Summary
Following intracellular replication, the apicomplexan parasites Plasmodium falciparum and Toxoplasma gondii cause host cell cytolysis to facilitate parasite release and disease progression. Parasite exit from infected cells requires the interplay of parasite-derived proteins and host actin cytoskeletal changes; however, the host proteins underlying these changes remain obscure. We report the identification of a Gαq-coupled host-signaling cascade required for the egress of both P. falciparum and T. gondii. Gαq-coupled signaling results in protein kinase C (PKC)-mediated loss of the host cytoskeletal protein adducin and weakening of the cellular cytoskeleton. This cytoskeletal compromise induces catastrophic Ca2+ influx mediated by the mechanosensitive cation channel TRPC6, which activates host calpain that proteolyzes the host cytoskeleton allowing parasite release. Reinforcing the feasibility of targeting host proteins as an antiparasitic strategy, mammalian PKC inhibitors demonstrated activity in murine models of malaria and toxoplasmosis. Importantly, an orally bioavailable PKC inhibitor prolonged survival in an experimental cerebral malaria model.
Introduction
The protozoan parasites Plasmodium falciparum and Toxoplasma gondii cause significant morbidity and mortality worldwide. Plasmodium species collectively cause over 500 million clinical cases of malaria per year, which result in approximately one million deaths (Hay et al., 2009), while the related apicomplexan parasite (T. gondii) infects over 30% of the world's population (Weiss and Dubey, 2009). Following host cell invasion, these intracellular parasites initiate a lytic cycle within human hosts, in which they grow and replicate in a specialized parasitophorous vacuole (PV) prior to host cell cytolysis at ∼48 hr postinvasion (hpi; Joiner et al., 1994). The PV and host membranes are destroyed upon cytolysis and parasite release, which results in infection of new host cells and continuation of the intracellular cycle. The molecular details governing parasite-mediated cytolysis are incompletely understood, and the elucidation of this pathway may provide drug targets since parasites trapped in the host cell cannot proliferate (Chandramohanadas et al., 2009; Dvorin et al., 2010; Kafsack et al., 2009).
Parasite-induced host cell cytolysis has been suggested to be a two-step, Ca2+-dependent process (Salmon et al., 2001; Wickham et al., 2003) requiring both host cell ion loss (Moudy et al., 2001) and increased membrane poration (Abkarian et al., 2011; Glushakova et al., 2010). Recent reports implicate the activity of parasite-derived proteins including a perforin-like protein (Kafsack et al., 2009), a kinase (Dvorin et al., 2010), and proteases (Arastu-Kapur et al., 2008; Yeoh et al., 2007), though reports of host protein contribution to this process are limited. We have recently shown that the host-derived protease calpain is required for exit of both P. falciparum and T. gondii and functions to proteolyze the actin cytoskeleton just prior to cytolysis (Chandramohanadas et al., 2009; Millholland et al., 2011). Furthermore, we have recently shown calpain-independent loss of adducin from the host erythrocyte actin cytoskeleton prior to P. falciparum exit (Millholland et al., 2011). Given the temporal separation of these striking changes to the host cytoskeleton during the last phase of the intracellular cycle, we hypothesized that a complex host pathway may connect these changes and result in the disintegration of the host plasma membrane. Identification of host proteins necessary for parasite infection may offer an untapped resource of antiparasitic targets.
Results
Host RNAi Screen Reveals a Pathway Necessary for T. gondii-Mediated Cytolysis
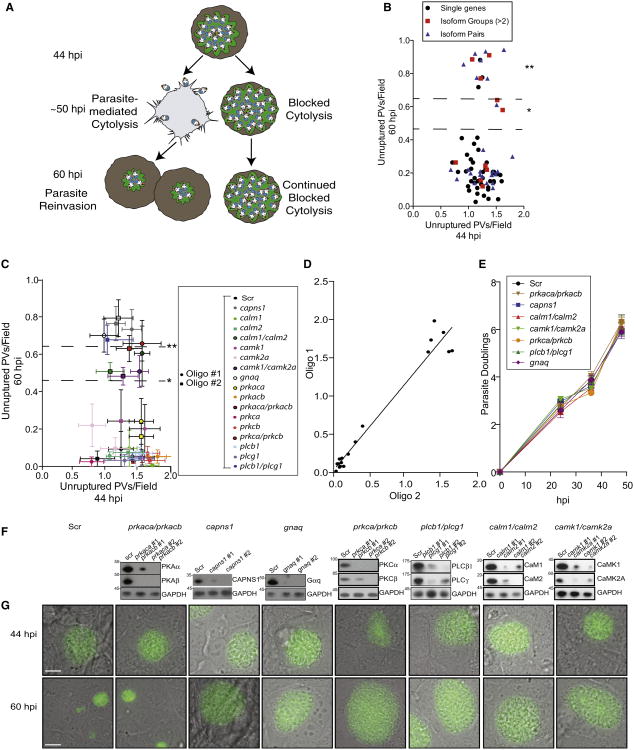
To identify host genes essential for parasite-mediated cytolysis, we performed an RNAi screen in U2OS cells (highly transfectable T. gondii host cells) focused on canonical Ca2+-signaling components, given our earlier studies that implicated host calpain in T. gondii and P. falciparum exit (Chandramohanadas et al., 2009; Millholland et al., 2011). To circumvent the potential for functional redundancy between host gene isoforms, we tested pooled small interfering RNAs (siRNAs) for simultaneous knockdown of gene families as well as individual genes present in both U2OS cells and erythrocytes. This primary screen included 45 individual-gene knockdowns and 11 multi-isoform knockdowns (Table S1, sheets 1 and 2). Pooled siRNAs (three siRNAs/gene) were arrayed in quadruplicate, reverse transfected into U2OS cells, and infected 24 hr posttransfection with transgenic T. gondii tachyzoites (moi of 0.1) that constitutively secrete GFP into the PV space, allowing for facile identification of parasite doublings as a function of life cycle progression (P30-GFP; Figure 1A; Figure S1 available online) (Striepen et al., 1998). siRNAs against calpain small subunit 1 (capns1) and a scrambled oligo (Scr) were included as positive and negative controls, respectively. Knockdown efficiency was maximal at 72 hr posttransfection, which corresponded with parasite-mediated cytolysis in this cell type (∼50 hpi). Following synchronous T. gondii infection (Kafsack et al., 2004), plates were fixed and imaged at 6, 24, 44, or 60 hpi to assess parasite life cycle progression through cytolysis. Genes involved in parasite-mediated cytolysis showed an accumulation of PVs containing >64 parasites at 60 hpi upon knockdown (Z score of +1.5, p < 0.05), ∼10 hr after host cell rupture typically occurs in this cell type.
Figure 1. siRNA Screen Identifies Signaling Components Required for T. gondii-Mediated Cytolysis.
(A) Model of host siRNA screen for mediators of parasite-induced cytolysis. Defects in T. gondii-mediated cytolysis are scored at 60 hpi. Parasites unable to exit persist as vacuoles with >64 parasites, while parasites able to exit host cells reinvade new cells.
(B) Primary siRNA screen in U2OS cells. Unruptured vacuoles (intact vacuoles with >64 parasites) are compared at 44 and 60 hpi to identify host gene involvement in parasite release (*p < 0.05; **p < 0.01). Data shown are mean unruptured vacuoles per field ± SEM.
(C) Secondary screen utilizing multiple oligos validates individual host genes and gene pairs whose knockdown resulted in accumulation of unruptured vacuoles by 60 hpi. Data shown are mean unruptured vacuoles per field ± SEM.
(D) Pearson correlation graph indicates strong relation between siRNA oligos (r = 0.91).
(E) Measurements of parasite doublings within stable shRNA knockdown cell lines.
(F) Western blot analysis confirms shRNA-mediated stable knockdown of target proteins, as compared to Scr oligo.
(G) Representative images of P30-GFP vacuoles in stable knockdown cell lines at 44 and 60 hpi. Also see Figure S1 and Table S1.
From this screen, we initially identified five gene families whose knockdown blocked parasite-mediated host cell cytolysis: calmodulin (CaM), Ca2+/calmodulin-dependent kinases (CaMK), Gα subunits (Gα), phospholipase C (PLC), and protein kinase C (PKC) (Figure 1B). To deconvolute the specific genes required for parasite-mediated cytolysis from these gene family hits, we tested two unique siRNAs targeting individual and pairs of genes (Figure 1C; Table S1, sheet 3). We determined that single knockdown of gnaq (p < 0.002) and knockdown of the gene pairs prkca/prkcb (p < 0.002), plcb1/plcg1 (p < 0.002), calm1/calm2 (p < 0.05), and camk1/camk2a (p < 0.05) showed a statistically significant and correlated accumulation of unruptured PVs at 60 hpi (r = 0.91; Figure 1D). Stable small hairpin RNA (shRNA)-mediated knockdown of these genes had no effect on parasite doublings, reinforcing their function in cytolysis rather than parasite replication (Figure 1E). shRNA knockdown efficiency using multiple oligos was measured by western blot analysis of total protein as compared to scr (Figure 1F). Representative images are shown for shRNA knockdown cell lines that resulted in an accumulation of unruptured PVs in comparison to controls (scr) or negatives (prkaca/prkacb) that show newly reinvaded parasites at 60 hpi (Figure 1G).
Immunodepletion Studies Indicate that Conserved Host Proteins Function in P. falciparum-Mediated Cytolysis
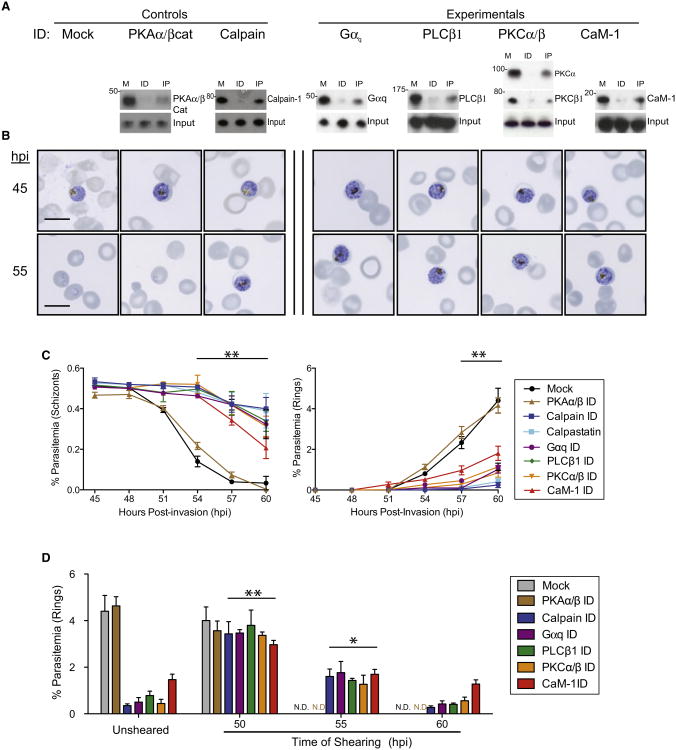
We next investigated whether the protein products of the validated gene hits in T. gondii functioned similarly in P. falciparum-induced erythrocyte cytolysis via antibody-mediated depletion studies. To do this, erythrocytes were hypotonically lysed prior to incubation with target antibodies preconjugated to sepharose beads. Removal of the target protein-antibody conjugates was achieved via centrifugation, and manipulated erythrocytes were hypertonically resealed prior to P. falciparum infection. Efficiency of target protein immunodepletion in erythrocytes was determined by western blot, and only immunodepletions showing >80% knockdown were further analyzed (Figure 2A; Chandramohanadas et al., 2009). Synchronous P. falciparum parasites were assessed for life cycle progression by Giemsa smear (Figure 2B) and flow cytometry of DNA content (Figure 2C; Figure S2) to distinguish blocked multinucleated schizonts from newly invaded, ring-form parasites with a single nucleus. Immunodepletion of Gαq, PLCβ1, CaM-1, or the simultaneous immunodepletion of both PKCα and PKCβ resulted in an accumulation of schizont-stage parasites and a lack of newly invaded rings at 60 hpi, indicating a block in parasite-mediated cytolysis and corroborating the results of our RNAi screen in T. gondii host cells. To assess parasite viability, trapped parasites were mechanically released from depleted host cells and assessed for their invasive capacity via flow cytometric quantitation of mononuclear ring-stage parasites 12 hr following mechanical release (Figure 2D). Parasites trapped within erythrocytes were able to recover invasive capacity upon needle shearing only up to 54 hpi, indicating that parasite death likely occurs following 6 hr of blocked cytolysis.
Figure 2. Conserved Function of Host Proteins in P. falciparum-Mediated Cytolysis.
(A) Immunodepletion studies of soluble erythrocyte protein hits confirmed by western blot (M, mock; ID, immunodepletion; IP, elution of target protein immunoprecipitation).
(B) Representative Giemsa images following immunodepletion of host Gαq, PLCβ1, PKCα/PKCβ, and CaM-1 results in persistence of schizont-stage parasites by 60 hpi versus mock or PKAα/PKAβcat depletion, which resulted in exit and reinvasion by 60 hpi.
(C) Flow cytometric quantitation of schizont-stage (left) and ring-stage (right) parasites in depleted erythrocytes. Data shown are means of at least three experiments ± SEM (**p < 0.01).
(D) Ring parasitemia assessed 12 hr following needle shearing of infected and depleted erythrocytes at the times indicated (ND, not determined; **p < 0.01; *p < 0.05). Data shown are means of at least three experiments ± SEM. Also see Figure S2.
Host PKC Is Activated during Schizogony to Remove Adducin from the Host Cytoskeleton
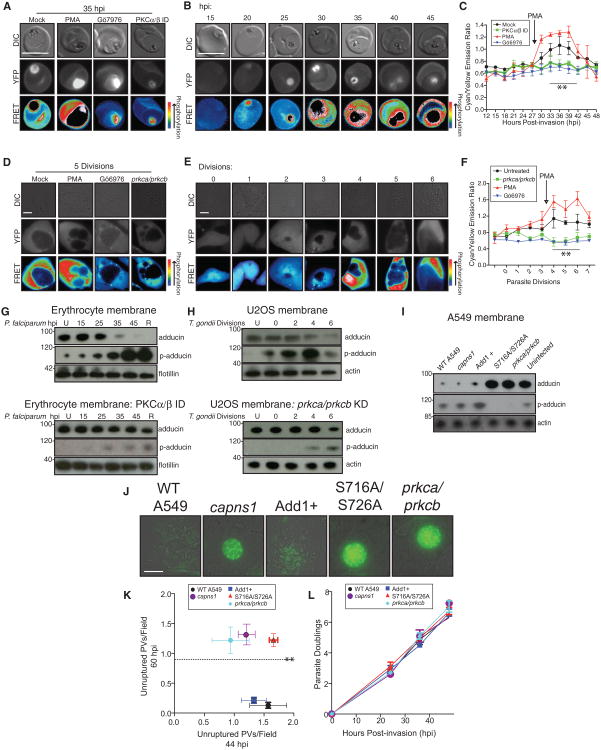
As PKC is a major downstream effector of Gαq-coupled GPCR signaling via PLC-mediated generation of diacylglycerol (DAG; Castagna et al., 1982; Rhee et al., 1989) and PKC inhibitors showed potent antiparasitic activity and the ability to block parasite-mediated cytolysis in vitro (Figure S3A), we were interested in understanding the role of this critical host enzyme in parasite life cycle progression. To measure host PKC activity during the P. falciparum life cycle, we generated a transgenic P. falciparum line that secretes a FRET-based PKC activity indicator (CKAR; Violin et al., 2003) into the infected erythrocyte cytoplasm (3D7-CKAR) via expression of a chimeric construct with an N-terminal signal peptide and Plasmodium export element (PEXEL) motif (Gallegos et al., 2006; Hiller et al., 2004; Marti et al., 2004). Figure 3A displays the specificity of the CKAR reporter for PKC activity with representative positive control FRET images (maximal FRET signal) of 3D7-CKAR-infected erythrocytes treated with the PKC agonist phorbol myristate acetate (PMA) and negative control images (minimal FRET signal) upon treatment with the PKC inhibitor Gö6976 or PKC immunodepletion prior to infection. As with any protein present in the erythrocyte cytoplasm, a significant amount of CKAR was taken up into the parasite digestive vacuole, causing an intense CKAR FRET signal within this organelle likely due to intermolecular FRET. In order to account for this artifact in our measurements of host PKC activity, 3D7-CKAR parasites were removed from their host cells and intracellular FRET signal was quantified to assess this background signal within the digestive vacuole (Figure S3C). By FRET microscopy (Figure 3B) and background-corrected fluorometry to remove digestive vacuole signal (Figure 3C), we show limited host PKC activity throughout the ring and trophozoite stages (0–25 hpi) with a sharp increase during schizogony (30–40 hpi). Immunodepletion of Gαq or PLCβ1 ablates PKC activity during the entire infectious cycle, reinforcing the importance of the host Gαq signaling pathway upstream of PKC activation (Figure S3D). PMA restored PKC activity in these parasite-infected erythrocytes depleted of signaling components and also rescued the associated exit defect (Figure S3E), further indicating a critical role for host PKC. PKC activity-reporter cell lines were also generated via stable expression of CKAR in U2OS host cells prior to T. gondii infection. The CKAR signal output throughout the T. gondii life cycle in these reporter U2OS cells largely mirrored the results obtained in P. falciparum, with maximal FRET signal occurring in the last third of the intracellular life cycle (Figures 3D–3F). Knockdown of host gnaq or plcb1/plcg1 ablates PKC activity, but this activity was restored by the PKC agonist PMA (Figures S3H and S3I).
Figure 3. Host PKC Is Activated Late in the Parasite Intracellular Cycle and Abrogates Host Adducin Cytoskeletal Association.
(A–C) Assessment of host PKC activity through imaging and fluorometry of 3D7-CKAR P. falciparum parasites. (A) Representative control FRET images at 35 hpi. Positive control, 100 nM PMA; negative controls, PKCα/PKCβ depletion or 500 nM Gö6976. (B) Representative FRET images through the P. falciparum intracellular cycle. (C) CFP/YFP FRET fluorometry as a measurement of host PKC activity throughout the P. falciparum cycle. Data shown are mean fluorescence of at least three experiments ± SEM (**p < 0.01).
(D–F) Assessment of host PKC activity through imaging and fluorometry of T. gondii-infected U2OS cells expressing cytoplasmic CKAR. (D) Representative control FRET images at five parasite divisions.Positive control, 100nM PMA; negative controls, PKCα/PKCβ knockdown and 500 nM Gö6976. (E) Representative FRET images through the T. gondii intracellular cycle. (F) CFP/YFP FRET fluorometry as a measurement of host PKC activity throughout the T. gondii cycle. Data shown are mean fluorescence of at least three experiments ± SEM (**p < 0.01).
(G) Western blot analysis of erythrocyte cytoskeletal fraction through P. falciparum cycle in mock-treated erythrocytes (top) or erythrocytes depleted of PKCα/PKCβ (bottom). Loading control, actin.
(H) Western blot analysis of U2OS cytoskeletal fraction through T. gondii cycle in mock-treated cells (top) or PKCα/PKCβ-stable knockdown cells (bottom). Loading control, actin.
(I) Western blot analysis of A549 cytoskeletal fractions upon adducin mutant expression, following T. gondii infection at 44 hpi, compared to capns1 or prkca/prkcb stable knockdown. Add1+, adducin overexpression; S716A/S726A, PKC phospho mutant adducin expression; loading control, actin.
(J) Representative 60 hpi images of T. gondii vacuoles at 60 hpi following host cell manipulation as in (I).
(K) Quantitation of unruptured vacuoles at 60 versus 44 hpi following host cell manipulation as in (I). Data shown are means of at least four experiments ± SEM (**p < 0.01).
(L) Parasite doublings throughout life cycle following host cell manipulation as in (I). Also see Figure S3.
We have previously shown that the host cytoskeletal protein adducin is lost from the erythrocyte actin cytoskeleton at ∼35 hpi of P. falciparum in a calpain-independent manner (Millholland et al., 2011). As adducin cytoskeletal association is regulated by the PKC-mediated phosphorylation of residues S716/S726 (Matsuoka et al., 1996), we assessed PKC depletion on host adducin cytoskeletal association during parasite infection. Western blot and immunofluorescence analysis of host cytoskeletal fractions confirms adducin disappearance from both P. falciparum-infected erythrocytes (Figure 3G, top; Figure S3F) and T. gondii-infected U2OS cells late in the intracellular cycle (Figure 3H, top; Figure S3G). However, PKCα/PKCβ immunodepletion from erythrocytes (Figure 3G, bottom) or shRNA knockdown in U2OS cells (Figure 3H, bottom) abrogates this loss and maintains adducin cytoskeletal association through the end of both parasite life cycles. The S716/S726 phosphorylated adducin species (p-adducin) accumulates in mock-treated cells in the last third of the life cycle by western blot while the unphosphorylated form is lost from cytoskeletal fractions, unless PKCα/PKCβ is depleted from host cells. Gαq or PLCβ1 immunodepletion from erythrocytes (Figure S3F) or knockdown in U2OS cells (Figure S3G) also caused adducin persistence within the host cytoskeleton, highlighting the function of this upstream cascade.
In order to examine the effect of adducin persistence on T. gondii life cycle progression, we overexpressed the PKC phosphomutant adducin (S716A/S726A; Matsuoka et al., 1998) as well as wild-type adducin in host A549 cells. S716A/S726A adducin expression resulted in minimal phosphorylation by western blot upon T. gondii infection (Figure 3I), while wild-type adducin overexpressors show an abundance of p-adducin. Similar to PKCα/PKCβ stable knockdown, S716A/S726A adducin expression resulted in the persistence of enlarged vacuoles by 60 hpi (Figures 3J and 3K), with no effect on parasite replication (Figure 3L), suggesting that PKC-induced adducin loss directly mediates host cell cytolysis. Tachyzoites trapped within host cells show reduced viability following 18–24 hr of blocked cytolysis within host cells expressing the adducin phosphomutant S716A/S726A, similar to PKC inhibition or knockdown (Figure S3B), highlighting the feasibility of targeting this phase of the parasite life cycle as an antiparasitic strategy.
Host Signaling Cascade Is Required for Calpain-Mediated Cytoskeletal Proteolysis
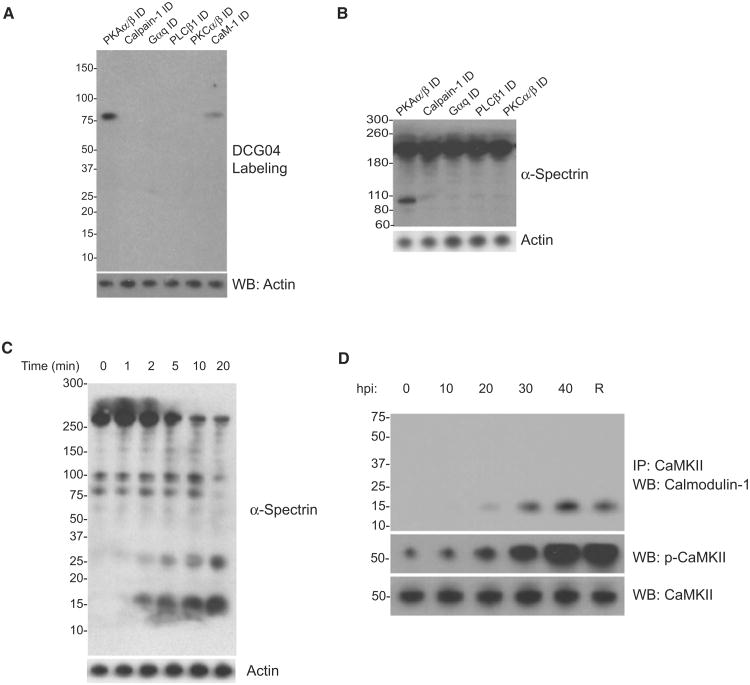
Host calpain activation has been shown to result in cytoskeletal proteolysis necessary for parasite exit (Chandramohanadas et al., 2009; Millholland et al., 2011). We thus assessed calpain activation following immunodepletion of signaling components from erythrocytes using an activity-based probe for cysteine proteases, DCG-04 (Figure 4A; Greenbaum et al., 2000). Similar to positive control calpain-immunodepleted erythrocytes, host cells lacking PKCα/PKAβ, PLCβ1, or Gαq show no active calpain labeling at the conclusion of the parasite intracellular cycle, suggesting that these proteins function upstream of host calpain in the signaling cascade. Negative control cells, which were immunodepleted of PKAα/PKAβ, show labeling of active calpain at the conclusion of the parasite intracellular cycle. CaM-1 immunodepletion resulted in diminished calpain activation, indicating that CaM-1 may facilitate this process. As a corollary to calpain activation, we examined spectrin cleavage via western blot, a key calpain substrate. Immunodepletion of pathway components abrogates spectrin proteolysis in P. falciparum-infected erythrocytes at 50 hpi, while PKAα/PKAβ-immunodepleted cells show multiple spectrin fragments (Figure 4B). Interestingly, in vitro incubation of U2OS cell membrane fractions with activated CaMKII prior to incubation with calpain resulted in multiple spectrin cleavage products (Figure 4C), suggesting that CaMKII phosphorylation of cytoskeletal substrates may enhance calpain-mediated proteolysis. CaMKII activation is shown by CaM-1 coimmunoprecipitation and autophosphorylation by p-CaMKII western blot analysis near the end of the T. gondii life cycle (Figure 4D), suggesting that host CaMKII activity may enhance cytoskeletal proteolysis to facilitate parasite release.
Figure 4. Host Signaling Cascade Enhances Calpain-Mediated Cytoskeletal Proteolysis Just Prior to Parasite Exit.
(A) DCG-04-labeling of active calpain in P. falciparum-infected erythrocyte fractions at 50 hpi in cells depleted of Gαq, PLCβ1, PKCα/PKCβ, and CaM-1 by immunodepletion. Biotin blot confirms calpain activity upon labeling. Negative control, Calpain-1 depletion; positive control, PKAα/PKAβ depletion; loading control, actin.
(B) α-Spectrin cleavage by western blot at 50 hpi in erythrocyte cytoskeletal fractions following depletion of signaling components.
(C) α-Spectrin cleavage by western blot following in vitro incubation of U2OS cell cytoskeletal fractions with activated CaMKII prior to incubation with recombinant human calpain-1.
(D) Host CAMKII/calmodulin-1 coimmunoprecipitation through the T. gondii life cycle and presence of p-CAMKII (the activated form) by western blot.
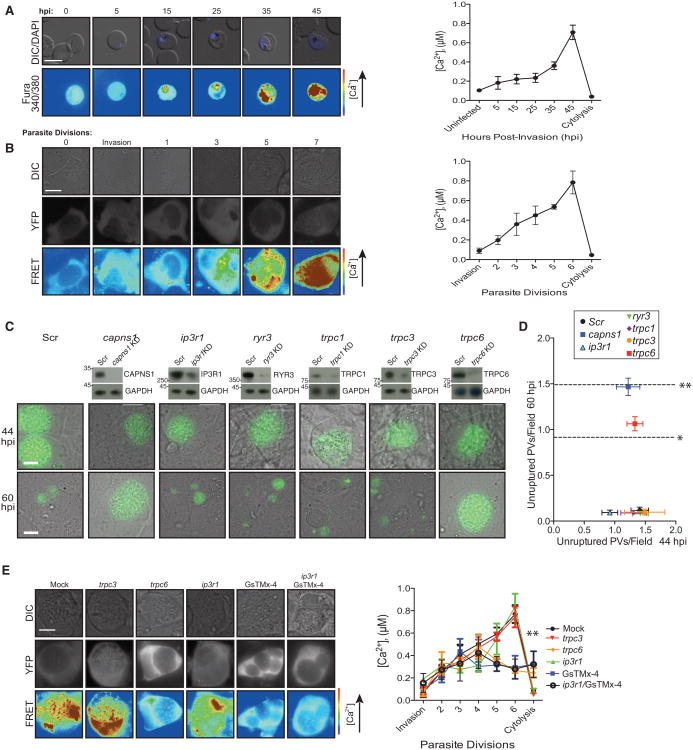
Analysis of Host Ca2+ Dynamics Implicates TRPC6 in Ca2+ Influx
Finally, we determined changes in host [Ca2+] that drive this network. To assess erythrocyte [Ca2+] during P. falciparum infection, we confined a Ca2+ indicator to the erythrocyte space via loading of a fura-2-dextran conjugate. Within the U2OS cell cytoplasm during T. gondii infection, Ca2+ measurements were achieved via expression of the ratiometric FRET-based Ca2+ indicator yellow cameleon 3.6 (YC3.6) in U2OS cells (Nagai et al., 2004; Palmer and Tsien, 2006). By microscopy and background-corrected fluorometry (Figures 5A and 5B), we show the relative changes in host cell [Ca2+] to be similar between both parasite systems, with host [Ca2+] hovering near resting levels (100 nM) throughout the first two-thirds of the life cycle, followed by a sharp increase in [Ca2+] at the point of parasite release.
Figure 5. Host [Ca2+] Increases throughout the Last Third of the Intracellular Cycle in a TRPC6-Dependent Manner.
(A) Representative images of erythrocytes loaded with fura-2-dextran prior to challenge with synchronous P. falciparum parasites (left). [Ca2+] as a function of 340 nm/380 nm emission ratio (right). Data shown are mean calcium measurements of at least three experiments ± SEM.
(B) Representative images of T. gondii-infected U2OS cells expressing YC3.6 (left). [Ca2+] as a function of CFP/YFP FRET (right). Data shown are mean Ca2+ measurements of at least three experiments ± SEM.
(C) Representative images at 44 and 60 hpi of siRNA-mediated knockdown of cation channels prior to T. gondii infection. Positive control, Capns1; negative control, scr oligo. Data shown are mean unruptured vacuoles per field ± SEM (*p < 0.05; **p < 0.01).
(D) Quantitation at 44 and 60 hpi of siRNA-mediated knockdown of cation channels prior to T. gondii infection. Positive control, Capns1; negative control, scr oligo. Data shown are mean unruptured vacuoles per field ± SEM (*p < 0.05; **p < 0.01).
(E) Representative images (left) and [Ca2+] measurements as a function of CFP/YFP FRET (right) at 5 T. gondii divisions (∼35 hpi) following siRNA-mediated knockdown of cation channels in U2OS cells expressing YC3.6. Data shown are mean Ca2+ measurements ± SEM. Also see Figure S4.
Given the large [Ca2+] increase seen in both T. gondii and P. falciparum host cells, we further hypothesized that host plasma membrane cation channels play a role in this influx. siRNA-mediated knockdown studies of mechanosensitive transient receptor potential (TRP) channels (trpc1, trpc3, trpc6) as well as canonical endoplasmic reticulum Ca2+ pumps (ryr3, ip3r1) identified TRPC6 as a specific mediator of T. gondii exit, as trpc6 knockdown resulted in an accumulation of unruptured vacuoles by 60 hpi (p < 0.05; Figures 5C and 5D). Trpc6 knockdown using multiple siRNA oligos abrogated the large increase in cytoplasmic [Ca2+], while ip3r1 knockdown diminished the initial minor increase in [Ca2+] observed earlier in T. gondii infection, indicating that PLC activity and generation of IP3 may be responsible for the minor increase in [Ca2+] seen only in T. gondii-infected cells (Figure 5E).
Pharmacological studies in P. falciparum-infected erythrocytes confirmed the importance of mechanosensitive cation channel function in parasite-mediated cytolysis (Figure S4A). [Ca2+] diminished in cells depleted of Gαq, PLCβ, or PKCα/PKCβ (Figures S4B and S4C), but was rescued by PKC activation with PMA in cells depleted of Gαq or PLCβ, indicating that PKC activity facilitates later Ca2+ influx, perhaps via adducing phosphorylation and loss. PMA could not rescue Ca2+ influx in cells depleted of PKCα/PKCβ, further confirming the necessity of PKC activity for this process. Taken together, these data highlight the importance of TRPC6-mediated cation influx in host cell cytolysis, perhaps as a mechanosensitive response to cytoskeletal rearrangement.
Mammalian PKC Inhibitor Demonstrates Antiparasitic Activity In Vivo
In order to assess the importance of this signaling cascade in parasitic disease progression, we endeavored to test inhibition of this cascade on parasite burden in vivo. Given that PKC has been targeted for drug development efforts in multiple disease contexts from cancer to transplant rejection, and there are no known orthologs of the classical PKC enzymes in apicomplexan genomes, we undertook studies of known PKC inhibitors in murine models of malaria and toxoplasmosis. Intravenous injection of 10 mg/kg Gö6976, an inhibitor of classical mammalian PKC enzymes, caused a significant decrease in parasitemia in a mouse P. yoelii malaria model in the classic Peter's 4-day suppressive test (Figure 6A). Similarly, intraperitoneal injection of 10 mg/kg Gö6976 limited T. gondii parasite burden in both the spleen (p < 0.0001; Figure 6B, top) and peritoneal exudate cells (p < 0.0001; Figure 6B, bottom). The consistency in antiparasitic activity in vivo further suggests the importance of host PKC function in both parasitic infections and underscores the conserved function of this host signaling pathway.
Figure 6. Mammalian PKC Inhibitors Show Antiparasitic Activity In Vivo.
(A) P. yoelii parasitemia quantified via Giemsa tail vein blood smear upon intravenous injection of 10 mg/kg Gö6976 once daily for 4 days. (n = 10; ***p < 0.001).
(B) T. gondii parasite burden in cells from the spleen (top) and peritoneal cavity (PECS; bottom) upon intraperitoneal injection of 10 mg/kg Gö6976, quantified by flow cytometry. Plots shown are gated on single, live cells by FSC and SSC. (n = 15 for all conditions; ***p < 0.0001).
(C) P. berghei ANKA parasitemia quantified via Giemsa tail vein blood smear upon 50 mg/kg sotrastaurin treatment via gastric gavage once daily for 4 days. (n = 7 for all conditions; ***p < 0.0001). Shown are mean parasitemias ± SEM.
(D) Survival curves following sotrastaurin treatment using the standard Mantel-Cox log rank test (**p = 0.0029).
As further corroboration of the central role of host PKC in severe malaria, we undertook studies of the orally bioavailable specific PKC inhibitor sotrastaurin in a mouse model of experimental cerebral malaria (Figures 6C and 6D). This inhibitor has passed phase I trials and is undergoing phase II trials for numerous indications (Budde et al., 2010; Friman et al., 2011; Wagner et al., 2009), underscoring its potential as an antimalarial drug candidate. Injection of 50 mg/kg by gastric gavage in a standard Peters' 4-day suppressive test (Knight and Peters, 1980)led to a significant decrease in P. berghei ANKA parasitemia (p < 0.0001; Figure 6C) and significantly increased survival versus vehicle-treated controls (p = 0.0029; Figure 6D). We show direct translation of biological discovery to therapeutic design: identification of this host signaling activity translates directly to a drug discovery effort, as known inhibitors of extensively-studied host PKC enzymes control parasitic disease in vivo. Given the multitude of other active proteins within the cascade, it is clear that the host mediators of parasite-induced cytolysis identified in this study represent an untapped resource of antiparasitic targets.
Discussion
Host cell cytolysis was thought to be largely parasite mediated, mainly due to increasing pressure on the host cell membrane and cytoskeleton by the growing parasite body (Glushakova et al., 2005, 2010). However, we have recently shown that complete host calpain-mediated proteolysis of host cytoskeletal proteins is necessary for parasite exit (Chandramohanadas et al., 2009; Millholland et al., 2011). In this work we present a complex host-derived signaling pathway that functions in parasite-mediated cytolysis, suggesting that host cell cytolysis is a highly regulated process requiring a complex interplay of host-derived components.
As Gαq, PLC, and PKC converge in established GPCR signaling networks, we hypothesized that GPCRs may play a role in initiation of this host pathway. Considering that parasites do not encode GPCRs, we suggest that a parasite-derived small molecule ligand may signal to GPCRs of host origin through direct interaction with the host cell membrane to mediate signaling through Gαq. We found it striking that several parasite metabolites peak during schizogony, especially the TCA cycle intermediate alphaketoglutarate (αKG; Olszewski et al., 2009). As αKG and other TCA cycle intermediates including succinate have recently been shown to signal through Gαq-coupled GPCRs, such as oxoglutarate receptor 1 (oxgr1) and succinate receptor 1 (sucnr1) (He et al., 2004; Qi et al., 2004), we undertook knockdown studies to assess their contribution to host cell cytolysis. We observed a delay in T. gondii exit from cells depleted of both GPCRs (Figures S5A and S5B). Furthermore, a mixture of exogenous αKG/succinate initiated a hastening of T. gondii exit (Figure S5C) and induced premature lysis of P. falciparum parasites leading to parasite death (Figure S5D). As parasites rely on glucose fermentation for their energy needs, the requirement for oxidative phosphorylation in the parasite mitochondrion has been widely disputed, though parasites express nearly all TCA enzymes. αKG as well as other TCA cycle intermediates have been shown to diffuse out as putative waste products (Olszewski et al., 2010) due to a branched TCA pathway. We now suggest that the diffusion of TCA metabolites and other small molecule GPCR ligands into the host cell space may serve to induce a host cascade to facilitate parasite release.
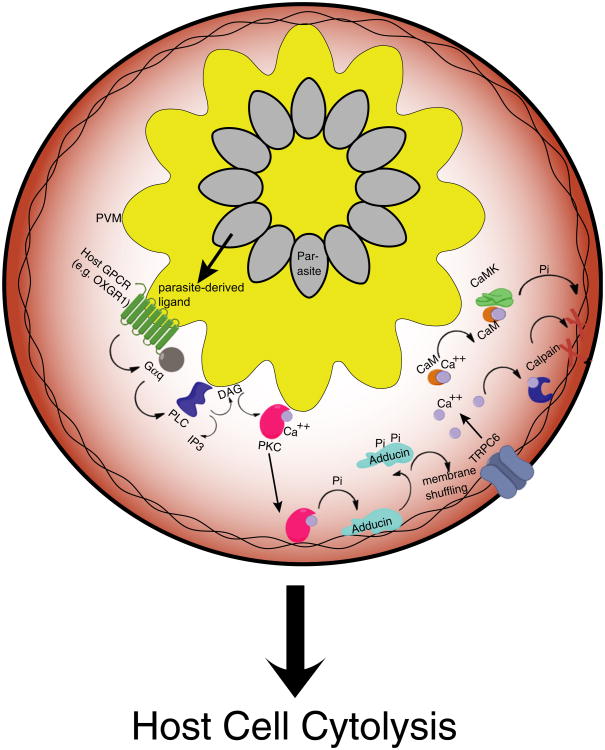
We present a model (Figure 7) in which parasite-derived GPCR ligands result in overstimulation of host GPCRs following diffusion from the parasite body into the PV space. These GPCRs engage a Gαq-mediated signaling pathway converging on PKC activation that results in the complete loss of a key cytoskeletal component and leads to pathological Ca2+ influx through mechanosensitive plasma membrane TRP channels. Ca2+-charged host CaM activates CaMK, which in turn phosphorylates cytoskeletal substrates, increasing their propensity for calpain-mediated proteolysis. Increasing cytoplasmic [Ca2+] induces global calpain activation, which results in the proteolysis of various substrates, and in turn causes the loss of host cell plasma membrane integrity to allow for parasite release.
Figure 7. Model of Host GPCR-Mediated Cytolysis.
Parasite ligands, perhaps TCA cycle intermediates generated during periods of high parasite replication, activate host GPCRs on the PV membrane and initiate a signaling cascade through host Gαq. PKC-mediated phosphorylation liberates adducin from the host cell cytoskeleton, activating the mechanosensitive plasma membrane cation channel TRPC6. CaMKII-activation following Ca2+ influx mediates phosphorylation of cytoskeletal substrates, which, upon rapid influx of Ca2+ from the extracellular media, are proteolyzed by calpain to allow for cytoskeletal dissolution and parasite release. Also see Figure S5.
PKC and calpain play key roles within this cascade, a partnership that has been underscored in other human pathologies related to GPCR overstimulation, including glutamate neuroexcitotoxicity and postmyocardial infarction cell death. PKC downregulation has been shown to protect against glutamate neurotoxicity (Ahlemeyer et al., 2002; Favaron et al., 1990; Hasham et al., 1997), while calpain inhibition has been shown to abrogate neuronal cell death (Witt et al., 1994) and block Ca2+ influx (Weiss et al., 1990). Similarly, PKC and calpain are thought to play a role in postmyocardial infarction ischemic cell death of multiple tissues (Bright et al., 2004; Padanilam, 2001; Piccoletti et al., 1992; Speechly-Dick et al., 1994), as inhibitors of these enzymes offer protective effects (Bevers et al., 2009, 2010; Koponen et al., 2003; Neuhof et al., 2008; Wagey et al., 2001; Wei et al., 2010). Though it is unclear whether the sequential activation of these enzymes is necessary for cell death in these disease contexts, we suggest that PKC activity is an essential upstream component of pathological calpain activation in the context of parasite infection. Given the antiparasitic activity of PKC inhibitors in mouse models of both toxoplasmosis and malaria, inhibition of multiple host proteins upstream of PKC activity will likely limit parasite growth with low propensity for generation of resistance in the field.
Experimental Procedures
Immunodepletion and Loading of Erythrocytes
Erythrocyte ghosts were prepared by hypotonic lysis as previously described utilizing 5 mM K2HPO4 at <4°C for 15 min (Chandramohanadas et al., 2009). To immunodeplete target proteins, target antibodies were preconjugated to Protein G-Sepharose (Upstate/Millipore) following a titration to achieve maximal immunodepletion (1–10 μg antibody per 106 erythrocytes). Conjugates were incubated with ghosts for 3 hr on ice with gentle mixing and repeated with fresh antibody-sepharose conjugates up to three times. The slurry was separated by centrifugation and followed by another round of immunodepletion for cells immunodepleted of multiple proteins. Ghosts were resealed by gradual addition of 5× resealing buffer (475 mM KOAc, 25 mM Na2HPO4, 25 mM MgCl2, 237.5 mM KCl [pH 7.5]) over 1 hr at 37°C. Parallel studies were carried out using anti-PKAcat, and mock-treated erythrocytes were incubated with G-Sepharose beads without antibodies.
Analysis of Host Protein Function during Parasite Exit from Immunodepleted Erythrocytes
Resealed erythrocytes were prepared as described above. Schizont stages were isolated from 50 ml parasite culture by magnet purification ∼40 hr after sorbitol synchronization and added to mock-treated, PKCα/PKCβ-depleted, PLCβ1-depleted, calmodulin-1-depleted, Gαq-depleted, or calpain-1-depleted erythrocytes to a final hematocrit of 4%. Parasite progress through the intraerythrocytic cycle was monitored by Giemsa smear. Schizont-stage parasites were followed to assess egress and the establishment of rings in newly-infected erythrocytes from 45–60 hpi. Flow cytometry was used for quantitative evaluation of P. falciparum development. A total of 106 events were collected per sample using an Accuri flow cytometer and analyzed using Accuri software with gating to exclude debris (defined by scatter characteristics) and uninfected erythrocytes (defined by low fluorescence). Rings and trophozoites were distinguished from schizonts based on DNA content. Data are presented as mean ± SEM; n = 4.
Mechanical Removal of P. falciparum from Erythrocytes
Needle shearing experiments were completed as previously described (Dvorin et al., 2010) with modifications. Parasites trapped within erythrocytes immunodepleted of calpain-1, PKCα/PKCβ, PLCβ1, or CaM-1 or negative control mock-immunodepleted or PKAα/PKAβcat-immunodepleted erythrocytes were plated in quadruplicate. At the indicated time point, cultures were sheared by 20 strokes through a 28.5 gauge needle. Cultures were incubated for an additional 12 hr, and ring-stage parasitemia was determined by Giemsa smear microscopy and flow cytometry of DNA content. Data are presented as mean ± SEM; n = 4.
P. berghei ANKA Mouse Studies
C57BL/6 mice were infected with 2.5 × 104 P. berghei ANKA parasitized erythrocytes via intravenous injection. Mice were dosed once per day by gastric gavage for 4 days with either 50 mg/kg sotrastaurin (in 0.1% HCl in water; n = 7) or vehicle control (n = 7). Parasitemias were determined on days 5 and 7 via Giemsa-stained blood smears. Mice were assessed for survival through day 20. Statistical significance was determined using a two-tailed Mann-Whitney U test. Experiments were performed at Johns Hopkins University (JHU) Bloomberg School of Public Health in accordance with the guidelines of the JHU Institutional Animal Care and Use Committees.
Western Blotting
U2OS cells or erythrocytes were fractionated using Triton X-100 prior to separation by SDS-PAGE, transfer to PVDF membrane, and probing for target proteins of interest using primary antibodies (Santa Cruz Biotechnology, Inc., Abcam) and secondary antibodies conjugated to HRP (Sigma-Aldrich).
Monitoring Calpain Activation and Membrane Binding
Immunodepleted cells were prepared as above and challenged with synchronous, magnet-purified schizont-stage parasites. Following 48 hr incubation, infected cells were incubated with DCG-04 (5 μM) and membrane fractions were prepared from each sample by ultracentrifugation (2 hr at 200,000 × g). Equal amounts of solubilized protein from each sample was separated by SDS-PAGE, transferred to PVDF membrane, and probed for biotin using streptavidin-HRP, revealing DCG-04 labeling of active calpain. As a loading control, the same western blots were probed with actin antibodies (Santa Cruz Biotechnology, Inc.).
CaMKII Studies
U2OS cells were fractionated using Triton X-100. The cytoskeletal fraction was incubated with 100 nM recombinant rat CAMKII (NEB) in 1× NEBuffer for Protein Kinases supplemented with 200 μM ATP, 1.2 μM calmodulin, and 2 mM CaCl2 and incubated for 1 hr at 37°C. Recombinant human calpain (100 nM; Biovision) was added to the mixture and incubated for 30 min at 37°C. Each sample was then separated by SDS-PAGE, transferred to a PVDF membrane, and probed for α-spectrin (primary α-spectrin antibody was a gift from David Speicher; anti-rabbit secondary antibody was purchased from Sigma-Aldrich).
T. gondii Invasion, Replication, and Egress in Knockdown Cultures
Intracellular parasite growth was measured by counting the number of parasites per parasitophorous vacuole at 6, 24, and 44 hr postinfection (prior to egress from the initial host cell). Confluent monolayers of ∼5 × 105 stable knockdown host cells in 60 mm dishes were infected with 106 T. gondii parasites. At least 100 vacuoles were counted per time point. Data are presented as mean ± SEM; n = 5.
siRNA Screen and shRNA Stable Knockdown Generation
Primary siRNA oligos were purchased from Santa Cruz Biotechnology, Inc., and oligos for follow up screens were purchased from Ambion. siRNAs were added at a final concentration of 20 nM in 96-well plates and titrated to achieve maximal knockdown by 72 hr posttransfection, along with 106 U2OS cells in culture medium lacking phenol red. Reverse transfection was carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Transfected host cells were inoculated with 105 P30-GFP-expressing T. gondii tachyzoites 24 hr posttransfection and followed throughout the intracellular cycle to assess invasion, replication, and egress rates at 6, 24, 44, and 60 hpi. Data are presented in Figures 1B and 1C as mean number of unruptured vacuoles at 60 hpi versus 44 hpi ± SEM; n = 4. siRNA knockdown was confirmed via western blot of mirrored cultures utilizing validated antibodies from Santa Cruz Biotechnology, Inc. Prior to isolation of clonal populations, stable knockdown cell lines were generated following transfection with HuSH shRNA plasmids (OriGene) encoding target shRNAs conjugated to soluble cytoplasmic RFP to affirm knockdown and selection with puromycin (1 μg/ml) for two passages. Double knockdown cells were generated utilizing pRS expression vectors engineered to include a blasticidin selection marker (OriGene) and selection with blasticidin. Following confirmation of knockdown via western blot, cells were cultured in the absence of selection pressure.
Ca2+ Studies
Erythrocytes were hypotonically lysed and loaded with 50 μM fura-2-dextran prior to resealing and incubation with synchronous P. falciparum schizonts. Parasites infecting fura-2-dextran-loaded erythrocytes were followed by fluorescent microscopy and plate-based fluorometry throughout the life cycle to assess host erythrocyte cytoplasmic [Ca2+] via the equation: [Ca2+] = Kd × Q (R−Rmin)/(Rmax−R), where R represents the fluorescence intensity ratio at 340 nm/380 nm excitation and emission at 510 nm. Data are presented as mean fluorescence ± SEM; n = 3. Representative images are shown.
U2OS cells were transduced using the Premo Cameleon Calcium Sensor (Life Technologies) according to manufacturer's instructions to facilitate cytoplasmic expression of YC3.6 prior to synchronous T. gondii infection. Host [Ca2+] was measured throughout the parasite life cycle via FRET microscopy on a Leica DMI6000B and FRET-based fluorometry to assess [Ca2+] as a function of the ratio of the emission measured at 480 nm (cyan fluorescent protein, CFP) divided by the emission measured at 535 nm (yellow fluorescent protein, YFP). Data are presented as mean fluorescence ± SEM; n = 3. Representative images are shown.
CKAR Transfection and PKC Activity Measurement
CKAR plasmid (Addgene) was transfected into U2OS cells using Lipofectamine 2000 (Life Technologies) according to manufacturer's instructions. 3D7-CKAR construct was generated via addition of a minimal export PEXEL motif N terminus to CKAR (Marti et al., 2004; Hiller et al., 2004) following PCR amplification from the CKAR plasmid. This was achieved via amplification of the N terminus of the exported protein KAHRP (PF3D7_0202000) from cDNA of asynchronous 3D7 parasite cultures and recombination with the CKAR motif into the P. falciparum expression vector p-HHVPatt using MultiSite Gateway (Marti et al., 2004). Constructs were transfected into the P. falciparum 3D7 line and selected using 10 nM WR99210 (an inhibitor of dihydrofolate reductase; Fidock and Wellems, 1997). 3D7-CKAR transgenic parasites and T. gondii-infected U2OS cells expressing CKAR were cultured under standard conditions and assessed for PKC activity via FRET-based fluorometry as a function of YFP/CFP emission ratio and FRET imaging on a Leica DMI6000B. Data are presented as mean ± SEM; n = 4. Representative images are shown.
Statistical Analyses
We analyzed the statistical significance of unruptured vacuole persistence, in vitro parasitemias, FRET-based signal for CKAR activity or YC3.6 Ca2+ studies, and fura-2 signal with a one-way ANOVA with Dunnett's post hoc test. In vivo mouse parasitemias were analyzed with one-tailed paired Student's t tests and mouse survival was analyzed using the log rank test.
Supplementary Material
Acknowledgments
We thank Vann Bennett, James Powers, and David Speicher for key reagents and the following funding sources: NIHT32AI007532 (M.G.M.), 1R01AI097273-01A1 (D.C.G.), University of Pennsylvania TAPITMAT Pilot Program (D.C.G.), UPenn Genome Frontiers Institute (D.C.G.), and NIH R01 AI056840 (P.S.).
Footnotes
Supplemental Information: Supplemental Information includes five figures, one table, and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.chom.2012.12.001.
References
- Abkarian M, Massiera G, Berry L, Roques M, Braun-Breton C. A novel mechanism for egress of malarial parasites from red blood cells. Blood. 2011;117:4118–4124. doi: 10.1182/blood-2010-08-299883. [DOI] [PubMed] [Google Scholar]
- Ahlemeyer B, Kölker S, Zhu Y, Hoffmann GF, Krieglstein J. Increase in glutamate-induced neurotoxicity by activated astrocytes involves stimulation of protein kinase C. J Neurochem. 2002;82:504–515. doi: 10.1046/j.1471-4159.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- Arastu-Kapur S, Ponder EL, Fonović UP, Yeoh S, Yuan F, Fonović M, Grainger M, Phillips CI, Powers JC, Bogyo M. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat Chem Biol. 2008;4:203–213. doi: 10.1038/nchembio.70. [DOI] [PubMed] [Google Scholar]
- Bevers MB, Lawrence E, Maronski M, Starr N, Amesquita M, Neumar RW. Knockdown of m-calpain increases survival of primary hippocampal neurons following NMDA excitotoxicity. J Neurochem. 2009;108:1237–1250. doi: 10.1111/j.1471-4159.2008.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevers MB, Ingleton LP, Che D, Cole JT, Li L, Da T, Kopil CM, Cohen AS, Neumar RW. RNAi targeting micro-calpain increases neuron survival and preserves hippocampal function after global brain ischemia. Exp Neurol. 2010;224:170–177. doi: 10.1016/j.expneurol.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright R, Raval AP, Dembner JM, Pérez-Pinzón MA, Steinberg GK, Yenari MA, Mochly-Rosen D. Protein kinase C delta mediates cerebral reperfusion injury in vivo. J Neurosci. 2004;24:6880–6888. doi: 10.1523/JNEUROSCI.4474-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde K, Sommerer C, Becker T, Asderakis A, Pietruck F, Grinyo JM, Rigotti P, Dantal J, Ng J, Barten MJ, Weber M. Sotrastaurin, a novel small molecule inhibiting protein kinase C: first clinical results in renal-transplant recipients. Am J Transplant. 2010;10:571–581. doi: 10.1111/j.1600-6143.2009.02980.x. [DOI] [PubMed] [Google Scholar]
- Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257:7847–7851. [PubMed] [Google Scholar]
- Chandramohanadas R, Davis PH, Beiting DP, Harbut MB, Darling C, Velmourougane G, Lee MY, Greer PA, Roos DS, Greenbaum DC. Apicomplexan parasites co-opt host calpains to facilitate their escape from infected cells. Science. 2009;324:794–797. doi: 10.1126/science.1171085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorin JD, Martyn DC, Patel SD, Grimley JS, Collins CR, Hopp CS, Bright AT, Westenberger S, Winzeler E, Blackman MJ, et al. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science. 2010;328:910–912. doi: 10.1126/science.1188191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaron M, Manev H, Siman R, Bertolino M, Szekely AM, DeErausquin G, Guidotti A, Costa E. Down-regulation of protein kinase C protects cerebellar granule neurons in primary culture from glutamate-induced neuronal death. Proc Natl Acad Sci USA. 1990;87:1983–1987. doi: 10.1073/pnas.87.5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock DA, Wellems TE. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc Natl Acad Sci USA. 1997;94:10931–10936. doi: 10.1073/pnas.94.20.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friman S, Arns W, Nashan B, Vincenti F, Banas B, Budde K, Cibrik D, Chan L, Klempnauer J, Mulgaonkar S, et al. Sotrastaurin, a novel small molecule inhibiting protein-kinase C: randomized phase II study in renal transplant recipients. Am J Transplant. 2011;11:1444–1455. doi: 10.1111/j.1600-6143.2011.03538.x. [DOI] [PubMed] [Google Scholar]
- Gallegos LL, Kunkel MT, Newton AC. Targeting protein kinase C activity reporter to discrete intracellular regions reveals spatiotemporal differences in agonist-dependent signaling. J Biol Chem. 2006;281:30947–30956. doi: 10.1074/jbc.M603741200. [DOI] [PubMed] [Google Scholar]
- Glushakova S, Yin D, Li T, Zimmerberg J. Membrane transformation during malaria parasite release from human red blood cells. Curr Biol. 2005;15:1645–1650. doi: 10.1016/j.cub.2005.07.067. [DOI] [PubMed] [Google Scholar]
- Glushakova S, Humphrey G, Leikina E, Balaban A, Miller J, Zimmerberg J. New stages in the program of malaria parasite egress imaged in normal and sickle erythrocytes. Curr Biol. 2010;20:1117–1121. doi: 10.1016/j.cub.2010.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum D, Medzihradszky KF, Burlingame A, Bogyo M. Epoxide electrophiles as activity-dependent cysteine protease profiling and discovery tools. Chem Biol. 2000;7:569–581. doi: 10.1016/s1074-5521(00)00014-4. [DOI] [PubMed] [Google Scholar]
- Hasham MI, Pelech SL, Krieger C. Glutamate-mediated activation of protein kinase C in hippocampal neurons. Neurosci Lett. 1997;228:115–118. doi: 10.1016/s0304-3940(97)00382-0. [DOI] [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Gething PW, Patil AP, Tatem AJ, Noor AM, Kabaria CW, Manh BH, Elyazar IR, Brooker S, et al. A world malaria map: Plasmodium falciparum endemicity in 2007. PLoS Med. 2009;6:e1000048. doi: 10.1371/journal.pmed.1000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- Hiller NL, Bhattacharjee S, van Ooij C, Liolios K, Harrison T, Lopez-Estraño C, Haldar K. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–1937. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- Joiner KA, Beckers CJ, Bermudes D, Ossorio PN, Schwab JC, Dubremetz JF. Structure and function of the parasitophorous vacuole membrane surrounding Toxoplasma gondii. Ann N Y Acad Sci. 1994;730:1–6. doi: 10.1111/j.1749-6632.1994.tb44233.x. [DOI] [PubMed] [Google Scholar]
- Kafsack BF, Beckers C, Carruthers VB. Synchronous invasion of host cells by Toxoplasma gondii. Mol Biochem Parasitol. 2004;136:309–311. doi: 10.1016/j.molbiopara.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Kafsack BF, Pena JD, Coppens I, Ravindran S, Boothroyd JC, Carruthers VB. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science. 2009;323:530–533. doi: 10.1126/science.1165740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DJ, Peters W. The antimalarial activity of N-benzyloxydi-hydrotriazines. I. The activity of clociguanil (BRL 50216) against rodent malaria, and studies on its mode of action. Ann Trop Med Parasitol. 1980;74:393–404. [PubMed] [Google Scholar]
- Koponen S, Kurkinen K, Akerman KE, Mochly-Rosen D, Chan PH, Koistinaho J. Prevention of NMDA-induced death of cortical neurons by inhibition of protein kinase Czeta. J Neurochem. 2003;86:442–450. doi: 10.1046/j.1471-4159.2003.01846.x. [DOI] [PubMed] [Google Scholar]
- Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–1933. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Hughes CA, Bennett V. Adducin regulation.Definition of the calmodulin-binding domain and sites of phosphorylation by protein kinases A and C. J Biol Chem. 1996;271:25157–25166. doi: 10.1074/jbc.271.41.25157. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Li X, Bennett V. Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol. 1998;142:485–497. doi: 10.1083/jcb.142.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millholland MG, Chandramohanadas R, Pizzarro A, Wehr A, Shi H, Darling C, Lim CT, Greenbaum DC. The malaria parasite progressively dismantles the host erythrocyte cytoskeleton for efficient egress. Mol Cell Proteomics. 2011;10:M111.010678. doi: 10.1074/mcp.M111.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudy R, Manning TJ, Beckers CJ. The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J Biol Chem. 2001;276:41492–41501. doi: 10.1074/jbc.M106154200. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca(+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhof C, Fabiunk V, Speth M, Möller A, Fritz F, Tillmanns H, Neuhof H, Erdogan A. Reduction of myocardial infarction by postis-chemic administration of the calpain inhibitor A-705253 in comparison to the Na(+)/H(+) exchange inhibitor Cariporide in isolated perfused rabbit hearts. Biol Chem. 2008;389:1505–1512. doi: 10.1515/BC.2008.172. [DOI] [PubMed] [Google Scholar]
- Olszewski KL, Morrisey JM, Wilinski D, Burns JM, Vaidya AB, Rabinowitz JD, Llinás M. Host-parasite interactions revealed by Plasmodium falciparum metabolomics. Cell Host Microbe. 2009;5:191–199. doi: 10.1016/j.chom.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski KL, Mather MW, Morrisey JM, Garcia BA, Vaidya AB, Rabinowitz JD, Llinás M. Branched tricarboxylic acid metabolism in Plasmodium falciparum. Nature. 2010;466:774–778. doi: 10.1038/nature09301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Padanilam BJ. Induction and subcellular localization of protein kinase C isozymes following renal ischemia. Kidney Int. 2001;59:1789–1797. doi: 10.1046/j.1523-1755.2001.0590051789.x. [DOI] [PubMed] [Google Scholar]
- Palmer AE, Tsien RY. Measuring calcium signaling using genetically targetable fluorescent indicators. Nat Protoc. 2006;1:1057–1065. doi: 10.1038/nprot.2006.172. [DOI] [PubMed] [Google Scholar]
- Piccoletti R, Bendinelli P, Arienti D, Bernelli-Zazzera A. State and activity of protein kinase C in postischemic reperfused liver. Exp Mol Pathol. 1992;56:219–228. doi: 10.1016/0014-4800(92)90038-d. [DOI] [PubMed] [Google Scholar]
- Qi AD, Harden TK, Nicholas RA. GPR80/99, proposed to be the P2Y(15) receptor activated by adenosine and AMP, is not a P2Y receptor. Purinergic Signal. 2004;1:67–74. doi: 10.1007/s11302-004-5069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SG, Suh PG, Ryu SH, Lee SY. Studies of inositol phospholipid-specific phospholipase C. Science. 1989;244:546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- Salmon BL, Oksman A, Goldberg DE. Malaria parasite exit from the host erythrocyte: a two-step process requiring extraerythrocytic proteolysis. Proc Natl Acad Sci USA. 2001;98:271–276. doi: 10.1073/pnas.011413198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speechly-Dick ME, Mocanu MM, Yellon DM. Protein kinase C. Its role in ischemic preconditioning in the rat. Circ Res. 1994;75:586–590. doi: 10.1161/01.res.75.3.586. [DOI] [PubMed] [Google Scholar]
- Striepen B, He CY, Matrajt M, Soldati D, Roos DS. Expression, selection, and organellar targeting of the green fluorescent protein in Toxoplasma gondii. Mol Biochem Parasitol. 1998;92:325–338. doi: 10.1016/s0166-6851(98)00011-5. [DOI] [PubMed] [Google Scholar]
- Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagey R, Hu J, Pelech SL, Raymond LA, Krieger C. Modulation of NMDA-mediated excitotoxicity by protein kinase C. J Neurochem. 2001;78:715–726. doi: 10.1046/j.1471-4159.2001.00459.x. [DOI] [PubMed] [Google Scholar]
- Wagner J, von Matt P, Sedrani R, Albert R, Cooke N, Ehrhardt C, Geiser M, Rummel G, Stark W, Strauss A, et al. Discovery of 3-(1H-indol-3-yl)-4-[2-(4-methylpiperazin-1-yl)quinazolin-4-yl]pyrrole-2,5-dione (AEB071), a potent and selective inhibitor of protein kinase C isotypes. J Med Chem. 2009;52:6193–6196. doi: 10.1021/jm901108b. [DOI] [PubMed] [Google Scholar]
- Wei L, Sun D, Yin Z, Yuan Y, Hwang A, Zhang Y, Si R, Zhang R, Guo W, Cao F, Wang H. A PKC-beta inhibitor protects against cardiac microvascular schemia reperfusion injury in diabetic rats. Apoptosis. 2010;15:488–498. doi: 10.1007/s10495-009-0439-2. [DOI] [PubMed] [Google Scholar]
- Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. Int J Parasitol. 2009;39:895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JH, Hartley DM, Koh J, Choi DW. The calcium channel blocker nifedipine attenuates slow excitatory amino acid neurotoxicity. Science. 1990;247:1474–1477. doi: 10.1126/science.247.4949.1474. [DOI] [PubMed] [Google Scholar]
- Wickham ME, Culvenor JG, Cowman AF. Selective inhibition of a two-step egress of malaria parasites from the host erythrocyte. J Biol Chem. 2003;278:37658–37663. doi: 10.1074/jbc.M305252200. [DOI] [PubMed] [Google Scholar]
- Witt MR, Dekermendjian K, Frandsen A, Schousboe A, Nielsen M. Complex correlation between excitatory amino acid-induced increase in the intracellular Ca2+ concentration and subsequent loss of neuronal function in individual neocortical neurons in culture. Proc Natl Acad Sci USA. 1994;91:12303–12307. doi: 10.1073/pnas.91.25.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh S, O'Donnell RA, Koussis K, Dluzewski AR, Ansell KH, Osborne SA, Hackett F, Withers-Martinez C, Mitchell GH, Bannister LH, et al. Subcellular discharge of a serine protease mediates release of invasive malaria parasites from host erythrocytes. Cell. 2007;131:1072–1083. doi: 10.1016/j.cell.2007.10.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.