Abstract
Generation of induced pluripotent stem (iPS) cells has revolutionized the field of regenerative medicine. With the exponential increase in iPS cell research in the past three years, human iPS cells have been derived with different technologies and from various cell types. From a translational perspective, however, a number of issues must be addressed before safe and high quality patient-specific iPS cells can be derived for clinical applications. In addition, iPS cell-based therapies also need to be thoroughly evaluated in pre-clinical animal models before they can be applied to human subjects.
It has been shown in the past two years that ectopic expression of defined transcriptional factors can reprogram human somatic cells to a pluripotent state 1, 2. These reprogrammed pluripotent cells, defined as induced pluripotent stem (iPS) cells by Shinya Yamanaka 3, closely resemble embryonic stem (ES) cells, which can differentiate into every somatic cell type of the human body and possess the capacity of unlimited replication. Because iPS cells can bypass the ethical concerns related to ES cell derivation and potentially issues of allogeneic immune rejection, they may represent a more ideal source to produce patient-specific and disease-specific adult cells for future clinical applications and drug development. As a result, these cells have been regarded as a leading candidate for donor cell source in regenerative medicine. However, a number of obstacles need to be cleared before patient-specific iPS cells can advance into the clinic. Here we focus our discussion on human iPS cell derivation as well as issues that should be addressed to generate clinically approved iPS cell products for regenerative therapy.
Choosing An Appropriate Cell Type
Choosing an appropriate cell type for reprogramming is a critical consideration for future autologous patient-specific iPS cell production and clinical therapy. The ideal cell source to be isolated from the patients and used for reprogramming must meet the criteria of easy accessibility with minimal risk procedures, availability in large quantities, relatively high reprogramming efficiency, and fast iPS cell derivation speed.
Skin Fibroblasts
The majority of published studies thus far have used skin fibroblasts as the starting population for reprogramming. The major advantages of these cells are their easy accessibility from the patients and easy maintenance in culture. Skin fibroblasts usually come from a single skin biopsy followed by 3–4 weeks of in vitro expansion to obtain a sufficient starting number of cells 4. However, the efficiency of reprogramming adult human skin fibroblasts is very low, typically under 0.01% when using Yamanaka 4 factors (Oct4, Sox2, Klf4, c-MYC) (OSKM) and even one to two magnitude lower with 3 factors without c-MYC 1, 5–7. It also takes a relatively long time, usually more than 3–4 weeks, for ES cell-like iPS cell colonies to appear from the reprogramming. In the model proposed by Shinya Yamanaka based on Conrad Waddington’s epigenetic landscape model 8, skin fibroblasts are considered terminally differentiated cells and therefore take higher energy to be reprogrammed back to a pluripotent stage.
Neural stem cells
Hans Scholer and colleagues reported that human fetal neural stem cells (NSCs) can be reprogrammed using only one factor, Oct4 9. Due to the highly invasive nature of deriving NSCs, they are not a readily available source of cells for generating human iPS cells. However, NSCs could represent a better and simpler platform to generate animal iPS cells as well as animal disease models that might prove useful in studying iPS cell transplantation, human disease mechanisms, and drug development.
Keratinocytes
Aasen et al. have used keratinocytes derived from human foreskin biopsies and plucked hairs as the starting population for reprogramming 10. These cells are easy to access but are also limited by the problem of requiring an extended period of time for in vitro expansion. Comparing to skin fibroblasts, these cells showed a ~100-fold improvement in reprogramming efficiency and ~3-fold improvement in reprogramming speed using retroviral OSKM. However, this improvement was calculated from reprogramming neonatal/juvenile keratinocytes (2-, 2-, 4- and 16-year old individuals). The reprogramming speed and efficiency of adult human keratinocytes were not reported in the study and thus remain unknown.
CD34+ cells from peripheral blood
Loh et al. reported generation of iPS cells from peripheral blood CD34+ cells using Yamanaka 4 factors 11. These cells are usually isolated from the peripheral blood of patients undergoing G-CSF mobilization for several days, taking up ~1% of the total cell counts. More than one million CD34+ cells can be isolated from 100 ml of mobilized peripheral blood, representing an abundant source of cells for reprogramming. However, G-CSF treatment often leads to uncomfortable side effects such as headache, nausea, and bone pain. Even more severe and fatal complications can happen in patients having cardiovascular and cerebrovascular diseases 12. Moreover, CD34+ cells gradually decrease in number over time when cultured in vitro, and thus need to be banked in the early days after isolation. The reprogramming efficiency of CD34+ cells was still relatively low at 0.01%–0.02%, which is close to the efficiency of reprogramming adult skin fibroblasts. A recent study also reported iPS cells derived from CD34+ cells mobilized from peripheral blood 13. However, the efficiency and speed of reprogramming were not reported.
Melanocytes
Utikal et al. used human primary melanocytes as their starting cell population for reprogramming 14. Like skin fibroblasts and keratinocytes, melanocytes are also derived from skin biopsies and expanded by in vitro culture under specific conditions. These cells express very high endogenous levels of Sox2 and thus can be reprogrammed with 3 factors (Oct4, Klf4, and c-MYC) (OKM). The efficiency of reprogramming melanocytes with Yamanaka 4 factors was reported at 0.05%, which is higher than reprogramming fibroblasts. Moreover, the melanocyte reprogramming speed seemed faster than that of fibroblast, with the generation of ES cell-like colonies taking place ~10 days after introducing the exogenous factors. However, the age of the patient from which the melanocytes were derived was not reported in this study, and thus the reprogramming efficiency and speed for adult human melanocytes are still unclear. Nevertheless, melanocytes provide an opportunity of improving the safety of derived iPS cells by using reduced factors as well as non-integrating techniques.
Adipose-derived stem cells
Our group has recently generated iPS cells from adult human adipose stem cells (hASCs) derived from lipoaspiration using Yamanaka 4 factors 15. Lipoaspiration is a relatively simple and minimally invasive procedure that can be routinely performed in outpatient clinics. Furthermore, hASCs can be derived from lipoaspirates of patients of all ages. A small amount of adipose tissue can yield a large number of donor cells used to derive iPS cells. From routinely processed 300 ml of fresh lipoaspirate, we typically collect approximately 100 million cells after a brief 48-hour in vitro culture. These cells can be directly reprogrammed thereafter, thereby bypassing the 4-week expansion period needed when using skin fibroblasts for reprogramming. In addition, even when adipose tissue was only available from “slender patients,” we have successfully processed 15–50 ml tissue samples without any remarkable differences in our procedure and cell yield. Virtually all individuals will have adequate amounts of abdominal, flank, thigh, and/or buttock adipose tissue. This small amount of adipose tissue can be quickly and easily harvested under local anesthesia without requiring any changes in patient consciousness.
hASCs are heterogeneous multipotent progenitor cells that have been shown to differentiate into multiple cell lineages, including bone, cartilage, muscle, and adipose tissues 16. Compared to reprogramming human fibroblasts with Yamanaka’s 4 factors 1, 2, 5, 10, reprogramming hASCs was ~20-fold higher in efficiency and ~2-fold faster. Expression of Klf4 and c-MYC, two of the reprogramming factors, is relatively high in hASCs compared with that in human ES cells. In addition, we have found that the reprogramming of hASCs does not require the support of mouse feeder cells, which may help to generate more defined, clinically qualified iPS cells in the future. With the unique properties of large quantities, very short expansion time, relatively high reprogramming efficiency, and fast reprogramming speed, hASCs represent one of the optimal cell sources for clinical derivation of patient-specific iPS cells in the future.
Cord blood cells
Two very recent reports described the generation of human iPS cells from cord blood cells. In the first study, Giorgetti et al. successfully reprogrammed CD133+ cells isolated from cord blood with only two factors, Oct4 and Sox2 17. They also reported generating iPS cells using cord blood units that were cryopreserved for more than 5 years. In the other study, Haase et al. generated iPS cells from cord blood-derived endothelial cells using lentiviruses with the Thomson factors (Oct4, Sox2, Nanog, and Lin28) 18. Cord blood is collected from the umbilical cord at childbirth and contains a mixed population of cells, including hematopoietic progenitor cells 19. Therefore, iPS cell derivation using cord blood cells is limited to the patients who had their cord blood banked at childbirth. Moreover, it is still unclear how long these cells can be cryopreserved to remain viable or amenable for reprogramming. Cord blood cells that have been cryopreserved for decades should be tested for reprogramming, as most patients who need regenerative therapy might be of relatively advanced age.
In summary, each cell type has its own advantages and limitations to serve as the origin for iPS cell derivation. The optimal cell source for generating patient-specific iPS cells should also be selected on a patient-specific basis, when it proves possible to evaluate specific conditions of individual patient in the future. Table 1 shows a comparison of the different cell origins that have been used for reprogramming.
Table 1.
Comparison of different cell origins used for reprogramming
| Cell Source | Derivation | In vitro Expansion | Reprogramming Efficiency (4Factor) | Reprogramming Speed | Reprogramming Factors | References |
|---|---|---|---|---|---|---|
| Skin Fibroblasts | Skin Biopsy | Yes | ~0.01% (Adult cells) | >21 days | OSKM, OSK, OSNL | 1–7 |
| Neural Stem Cells | NA | Yes | 0.004% (1Factor, Fetal cells) | >7–8 weeks (1Factor) | OK, O | 9 |
| Keratinocytes | Skin Biopsy | Yes | ~1% (neonatal and juvenile cells) | >10 days | OSKM, OSK | 10 |
| CD34 Blood Cells | Peripheral blood undergo G-CSF stimulation | No | ~0.01–0.02% (Adult cells) | >14 days | OSKM | 11 |
| Adipose Stem Cells | Lipoaspiration | No | ~ 0.2% (Adult cells) | >13–14 days | OSKM | 15 |
| Melanocytes | Skin Biopsy | Yes | ~0.05% (not known) | >10 days | OSKM, OKM | 14 |
| Cord Blood Cells | Collected at birth from cord blood | No | ~ 0.01% (neonatal cells) | >12–15 days | OSKM, OSNL, OSK, OS | 17, 18 |
O: Oct4, S: Sox2, K: Klf4, M: c-MYC, N: Nanog, L: Lin-28, NA: Not Applicable
iPS Cell Derivation Methods Need to Be Improved
Most of the studies in current literature have used lentiviruses or retroviruses containing reprogramming factors to generate iPS cells. Both lentiviruses and retroviruses lead to genomic integration of the transgenes that may not be completely silenced in the host cells. Reactivation of the silenced transgenes in reprogrammed cells can also occur and lead to undesirable side effects. Further, both Klf4 and c-MYC are oncogenes. These factors raise the specter that even terminally differentiated cells derived from parent iPS cells with leaky expression of Klf4 or C-MYC may induce cancers in the host. In addition, insertional mutagenesis may be associated with transgene integration in the host genome, which may also lead to tumorigenicity in patients 20.
Several recent studies have used different approaches to avoid genomic integration of the reprogramming genes. Soldner et al. generated transgene-free human iPS cells from Parkinson’s disease patients using Cre-recombinase excisable lentiviruses 21. However, Cre-recombinase leaves residual loxP sequences within the genome after excision of the transgene, raising the possibility of inducing abnormal genomic activities in the reprogrammed cells. Yu et al. reported virus-free and transgene-free human iPS cell derivation using oriP/EBNA1 (Epstein-Barr nuclear antigen-1)-based episomal vectors. The oriP/EBNA1 vectors are able to stably replicate as episomes and can be gradually removed from the reprogrammed cells in the absence of drug selection 22. However, this plasmid-based technique not only required a total combination of 7 reprogramming factors (Oct4, Sox2, Klf4, c-MYC, Nanog, Lin28, and SV40LT) but also had very low efficiency compared to viral reprogramming methods. In a very recent study, Woltjen and colleagues reported a virus-free reprogramming technique using piggyBac transposon expression vectors carrying a polycistronic transgene of Yamanaka 4 factors 23. This polycistronic transgene can be excised using transposase after successful reprogramming without leaving residual exogenous sequences. However, genomic alteration during transposon insertion and excision may still occur. In addition, it is cumbersome to identify the specific iPS cell clone with the transgenes correctly excised. In another recent study, by conjugating cell-penetrating peptide (cpp) with recombinant proteins of OSKM, Kim et al. successfully generated iPS cells from human newborn fibroblasts, although at a very low efficiency (~0.001%) and with prolonged time (~8 weeks) 24. However, with improved speed and efficiency in the future, recombinant protein-based technique may become a practical method to generate virus-free and transgene-free human iPS cells for clinical use.
Instead of using genetic-based techniques, reprogramming strategies relying on adding small molecules, small interfering RNAs (siRNAs), or micro RNAs (miRNAs) to the reprogramming cocktails represent an important alternative future direction to generate safer iPS cells. These small molecules promote reprogramming through different mechanisms, either replacing some of the reprogramming factors or increasing reprogramming efficiency and speed 25. For example, Huangfu et al. showed that valproic acid, a histone deacetylase inhibitor, helped increase the reprogramming efficiency more than 100-fold 26 and facilitate reprogramming human neonatal fibroblasts with Oct4 and Sox2 only 7. Zhao et al. showed that p53 siRNAs dramatically increased reprogramming efficiency when added to Yamanaka 4 factors 27. Ding and colleagues found that a number of small molecules, such as glycogen synthase kinase 3 (GSK-3), MEK-ERK pathway, and TGFβ pathway inhibitors, can promote reprogramming efficiency or replace some of the reprogramming factors 28–30. Ichida et al. also showed recently that a small molecule inhibitor of TGFβ signaling can replace Sox2 and induce Nanog expression in reprogramming 31. miRNAs that were upregulated in iPS cells 32 may also have potential effects in promoting or inhibiting reprogramming. For a more detailed review on small molecules that can enhance or promote reprogramming, please see a recent review article 25. Overall, with the rapid progress and improvements in iPS cell derivation methodologies, it should be feasible to reach the final objective of generating safe, virus-free, and transgene-free autologous iPS cells at a relatively high efficiency for human patients in the future.
Need for Consensus in Identification of iPS Cells
A single reprogramming experiment usually generates multiple iPS cell lines that are not always identical. Each individual iPS cell line needs to be fully characterized to ensure safety and pluripotency capacity, which currently is a cumbersome and time-consuming process. In addition, the criterion that investigators use to select the fully reprogrammed iPS cells also varies significantly. These problems are largely due to the limited understanding of the underlying reprogramming mechanism. With further elucidation of the reprogramming mechanism and improvements in iPS cell derivation technologies, new methods to simplify and facilitate characterization of iPS cell lines will become possible in the future. The final goal is to establish a fast and reliable standard protocol for identifying bona fide iPS cells for future clinical regenerative therapies. Recently, by following the reprogrammed cells using in situ live cell imaging, Chan et al. attempted to identify bona fide iPS cells during the reprogramming process 33. They showed that transgene silencing and activation of TRA-1-60, DNMT3B, and Rex1 expression marked the fully reprogrammed cells, whereas alkaline phosphatase, SSEA-4, and Nanog were insufficient as markers. This study revealed some of the molecular events during reprogramming and is a step forward toward the establishment of a set of standard markers for identifying true iPS cells in the future.
Improvement of iPS Cell Cultivation and Differentiation Methods
Current iPS cell cultivation utilizes almost the same culturing conditions as those for human ES cells. A feeder layer of inactivated mouse embryonic fibroblasts (MEFs) is required to support the proliferation of iPS cells and to maintain their undifferentiated state. However, the use of MEFs adds the possibility of contaminating the derived patient-specific iPS cells with animal pathogens. Rather than using MEFs as the feeder cells, autologous skin fibroblasts derived from the same patient may serve as a better source of feeder cells, as similar culturing methods for human ES cells have been reported 34, 35. An alternative feeder-free culture method for iPS cells and human ES cells utilizes surfaces coated with Matrigel 36, which is a mixture of different extracellular matrix proteins and growth factors secreted by mouse Engelbreth-Holm-Swarm (EHS) sarcoma 37. However, using Matrigel may still bring animal pathogens to iPS cells. Thus a more defined pathogen-free and feeder-free culture condition is required for iPS cell cultivation in the future. For the purposes of regenerative medicine, large scale cultivation of iPS cells and subsequent efficient differentiation into specific cell lineages remain as challenges for researchers.
Pre-Clinical iPS Cell Therapies Still Need to Be Validated
iPS cells hold great promise for the future of regenerative medicine. It is exciting to see that many patient-specific and disease-specific iPS cells have been generated recently 38–42, including a human sickle cell anemia mouse model that was successfully treated with genetically corrected autologous iPS cells 43. However, the safety and therapeutic applications of iPS and iPS-derived cells must be rigorously tested in appropriate animal models before advancing to any clinical trial. First, the minimum number of undifferentiated iPS cells that can cause teratoma or teratocarcinoma needs to be thoroughly studied in autologous transplantation animal models, as residual undifferentiated cells may still remain after iPS cells are differentiated to specific cell lineages and may lead to tumorigenicity after delivery into patients. This is a problem that also exists with ES cell transplantation as demonstrated elsewhere 44, 45. Second, as oncogenic transgene integration and insertional mutagenesis may be associated with many of the currently established iPS cell lines, the questions of whether iPS cells generated with different reprogramming technologies as well as their derivatives can induce cancer in the host also need to be rigorously evaluated. Even with improvements in the virus-free and transgene-free reprogramming technologies, the cancer-causing possibility of the derived “safe” iPS cells/derivatives still needs to be evaluated in animal models before these products can be used clinically for regenerative treatment. Third, iPS cell therapies need to be validated not only in small animals (mice and rats) but also in large animal models that are anatomically and physiologically more similar to humans. Both monkey 46 and pig 47–49 iPS cells have been generated, providing excellent models for iPS cell/derivatives transplantation studies. Although the thorough pre-clinical evaluation of iPS cells would be laborious, it is necessary to ensure their safe applications in the future.
Clinical iPS Cell Therapies Face Regulatory and Business Hurdles
Given the many potential risks of applying autologous iPS cell treatment to human subjects, iPS cell therapies may encounter strict regulatory restrictions in some parts of the world, including in the United States. For instance, it took Geron Corporation more than 6 years to receive approval from the Food and Drug Administration (FDA) for its human ES cell-derived neuronal cell (GRNOPC1) therapies in terms of cell product safety and reliability. (Note: the trial was recently placed on clinical hold due to one preclinical study showing a higher frequency of developing cysts in the injury site in animals treated with GRNOPC1). More recently, a second company has presented an investigational new drug (IND) for a phase I/II trial using human ES cell-derived retinal pigment epithelial (RPE) cells to treat patients with Stargardt’s Macular Dystrophy (SMD), one of the most common causes of juvenile blindness. The sponsoring company, Advanced Cell Technology (ACT), has performed years of testing to show that differentiated RPE cells can improve the visual performance of rats without adverse effects (e.g., teratomas) in hundreds of treated animals.
For patient-specific iPS cells, there is still a lack of consensus in deriving, culturing, and differentiating iPS cells among different laboratories as highlighted earlier. Furthermore, individual iPS cell lines may vary in their ability to differentiate into different cell lineages, making testing and approval by the FDA in a timely fashion even more difficult. Another issue that may hinder the clinical translation of iPS cell therapies is the economic feasibility of producing individualized iPS cell therapeutic products. The viability of a business model for patient-specific iPS treatment is still unknown. It may well be the case that few if any pharmaceutical companies will be able to produce cost-effective individualized iPS cell products tailored for a single patient at a time. On the other hand, the unique potential of iPS therapies cannot be denied. For instance, if researchers are able to solve the immune tolerance problem 50, then one can foresee allogeneic transplantation of iPS cell products (which can bypass the traditional ethical concerns that still plague ES cell products). To be commercially feasible, these cells will need to be made in standardized, large-scale production, and the individual needs or profiles of patients will need to be easily assessed to allow matching and wide distribution.
Conclusion
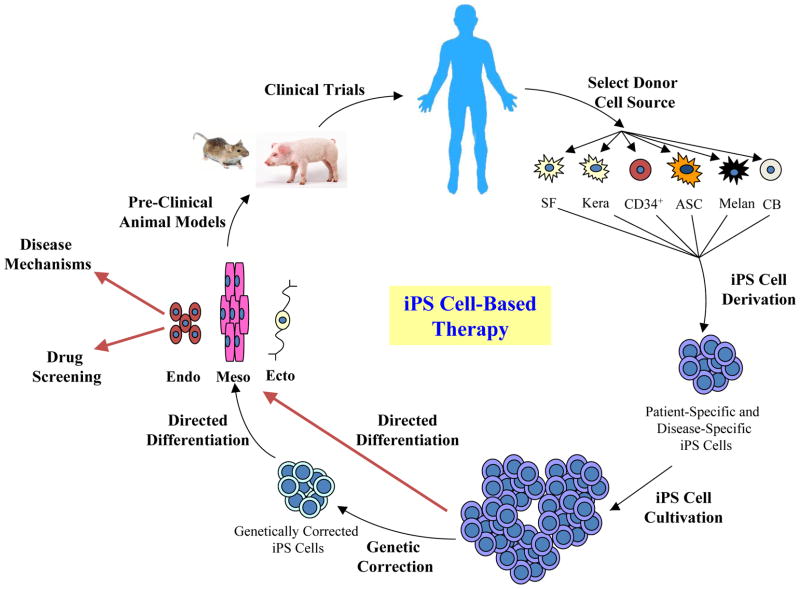
In summary, iPS cell-based therapies are still in their infancy, and many hurdles remain to be overcome before their clinical applications become a reality (Figure 1). With further improvements in derivation technologies, characterization methods, cultivation and differentiation protocols, and a better understanding of the reprogramming mechanisms, therapies using patient-specific iPS cells have the potential to revolutionize regenerative medicine and benefit patients for decades to come.
Figure 1. The idea of iPS cell-based regenerative therapy.
There remain significant hurdles to be overcome in each step, from iPS cell derivation to pre-clinical trials, before iPS cell-based clinical applications can become a reality. SF, skin fibroblasts; Kera, keratinocytes; CD34+, CD34+ cells from peripheral blood; ASC, adipose stem cell; CB, cord blood cell; Endo, endoderm; Meso, mesoderm; Ecto, ectoderm.
Acknowledgments
We would like to thank funding support from NIH DP2OD004437, Edward Mallinckrodt Jr. Foundation, AHA 0970394 (JCW), and RC1 HL100490 (MTL, JCW).
Abbreviations
- iPS cell
induced pluripotent stem cell
- hASC
human adipose stem cell
- ES cell
embryonic stem cell
- MEF
mouse embryonic fibroblasts
- NSC
neural stem cell
References
- 1.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Park IH, Lerou PH, Zhao R, Huo H, Daley GQ. Generation of human-induced pluripotent stem cells. Nat Protoc. 2008;3:1180–6. doi: 10.1038/nprot.2008.92. [DOI] [PubMed] [Google Scholar]
- 5.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–6. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 7.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–75. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 8.Yamanaka S. Elite and stochastic models for induced pluripotent stem cell generation. Nature. 2009;460:49–52. doi: 10.1038/nature08180. [DOI] [PubMed] [Google Scholar]
- 9.Kim JB, Greber B, Arauzo-Bravo MJ, Meyer J, Park KI, Zaehres H, et al. Direct reprogramming of human neural stem cells by OCT4. Nature. 2009;461:649–3. doi: 10.1038/nature08436. [DOI] [PubMed] [Google Scholar]
- 10.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–84. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 11.Loh YH, Agarwal S, Park IH, Urbach A, Huo H, Heffner GC, et al. Generation of induced pluripotent stem cells from human blood. Blood. 2009;113:5476–9. doi: 10.1182/blood-2009-02-204800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cashen AF, Lazarus HM, Devine SM. Mobilizing stem cells from normal donors: is it possible to improve upon G-CSF? Bone Marrow Transplant. 2007;39:577–88. doi: 10.1038/sj.bmt.1705616. [DOI] [PubMed] [Google Scholar]
- 13.Ye Z, Zhan H, Mali P, Dowey S, Williams DM, Jang YY, et al. Human induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood. 2009 doi: 10.1182/blood-2009-04-217406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Utikal J, Maherali N, Kulalert W, Hochedlinger K. Sox2 is dispensable for the reprogramming of melanocytes and melanoma cells into induced pluripotent stem cells. J Cell Sci. 2009;122:3502–10. doi: 10.1242/jcs.054783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun N, Panetta NJ, Gupta DM, Wilson KD, Lee A, Jia F, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci U S A. 2009;106:15720–5. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giorgetti A, Montserrat N, Aasen T, Gonzalez F, Rodriguez-Piza I, Vassena R, et al. Generation of induced pluripotent stem cells from human cord blood using OCT4 and SOX2. Cell Stem Cell. 2009;5:353–7. doi: 10.1016/j.stem.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haase A, Olmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, et al. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5:434–41. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Cairo MS, Wagner JE. Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood. 1997;90:4665–78. [PubMed] [Google Scholar]
- 20.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 21.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, et al. Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–77. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hamalainen R, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–70. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–6. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng B, Ng JH, Heng JC, Ng HH. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell. 2009;4:301–12. doi: 10.1016/j.stem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–7. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Yin X, Qin H, Zhu F, Liu H, Yang W, et al. Two supporting factors greatly improve the efficiency of human iPSC generation. Cell Stem Cell. 2008;3:475–9. doi: 10.1016/j.stem.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Lin T, Ambasudhan R, Yuan X, Li W, Hilcove S, Abujarour R, et al. A chemical platform for improved induction of human iPSCs. Nat Methods. 2009;6:805–8. doi: 10.1038/nmeth.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, et al. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–9. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Do JT, Desponts C, Hahm HS, Scholer HR, Ding S. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2:525–8. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, et al. A Small-Molecule Inhibitor of Tgf-beta Signaling Replaces Sox2 in Reprogramming by Inducing Nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson KD, Venkatasubrahmanyam S, Jia F, Sun N, Butte AJ, Wu JC. MicroRNA profiling of human-induced pluripotent stem cells. Stem Cells Dev. 2009;18:749–58. doi: 10.1089/scd.2008.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan EM, Ratanasirintrawoot S, Park IH, Manos PD, Loh YH, Huo H, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–7. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- 34.Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–6. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- 35.Unger C, Felldin U, Nordenskjold A, Dilber MS, Hovatta O. Derivation of human skin fibroblast lines for feeder cells of human embryonic stem cells. Curr Protoc Stem Cell Biol. 2008;Chapter 1(Unit 1C):7. doi: 10.1002/9780470151808.sc01c07s5. [DOI] [PubMed] [Google Scholar]
- 36.Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–46. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 37.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, et al. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–8. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 38.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–21. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 39.Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–80. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–6. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raya A, Rodriguez-Piza I, Guenechea G, Vassena R, Navarro S, Barrero MJ, et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature. 2009;460:53–9. doi: 10.1038/nature08129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanna J, Wernig M, Markoulaki S, Sun CW, Meissner A, Cassady JP, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–3. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 44.Lee AS, Tang C, Cao F, Xie X, van der Bogt K, Hwang A, et al. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle. 2009;8:2608–12. doi: 10.4161/cc.8.16.9353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao F, Drukker M, Lin S, Sheikh AY, Xie X, Li Z, et al. Molecular imaging of embryonic stem cell misbehavior and suicide gene ablation. Cloning Stem Cells. 2007;9:107–17. doi: 10.1089/clo.2006.0E16. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Zhu F, Yong J, Zhang P, Hou P, Li H, et al. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell. 2008;3:587–90. doi: 10.1016/j.stem.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Esteban MA, Xu J, Yang J, Peng M, Qin D, Li W, et al. Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J Biol Chem. 2009;284:17634–40. doi: 10.1074/jbc.M109.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ezashi T, Telugu BP, Alexenko AP, Sachdev S, Sinha S, Roberts RM. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci U S A. 2009;106:10993–8. doi: 10.1073/pnas.0905284106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Z, Chen J, Ren J, Bao L, Liao J, Cui C, et al. Generation of pig induced pluripotent stem cells with a drug-inducible system. J Mol Cell Biol. 2009;1:46–54. doi: 10.1093/jmcb/mjp003. [DOI] [PubMed] [Google Scholar]
- 50.Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci U S A. 2008;105:12991–6. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]