Abstract
A novel method for the simultaneous quantification of 16 antiretroviral (ARV) drugs and 4 metabolites in meconium was developed and validated. Quantification of 6 nucleoside/nucleotide reverse transcriptase inhibitors, 2 non-nucleoside reverse transcriptase inhibitors, 7 protease inhibitors and 1 integrase inhibitor was achieved in 0.25g of meconium. Specimen preparation included methanol homogenization and solid phase extraction. Separate positive and negative polarity multiple reaction monitoring mode injections were required to achieve sufficient sensitivity. Linearity ranged from 10–75ng/g up to 2500ng/g for most analytes and 100–500ng/g up to 25000ng/g for some; all correlation coefficients were ≥0.99. Extraction efficiencies from meconium were 32.8–119.5% with analytical recovery 80.3–108.3% and total imprecision 2.2–11.0% for all quantitative analytes. Two analytes with analytical recovery (70.0–138.5%) falling outside the 80–120% criteria range were considered semiquantitative. Matrix effects were −98.3–47.0% and −98.0–67.2% for analytes and internal standards, respectively. Analytes were stable (>75%) at room temperature for 24 h, 4°C for 3 days, −20°C for 3 freeze-thaw cycles over 3 days and on the autosampler. Method applicability was demonstrated by analyzing meconium from HIV-uninfected infants born to HIV-positive mothers on ARV therapy. This method can be used as a tool to investigate the potential effects of in utero ARV exposure on childhood health and neurodevelopmental outcomes.
Keywords: Meconium, Antiretrovirals, HIV, Liquid chromatography, Mass spectrometry
INTRODUCTION
Prevention and treatment of HIV/AIDS is a major global health priority, with prevention of perinatally acquired HIV a key strategy in eradication of the disease. In 2009, in low- and middle-income countries, an estimated 1.4 million HIV-infected pregnant women gave birth, with women from Africa’s sub-Saharan region accounting for 91% of all pregnant women living with HIV.1 Approximately 9,000 HIV-positive women give birth each year in the United States.2 Antiretroviral (ARV) administration to HIV-positive pregnant women and neonates reduces perinatal HIV transmission to less than 2% worldwide.3 However, concerns have been raised about potential toxicity in some neonates following gestational ARV exposure including mitochondrial dysfunction, increased bone porosity, growth deficits, and hearing and language impairments.4–9 Accurate quantification of ARV exposure by maternal ARV history is difficult as maternal pharmacokinetics, placental transfer and fetal metabolism vary.
Prenatal highly active anti-retroviral therapy (HAART) exposure increased from 19 to 88% from 1997 to 2009 and children born to HIV-infected women are exposed to a wide variety of ARV regimens.10 The increasing trend and diversity of ARV in utero exposures demand a method that can simultaneously quantify a large variety of exposures.
Meconium is the first neonatal fecal sample. It begins to form in the fetus during the 12th–13th week of gestation and accumulates thereafter.11–12 It is usually passed within the first 24–72h after birth and collection from diapers is easy and non-invasive.12 Meconium drug analysis is advantageous as disposition in meconium reflects fetal drug exposure during the 3rd and perhaps 2nd trimesters.13–15
Previous investigations demonstrated meconium’s utility in detecting in utero drug exposure and concentrations can correlate to maternal self-reported drug use and/or neonatal outcomes.13–16 Assessment of in utero tobacco exposure with meconium showed reduced infant birth weight, gestational age and head circumference in infants with positive meconium specimens.13 Analysis of buprenorphine in meconium suggested that buprenorphine marker concentrations predicted the onset and frequency of neonatal abstinence syndrome (NAS) in infants born to women on buprenorphine opioid replacement medication.17
Children exposed in utero to the same ARV regimen exhibit different developmental outcomes.4–9 It is unclear why some children manifest abnormalities and others do not despite the mother reportedly taking similar doses of ARVs. This may be because there is no causal association between in utero ARV exposure and certain developmental abnormalities, or alternatively, the inconsistent results may be because current methods quantifying fetal exposure are inadequate to examine this association. We believe meconium ARV drug and metabolite concentrations may better predict children likely to manifest developmental abnormalities than reported maternal dose. Therefore, we developed and validated the first liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for simultaneous quantification of ARVs and metabolites in meconium.
There is value to assaying all drug exposures from a single specimen. Pregnant women on modern therapy usually receive 3–4 ARVs from at least 2 different drug classes. Therefore, simultaneous extraction and quantification of several ARVs from different classes is needed to reduce the required specimen amount as small amounts of meconium (<1g) are available from infants. There are several published assays for quantification of specific ARV drug classes (protease inhibitors, PIs; non-nucleoside reverse transcriptase inhibitors, NNRTIs; and nucleoside/nucleotide reverse transcriptase inhibitors, NRTIs) in plasma while there are only three assays simultaneously quantifying multiple ARV classes in plasma.18–20
ARV analytical methods have been reported for blood, plasma or serum; however, quantitative methods are available for ARVs in amniotic fluid, breast milk, placental and fetal tissues, umbilical cord blood, cervicovaginal secretions, and hair.21–25 To date, there are no analytical methods for ARV drugs in meconium. This novel method provides a valuable tool for identifying and quantifying ARV exposure in children born to infected women in order to better evaluate the effect of gestational ARV exposure on health and neurodevelopmental outcomes.
EXPERIMENTAL SECTION
Meconium
A homogenous lot of ARV-negative meconium was prepared from meconium pools confirmed negative for amphetamines, opioids, cocaine, and cannabinoids. Prior to method validation, the meconium pool was confirmed negative for all ARV analytes at the assay’s limits of quantification (LOQs). To demonstrate method applicability, 32 meconium specimens were obtained through the Surveillance Monitoring for Antiretroviral Toxicities Study in HIV-uninfected Children Born to HIV-infected Women (SMARTT) protocol of the Pediatric HIV/AIDS Cohort Study (PHACS). Beginning in 2007, this study enrolls HIV-exposed but uninfected children of HIV-infected women administered ARVs during pregnancy in the United States to study the long-term effects of prenatal exposure to ARVs. PHACS study design and enrollment criteria are described by Williams et al.26 Infant follow-up through childhood and adolescence has a trigger-based design; initial assessments are conducted on all children, with more intensive evaluations on those meeting certain thresholds, to determine whether there are adverse developmental outcomes.26 Meconium samples were collected from infants within 72h of delivery at the hospital and stored at −20°C prior to analysis.
Reagents
The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (Bethesda, MD): lamivudine (3TC), abacavir (ABC), amprenavir (APV), atazanavir (ATV), zidovudine (3'-azidothymidine; AZT), stavudine (2'-3'-didehydro-2'-3'-dideoxythymidine; d4T), darunavir (DRV), efavirenz (EFV), emtricitabine (FTC), lopinavir (LPV), nelfinavir (NFV), nevirapine (NVP), raltegravir (RAL), ritonavir (RTV), saquinavir (SQV), and tenofovir (TFV). Metabolite standards abacavir carboxylate (ABC-C), abacavir glucuronide (ABC-G), zidovudine glucuronide (AZT-G), and nelfinavir hydroxy-tert-butylamide (M8), and deuterated or isotopically labeled internal standards were purchased from Toronto Research Chemicals (North York, Ontario, Canada). Methanol and formic acid were from Fisher Scientific (Fair Lawn, NJ). Acetonitrile and trifluoroacetic acid were from Sigma-Aldrich (St. Louis, MO). Water was purified in-house by an ELGA Purelab Ultra Analytic purifier (Siemens Water Technologies, Lowell, MA). All solvents were high-performance liquid chromatography (HPLC) grade or better. Strata-X solid-phase extraction (SPE) cartridges (60mg/3mL) were used in sample preparation (Phenomenex, Torrance, CA).
Instrumentation
Experiments were performed on an AB Sciex 3200 Qtrap mass spectrometer with a TurboV electrospray ionization (ESI) source (AB Sciex, Foster City, CA). The mass spectrometer was interfaced to a Shimadzu UFLCXR system with two LC-20ADXR pumps, a SIL-20ACXR autosampler, and a CTO-20 AC column oven (Shimadzu Corporation, Columbia, MD). A TurboVap LV evaporator from Zymark (Hopkinton, MA) was used to evaporate samples under nitrogen. Samples were centrifuged with an Eppendorf 5804R centrifuge. SPE was performed with a CEREX System-48 positive-pressure manifold (SPEware Corp, Baldwin Park, CA).
Preparation of Standard Solutions
Powdered standards were reconstituted in the manufacturer’s recommended solvent. Individual stock solutions of 2mg/mL 3TC, ABC, APV, ATV, AZT, d4T, DRV, EFV, FTC, LPV, NFV, NVP, RAL, RTV, SQV, and TFV; and 1mg/mL ABC-C, ABC-G, AZT-G and M8 were diluted with methanol to prepare calibrator solutions. A high calibrator solution containing 6250ng/mL of ARV standards (62500ng/mL for DRV, APV, d4T, AZT, AZT-G and EFV) was prepared in methanol and serial dilutions created subsequent calibrator stock solutions of 625 and 50ng/mL (6250 and 500ng/mL for DRV, APV, d4T, AZT, AZT-G and EFV). Appropriate volumes ranging from 20–100µL were added to 0.25g meconium yielding 10, 50, 75, 100, 250, 500, 1000, and 2500ng/g calibrators for all analytes except DRV, APV, d4T, AZT, AZT-G and EFV (100, 500, 750, 1000, 2500, 5000, 10000, and 25000ng/g).
Quality control (QC) solutions were prepared in methanol using different stock solutions than for preparing calibration standards. Low, medium and high quality control samples were prepared across the linear dynamic range of each analyte. Stock internal standard solution preparation is described in supplementary material. Internal standards for each analyte are listed in Tables 1 and 2. All calibrator, QC and internal standard solutions were stored at −20°C.
Table 1.
LC-MS/MS parameters for antiretrovirals and metabolites in meconium
| Analyte | Q1 Mass (m/z) |
Q3 Mass (m/z) |
Dwell (msec) |
DP (V) |
CE (V) |
Internal Standard |
Q1 Mass (m/z) |
Q3 Mass (m/z) |
Dwell (msec) |
DP (V) |
CE (V) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TFV | 288.0 | 176.2 | 40 | 61 | 31 | TFV-d6 | 294.1 | 182.2 | 40 | 66 | 33 |
| 288.0 | 159.0 | 40 | 61 | 41 | 294.1 | 164.2 | 40 | 66 | 41 | ||
| 3TC | 230.1 | 112.0 | 60 | 31 | 19 | 3TC-13C,15N2 | 233.0 | 115.0 | 60 | 26 | 21 |
| 230.1 | 95.0 | 60 | 31 | 51 | 233.0 | 97.0 | 60 | 26 | 51 | ||
| FTC | 248.1 | 130.2 | 80 | 26 | 19 | FTC-13C,15N2 | 251.0 | 133.2 | 80 | 21 | 19 |
| 248.1 | 113.0 | 80 | 26 | 53 | 251.0 | 114.9 | 80 | 21 | 49 | ||
| ABC | 287.1 | 191.2 | 80 | 46 | 25 | ABC-d4 | 291.2 | 195.1 | 80 | 46 | 27 |
| 287.1 | 150.2 | 80 | 46 | 41 | 291.2 | 152.2 | 80 | 46 | 41 | ||
| ABC-G | 463.3 | 191.3 | 80 | 36 | 37 | ABC-d4 | 291.2 | 195.1 | 80 | 46 | 27 |
| 463.3 | 287.3 | 80 | 36 | 29 | 291.2 | 152.2 | 80 | 46 | 41 | ||
| ABC-C | 301.7 | 191.3 | 80 | 26 | 25 | ABC-d4 | 291.2 | 195.1 | 80 | 46 | 27 |
| 301.7 | 150.2 | 80 | 26 | 41 | 291.2 | 152.2 | 80 | 46 | 41 | ||
| NVP | 267.2 | 226.2 | 80 | 56 | 33 | NVP-d5 | 272.1 | 227.2 | 80 | 61 | 35 |
| 267.2 | 80.2 | 80 | 56 | 53 | 272.1 | 198.0 | 80 | 61 | 51 | ||
| RAL | 445.2 | 109.0 | 60 | 61 | 41 | RAL-d3 | 448.2 | 109.2 | 60 | 51 | 39 |
| 445.2 | 361.2 | 60 | 61 | 25 | 448.2 | 364.2 | 60 | 51 | 25 | ||
| M8 | 584.3 | 330.2 | 60 | 71 | 45 | NFV-d3 | 571.3 | 110.0 | 60 | 71 | 99 |
| 584.3 | 135.3 | 60 | 71 | 67 | 571.3 | 82.2 | 60 | 71 | 113 | ||
| APV | 506.2 | 245.2 | 60 | 56 | 25 | APV-d4 | 510.2 | 245.2 | 60 | 41 | 27 |
| 506.2 | 156.1 | 60 | 56 | 35 | 510.2 | 156.2 | 60 | 41 | 35 | ||
| DRV | 548.2 | 392.3 | 60 | 36 | 25 | DRV-d9 | 557.2 | 401.4 | 60 | 36 | 25 |
| 548.2 | 69.1 | 60 | 36 | 51 | 557.2 | 68.9 | 60 | 36 | 51 | ||
| NFV | 568.3 | 330.2 | 60 | 66 | 41 | NFV-d3 | 571.3 | 110.0 | 60 | 71 | 99 |
| 568.3 | 135.2 | 60 | 66 | 67 | 571.3 | 82.2 | 60 | 71 | 113 | ||
| SQV | 671.3 | 570.1 | 60 | 71 | 39 | SQV-d9 | 680.4 | 128.2 | 60 | 81 | 119 |
| 671.3 | 128.2 | 60 | 71 | 125 | 680.4 | 225.2 | 60 | 81 | 75 | ||
| ATV | 705.3 | 168.2 | 60 | 71 | 61 | ATV-d5 | 710.3 | 168.0 | 60 | 81 | 61 |
| 705.3 | 335.1 | 60 | 71 | 39 | 710.3 | 340.2 | 60 | 81 | 41 | ||
| RTV | 721.2 | 140.2 | 60 | 51 | 83 | RTV-d6 | 727.3 | 146.2 | 60 | 51 | 83 |
| 721.2 | 296.2 | 60 | 51 | 29 | 727.3 | 302.2 | 60 | 51 | 29 | ||
| LPV | 629.2 | 155.2 | 60 | 41 | 59 | LPV-d8 | 637.3 | 163.1 | 60 | 36 | 63 |
| 629.2 | 447.2 | 60 | 41 | 25 | 637.3 | 447.2 | 60 | 36 | 25 | ||
| d4T | 223.0 | 42.0 | 60 | −35 | −30 | d4T-d3 | 226.0 | 42.0 | 60 | −35 | −32 |
| 223.0 | 192.9 | 60 | −35 | −16 | 226.0 | 195.9 | 60 | −35 | −12 | ||
| AZT | 266.0 | 223.1 | 60 | −35 | −14 | AZT-d3 | 269.0 | 226.2 | 60 | −35 | −14 |
| 266.0 | 42.1 | 60 | −35 | −30 | 269.0 | 42.0 | 60 | −35 | −32 | ||
| AZT-G | 442.1 | 125.0 | 60 | −105 | −32 | GAZT-d3 | 446.0 | 128.0 | 60 | −55 | −32 |
| 442.1 | 42.1 | 60 | −105 | −70 | 446.0 | 42.1 | 60 | −55 | −86 | ||
| EFV | 314.0 | 69.0 | 60 | −60 | −42 | EFV-d5 | 318.9 | 69.0 | 40 | −50 | −46 |
| 314.0 | 244.1 | 60 | −60 | −24 | 318.9 | 248.0 | 40 | −50 | −24 |
Bold denotes quantifier transition
Q1 quadrupole 1, Q3 quadrupole 3, DP declustering potential, CE collision energy, TFV tenofovir, 3TC lamivudine, FTC emtricitabine, ABC abacavir, ABC-G ABC-glucuronide, ABC-C ABC carboxylate, NVP nevirapine, RAL raltegravir, APV amprenavir, DRV darunavir, NFV nelfinavir, M8 nelfinavir hydroxy-tert-butylamide, SQV saquinavir, ATV atazanavir, RTV ritonavir, LPV lopinavir, d4T stavudine, AZT zidovudine, AZT-G AZT-glucuronide, EFV efavirenz
Table 2.
Limits of detection (LOD), quantification (LOQ), calibration results and retention times for ARV drugs and metabolites in meconium by LC-MS/MS
| Analyte | Internal Standard |
LOD (ng/g) |
LOQ (ng/g) |
Slope (mean ± SD, n=5) |
y-intercept (mean ± SD, n=5) |
R2 (range, n=5) |
Linear range (ng/g) |
Retention Time (min) |
|---|---|---|---|---|---|---|---|---|
| TFV | TFV-d6 | 5 | 10 | 1.812 ± 0.087 | 0.002 ± 0.031 | 0.997–0.999 | 10–2500 | 4.3 |
| 3TC | 3TC-13C,15N2 | 25 | 50 | 1.200 ± 0.112 | 0.003 ± 0.004 | 0.995–0.998 | 50–2500 | 5.5 |
| FTC | FTC-13C,15N2 | 5 | 10 | 1.306 ± 0.046 | −0.003 ± 0.004 | 0.998–1.000 | 10–2500 | 6.5 |
| ABC | ABC-d4 | 1 | 10 | 0.480 ± 0.028 | 0.005 ± 0.004 | 0.993–0.999 | 10–2500 | 11.5 |
| ABC-G | ABC-d4 | 5 | 10 | 0.148 ± 0.043 | 0.000 ± 0.001 | 0.990–0.995 | 10–2500 | 10.5 |
| ABC-C | ABC-d4 | 25 | 75 | 0.015 ± 0.003 | 0.002 ± 0.001 | 0.990–0.998 | 75–2500 | 11.6 |
| NVP | NVP-d5 | 5 | 10 | 1.65 ± 0.336 | 0.523 ± 0.032 | 0.990–0.999 | 10–2500 | 13.9 |
| RAL | RAL-d3 | 10 | 10 | 1.628 ± 0.106 | −0.031 ± 0.015 | 0.998–1.000 | 10–2500 | 17.5 |
| APV | APV-d4 | 10 | 100 | 0.514 ± 0.033 | 0.009 ± 0.004 | 0.994–1.000 | 100–25000 | 18.6 |
| DRV | DRV-d9 | 50 | 100 | 0.940 ± 0.337 | 0.002 ± 0.002 | 0.996–0.998 | 100–25000 | 18.7 |
| M8 | NFV-d3 | 5 | 10 | 7.368 ± 0.060 | 0.107 ± 0.060 | 0.994–0.998 | 10–2500 | 18.9 |
| NFV | NFV-d3 | 5 | 10 | 5.020 ± 0.360 | 0.105 ± 0.036 | 0.996–0.999 | 10–2500 | 20.1 |
| SQV | SQV-d9 | 5 | 10 | 0.727 ± 0.048 | 0.006 ± 0.009 | 0.994–0.999 | 10–2500 | 20.9 |
| ATV | ATV-d5 | 1 | 10 | 1.898 ± 0.136 | 0.175 ± 0.009 | 0.995–0.999 | 10–2500 | 22.4 |
| RTV | RTV-d6 | 1 | 10 | 1.684 ± 0.033 | 0.052 ± 0.033 | 0.996–0.999 | 10–2500 | 23.3 |
| LPV | LPV-d8 | 5 | 10 | 0.795 ± 0.014 | 0.139 ± 0.005 | 0.996–0.999 | 10–2500 | 24.1 |
| d4T | d4T-d3 | 250 | 500 | 0.531 ± 0.172 | 0.007 ± 0.007 | 0.994–0.998 | 500–25000 | 4.3 |
| AZT | AZT-d3 | 50 | 100 | 0.860 ± 0.170 | 0.005 ± 0.009 | 0.996–0.999 | 100–25000 | 4.9 |
| AZT-G | AZT-G-d3 | 500 | 500 | 2.072 ± 0.132 | −0.117 ± 0.036 | 0.990–0.993 | 500–25000 | 5.3 |
| EFV | EFV-d5 | 50 | 100 | 1.698 ± 0.515 | 0.003 ± 0.004 | 0.997–0.999 | 100–25000 | 8.0 |
Procedures
Sample Preparation
Blank meconium (0.25g±0.003g) was weighed into a 1.5mL microcentrifuge tube and fortified with calibrator or QC solution and internal standard. Methanol (1mL) was added and specimens homogenized with wooden applicator sticks, vortexed vigorously and centrifuged at 15000×g for 10min at 4°C. The supernatant was transferred to a clean 13×100mm glass tube. An additional 1mL of methanol was added to the specimens and mixed vigorously for 10min on a multi-tube vortexer. Specimens were centrifuged and the supernatant was added to the previous aliquot and evaporated to 0.1mL under nitrogen at 42°C. The concentrated supernatant was reconstituted in 3mL 0.025% (12.3mM) formic acid in deionized water (v/v), pH 2.9, and vortexed.
Solid-Phase Extraction
Strata-X extraction columns were conditioned with 1.25mL methanol and 1.25mL 0.025% formic acid in deionized water (v/v), pH 2.9. Samples were decanted onto conditioned columns with collection of the fraction passing through the column in 10mL conical polypropylene tubes. These fractions were removed and SPE columns washed with 1.25mL 5% methanol in de-ionized water (v/v). Columns were dried via vacuum at 20psi for 5min prior to eluting analytes with 1.5mL elution solvent (0.025% formic acid in acetonitrile (v/v)) into clean 15mL conical polypropylene tubes. Three to 5 psi was applied to each column to assist flow during all SPE steps.
A second SPE procedure was required to recover TFV, which was not retained on the SPE columns at pH 2.9. After the initial elution that collected all other analytes, the same SPE columns were re-equilibrated with 1.25mL deionized water containing 0.025% formic acid and 1% trifluoroacetic acid (v/v), pH 1.1. The pH of the initial sample was adjusted to 1.1 by adding 40µL trifluoroacetic acid prior to applying to re-conditioned columns. Columns were washed with 0.25mL de-ionized water prior to eluting with 1.5mL elution solvent into 15mL conical polypropylene tubes containing initial eluates. Combined eluates from the pH 2.9 and 1.1 extractions were dried under nitrogen at 42°C in a Zymark evaporator. Samples were reconstituted in 150µL of 0.025% formic acid in deionized water, vortexed for 30s, centrifuged at 4°C at 10,500×g for 5min and transferred to autosampler vials.
Liquid Chromatography
To achieve maximum sensitivity, 2 injections, 1 in positive and 1 in negative ionization mode were required for quantification of 20 target analytes. Most analytes optimally ionized in positive mode; however, d4T, AZT, AZT-G, and EFV required negative mode acquisition. Both runs were performed on a Poroshell 120 end-capped C18 column (150×2.1mm, 2.7µm) fitted with a Zorbax Eclipse Plus C18 guard column (2.1×12.5mm, 5µm) and a 0.2µm in-line frit (Agilent Technologies, Santa Clara, CA). The column oven and autosampler temperatures were 40 and 4°C, respectively. Gradient elution was performed using water (A) and methanol (B), both adjusted to pH 2.70 (±0.05) with formic acid (0.05%/volume), at a flow rate of 0.3mL/min. The first acquisition method (method 1) utilized a gradient program beginning with 0%B, increased to 90%B over 24min, increased to 100%B at 26min, held for 4min, decreased to 0%B in 0.5min and held for 4min; total run time was 34min. The second acquisition method (method 2) quantifying d4T, AZT-G, AZT, and EFV used a gradient beginning with 0%B, increased to 90%B over 6min, increased to 100%B at 9min, held for 3min, decreased to 0%B in 0.5min and held for 4min; total run time was 16min. In both methods, HPLC eluent was diverted to waste for the first 2min and the final 8.5min of analysis; injection volumes were 10µL.
Mass spectrometry
Mass spectrometric data were acquired with ESI operating in positive mode for method 1 and negative mode for method 2. Table 1 shows the compound-specific optimized MS/MS parameters achieved via direct infusion at 10µL/min of 250ng/mL (4µg/mL required for analytes in negative mode) reference solutions in 50% aqueous methanol solution. Method 1 was divided into 5 periods as follows: period 1-TFV, 3TC, FTC; period 2-ABC, ABC-G, ABC-C; period 3-NVP; period 4-RAL, M8, DRV, APV, NFV, SQV; period 5-ATV, RTV, LPV. Optimized source parameters were as follows: gas (1), 0.41MPa; gas (2), 0.52MPa; curtain gas, 0.21MPa (0.17MPa periods 4 and 5); source temperature, 600 °C; and ion source voltage 5500V (5000V for periods 1 and 2). Method 2 was divided into 2 periods as follows: period 1-d4T, AZT, AZT-G; period 2-EFV. Optimized source parameters for method 2 were the same as method 1 except ion spray voltage was −4500V.
Data Analysis
Linear regression with 1/x2 weighting was employed for all analytes. Peak area ratios of target analytes and respective internal standards were calculated for each concentration. Analyst 1.5 (AB Sciex, Foster City, CA) was utilized for all data collection and processing.
Validation
Specificity, sensitivity, linearity, intra- and inter-day imprecision, analytical recovery, extraction efficiency, matrix effect, carryover, dilution integrity, endogenous and exogenous interferences, and analyte stability were evaluated. Specificity was assessed by relative retention time, precursor mass, and fragment ion. Retention times for QC and authentic specimens were required to be within ±0.2min of the mean calibrator retention time. Transition peak area ratios for QC and authentic specimens were required to be within ±20% of the mean peak area ratios for calibrators of each analyte. Sensitivity was defined by limits of detection (LOD) and LOQ. Decreasing concentrations of drug-fortified meconium were analyzed to empirically determine LOD and LOQ. LOD was defined as the concentration with a signal-to-noise ratio of at least 3, transition peak area ratios within 20% of the mean calibrator ratios, retention time within ±0.2min of the mean calibrator retention time, and acceptable peak shape. LOQ was defined by LOD criteria in addition to a signal-to-noise ratio of at least 10 and acceptable bias and imprecision (calculated target concentration and relative standard deviation within ±20%, n=5). Any analyte for which accuracy did not fall within 80%–120% of the target concentration was considered semiquantitative.
Linearity, expressed by the squared correlation coefficient (R2), was evaluated with calculation of a least squares regression line. Heteroscedasticity was compensated with a 1/x2 weighting factor. Linearity of each analyte was determined with at least 6 concentration levels, not including the blank matrix, on 5 separate days.
Intra-day and inter-day analytical recovery (accuracy) and imprecision were determined from 4 replicates at 3 QC concentrations analyzed in 5 batches with separate calibration curves. Percent accuracy was assessed by comparing the mean QC concentration to the expected concentration value. Inter-day accuracy and imprecision were assessed with 4 replicates on 5 different days (n=20). The recommendations of Krouwer and Rabinowitz27 were used to calculate pooled intra-day, inter-day and total imprecision, expressed as % relative standard deviation.
Extraction efficiency and matrix effect were determined according to Matuszewski et al.28 Extraction efficiency was calculated by dividing the mean analyte peak areas of samples with QC and internal standard solutions added prior to SPE by the mean analyte peak area of blank samples fortified with these solutions after SPE (n=5). Matrix effect was examined by dividing the mean analyte peak area of extracted blank samples fortified after SPE to the mean analyte peak area of neat samples prepared in initial mobile phase at equivalent concentrations (n=5). The value was converted to a percentage and subtracted from 100 to represent the amount of signal suppression or enhancement due to matrix presence.
Carryover was determined by injecting a negative specimen containing internal standard after a specimen containing 2 and 5 times the upper limit of quantification (n=3). High concentrations of ARVs may be observed in meconium, making dilution necessary. Dilution integrity (1:5) was assessed with 3 meconium specimens fortified with high QC solution and internal standards and homogenized in methanol as previously described. After each centrifugation, 200µL supernatant was combined with 800µL supernatant from meconium fortified with only internal standard and the procedure was followed as usual.
Interference from endogenous substances was evaluated through analysis of 10 blank meconium pools fortified with internal standards. Interferences from 96 illicit and common therapeutic drugs and metabolites and other ARVs (Table S-1) were evaluated by adding potential interferents to meconium samples fortified with low QC solution. For most compounds investigated, expected concentrations in meconium were unavailable; therefore a 20,000ng/g concentration was chosen for all interferents to far exceed observed concentrations seen for drugs of abuse.29–31 A compound did not interfere if the low QC quantified within 20% of target and had stable retention time and correct transition ratios.
Analyte stability in meconium was evaluated with 4 replicates at each QC concentration under 3 conditions: 24h at room temperature (RT), 72h at 4°C, and 3 freeze–thaw cycles at −20°C (23h freeze, 1h thaw at RT). Calculated concentrations of stability specimens were compared to 4 QC specimens prepared on the day of analysis. Autosampler stability was assessed by reinjecting QC specimens after 48h and comparing calculated concentrations to original values using the initial calibration curve.
RESULTS AND DISCUSSION
We present the first validated LC-MS/MS method for the analysis of ARV drugs and metabolites in meconium. Meconium ARV drug and metabolite concentrations may better predict children likely to manifest developmental abnormalities than reported maternal ARV dose as meconium objectively reflects fetal drug exposure during late pregnancy. This validated assay will be employed to compare meconium concentrations to maternal antiretroviral regimens and child developmental outcomes.
Calibration and Validation
This method was validated according to the criteria described in the Experimental Section. Calibration results are detailed in Table 2. LODs ranged from 1–500ng/g and LOQs from 10–500ng/g. The high 500ng/g LOQ for d4T and AZT-G was due to poor fragmentation that failed to yield qualifier product ions of sufficient sensitivity for a lower LOQ; fragmentation of d4T and parent AZT are described by Gehrig et al.18 For many analytes, linear ranges spanned more than 2 orders of magnitude.
Two analytical runs were required to quantify all 20 analytes in meconium. Poor sensitivity was observed with positive mode ionization for EFV, AZT, AZT-G, and d4T native and deuterated standards and they could not be chromatographically isolated in method 1 to allow for polarity switching. A second injection of the same extract with a shorter run time was developed for these analytes.
Analytical recovery and imprecision were evaluated at 3 concentrations spanning the linear dynamic range of each analyte. ABC-C and ABC-G failed to meet quantitative criteria as the mean intra-day analytical recovery was >120% at some of the tested concentrations and the range of inter-day analytical recovery was >120% at the tested concentrations (Table 3). Therefore, we regard any data for ABC-C and ABC-G as semiquantitative. Accurate quantitation issues for these metabolites may stem from the poor and variable recovery (23.8–50.2%, Table 4) and the lack of deuterated ABC-C and ABC-G standards. ABC-d4 was chosen as an internal standard for ABC-C and ABC-G as it has a similar retention time and is the most structurally similar. ABC and ABC-d4 had higher extraction efficiencies (76.6–87.2%, Table 4) than ABC-C and ABC-G. Matrix effect of ABC-d4 matched ABC-C well, but this was not the case for ABC-G. A better-suited internal standard might improve quantification of these metabolites. Another metabolite included in this method, M8, also lacked a deuterated internal standard; however use of NFV-d3 proved to be sufficient for accurate quantification of this metabolite.
Table 3.
Analytical recovery (bias) and imprecision data for ARVs in meconium
| Analytical Recovery (% of target concentration) | Imprecision (% RSD, n=20) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Analyte | Expected conc. (ng/g) |
Intra-day, n=4 | Inter-day, n=20 | ||||||
| Mean | Range | Mean | Range | Pooled Intra-day |
Inter- day |
Total | |||
| 30 | 107.5 | 100.7 – 117.0 | 105.0 | 86.3 – 117.0 | 4.7 | 7.7 | 9.0 | ||
| TFV | 300 | 106.2 | 101.0 – 114.0 | 107.9 | 101.0 – 117.0 | 3.5 | 3.8 | 5.2 | |
| 2000 | 101.4 | 98.0 – 105.5 | 103.8 | 98.0 – 115.0 | 4.1 | 1.0 | 4.2 | ||
| 150 | 110.3 | 106.7 – 114.7 | 106.4 | 98.0 – 114.7 | 3.5 | 2.0 | 4.0 | ||
| 3TC | 750 | 107.9 | 102.0 – 111.3 | 106.7 | 100.4 – 112.0 | 3.7 | 0.8 | 3.8 | |
| 2000 | 106.5 | 105.0 – 107.5 | 103.7 | 96.0 – 114.5 | 3.5 | 3.1 | 4.7 | ||
| 30 | 105.7 | 104.7 – 106.7 | 103.0 | 96.3 – 111.0 | 2.7 | 1.4 | 3.0 | ||
| FTC | 300 | 103.8 | 101.0 – 108.0 | 104.5 | 96.3 – 111.3 | 3.0 | 1.4 | 3.3 | |
| 2000 | 98.6 | 96 – 101.5 | 99.6 | 90.5 – 108.0 | 3.8 | 3.1 | 5.0 | ||
| 30 | 107.8 | 102.0 – 114.7 | 111.9 | 98.7 – 119.0 | 4.6 | 1.1 | 4.7 | ||
| ABC | 300 | 110.9 | 108.3 – 116.7 | 109.2 | 97.0 – 118.3 | 3.6 | 5.3 | 6.4 | |
| 2000 | 96.9 | 84.0 – 102.5 | 95.3 | 84.0 – 104.5 | 5.4 | 3.3 | 6.3 | ||
| 30 | 120.3 | 105.7 – 127.7 | 109.7 | 88.0 – 127.7 | 8.1 | 8.6 | 11.9 | ||
| ABC-Ga | 300 | 118.8 | 104.0 – 124.3 | 110.8 | 81.3 – 129.3 | 12.4 | 4.1 | 13.1 | |
| 2000 | 114.1 | 96.5 – 122.0 | 102.8 | 72.5 – 137.5 | 15.5 | 9.0 | 17.9 | ||
| 150 | 120.2 | 108.7 – 128.0 | 105.1 | 70.0 – 128.0 | 10.2 | 13.0 | 16.5 | ||
| ABC-Ca | 750 | 120.6 | 112.0 – 133.3 | 111.8 | 91.5 – 137.3 | 11.9 | 5.8 | 13.2 | |
| 2000 | 111.8 | 104.5 – 117.0 | 103.4 | 73.5 – 138.5 | 12.8 | 8.7 | 15.5 | ||
| 30 | 108.3 | 104.3 – 115.3 | 111.0 | 104.3 – 116.7 | 3.6 | 0.7 | 3.7 | ||
| NVP | 300 | 109.4 | 100.7 – 117.7 | 109.7 | 98.3 – 117.7 | 4.5 | 1.1 | 4.7 | |
| 2000 | 90.8 | 86.5 – 95.0 | 92.0 | 84.0 – 99.5 | 2.9 | 3.1 | 4.2 | ||
| 30 | 84.5 | 80.7 – 90.0 | 89.3 | 80.3 – 108.0 | 4.2 | 6.2 | 7.5 | ||
| RAL | 300 | 103.0 | 93.0 – 112.3 | 104.4 | 93.0 – 115.0 | 3.9 | 5.4 | 6.7 | |
| 2000 | 95.0 | 94.0 – 96.0 | 97.3 | 93.5 – 108.0 | 4.0 | 2.6 | 4.8 | ||
| 30 | 105.4 | 84.0 – 119.3 | 104.8 | 84.0 – 119.3 | 6.8 | 8.6 | 11.0 | ||
| M8 | 300 | 103.9 | 86.0 – 119.7 | 103.3 | 86.0 – 119.7 | 6.3 | 7.4 | 9.7 | |
| 2000 | 91.8 | 80.5 – 112.0 | 95.8 | 80.5 – 112.0 | 9.2 | 3.7 | 9.9 | ||
| 300 | 102.8 | 94.3 – 111.7 | 106.1 | 94.0 – 116.0 | 5.3 | 3.4 | 6.3 | ||
| APV | 3000 | 105.6 | 100.3 – 110.0 | 104.7 | 94.3 – 110.0 | 2.9 | 2.2 | 3.6 | |
| 20000 | 89.9 | 86.5 – 93.0 | 92.3 | 84.0 – 104.0 | 3.9 | 0 | 3.9 | ||
| 300 | 96.5 | 88.7 – 108.7 | 101.0 | 88.7 – 108.7 | 4.4 | 2.3 | 4.9 | ||
| DRV | 3000 | 100.1 | 92.7 – 105.3 | 99.7 | 92.7 – 105.3 | 2.4 | 0 | 2.4 | |
| 20000 | 85.5 | 81.0 – 94.5 | 89.2 | 81.0 – 100.0 | 2.8 | 4.3 | 5.1 | ||
| 30 | 105.8 | 102.7 – 109.7 | 107.1 | 93.7 – 116.0 | 4.5 | 2.2 | 5.0 | ||
| NFV | 300 | 101.2 | 94.0 – 107.7 | 106.3 | 94.0 – 118.7 | 3.9 | 4.0 | 5.6 | |
| 2000 | 91.0 | 83.5 – 100.0 | 94.9 | 83.5 – 107.5 | 5.3 | 5.4 | 7.5 | ||
| 30 | 95.5 | 86.7 – 109.7 | 103.0 | 86.7 – 119.0 | 8.1 | 3.6 | 8.9 | ||
| SQV | 300 | 99.4 | 93.7 – 101.7 | 105.5 | 92.0 – 117.0 | 6.0 | 3.4 | 6.9 | |
| 2000 | 101.8 | 90.0 – 103.5 | 99.0 | 90.0 – 114.5 | 6.9 | 0 | 6.9 | ||
| 30 | 107.0 | 101.3 – 114.7 | 110.3 | 101.3 – 119.3 | 3.2 | 3.7 | 4.9 | ||
| ATV | 300 | 103.9 | 100 – 110.7 | 107.0 | 100.0 – 116.7 | 3.1 | 3.7 | 4.8 | |
| 2000 | 89.5 | 84.5 – 93.5 | 91.9 | 84.5 – 101.0 | 2.7 | 2.0 | 3.4 | ||
| 30 | 102.1 | 97.3 – 105.0 | 104.4 | 94.7 – 115.3 | 3.5 | 4.8 | 5.9 | ||
| RTV | 300 | 105.2 | 98.0 – 118.7 | 107.9 | 98.0 – 118.7 | 3.9 | 0 | 3.9 | |
| 2000 | 93.9 | 87.0 – 102.0 | 97.5 | 87.0 – 103.5 | 4.2 | 2.5 | 4.9 | ||
| 30 | 107.3 | 100.7 – 112.3 | 107.7 | 99.3 – 117.7 | 3.7 | 1.3 | 3.9 | ||
| LPV | 300 | 108.8 | 106.3 – 113.3 | 106.5 | 101.3 – 113.3 | 2.3 | 1.3 | 2.7 | |
| 2000 | 98.8 | 90.5 – 103.0 | 99.0 | 90.5 – 103.0 | 3.0 | 0.3 | 3.0 | ||
| 500 | 97.3 | 96.0 – 99.3 | 97.7 | 91.3 – 106.0 | 3.3 | 1.6 | 3.7 | ||
| d4T | 7500 | 100.0 | 98.7 – 103.2 | 100.0 | 92.9 – 105.3 | 2.6 | 2.0 | 3.3 | |
| 20000 | 93.5 | 87.0 – 96.5 | 95.8 | 87.0 – 101.5 | 3.0 | 1.8 | 3.5 | ||
| 300 | 102.2 | 96.0 – 108.3 | 102.0 | 91.3 – 110.0 | 4.1 | 0.9 | 4.2 | ||
| AZT | 3000 | 104.7 | 101.0 – 108.0 | 103.0 | 95.0 – 112.3 | 3.6 | 2.2 | 4.2 | |
| 20000 | 95.5 | 93.0 – 97.5 | 97.8 | 87.5 – 109.0 | 3.2 | 4.4 | 5.4 | ||
| 500 | 95.8 | 92.0 – 100.0 | 98.2 | 92.0 – 101.3 | 1.6 | 1.4 | 2.2 | ||
| AZT-G | 7500 | 102.9 | 96.9 – 107.9 | 107.8 | 96.9 – 117.5 | 4.0 | 1.6 | 4.3 | |
| 20000 | 108.9 | 97.5 – 116.5 | 112.9 | 93.0 – 118.0 | 6.9 | 0 | 6.9 | ||
| 300 | 98.4 | 97.0 – 102.3 | 103.4 | 97.0 – 111.0 | 3.0 | 2.7 | 4.0 | ||
| EFV | 3000 | 103.5 | 101.7 – 106.0 | 104.6 | 99.0 – 111.7 | 2.1 | 2.1 | 3.0 | |
| 20000 | 95.8 | 90.0 – 101.5 | 93.7 | 85.5 – 101.5 | 3.6 | 1.1 | 3.8 | ||
Since accuracy fell outside 80–120% target concentrations for ABC-G and ABC-C QC concentrations, these analytes are considered semiquantitative.
Table 4.
Mean extraction efficiency and matrix effect for ARVs in meconium
| Analyte | Extraction Efficiency (%, n=4) |
Matrix Effect (%, n=4) |
||||
|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | |
| TFV | 44.0 | 37.9 | 32.8 | −7.3 | −13.5 | −24.9 |
| 3TC | 68.7 | 79.7 | 74.5 | 36.6 | 26.4 | 47.0 |
| FTC | 97.2 | 80.4 | 73.3 | 2.6 | 13.1 | 11.0 |
| ABC | 76.9 | 87.2 | 76.6 | −60.7 | −54.9 | −49.8 |
| ABC−G | 40.6 | 31.6 | 23.8 | −15.5 | −5.1 | −29.9 |
| ABC−C | 41.7 | 50.2 | 33.3 | −68.8 | −59.2 | −69.5 |
| NVP | 84.4 | 87.1 | 90.0 | −11.3 | −9.2 | −10.2 |
| RAL | 58.7 | 57.1 | 62.1 | −6.1 | −15.6 | −35.6 |
| APV | 78.7 | 97.6 | 107.2 | −94.4 | −93.3 | −94.8 |
| DRV | 93.7 | 106.7 | 111.5 | −95.6 | −94.5 | −94.8 |
| M8 | 82.2 | 97.9 | 76.9 | −91.3 | −90.0 | −90.3 |
| NFV | 100.8 | 119.5 | 76.9 | −92.9 | −92.7 | −95.7 |
| SQV | 103.0 | 98.8 | 99.8 | −95.8 | −95.1 | −97.1 |
| ATV | 97.6 | 104.7 | 78.4 | −66.5 | −63.5 | −74.8 |
| RTV | 106.1 | 84.2 | 99.2 | −78.1 | −75.4 | −87.5 |
| LPV | 103.6 | 119.5 | 95.9 | −86.6 | −89.5 | −91.6 |
| d4T | 91.0 | 81.3 | 77.0 | −86.3 | −82.0 | −56.8 |
| AZT−G | 57.7 | 71.3 | 64.4 | −91.3 | −90.7 | −68.5 |
| AZT | 86.9 | 96.1 | 80.6 | −96.4 | −95.1 | −83.1 |
| EFV | 111.7 | 101.1 | 113.6 | −98.3 | −96.8 | −91.5 |
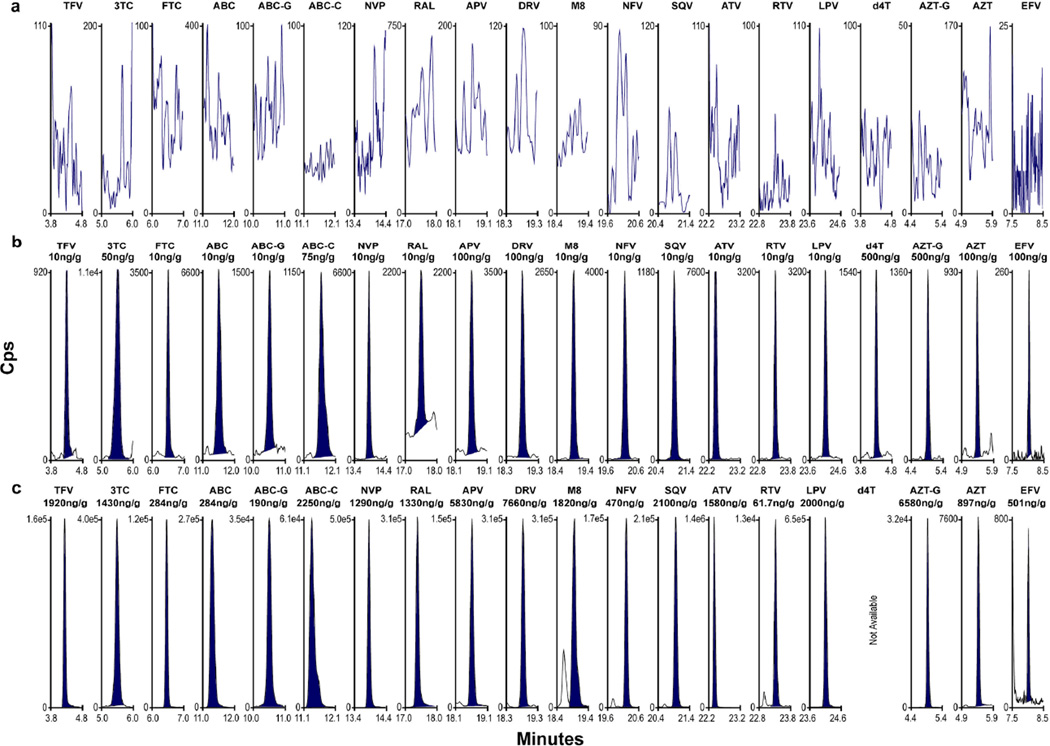
For all quantitative analytes, intra- and inter-day analytical accuracy in meconium ranged from 80.3–108.3%, inter-day imprecision from 0–8.6% and total imprecision from 2.2–11.0% (Table 3). Semiquantitative ABC-C and ABC-G showed greater variability with total imprecision from 11.9–17.9%. Representative chromatograms of extracted blank meconium, LOQ and positive meconium specimens collected through the PHACS study are shown in Figure 1.
Figure 1.
MRM chromatograms of a) extracted blank meconium, b) ARV limits of quantification, and c) ARVs detected in authentic meconium specimens from ARV-exposed, HIV-uninfected infants.
For all quantitative analytes except TFV, extraction efficiency of native and deuterium- and isotopically-labeled analytes ranged from 55.6–119.5% (Table 4). The dual SPE procedure described in this method was specifically developed to optimize recovery of TFV as approximately 30% of the study population is administrated this medication and it is part of the recommended first-line ARV regimen for pregnant women.10 TFV and TFV-d6 were not well recovered (<10%) by SPE at pH 2.9, an optimal pH for extraction of all other analytes with recovery ranging from 55–80%. An initial pH of 1.1 demonstrated poor extraction efficiency (22–39%) for NFV, M8, SQV, RTV and LPV; these analytes were easily recovered with an initial pH of 2.9. Therefore, we collected the TFV fraction during the initial pH 2.9 sample loading and adjusted the TFV fraction pH to 1.1 to improve SPE phase binding. Application of this fraction to the same SPE column after initial elution followed by re-equilibration achieved maximum extraction efficiencies while minimizing cost and required specimen for our complete analyte panel. This procedure improved TFV extraction efficiency to 32.8–44.0% across the linear range. Similarly low TFV extraction efficiencies of 40.6–46.5% were reported in plasma32–33 and these methods targeted TFV with only 1–2 additional compounds included.
Matrix effects ranged from −98.3–47% (Table 4). Significant matrix effects occurred for several analytes; similar matrix effects also occurred for corresponding internal standards enabling accurate quantification. Significant matrix effect is not uncommon in meconium analysis.34 This method is unique among ARV quantification methods in other matrices in its utilization of 17 separate deuterated or isotopically labeled internal standards. Inclusion of appropriately matched internal standards is critical to achieve accurate quantification despite matrix effects encountered during meconium analysis.
Meconium from 10 different ARV-negative pools contained no interfering compounds with the analytes of interest. None of the 96 potential exogenous interferences fortified at 20,000ng/g into low QC samples caused quantitation criteria or transition ratios to fail for any quantitative analyte. No carryover was detected after a specimen containing 2 and 5 times the upper LOQ. Dilutions (1:5) of specimens fortified with high QC were within 89.9–113.0% of expected diluted concentrations. Quantitative analytes proved to be >75% stable at all concentrations under the four tested conditions: 24h RT, 72h 4°C, −20°C freeze/thaw cycles, and 48h on the 4°C autosampler (Table S-2).
Application of method
Within the PHACS study population, there are a wide range of maternal ARV regimens with the 16 parent drugs quantified in this method representing 99% of neonatal in utero ARV exposures. Metabolites ABC-G, ABC-C, M8, and AZT-G were chosen for inclusion in this method due to prevalence of parent drug use, commercial availability of standards and published literature suggesting extensive metabolism of the parent compound to these analytes (ABC-G and ABC-G),35 high placental transfer (M8),25 and/or metabolite presence in fetal animal tissue (AZT-G).36 Authentic meconium specimens from 32 HIV-uninfected infants born to HIV-positive mothers enrolled in the PHACS study who received diverse combination ARV therapy during pregnancy were tested to demonstrate method applicability. Analysts did not know what ARV medications the infants’ mothers received. Results from authentic PHACS specimens are shown in Figure 1c, indicating the method correctly identified authentic ARV exposures. This objective information on fetal ARV exposure can be used to further assess ARV-related toxicities in exposed uninfected children.
CONCLUSION
This method is the first to quantify ARV drugs and metabolites in meconium. Utilization of this method yields a comprehensive profile of in utero ARV exposure. Quantitative meconium analysis for ARV drugs and metabolites may better reflect fetal exposure during the third and perhaps second trimesters than self-report and medical histories, as maternal pharmacokinetics, placental transfer and fetal metabolism vary. This sensitive and specific LC-MS/MS method for quantifying ARV drugs and metabolites in meconium can be employed to assess fetal ARV exposure and evaluate whether meconium ARV concentrations better predict developmental toxicities than drug regimen alone. This assay can identify ARV-exposed children at risk of developing toxicities. Correlations of meconium concentrations to maternal ARV dose and infant toxicities may offer an opportunity for dose adjustment and prevention of toxicities in exposed children
Supplementary Material
ACKNOWLEDGMENT
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104, 3U01HD052104-06S1) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support provided by Westat, Inc (PI: Julie Davidson).
The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2011, in alphabetical order: Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Emma Stuard, Anna Cintron; Children's Diagnostic & Treatment Center: Ana Puga, Dia Cooley, Doyle Patton, Deyana Leon; Children’s Memorial Hospital: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; New York University School of Medicine: William Borkowsky, Sandra Deygoo, Helen Rozelman; St. Jude Children's Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Lourdes Angeli-Nieves, Vivian Olivera; SUNY Downstate Medical Center: Hermann Mendez, Ava Dennie, Susan Bewley; Tulane University Health Sciences Center: Russell Van Dyke, Karen Craig, Patricia Sirois; University of Alabama, Birmingham: Marilyn Crain, Newana Beatty, Dan Marullo; University of California, San Diego: Stephen Spector, Jean Manning, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Emily Barr, Robin McEvoy; University of Florida/Jacksonville: Mobeen Rathore, Kristi Stowers, Ann Usitalo; University of Illinois, Chicago: Kenneth Rich, Delmyra Turpin, Renee Smith; University of Medicine and Dentistry of New Jersey: Arry Dieudonne, Linda Bettica, Susan Adubato; University of Miami: Gwendolyn Scott, Claudia Florez, Elizabeth Willen; University of Southern California: Toinette Frederick, Mariam Davtyan, Maribel Mejia; University of Puerto Rico Medical Center: Zoe Rodriguez, Ibet Heyer, Nydia Scalley Trifilio.
Footnotes
Supporting Information Available: This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.World Health Organization. Towards universal access. Progress report 2010. Available at http://www.who.int/hiv/pub/2010progressreport/en/index.html.
- 2.Whitmore SK, Zhang X, Taylor AW, Blair JM. J Acquir Immune Defic Syndr. 2011;57:218–222. doi: 10.1097/QAI.0b013e3182167dec. [DOI] [PubMed] [Google Scholar]
- 3.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States (Updated July 2012). Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/perinatalgl.pdf.
- 4.Barret B, Tardieu M, Rustin P, Lacroix C, Chabrol B, Desguerre I, Dollfus C, Mayaux MJ, Blanche S. AIDS. 2003;17:1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 5.Castillo AB, Tarantal AF, Watnik MR, Martin RB. J Orthop Res. 2002;20:1185–1189. doi: 10.1016/S0736-0266(02)00074-8. [DOI] [PubMed] [Google Scholar]
- 6.Rice ML, Buchanan AL, Siberry GK, Malee KM, Zeldow B, Frederick T, Purswani MU, Hoffman HJ, Sirois PA, Smith R, Torre P, 3rd, Allison SM, Williams PL. J Dev Behav Pediatr. 2011;33:112–123. doi: 10.1097/DBP.0b013e318241ed23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siberry GK, Williams PL, Mendez H, Seage GR, 3rd, Jacobson DL, Hazra R, Rich KC, Griner R, Tassiopoulos K, Kacanek D, Mofenson LM, Miller T, DiMeglio LA, Watts DH. AIDS. 2012;26:1151–1159. doi: 10.1097/QAD.0b013e328352d135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torre P, 3rd, Zeldow B, Hoffman HJ, Buchanan A, Siberry GK, Rice M, Sirois PA, Williams PL. Pediatr Infect Dis J. 2012;31:835–841. doi: 10.1097/INF.0b013e31825b9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, Ciraru-Vigneron N, Lacroix C, Rouzioux C, Mandelbrot L, Desguerre I, Rotig A, Mayaux MJ, Delfraissy JF. Lancet. 1999;354:1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- 10.Griner R, Williams PL, Read JS, Seage GR, 3rd, Crain M, Yogev R, Hazra R, Rich K. AIDS Patient Care STDS. 2011;25:385–394. doi: 10.1089/apc.2011.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bearer CF, Lee S, Salvator AE, Minnes S, Swick A, Yamashita T, Singer LT. Alcohol Clin Exp Res. 1999;23:487–493. [PMC free article] [PubMed] [Google Scholar]
- 12.Gray T, Huestis M. Anal Bioanal Chem. 2007;388:1455–1465. doi: 10.1007/s00216-007-1228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray TR, Eiden RD, Leonard KE, Connors G, Shisler S, Huestis MA. Nicotine Tob Res. 2010;12:658–664. doi: 10.1093/ntr/ntq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moller M, Karaskov T, Koren G. Ther Drug Monit. 2010;32:318–323. doi: 10.1097/FTD.0b013e3181dca48b. [DOI] [PubMed] [Google Scholar]
- 15.Ostrea EM, Knapp K, Tannenbaum L, Ostrea AR, Romero A, Salari V, Ager J. J Pediatr. 2001;138:344–348. doi: 10.1067/mpd.2001.111429. [DOI] [PubMed] [Google Scholar]
- 16.Tassiopoulos K, Read JS, Brogly S, Rich K, Lester B, Spector SA, Yogev R, Seage GR., 3rd AIDS Behav. 2010;14:1269–1278. doi: 10.1007/s10461-010-9705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kacinko SL, Jones HE, Johnson RE, Choo RE, Huestis MA. Clin Pharmacol Ther. 2008;84:604–612. doi: 10.1038/clpt.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gehrig AK, Mikus G, Haefeli WE, Burhenne J. Rapid Commun Mass Spectrom. 2007;21:2704–2716. doi: 10.1002/rcm.3138. [DOI] [PubMed] [Google Scholar]
- 19.Jung BH, Rezk NL, Bridges AS, Corbett AH, Kashuba AD. Biomed Chromatogr. 2007;21:1095–1104. doi: 10.1002/bmc.865. [DOI] [PubMed] [Google Scholar]
- 20.Volosov A, Alexander C, Ting L, Soldin SJ. Clin Biochem. 2002;35:99–103. doi: 10.1016/s0009-9120(02)00286-2. [DOI] [PubMed] [Google Scholar]
- 21.Clark TN, White CA, Bartlett MG. Biomed Chromatogr. 2006;20:605–611. doi: 10.1002/bmc.651. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Gandhi M, Greenblatt RM, Gee W, Lin ET, Messenkoff N. Rapid Commun Mass Spectrom. 2008;22:3401–3409. doi: 10.1002/rcm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezk NL, White N, Bridges AS, Abdel-Megeed MF, Mohamed TM, Moselhy SS, Kashuba AD. Ther Drug Monit. 2008;30:611–619. doi: 10.1097/FTD.0b013e318186e08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh RF, Rezk NL, Kashuba AD, Dumond JB, Tappouni HL, Tien HC, Chen YC, Vourvahis M, Horton AL, Fiscus SA, Patterson KB. Antimicrob Agents Chemother. 2009;53:2367–2374. doi: 10.1128/AAC.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirt D, Urien S, Jullien V, Firtion G, Chappuy H, Rey E, Pons G, Mandelbrot L, Treluyer JM. Brit J Clin Pharmaco. 2007;64:634–644. doi: 10.1111/j.1365-2125.2007.02885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams PL, Seage GR, 3rd, Van Dyke RB, Siberry GK, Griner R, Tassiopoulos K, Yildirim C, Read JS, Huo Y, Hazra R, Jacobson DL, Mofenson LM, Rich K. Am J Epidemiol. 2012;175:950–961. doi: 10.1093/aje/kwr401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krouwer JS, Rabinowitz R. Clin Chem. 1984;30:290–292. [PubMed] [Google Scholar]
- 28.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 29.Gray TR, Choo RE, Concheiro M, Williams E, Elko A, Jansson LM, Jones HE, Huestis MA. Addiction. 2010;105:2151–2159. doi: 10.1111/j.1360-0443.2010.03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray TR, LaGasse LL, Smith LM, Derauf C, Grant P, Shah R, Arria AM, Della Grotta SA, Strauss A, Haning WF, Lester BM, Huestis MA. Ther Drug Monit. 2009;31:769–775. doi: 10.1097/FTD.0b013e3181bb438e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia Y, Wang P, Bartlett MG, Solomon HM, Busch KL. Anal Chem. 2000;72:764–771. doi: 10.1021/ac990201p. [DOI] [PubMed] [Google Scholar]
- 32.D'Avolio A, Sciandra M, Siccardi M, Baietto L, Gonzalez de Requena D, Bonora S, Di Perri G. J Chromatogr Sci. 2008;46:524–528. doi: 10.1093/chromsci/46.6.524. [DOI] [PubMed] [Google Scholar]
- 33.Nirogi R, Bhyrapuneni G, Kandikere V, Mudigonda K, Komarneni P, Aleti R, Mukkanti K. Biomed Chromatogr. 2009;23:371–381. doi: 10.1002/bmc.1125. [DOI] [PubMed] [Google Scholar]
- 34.Gray TR, Shakleya DM, Huestis MA. Anal Bioanal Chem. 2009;393:1977–1990. doi: 10.1007/s00216-009-2680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDowell JA, Chittick GE, Ravitch JR, Polk RE, Kerkering TM, Stein DS. Antimicrob Agents Chemother. 1999;43:2855–2861. doi: 10.1128/aac.43.12.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patterson TA, Binienda ZK, Lipe GW, Gillam MP, Slikker W, Jr, Sandberg JA. Drug Metab Dispos. 1997;25:453–459. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.