Abstract
A growing number of exciting animal and preclinical studies are beginning to reveal the immense potential in stem cell-based therapies, particularly in the area of treating cardiovascular diseases. However, in order to evaluate the efficacy of these treatments in clinical trials, the transplanted stem cells must be monitored quantitatively and qualitatively in vivo. To date, several non-invasive imaging approaches have been used to follow stem cell fate in vivo. Here we review the basic principles of the current techniques for cardiac stem cell tracking, compare the relative advantages and disadvantages of these imaging modalities, and discuss the future prospect of cardiac stem cell trafficking.
Keywords: stem cell imaging, magnetic resonance, radionuclide, quantum dot, reporter gene
Introduction
Despite recent advances in medical and surgical treatment, cardiovascular diseases (CVD) remain the number one cause of morbidity and mortality in the US. The societal and financial consequences are tremendous. For example, the American Heart Association estimates the economic costs of cardiovascular diseases in the United States for 2007 at $431.8 billion including direct and indirect costs incurred by CVD (1). In adult tissues such as those in the heart, the capacity for self-regeneration is limited. One promising approach is to inject stem cells into damaged hearts, which could potentially repopulate the myocardium, induce neovascularization, and lead to significant functional improvement (2). Encouraging animal studies from the late 1990s and early 2000s have led to initiation or completion of several human clinical trials involving transplantation of bone marrow stem cells, skeletal myoblasts, or circulating progenitor cells into the heart (3). However, contradictory results from recent studies that used different origins of therapeutic stem cells and different routes of cell delivery highlight the need to elucidate the molecular mechanisms by which stem cells actually contribute to cardiac functional recovery (4). For instance, Lunde et al. showed that at six months post intracoronary injection of mononuclear bone marrow cells (BMC) into infarcted heart, no significant improvement in left ventricular ejection fraction (LVEF) was observed (5). Similarly, Jassen et al. failed to detect any considerable improvement in ventricular function at four months after injection of mononuclear BMC (6). By contrast, Schachinger et al. found that 59 patients suffering from acute myocardial infarction who were treated with direct intracoronary infusion of either circulating progenitor cells or bone marrow-derived progenitor cells showed significant improvements in LVEF and end-systolic volume (7). Other trials are summarized in Table 1. Clearly, these mixed results on cardiac stem cell therapy are both perplexing to scientists and frustrating to patients. Further studies are therefore urgently needed to clarify the discrepancies in clinical trials and validate the efficacy of cardiac repair using therapeutic stem cells.
Table 1.
Intracoronary Injection of Bone Marrow Cells in Patients with Ischemic Heart Disease.
| Study | Patients, controls/subjects |
Cell No. | Duration of Follow-up |
Main Findings | Technique Used |
|---|---|---|---|---|---|
| Strauer et al. (31) | 10/10 | 2.8×107 | 3 months | Improvement in LVEF | T1-201 SPECT |
| BOOST (32) | 30/30 | 2.4×109 | 6 months | No significant change in LVEF | MRI |
| TOPCARE-AMI (7, 33) | 29 BMC | 2.1×108 | 4 months | Improvement in LVEF | TI-201 SPECT |
| TOPCARE-CHD (34) | 28/23 | 2.1×108 | 4 months | Improvement in LVEF | TI-201 SPECT |
| Janssens et al. (6) | 33/34 | 4.8×108 | 4 months | No significant change in LVEF | C-11 acetate PET |
| IACT (35) | 18/18 | 9×107 | 3 months | Improvement in LVEF | Tc-99m tetrofosmin |
| ASTAMI (36) | 50/47 | 6.8×107 | 6 months | No significant change in LVEF | MRI, SPECT, echocardiography |
| REPAIR-AMI (37) | 98/101 | 2.4×108 | 4 months | Improvement in LVEF | Left ventricular angiography |
BOOST, Bone Marrow Transfer to Enhance ST-Elevation Infarct Regeneration; TOPCARE-AMI, Transplantation of Progenitor Cells and Recovery of LV Function in Patients with acute myocardial infarction; TOPCARE-CHD, Transplantation of Progenitor Cells and Regeneration Enhancement in Congenital Heart Disease; IACT, Regeneration of Human Infarcted Heart Muscle by Intracoronary Autologous Bone Marrow Cell Transplantation in Chronic Coronary Artery Disease; ASTAMI, Evaluation of Treatment with Intracoronary Mononuclear Autologous Bone Marrow Cell Therapy Among Patients with Acute Anterior Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention; REPAIR-AMI, Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction.
Principles of Stem Cell Imaging
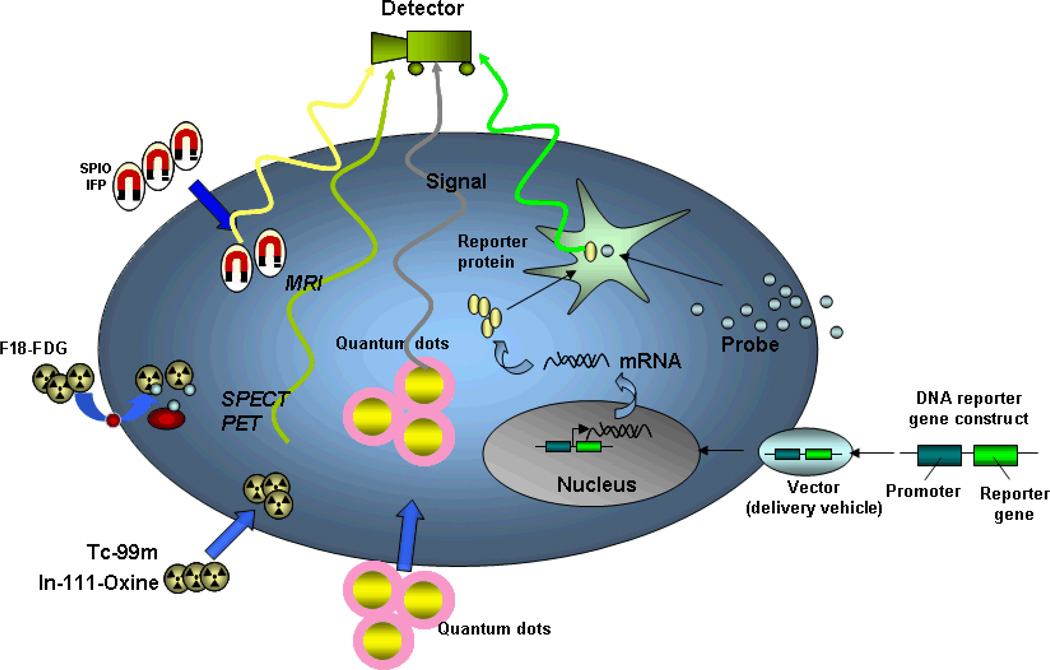
The introduction of potentially therapeutic stem cells in patients requires concurrent techniques that provide noninvasive assessment of the survival, distribution, and differentiation of these cells. Molecular imaging is a multi-disciplinary field that covers several disciplines including cell and molecular biology, imaging technology, and molecular medicine. It provides integrated information of specific molecules of interest within cells in living subjects and thus holds great promise as an effective way to track the transplanted cells in vivo. The unique information obtained from molecular imaging technique is particularly helpful to evaluate the functional outcomes of cell engraftment and may shed insight to the mixed results listed in Table 1. The current non-invasive imaging approaches for tracking stem cells in vivo include magnetic resonance, radionuclide, quantum dot, and reporter gene imaging. Schematic of these approaches is shown in Figure 1. The ideal stem cell tracking modality requires the imaging agents to be non-toxic, biocompatible, and highly specific to reduce perturbation to the target cells. In addition, the ideal imaging agents should possess properties of single-cell detection, decrease in signals with cell loss, and increase in signals with cell proliferation. At this point, none of the extant imaging modalities possesses all of these characteristics. In this review, we will highlight the current imaging modalities and evaluate their relative advantages and limitations.
Figure 1.
Schematic for non-invasive imaging of stem cell fate in the myocardium. The four different techniques include iron labeling, radioactive labeling, quantum dot labeling, and reporter gene labeling. SPIO, superparamagnteic iron oxide; IFP, iron fluorescent particles; F18-FDG, F18-fluorodeoxyglucose; MRI, magnetic resonance imaging; PET, positron emission tomography; SPECT, single-photon emission computed tomography.
Magnetic Particle Labeling
MRI is a widely used imaging modality for in vivo cell tracking in preclinical and clinical cases. Traditional MRI manipulates hydrogen nuclei in fluid under a static magnetic field to obtain regional contrasts based on differences in proton density, flow, and/or biochemical structure. To track stem cells in infarcted myocardium, cells need to be enriched with a contrast agent that is able to produce sufficient positive or negative signals to distinguish them from the background. Two major classes of contrast imaging materials have been developed. One uses lanthanide gadolinium (Gd3+) to generate signals on T1-weighted contrast images. The other type employs superparamagnetic iron oxide (SPIO) particles to generate signals on T2-weighted images. Several studies have demonstrated that incorporation of a gadolinium-based contrast agent into stem cells allows the tracking of magnetically labeled cells for up to six weeks (reviewed by (8)). However, at present, SPIO particles are the preferred agent for cell tracking as it gives three-dimensional imaging capabilities at low concentration loadings (5–10 µm/L) (9). The sensitivity of SPIO is achieved through a large dipolar magnetic field gradient experienced by protons near the particles. The detectability of labeled cells also depends on the number of cells injected into myocardium. For instance, 107 to 108 Feridex-labeled mesenchymal stem cells can be readily detected by MRI when injected directly into myocardium of a swine as shown in Figure 2 (10). Using SPIO imaging contrast (Feridex), Kraitchman et al. successfully demonstrated long-term evaluation of magnetically labeled mesenchymal stem cells following myocardial infarction in a swine model (11). The same group later used Feridex co-labeled with In-111 oxine to compare the relative sensitivities of MRI versus radionuclide imaging and observed that single photon emission computed tomography/computed tomography (SPECT/CT) imaging was more sensitive for detecting cell homing in the heart than MRI (12). Furthermore, intrinsic problems exist with MRI contrast agents, including the difficulty in quantification with cell division and possible transfers of contrast agents to neighboring cells. Another limitation with MRI is the contraindications for patients with implantable devices (e.g., pacemakers and defibrillators), who often are the very patients with refractory symptoms requiring novel stem cell therapy.
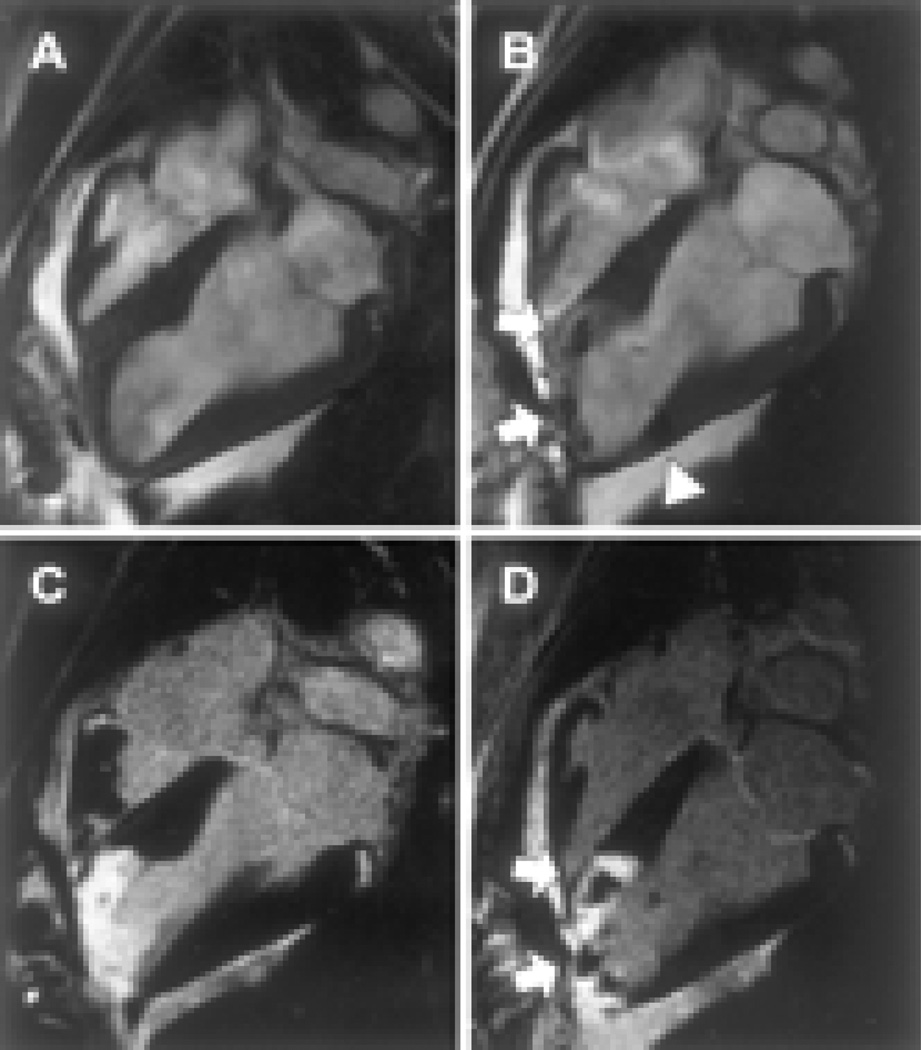
Figure 2. MRI of transplanted cardiac stem cells in pig models.
Long-axis MRI view of left ventricle before (A) and after (B) transcatheter injection of 4×106 iron fluorescent particle (IFP) labeled mesenchymal stem cells (MSCs) into infarct at apex (arrows) and into adjoining normal myocardium (arrowhead). Delayed hyperenhancement inversion recovery MRI highlights areas of nonviable infarcted myocardium using same views as above, before (C) and after (D) injection of IFP-labeled MSCs. MSCs appear dark against hyperenhanced infarct. Reprint with permission from ref (10).
Radionuclide Labeling
Radionuclide imaging techniques including positron emission tomography (PET) and SPECT have garnered intense research interest over the past decade. Compared to MRI, PET and SPECT provide high intrinsic sensitivity and can use a variety of clinically tested imaging agents. Recent improvements in spatial resolution (1–2 mm) have made radionuclide imaging particularly suitable for cell tracking (13). Direct labeling of cells with radioisotopes in clinical practice has used [In-111] oxyquinoline and Tc-99m hexamethylprophylene amine oxime. Labeling cells with [In-111] oxyquinoline is a well-established method that has been deployed in endothelial progenitor cells, hematopoietic stem cells, and mesencymal stem cells (14, 15). For instance, using [In-111] radiotracer, Aicher et al. observed that only 4.7% of the injected endothelial and hematopoietic progenitor cells were retained in the infarcted myocardium (15). Similar observation of cell targeting was also found using Tc-99m labeled mesenchymal stem cells (16). Furthermore, in the first human study, the 18F-fluorodeoxyglucose (FDG) PET radiotracer was used to follow the intracoronary delivery of bone marrow cells in patients after myocardial infarction (17). These investigators found that 14% to 39% of CD-34 positive cells accumulated in the infarcted myocardium approximately 1 hour after intracoronary delivery (Figure 3).
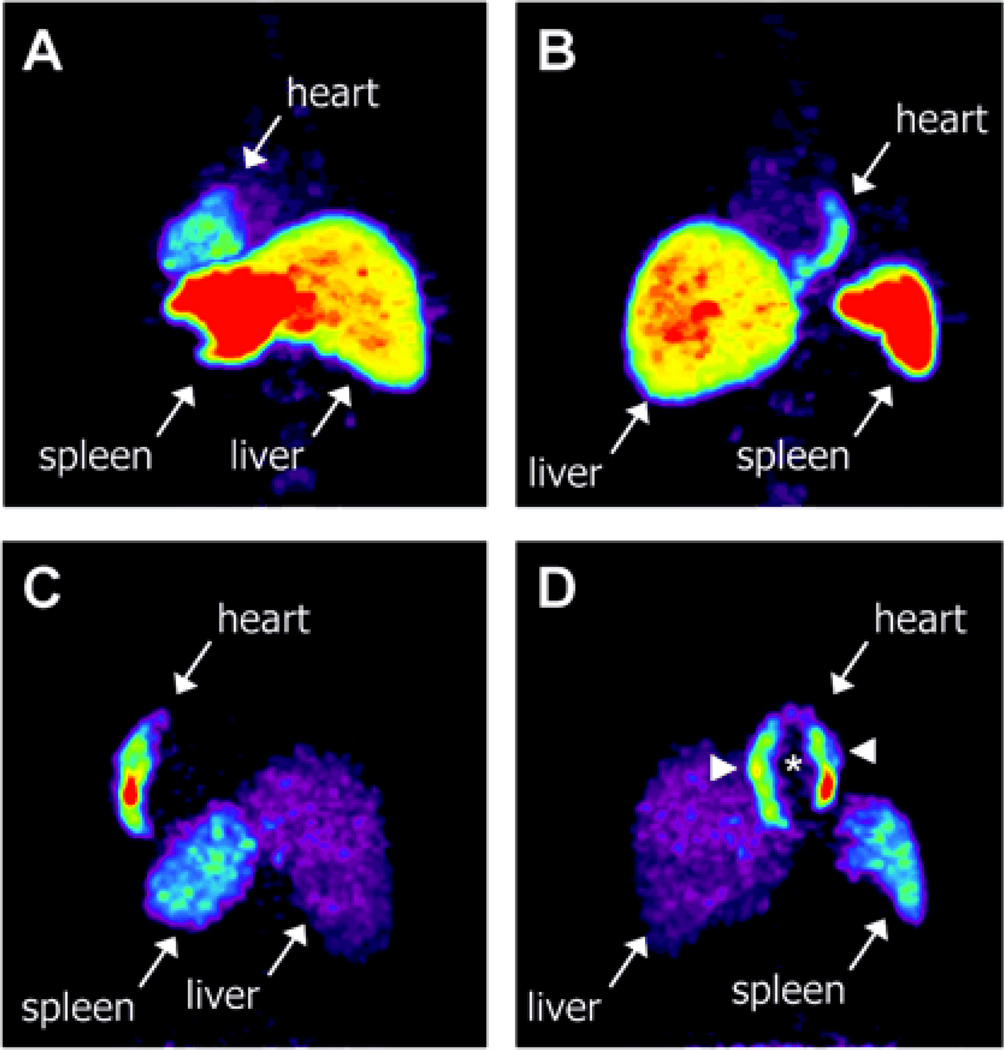
Figure 3.
Evaluation of myocardial homing and biodistribution of [18F]FDG-labeled bone marrow cells after intracoronary delivery in patient after myocardial infarction. Representative images of (A) left posterior oblique and (B) left anterior oblique at 65 minutes after injection of [18F]FDG-labeled unselected bone marrow cells into the left circumflex coronary artery. Representative images of (C) left posterior oblique and (D) left anterior oblique at 70 minutes after injection of [18F]FDG-labeled CD34+ subpopulation of bone marrow cells into the left anterior descending coronary artery. Reprint with permission from ref (17).
Although nuclear imaging has major advantages, it is limited by concerns such as the potential transfer of radiotracer to non-targeted cells and potential adverse effects of radiotracer on stem cell viability, function, and differentiation capacity. For instance, [In-111] radiotracer has been shown to impair proliferation and differentiation in CD34 positive hematopoietic progenitor cells (18). Additional studies thus are needed to examine the effects of labeling on differentiation capacity of stem cell from various origins.
Nanoparticle labeling
Semiconductor quantum dots (QDs) are a new class of fluorescent probes that have been used in non-invasive imaging in recent years (19, 20). This technique makes use of fluorescent semiconductor nanocrystals to detect membrane molecules of interest. The excitation wavelengths of the QDs can be manipulated, ranging from ultraviolet to near-infrared ranges. Depending on the size and composition of these probes, they can be designed to emit different wavelengths of light. The photostability of QDs, as seen in their resistance to photobleaching and long-lasting fluorescence, makes them appealing for tracking stem cells in vivo (21). However, the effects of QDs on stem cell proliferation and differentiation remain unclear as mixed results have been reported using different origins of stem cells or experimental protocols (19, 22). Finally, several other obstacles, including the tendency for aggregation of QDs in the cytosol, difficulty in delivery of QDs into cells, and non-specific binding to multiple molecules (23) must be overcome before QDs can realize its full potential in clinical imaging.
Reporter Gene Labeling
Reporter gene imaging has emerged as a unique imaging tool to confer the localization of specific molecules of interest within living subjects. The reporter gene of interest usually encodes a specific protein which, when it interacts with an imaging probe, generates some form of signal that can be captured and quantified by an imaging modality such as MRI, PET, SPECT, or optical charge-coupled device (CCD). Several studies have successfully demonstrated the tracking of the distribution and engraftment of stem cells in mice and rats using bioluminescence-based optical imaging (24, 25). Although the signals generated by firefly luciferase is more sensitive than other imaging modalities in small animals, luciferase proteins and their substrates have a high absorption rate in hemoglobin and can be easily scattered within deep tissues. For now, this current inability to accurately determine cell depth is a major limitation.
Fluorescence imaging is another widely used optical imaging which utilizes fluorescent protein for in vivo imaging. Compared to bioluminescence, near-infrared (NIR) fluorescence imaging has reduced absorption and scatter of photons at 700–1000 nm. However, similar to bioluminescence, NIR fluorescence imaging is restricted by shallow tissue depth (between 4–10 cm). In addition to the bioluminescence and fluorescence reporter genes, other types of reporter genes are available, including herpes simplex virus thymidine kinase (HSV-tk) for PET (24, 26) (Figure 4), sodium iodide symporter for SPECT (27), and transferrin receptor for MRI (28).
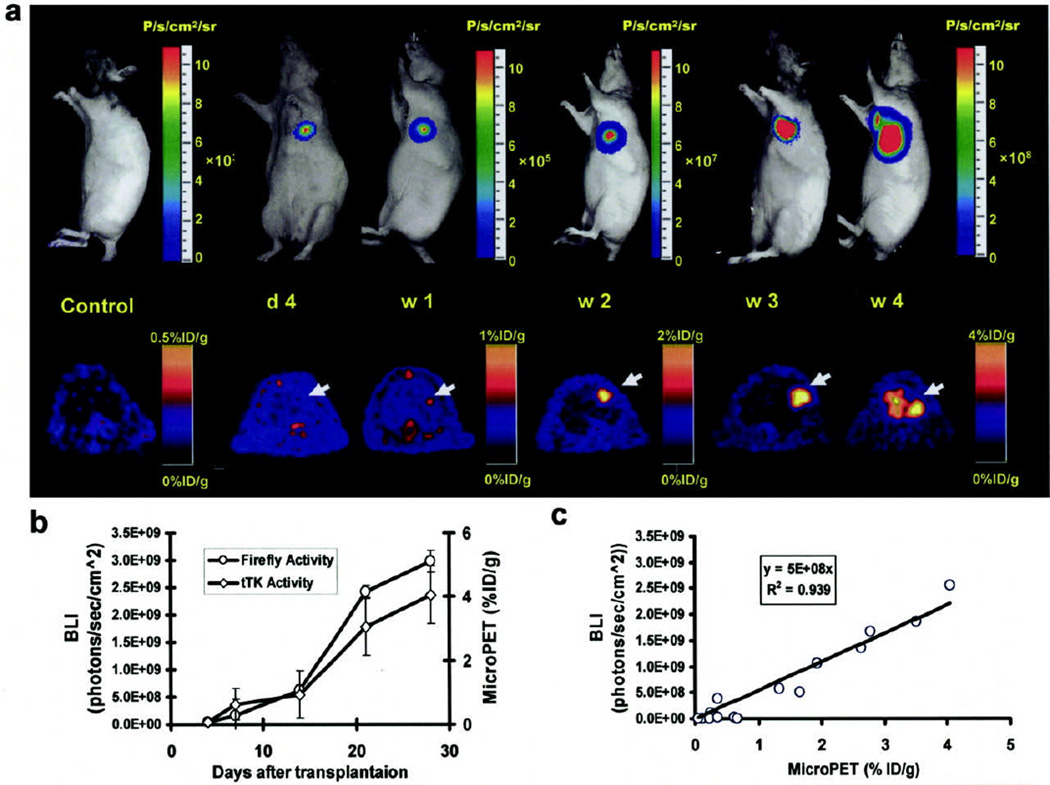
Figure 4.
Tracking of transplanted embryonic stem cells in rats by multimodality molecular imaging technique. (A) Bioluminescence (top) and microPET (bottom) imaging of embryonic stem cell fate in living animals. Representative images demonstrate imaging signal activities in these mouse embryonic stem cells that stably express firefly luciferase (Fluc; bioluminescence), monomeric red fluorescence protein (mRFP; fluorescence), and herpes simplex virus truncated thymidine kinase (HSVttk; PET) triple fusion reporter gene. (B) A significant increase of Fluc and HSV-ttk activities were seen from week 2 to week 4, indicative of in vivo teratoma formation. Reprint with permission from ref (24).
Applying the concept of reporter gene labeling has a significant potential to furnish greater insights into the therapeutic mechanisms of stem cell therapy. For instance, inducible promoters can be used to modulate the level of reporter gene as well as therapeutic gene expression (29). Tissue-specific promoters may be used as sensors of the cell differentiation state. In this approach, a cardiac-specific promoter such as α-cardiac myosin heavy chain promoter driving the expression of an antibiotic resistance gene can be used to select for cardiomyocytes in the mixed culture (30). Development of a similar system for monitoring stem cell differentiation in vivo using Fluc (bioluminescence) or HSV-tk (PET) would yield obvious benefits. However, several problems must be solved before reporter gene imaging can be fully and safely applied clinically. These issues include (a) finding appropriate probes that elicit minimal or zero immunogenic response, (b) enhancing transfection stability, and (c) minimizing the potential interference of the stem cell function and differentiation from vector transfection or transduction.
Future Perspectives
Stem cell tracking requires high sensitivity and spatial resolution. Currently, no imaging modality is perfect in all aspects. Although MRI provides a safe profile and three-dimensional capacity, it has the lowest sensitivity at 10−2 µm/L and is contraindicated in patients with implantable devices. Radiolabeling imaging has a fair sensitivity (10−8 to 10−9 µm/L) but is not suitable for long-term cell tracking due to radioisotope decay. QDs provide superior photostability and long-term multi-color in vivo imaging, but concerns such as possible toxicity to cells and dispersals of QDs in the cytosol need to be resolved. Bioluminescence and near-infrared fluorescence imaging are the most sensitive (10−12 to 10−15 µm/L) among available imaging modalities, but both techniques are constrained by relatively shallow tissue depth. Reporter gene imaging, though able to assess cell fate (viability & proliferation) more accurately, will need to demonstrate cell safety due to the genetic modification.
Given that each imaging modality presents its unique set of advantages and drawbacks, one should ask what biological questions need to be answered before choosing an appropriate imaging modality. If the exact location of cell delivery must be identified, then MRI is the preferred choice. If only short-term fate of transplanted cells needs to be determined, then radiolabeling techniques is suitable. If knowledge of the long-term fate of transplanted stem cells is desired, then reporter gene imaging is best. Finally, future efforts should also focus on multi-modality imaging approaches which may minimize potential drawbacks of using each imaging modality alone, as it is possible that a tailored combination of two or more techniques may provide the most ideal information profile for clinical applications.
Reference
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics-2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007 Feb 6;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001 Apr 5;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 3.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005 Feb 4;96(2):151–163. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 4.Rosenzweig A. Cardiac cell therapy--mixed results from mixed cells. N Engl J Med. 2006 Sep 21;355(12):1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 5.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006 Sep 21;355(12):1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 6.Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006 Jan 14;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 7.Schachinger V, Assmus B, Britten MB, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004 Oct 19;44(8):1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Kim D, Hong KS, Song J. The present status of cell tracking methods in animal models using magnetic resonance imaging technology. Mol Cells. 2007 Apr 30;23(2):132–137. [PubMed] [Google Scholar]
- 9.Rogers WJ, Meyer CH, Kramer CM. Technology insight: in vivo cell tracking by use of MRI. Nat Clin Pract Cardiovasc Med. 2006 Oct;3(10):554–562. doi: 10.1038/ncpcardio0659. [DOI] [PubMed] [Google Scholar]
- 10.Hill JM, Dick AJ, Raman VK, et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003 Aug 26;108(8):1009–1014. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraitchman DL, Heldman AW, Atalar E, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003 May 13;107(18):2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 12.Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005 Sep 6;112(10):1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acton PD, Kung HF. Small animal imaging with high resolution single photon emission tomography. Nucl Med Biol. 2003 Nov;30(8):889–895. doi: 10.1016/s0969-8051(03)00112-4. [DOI] [PubMed] [Google Scholar]
- 14.Berman DS, Hachamovitch R, Shaw LJ, et al. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: assessment of patients with suspected coronary artery disease. J Nucl Med. 2006 Jan;47(1):74–82. [PubMed] [Google Scholar]
- 15.Aicher A, Brenner W, Zuhayra M, et al. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003 Apr 29;107(16):2134–2139. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 16.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003 Aug 19;108(7):863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005 May 3;111(17):2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 18.Brenner W, Aicher A, Eckey T, et al. 111In-labeled CD34+ hematopoietic progenitor cells in a rat myocardial infarction model. J Nucl Med. 2004 Mar;45(3):512–518. [PubMed] [Google Scholar]
- 19.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science (New York, NY. 2002 Nov 29;298(5599):1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat Biotechnol. 2003 Jan;21(1):47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 21.Lin S, Xie X, Patel MR, et al. Quantum dot imaging for embryonic stem cells. BMC Biotechnol. 2007 doi: 10.1186/1472-6750-7-67. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh SC, Wang FF, Lin CS, Chen YJ, Hung SC, Wang YJ. The inhibition of osteogenesis with human bone marrow mesenchymal stem cells by CdSe/ZnS quantum dot labels. Biomaterials. 2006 Mar;27(8):1656–1664. doi: 10.1016/j.biomaterials.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Jaiswal JK, Simon SM. Potentials and pitfalls of fluorescent quantum dots for biological imaging. Trends Cell Biol. 2004 Sep;14(9):497–504. doi: 10.1016/j.tcb.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Cao F, Lin S, Xie X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006 Feb 21;113(7):1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheikh AY, Lin SA, Cao F, et al. Molecular Imaging of Bone Marrow Mononuclear Cell Homing and Engraftment in Ischemic Myocardium. Stem Cells. 2007 Jul 12; doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu JC, Chen IY, Sundaresan G, et al. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003 Sep 16;108(11):1302–1305. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyagawa M, Beyer M, Wagner B, et al. Cardiac reporter gene imaging using the human sodium/iodide symporter gene. Cardiovasc Res. 2005 Jan 1;65(1):195–202. doi: 10.1016/j.cardiores.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Weissleder R, Moore A, Mahmood U, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med. 2000 Mar;6(3):351–355. doi: 10.1038/73219. [DOI] [PubMed] [Google Scholar]
- 29.Xie X, Cao F, Sheikh AY, et al. Genetic modification of embryonic stem cells with VEGF enhances cell survival and improves cardiac function. Cloning Stem Cells. 2007 doi: 10.1089/clo.2007.0032. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embronic stem cells form stable intracardiac grafts. J Clin Invest. 1996 Jul 1;98(1):216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002 Oct 8;106(15):1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 32.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006 Mar 14;113(10):1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 33.Assmus B, Schachinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002 Dec 10;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 34.Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006 Sep 21;355(12):1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 35.Strauer BE, Brehm M, Zeus T, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J Am Coll Cardiol. 2005 Nov 1;46(9):1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 36.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Forfang K. Autologous stem cell transplantation in acute myocardial infarction: The ASTAMI randomized controlled trial. Intracoronary transplantation of autologous mononuclear bone marrow cells, study design and safety aspects. Scand Cardiovasc J. 2005 Jul;39(3):150–158. doi: 10.1080/14017430510009131. [DOI] [PubMed] [Google Scholar]
- 37.Cleland JG, Freemantle N, Coletta AP, Clark AL. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail. 2006 Jan;8(1):105–110. doi: 10.1016/j.ejheart.2005.12.003. [DOI] [PubMed] [Google Scholar]