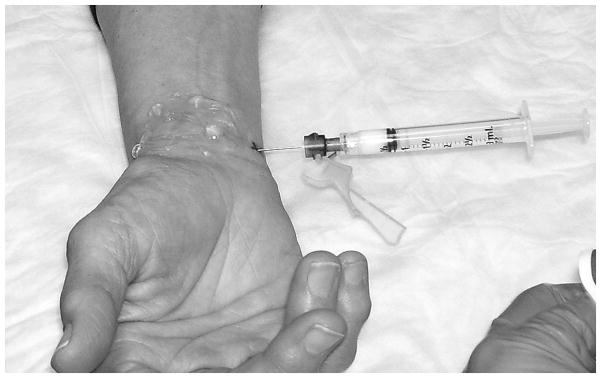
After hydrodissection with lidocaine has been performed, and the flexor retinaculum has been lifted by the lidocaine, and the tendon structures and median nerve have been pushed away from the needle tip by injected lidocaine, a syringe exchange is performed and 80 mg of triamcinolone acetonide is injected. This position represents the transverse (short-axis) ulnar approach.
Outcome Data of Clinical Procedures
Patient pain was measured with the standardized and validated 0–10 cm Visual Analogue Pain Scale (VAS), where 0 cm = no pain and 10 cm = unbearable pain [25,30,31]. At the time of the initial procedure, the patient was given a printed VAS scale (1–10 cm) to take home with them. The outcome inquiries at 2 weeks and 6 months was performed by a telephone call where the patient would look at the VAS and rate their pain accordingly. The inquirer was blinded as to diagnosis. Significant pain was defined as a VAS ≥ 5 cm [25]. Pain by VAS was determined 1) prior to the procedure (baseline pain), 2) during the insertion of the needle (procedural pain), 3) during injection of the treatment drug (injection pain), 4) 2 weeks post procedure (pain at primary outcome), and 5) 6 months post procedure (secondary outcome). Digital ulcers were counted and considered active if there were hemorrhage, a scab or lack of overlying epidermis [2,3,37]. Raynaud’s phenomenon activity was determined by number of attacks per day [5–8,10]. Two weeks has been demonstrated as the outcome measurement time most likely to detect maximum clinical effect of injected corticosteroid; thus, the 2 week observation was considered the primary outcome measure [15,25]. Responders were defined as an asymptomatic hand (0–10 cm VAS < 2 cm) at 2 weeks; non-responders were defined as a symptomatic hand (0–10 cm VAS ≥ 2 cm) at 2 weeks. Duration of therapeutic response was defined as the time interval in months when the hand became symptomatic (VAS ≥ 2 cm). If the hand remained asymptomatic at 6 months or longer, the duration was defined as 6 months. Time to next injection or referral for surgery was determined by both chart review and patient telephone interview at 12 months after the initial injection and expressed in months. If the next injection or referral to surgery occurred at a time greater than 12 months, the time to next injection was defined as 12 months.
Statistical Analysis
Data were entered into Excel (Version 5, Microsoft, Seattle, WA), and analyzed in SAS (SAS/STAT Software, Release 6.11, Cary, NC). Differences between parametric two group data were determined with the t-test. Differences in categorical data were determined with Fisher’s Exact Test, while differences between multiple parametric data sets were determined with Fishers Least Significant Difference Method.
Results
Patient characteristics are shown in Table 1. As can be seen, the scleroderma and RA/CTS patients were similar in age (p=0.96), proportion of female gender (p=0.82), subjects who completed the study (p=0.5), and pre-procedure baseline hand pain (p=0.65). Table 2 demonstrates the outcome of carpal tunnel injections. RA/CTS patients and scleroderma patients had similar low levels of procedural pain (10 cm VAS; RA: 2.8±4.1 cm, Scleroderma: 2.0±1.8 cm, p = 0.13). Injection of the carpal tunnel was highly efficacious in reducing hand pain scores in both RA and scleroderma: RA/CTS patients experienced a 46.8% reduction in pain scores from baseline (p=0.002) and scleroderma subjects a 66.7% reduction in pain from baseline (p=0.001). The intensity of the reduced pain scores between RA/CTS and scleroderma subjects were similar: absolute pain at outcome (p = 0.13), reduction in pain from baseline (p= 0.47), proportion of responders (p=0.15), proportion of non-responders (p=0.15), and duration of therapeutic effect (p=0.07). However, time to next procedure (re-injection or referral for surgery) was significantly longer in the scleroderma patients compared to the RA/CTS patients (RA/CTS: 5.2±3.1 months, Scleroderma: 8.5±3.0 months, p =0.011). 83% (10/12) scleroderma patients with painful hand noted reduced Raynaud’s phenomenon at 2 weeks, and 33% (4/12) noted stabilization and partial healing of digital ulcers. There were no complications in either group, and specifically no renal crisis in the scleroderma group.
Table 1.
Characteristics of Subjects with Painful Hand
| Rheumatoid Arthritis with Carpal Tunnel Syndrome | Painful Scleroderma Hand | Percent Difference | 95% Confidence Interval | P Value | |
|---|---|---|---|---|---|
| Number of Subjects | 14 | 12 | −14.3% | −24.3% to +21.5% | 0.5 |
| Age* | 43.0±8.1 | 42.7±8.3 | −6.9% | −16.2% to +14.8% | 0.96 |
| Female Gender | 78.6% (11/14) | 83.3% (10/12) | +6.0% | −33.9% to +42.7% | 0.82 |
| Subjects who Completed Study | 100% (14/14) | 100% (12/12) | 0% | −24.3% to +21.5% | 0.5 |
| Pre-Procedure Baseline Hand Pain (10 cm VAS)* | 7.9±1.9 | 6.6±2.5 | −16.5% | −64.6% to +31.6% | 0.65 |
Mean±Standard Deviation
Table 2.
Outcome of Injection of the Carpal Tunnel in Rheumatoid Arthritis and Scleroderma
| Rheumatoid Arthritis with Carpal Tunnel Syndrome | Painful Scleroderma Hand | ||||
|---|---|---|---|---|---|
| Number of Subjects | 14 | 12 | Percent Difference | 95% Confidence Interval | P Value |
| Pre-Procedure Baseline Pain (10 cmVAS)* | 7.9±1.9 cm | 6.6±2.5 cm | −16.5% | −64.6% to +31.6% | 0.16 |
| Procedural Pain (10 cmVAS)* | 2.8±4.1 cm | 2.0±1.8 cm | −28.6% | −123% to +65.7% | 0.52 |
| Pain at Outcome (2 weeks) (10 cm VAS)* | 4.4±4.2 cm | 2.2±2.8 cm | −46.9% | −117% to +16.8% | 0.13 |
| Reduction in Pain from Baseline (at 2 weeks) (10 cm VAS)* | 3.7±3.2 cm (−47% from baseline, p = 0.002) | 4.4±1.4 cm (−67% from baseline, 53.2%, p < 0.001) | +18.9% | −36.8% to +74.6% | 0.47 |
| Responders (10 cm VAS < 2 cm at 2 weeks) | 57.1% (8/14) | 83.3% (10/12) | +45.9 | −16.1% to +93.6% | 0.15 |
| Non- Responders (10 cm VAS ≥ 2 cm at 2 weeks) | 42.9% (6/14) | 16.7% (2/12) | −61.1% | −214% to +125% | 0.15 |
| Pain at Outcome (at 6 months) (10 cm VAS)* | 6.4±3.0 cm | 4.7±2.9 cm | −26.6% | −64.1% to +10.9% | 0.15 |
| Duration of Therapeutic Effect (months)* | 2.4±2.1 months | 4.0±2.2 months | +66.7% | −5.8% to +139% | 0.07 |
| Time to Next Procedure (months) (reinjection or referral to surgery)* | 5.2±3.1 months | 8.5±3.0 months | +53.5% | +15.8% to +111% | 0.011 |
Mean±Standard Deviation
Discussion
Presently, the painful scleroderma hand is a major cause of disability in scleroderma with few satisfactory therapeutic approaches [1–3,37]. The present study demonstrates that and scleroderma patients with painful hand respond well to hydrodissection and corticosteroid injection, resulting in a 66.7% reduction in pain scores at outcome with high proportion of responders (83.3%) and a low proportion of non-responders (16.7%) (Table 2). These results demonstrate that scleroderma patients with painful hand respond at least as well as RA/CTS patients do to hydrodissection and injection of the carpal tunnel (Table 2). It should be noted that RA/CTS patient had higher preinjection pain scores which potentially could have exaggerated the relative therapeutic effect in scleroderma patients; however, the majority of scleroderma patients noted not only a significant drop in the level hand pain, but 83% also noted reduced Raynaud’s phenomenon and 33% noted healing of digital ulcers. These findings are clinically important as Raynaud’s phenomenon and digital ulcers negatively impact hand function, disability and quality of life [2,3,37]. Furthermore, no complications were noted, and specifically no incidences of scleroderma renal crisis were associated with the procedure. This study suggests that hydrodissection with lidocaine followed by injection of triamcinolone acetonide is an alternative, but safe therapy for intractable hand pain in scleroderma.
As early as 1957 oral corticosteroids were shown to be effective to treat the painful sclerodermatous hand and carpal tunnel syndrome [18]. Subsequently, the use of injectable corticosteroids to treat primary or secondary carpal tunnel syndrome has become an established outpatient therapy, and has been shown to be effective for relief of pain and improvement in function, and in some cases can delay or obviate surgical decompression of the nerve [15, 9–22,38]. However, hydrodissection and corticosteroid injection of the scleroderma hand has not been used to any appreciable extent. Corticosteroid injection into the hand and wrist has been shown to be very safe, but rarely complications have been reported, including median nerve injury, vascular occlusion, digital necrosis, tendon rupture, cutaneous atrophy, inflammatory reaction, and injection pain [38–44]. Moreover, antecedent oral corticosteroid use in scleroderma has been associated with scleroderma renal crisis, although renal crisis from musculoskeletal injections has not been reported to date [16,17]. Fortunately, neither musculoskeletal or systemic complications were observed in either treatment group in the present study probably because the systemic concentration of injected corticosteroid remained well below the equivalent of the critical 20 to 30 mg prednisone daily dose most commonly associated with precipitating scleroderma renal crisis [16,17,44]. Thus, in this series corticosteroid injection of the carpal tunnel appeared to be safe.
The etiology of hand pain in scleroderma is believed to be complex and multifactorial, resulting from vasospasm, ischemia, arthritis, tenosynovitis, dysautonomia, and nerve entrapment [1–3,10–14]. Each of these individual etiologies might respond to hydrodissection and corticosteroid injection, although for different reasons. First, in 1957 Keith described that corticosteroids were useful in relieving the pain and paresthesias associated with the painful scleroderma hand, suggesting a real role for injection therapy, although this treatment does not seem to be used extensively subsequently [18]. Primary carpal tunnel syndrome itself is frequently associated with vasospasm and Raynaud’s phenomenon, occurring in as many as 50–60% of carpal tunnel syndrome patients, and these vasospastic symptoms appear to resolve with injection or decompressive therapy [12–14]. Similarly, there is a high incidence of carpal tunnel syndrome in patients with primary Raynaud’s phenomenon [12]. Furthermore, scleroderma, which is associated with fibrinous tenosynovitis and sclerosis of the dermal and subcutaneous tissues through which nerves and blood vessels travel has a definite incidence of carpal tunnel syndrome, entrapment of the small blood vessels, and fibrosis-associated dysautonomia [9–15]. Since hydrodissection frees fibrotically entrapped nerves, tendons, and blood vessels and corticosteroid injection atrophies this same resident connective tissue, and because corticosteroid-responsive tenosynovitis is common in scleroderma hand, it is not surprising that hydrodissection and corticosteroid injection might be effective for the treatment of the painful scleroderma hand [15,43,44] (Tables 1 and 2).
It is also possible that the beneficial effect of hydrodissection with lidocaine followed by corticosteroid injection in the painful scleroderma hand in the present study is not due to freeing entrapped structures, reducing compressive tenosynovitis, or reducing local inflammation, rather there could be a direct or indirect vasodilator response. This etiology is supported by the reduced incidence of Raynaud’s phenomenon after injection therapy. Other therapies directed at treating the vasospastic aspect of the painful scleroderma hand have been somewhat effective and have included calcium channel blockers, angiotensin converting enzyme inhibitors, prostanoids and prostacyclin analogs, endothelin receptor antagonists, and phosphodiesterase type-5 inhibitors as well as sympathetic blockage, sympathetectomy, microsurgical revascularization, arteriolysis, nerve blocks, neural stimulators, intrathecal infusion of analgesics, N-acetylcyseine, and botulinum toxin [1–11]. Thus, it may be that injected corticosteroids have a vasodilator effect due to a concentration-dependent vasodilator action on the peripheral vasculature [45].
It is also possible that the beneficial responses in the present study were not due to corticosteroid therapy of carpal tunnel syndrome or corticosteroid-induced vasodilation, but rather from the injected anesthesia, in this case injected lidocaine. Sympathectomy and nerve blocks have been shown to be beneficial for both Raynaud’s phenomenon and scleroderma hand, and it is possible that the beneficial effects noted were due to a local medical sympathectomy or nerve block caused by the lidocaine injected into the carpal tunnel [4–11]. Alternatively, since the scleroderma hand is characterized by contracture, fibrotic adhesions, and accumulation of dense connective tissue, this study used high pressure injection of fluid to lyse adhesions, open connective tissue spaces, define normal anatomic planes, and to separate and push away the tendinous, neural, and vascular structures from the needle tip (Figures 4 and 5). This maneuver, called hydrodissection, has been demonstrated to dilate the injection space at the needle tip freeing entrapped structures and, using a saline or lidocaine injectant, dissect and push critical structures (vein, artery, nerves, and tendons) away from the needle tip that thus preventing damage caused by direct injection of corticosteroid ester crystals [22–25].
The scleroderma hand is characterized by tendinous, arterial, and neural adhesions, fibrinous tenosynovitis, contracture, and accumulation of dense connective tissue [4,9,14,19]. For the flexors tendons of the hand and the median nerve, a mechanical choke point is the carpal tunnel, and thus the carpal tunnel is a natural target for mechanically freeing structures by hydrodissection and for atrophying excessive connective tissue with injected corticosteroid. Hydrodissection has two forms: 1) simply injecting larger amounts of fluid that force connective tissue planes open, or 2) the more sophisticated form where the needle tip positioning is frequently readjusted under sonographic guidance to separate individual structures and push away structures that could be damaged by the needle. Adjustment of the positioning of the needle tip should necessarily conservative; any movement that is tip to tissue causes direct cutting and tissue damage; with conservative hydrodissection the fluid injectate moves the tissue so there is minimal needle tip to tissue contact and thus much less tissue damage. Needle damage to tissues in the carpal tunnel must be minimized because of the concentrated critical structures (tendons, nerves, vascular structures) that reside in close proximity. The hydrodissection in this study was limited to the carpal tunnel, but we do have experience with more extensive hydrodissection of the individual flexor tendon sheaths in the palmar hand, of obstructing calcium deposits (calcinosis), and of contracted, severely fibrotic skin, and we are in the process of extending hydrodissection-injection studies to determine a potential larger role for hydrodissection in the treatment of the scleroderma hand.
Since peripheral nerves and vascular structures are specifically avoided visually by the ultrasound-guided hydrodissection technique, nerve or vascular injury being masked by the local anesthetic is much less a concern compared to palpation-guided anatomic landmark injections. The study design did not permit a differentiation of the relative contributions of hydrodissection, injected lidocaine, injected triamcinolone, or sonographic guidance in the scleroderma hand, but it is possible that a reduction in inflammation and tenosynovitis, atrophy of excessive connective tissue, freeing entrapped of nerves and blood vessels, and the vasodilator effects of corticosteroids and local anesthetic were all additive to the observed improved outcomes.
Although a number of proximal and distal variants exist, there are two basic approaches to injection of the carpal tunnel with ultrasound the transverse (short-axis or axial) ulnar approach as described by Smith et al (Figures 4–6), or the classic sagittal (long-axis or sagittal) approach by Grassi et al [20, 21] (Figures 1 and 2). The sagittal approach has the advantage of being able to dissect individual contracted tendon sheaths; the transverse ulnar approach has the advantage of visualization of anatomic structures of interest and the long axis of the entire needle including needle tip simultaneously. In the sagittal approach the needle can be visualized sagittally in the long-axis of the needle; however, the entire anatomy of the wrist cannot be visualized. The sagittal needle can also be viewed in a transverse image to see important anatomy where the needle appears as a pin-point; however, the position of the needle tip is uncertain and must be inferred, or the probe must be moved proximally and distally to locate the tip [21]. Although excellent results can achieved with either method, after considerable experience with both techniques, we feel that the transverse ulnar approach facilitates simultaneous precise imaging of relevant anatomy and an excellent view of the needle tip and we have adopted the transverse ulnar approach exclusively [20,21]. When using the transverse ulnar approach the operator must be careful to avoid the ulnar artery during the initial insertion, and make certain the needle is angled deeply enough to avoid the median nerve (Figures 3–5) [20].
In summary, hydrodissection of the carpal tunnel with 1% lidocaine followed by injection of 80 mg of triamcinolone acetonide markedly reduce pain and vasomotor changes in the scleroderma hand. Intractable hand pain, Raynaud’s phenomenon, and digital ulcerations in scleroderma may respond to hydrodissection of the carpal tunnel followed by injection with corticosteroid under sonographic guidance.
Supplementary Material
Under direct sonographic visualization the needle attached to a mechanical syringe is introduced to the ulnar side of the palmaris longus tendon. This is the sagittal (long-axis) injection position. The mechanical syringe, here held in the reverse position, injects by depressing the large plunger and aspirates by depressing the accessory plunger.
Footnotes
Author Disclosures: There was no industry-sponsored support for this study.
Disclosures: There was no industry support for this research. All devices and drugs were purchased, not donated to this study. Drs. Chavez-Chiang, Norton, DeLea, and Poole-none. Dr. Bankhurst is funded by a research grant from the Robert Wood Johnson Foundation. Dr. Wilmer L. Sibbitt, Jr. is funded by research grant RO1 HLO77422-01-A3 from the US National Institutes of Health and is an employee of the University of New Mexico. Dr. W. Sibbitt also is an expert consultant for Becton Dickinson, Inc., Intelligence Management Solutions, Inc., Ferring Pharmaceuticals, Inc., Avanca Medical Devices, Inc., Avasca Medical, Inc., and MediTech Duopross, Inc. Dr. Sibbitt holds stock in Apple Inc, Celgene Corp, Inc, Avanca, Inc, Avasca, Inc., Sun Microsystems, Inc, Symantec Corp, and Java, Inc. In the preceding 12 months Dr. Sibbitt has had 4 patents acquired by Abbott Vascular, Inc., but these patents do not relate to the present research.
References
- 1.Cuomo G, Zappia M, Abignano G, Iudici M, Rotondo A, Valentini G. Ultrasonographic features of the hand and wrist in systemic sclerosis. Rheumatology (Oxford) 2009;48:1414–7. doi: 10.1093/rheumatology/kep250. [DOI] [PubMed] [Google Scholar]
- 2.Avouac J, Walker U, Tyndall A, Kahan A, et al. Characteristics of joint involvement and relationships with systemic inflammation in systemic sclerosis: results from the EULAR Scleroderma Trial and Research Group (EUSTAR) database. J Rheumatol. 2010;37:1488–501. doi: 10.3899/jrheum.091165. [DOI] [PubMed] [Google Scholar]
- 3.Tagliafico A, Panico N, Serafini G, Ghio M, Martinoli C. The thickness of the A1 pulleys reflects the disability of hand mobility in scleroderma. A pilot study using high-frequency ultrasound. Eur J Radiol. 2010 doi: 10.1016/j.ejrad.2010.05.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Dhillon S. Bosentan: a review of its use in the management of digital ulcers associated with systemic sclerosis. Drugs. 2009;69:2005–24. doi: 10.2165/10489160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Fregene A, Ditmars D, Siddiqui A. Botulinum toxin type A: a treatment option for digital ischemia in patients with Raynaud’s phenomenon. J Hand Surg Am. 2009;34:446–52. doi: 10.1016/j.jhsa.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Hashem M, Lewis R. Successful long-term treatment of a patient with longstanding Raynaud’s disease by an extradural bupivacaine block. Anaesth Intensive Care. 2007;35:618–9. [PubMed] [Google Scholar]
- 7.Sylaidis P, Logan A. Local injection of phentolamine to treat digital ischaemic necrosis in Raynaud’s syndrome. J Wound Care. 1997;6:356–7. doi: 10.12968/jowc.1997.6.8.356. [DOI] [PubMed] [Google Scholar]
- 8.Willerson JT, Thompson RH, Hookman P, Herdt J, Decker JL. Reserpine in Raynaud’s disease and phenomenon. Short-term response to intra-arterial injection. Ann Intern Med. 1970;72:17–27. doi: 10.7326/0003-4819-72-1-17. [DOI] [PubMed] [Google Scholar]
- 9.Jones NF, Imbriglia JE, Steen VD, Medsger TA. Surgery for scleroderma of the hand. J Hand Surg Am. 1987;12:391–400. doi: 10.1016/s0363-5023(87)80012-6. [DOI] [PubMed] [Google Scholar]
- 10.Tomaino MM, Goitz RJ, Medsger TA. Surgery for ischemic pain and Raynaud’s’ phenomenon in scleroderma: a description of treatment protocol and evaluation of results. Microsurgery. 2001;21:75–9. doi: 10.1002/micr.1013. [DOI] [PubMed] [Google Scholar]
- 11.Klyscz T, Jünger M, Meyer H, Rassner G. Improvement of acral circulation in a patient with systemic sclerosis with stellate blocks. Vasa. 1998;27:39–42. [PubMed] [Google Scholar]
- 12.Mondelli M, Romano C, De Stefano R, Cioni R. Nerve conduction velocity study of the upper limb in Raynaud’s phenomenon. Rheumatol Int. 2000;19:165–9. doi: 10.1007/s002960000049. [DOI] [PubMed] [Google Scholar]
- 13.Pal B, Keenan J, Misra HN, Moussa K, Morris J. Raynaud’s phenomenon in idiopathic carpal tunnel syndrome. Scand J Rheumatol. 1996;25:143–5. doi: 10.3109/03009749609080004. [DOI] [PubMed] [Google Scholar]
- 14.Medsger TA., Jr Natural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-being. Rheum Dis Clin North Am. 2003;29:255–73. doi: 10.1016/s0889-857x(03)00023-1. [DOI] [PubMed] [Google Scholar]
- 15.Ly-Pen D, Andreu JL, de Blas G, Sanchez-Olaso A, Millan I. Surgical decompression versus local steroid injection in carpal tunnel syndrome: a one-year, prospective, randomized, open, controlled clinical trial. Arthritis Rheum. 2005;52:612–9. doi: 10.1002/art.20767. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira L, Mouthon L, Mahr A, Berezné A, Agard C, Mehrenberger M, Noël LH, Trolliet P, Frances C, Cabane J, Guillevin L Group Français de Recherche sur le Sclérodermie (GFRS) . Mortality and risk factors of scleroderma renal crisis: a French retrospective study of 50 patients. Ann Rheum Dis. 2008;67:110–6. doi: 10.1136/ard.2006.066985. [DOI] [PubMed] [Google Scholar]
- 17.Steen VD, Medsger TA., Jr Case-control study of corticosteroids and other drugs that either precipitate or protect from the development of scleroderma renal crisis. Arthritis Rheum. 1998;41:1613–9. doi: 10.1002/1529-0131(199809)41:9<1613::AID-ART11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 18.Keith MA. Acroparaesthesiae in sclerodactyly and carpal tunnel syndrome treated with prednisolone. Scott Med J. 1957;2:435–8. doi: 10.1177/003693305700201106. [DOI] [PubMed] [Google Scholar]
- 19.Tagliafico A, Panico N, Resmini E, Derchi LE, Ghio M, Martinoli C. The role of ultrasound imaging in the evaluation of peripheral nerve in systemic sclerosis (scleroderma) Eur J Radiol. 2009 doi: 10.1016/j.ejrad.2009.08.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Smith J, Wisniewski SJ, Finnoff JT, Payne JM. Sonographically guided carpal tunnel injections: the ulnar approach. J Ultrasound Med. 2008;27:1485–90. doi: 10.7863/jum.2008.27.10.1485. [DOI] [PubMed] [Google Scholar]
- 21.Grassi W, Farina A, Filippucci E, Cervini C. Intralesional therapy in carpal tunnel syndrome: a sonographic-guided approach. Clin Exp Rheumatol. 2002;20:73–6. [PubMed] [Google Scholar]
- 22.McNally E. Musculoskeletal interventional ultrasound. In: McNally E, editor. Practical Musculoskeletal Ultrasound. New York, NY: Elsevier; 2005. pp. 293–94. [Google Scholar]
- 23.Gimbel HV. Hydrodissection and hydrodelineation. Int Ophthalmol Clin. 1994;34:73–90. doi: 10.1097/00004397-199403420-00006. [DOI] [PubMed] [Google Scholar]
- 24.Mejia R, Saxena P, Tam RK. Hydrodissection in redo sternotomies. Ann Thorac Surg. 2005;79:363–4. doi: 10.1016/j.athoracsur.2003.10.096. [DOI] [PubMed] [Google Scholar]
- 25.Sibbitt WL, Jr, Peisajovich A, Michael AA, Park KS, Sibbitt RR, Band PA, Bankhurst AD. Does sonographic guidance influence the outcome of intraarticular injections? J Rheumatol. 2009;36:1892–902. doi: 10.3899/jrheum.090013. [DOI] [PubMed] [Google Scholar]
- 26.Moorjani GR, Bedrick EJ, Michael AA, Peisjovich A, Sibbitt WL, Jr, Bankhurst AD. Integration of safety technologies into rheumatology and orthopedic practices A randomized controlled trial. Arthritis Rheum. 2008;58:1907–14. doi: 10.1002/art.23499. [DOI] [PubMed] [Google Scholar]
- 27.Lonzetti LS, Joyal F, Raynauld JP, Roussin A, Goulet JR, Rich E, Choquette D, Raymond Y, Senécal JL. Updating the American College of Rheumatology preliminary classification criteria for systemic sclerosis: addition of severe nailfold capillaroscopy abnormalities markedly increases the sensitivity for limited scleroderma. Arthritis Rheum. 2001;44:735–6. doi: 10.1002/1529-0131(200103)44:3<735::AID-ANR125>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.The American College of Rheumatology. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 29.Miller MD, Ferris DG. Measurement of subjective phenomena in primary care research: the Visual Analogue Scale. Fam Pract Res J. 1993;13:15–24. [PubMed] [Google Scholar]
- 30.Katz J, Melzack R. Measurement of pain. Surg Clin North Am. 1999;79:231–52. doi: 10.1016/s0039-6109(05)70381-9. [DOI] [PubMed] [Google Scholar]
- 31.Moorjani GR, Michael AA, Peisjovich A, Park KS, Sibbitt WL, Jr, Bankhurst AD. Patient pain and tissue trauma during syringe procedures: A randomized controlled trial. J Rheumatology. 2008;35:1124–9. [PubMed] [Google Scholar]
- 32.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 33.El Miedany Y, Ashour S, Youssef S, Mehanna A, Meky FA. Clinical diagnosis of carpal tunnel syndrome: Old tests-new concepts. Joint Bone Spine. 2008;75:451–7. doi: 10.1016/j.jbspin.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Wilder-Smith EP, Seet RC, Lim EC. Diagnosing carpal tunnel syndrome--clinical criteria and ancillary tests. Nat Clin Pract Neurol. 2006;2:366–74. doi: 10.1038/ncpneuro0216. [DOI] [PubMed] [Google Scholar]
- 35.Sibbitt WL, Jr, Sibbitt RR, Michael AA, Fu DI, Draeger HT, Twining JM, Bankhurst AD. Physician control of needle and syringe during traditional aspiration-injection procedures with the new reciprocating syringe. J Rheumatol. 2006;33:771–8. [PubMed] [Google Scholar]
- 36.Sibbitt RR, Sibbitt WL, Jr, Nunez SE, Kettwich LG, Kettwich SC, Bankhurst AD. Control and performance characteristics of eight different suction biopsy devices. J Vasc Interv Radiol. 2006;17:1657–69. doi: 10.1097/01.RVI.0000236837.47302.8E. [DOI] [PubMed] [Google Scholar]
- 37.Mouthon L, Mestre-Stanislas C, Berezne A, Rannou F, Guilpain P, Revel M, et al. Impact of digital ulcers on disability and health-related quality of life in systemic sclerosis. Ann Rheum Dis. 2010;69:214–217. doi: 10.1136/ard.2008.094193. [DOI] [PubMed] [Google Scholar]
- 38.Kasten SJ, Louis DS. Carpal tunnel syndrome: a case of median nerve injection injury and a safe and effective method for injecting the carpal tunnel. J Fam Pract. 1996;43:79–82. [PubMed] [Google Scholar]
- 39.Frederick HA, Carter PR, Littler JW. Injection injuries to the median and ulnar nerves at the wrist. J Hand Surg [Am] 1992;17:645–7. doi: 10.1016/0363-5023(92)90309-d. [DOI] [PubMed] [Google Scholar]
- 40.Payne JM, Brault JS. Digital ischemia after carpal tunnel injection: a case report. Arch Phys Med Rehabil. 2008;89:1607–10. doi: 10.1016/j.apmr.2007.11.064. [DOI] [PubMed] [Google Scholar]
- 41.Cherasse A, Kahn MF, Mistrih R, Maillard H, Strauss J, Tavernier C. Nicolau’s syndrome after local glucocorticoid injection. Joint Bone Spine. 2003;70:390–2. doi: 10.1016/s1297-319x(03)00137-4. [DOI] [PubMed] [Google Scholar]
- 42.Friedman SJ, Butler DF, Pittelkow MR. Perilesional linear atrophy and hypopigmentation after intralesional corticosteroid therapy. Report of two cases and review of the literature. J Am Acad Dermatol. 1988;19:537–41. doi: 10.1016/s0190-9622(88)70209-1. [DOI] [PubMed] [Google Scholar]
- 43.Derendorf H, Möllmann H, Grüner A, Haack D, Gyselby G. Pharmacokinetics and pharmacodynamics of glucocorticoid suspensions after intra-articular administration. Clin Pharmacol Ther. 1986;39:313–7. doi: 10.1038/clpt.1986.45. [DOI] [PubMed] [Google Scholar]
- 44.Frey BM, Walker C, Frey FJ, de Weck AL. Pharmacokinetics and pharmacodynamics of three different prednisolone prodrugs: effect on circulating lymphocyte subsets and function. J Immunol. 1984;33:2479–87. [PubMed] [Google Scholar]
- 45.Sellevold OF, Jynge P. Steroids and cardioplegia: effects of glucocorticoids upon vascular resistance during cardioplegic perfusion. Thorac Cardiovasc Surg. 1987;35:307–11. doi: 10.1055/s-2007-1020252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Under direct sonographic visualization the needle attached to a mechanical syringe is introduced to the ulnar side of the palmaris longus tendon. This is the sagittal (long-axis) injection position. The mechanical syringe, here held in the reverse position, injects by depressing the large plunger and aspirates by depressing the accessory plunger.