Abstract
Background
Prescription opioid dependence is a growing problem, but little research exists on its treatment, including patient characteristics that predict treatment outcome.
Methods
A secondary analysis of data from a large multisite, randomized clinical trial, the National Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study (POATS) was undertaken to examine baseline patient characteristics (N=360) associated with success during 12-week buprenorphine/naloxone treatment for prescription opioid dependence. Baseline predictor variables included self-reported demographic and opioid use history information, diagnoses assessed via the Composite International Diagnostic Interview, and historical opioid use and related information from the Pain And Opiate Analgesic Use History.
Results
In bivariate analyses, pre-treatment characteristics associated with successful opioid use outcome included older age, past-year or lifetime diagnosis of major depressive disorder, initially obtaining opioids with a medical prescription to relieve pain, having only used opioids by swallowing or sublingual administration, never having used heroin, using an opioid other than extended-release oxycodone most frequently, and no prior opioid dependence treatment. In multivariate analysis, age, lifetime major depressive disorder, having only used opioids by swallowing or sublingual administration, and receiving no prior opioid dependence treatment remained as significant predictors of successful outcome.
Conclusions
This is the first study to examine characteristics associated with treatment outcome in patients dependent exclusively on prescription opioids. Characteristics associated with successful outcome after 12 weeks of buprenorphine/naloxone treatment include some that have previously been found to predict heroin-dependent patients’ response to methadone treatment and some specific to prescription opioid-dependent patients receiving buprenorphine/naloxone.
Keywords: prescription opioids, opioid analgesics, drug dependence, substance abuse, buprenorphine, treatment outcome, predictors, heroin
1. INTRODUCTION
Misuse of prescription opioids is a growing problem in the United States, with nonmedical prescription opioid use second only to marijuana in prevalence of use among illicit drugs. In 2010, 5.1 million Americans aged 12 or older (2.0%) reported past-month nonmedical prescription opioid use. After marijuana, prescription opioids had the second highest levels of past-year dependence or abuse, with 1.9 million people meeting criteria for these disorders (Substance Abuse and Mental Health Services Administration, 2011). Prescription opioid abuse is well-represented in treatment-seeking populations, with 9.8% of substance abuse treatment admissions reporting prescription opioid abuse, representing more than a fourfold increase between 1998 and 2008. More than a quarter of patients entering medication-assisted opioid therapy in 2008 were primarily using prescription opioids (Substance Abuse and Mental Health Services Administration, 2010).
Despite increasing rates of prescription opioid abuse and dependence (Arfken et al., 2010), most research on opioid dependence treatment has focused primarily on heroin-dependent patients receiving methadone maintenance treatment. Research has suggested, though, that compared to patients dependent on heroin, prescription opioid-dependent patients have characteristics associated with a more favorable response to treatment; these include shorter opioid use histories, less prior treatment, higher income, greater social stability, and less opioid use per day (Moore et al., 2007; Sigmon, 2006). Indeed, Moore et al. (2007) found that patients dependent on prescription opioids fared better than heroin-dependent patients with office-based buprenorphine/naloxone pharmacotherapy. Given this potential differential response to treatment between prescription opioid and heroin users, it is important to identify factors associated with better treatment outcome for patients dependent on prescription opioids.
Studies of predictors of treatment outcome in opioid dependence have employed various measures of outcome, including treatment retention (Mancino et al., 2010), rates of positive urine drug screens (Alterman et al., 1998), continuous opioid abstinence (Darke et al., 2005), and Addiction Severity Index composite scores (Cacciola et al., 2001). Studies of patient characteristics associated with treatment outcome have often focused on sociodemographic factors, drug use history, psychiatric history, and other areas of functioning. Although not all studies agree, characteristics most consistently associated with poorer outcome among heroin-dependent patients in methadone maintenance treatment include younger age (Mancino et al., 2010; Strain, 1998), Black or non-White race (Iguchi and Stitzer, 1991; Marsch et al., 2005), male gender (Iguchi and Stitzer, 1991; Schottenfeld et al., 1998), being unmarried/not living with a stable partner (McLellan, 1983; Torrens et al., 1996), cocaine use (Joe et al., 1999; Williamson et al., 2007), problematic and/or frequent alcohol use (Flynn et al., 2003; Stenbacka et al., 2007), more frequent baseline opioid use (Darke et al., 2005; Strain, 1998), more frequent injection use (Darke et al., 2005; Simpson et al., 1997), more previous treatment (Hser et al., 1999; Teesson et al., 2008), comorbid substance use disorders (Marsch et al., 2005; Peles et al., 2010), more severe psychiatric problems (Gelkopf et al., 2006; Joe et al., 1994), personality disorders (Alterman et al., 1996; Cacciola et al., 2001), poorer psychosocial functioning (Gerra et al., 2004; Hser et al., 1999), and more severe criminal and legal involvement (Favrat et al., 2002; Flynn et al., 2003). There have been conflicting results about depression as a predictor of treatment outcome; a diagnosis of depression has alternately been associated with both better (Rao et al., 2004) and worse (Teesson et al., 2008) outcome of methadone maintenance treatment.
Despite its demonstrated efficacy (Johnson et al., 2000) and increasingly wide use (Arfken et al., 2010) in opioid dependence treatment, the literature on characteristics associated with outcome of buprenorphine (as opposed to methadone) treatment is more limited, as buprenorphine was not available to treat opioid dependence until 1996 in Europe and 2003 in the United States. Studies examining characteristics associated with buprenorphine treatment outcome have primarily concurred with those examining methadone maintenance treatment. Younger age (Marsch et al., 2005; Soyka et al., 2008), male gender (Marsch et al., 2005; Schottenfeld et al., 1998), cocaine use (Sullivan et al., 2010) or dependence (Marsch et al., 2005), longer histories of opioid use (Soyka et al., 2008), more severe psychiatric problems (Pani et al., 2000; Petry and Bickel, 1999), poorer psychosocial functioning (Pani et al., 2000; Resnick et al., 1991), and more severe legal problems (Petry and Bickel, 2000) have been associated with poorer buprenorphine treatment outcome. Depression has been found to be associated with better outcome of buprenorphine treatment (Gerra et al., 2004; Marsch et al., 2005), corroborating Rao and colleagues’ (2004) results in their study of methadone treatment.
Given the growing problem of prescription opioid dependence and the potential differences between prescription opioid and heroin users in response to treatment, there is a need to determine characteristics of patients dependent on prescription opioids that may predict their response to buprenorphine treatment. Patient characteristics associated with treatment outcome in studies of heroin users receiving methadone or buprenorphine treatment may not be the same in prescription opioid-dependent patients, who differ in their sociodemographic characteristics as well as their drug use patterns (e.g., obtaining opioid prescriptions from physicians for pain, less injection use). The current study thus examined data from the National Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study (POATS; Weiss et al., 2011), a large multisite randomized clinical trial, to identify patient characteristics associated with success during a 12-week buprenorphine treatment period.
2. METHODS
2.1. Main Study Objectives and Design
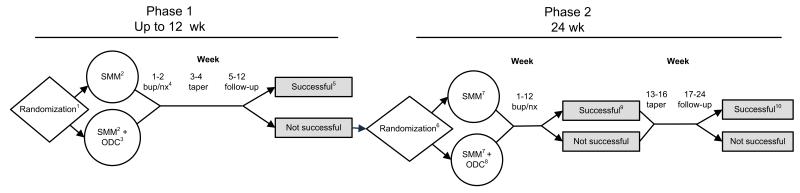
The primary objectives of POATS were to examine the added benefit of counseling to buprenorphine/naloxone treatment of prescription opioid dependence and to help identify the optimal length of pharmacological treatment for this population (detoxification versus maintenance treatment). The main study used a randomized, two-phase adaptive treatment research design (Figure 1; Murphy et al., 2007) at ten sites across the United States. Following brief buprenorphine/naloxone treatment, consisting of induction, 2 weeks of stabilization, and a 2-week taper (Phase 1), participants who returned to opioid use were invited to enter Phase 2, consisting of 12 weeks of buprenorphine/naloxone stabilization, followed by a 4-week taper and 8-week post-treatment follow-up (Weiss et al., 2010b). In both phases, participants were randomized to either 1) Standard Medical Management alone (SMM; Fiellin et al., 1999), or 2) SMM plus individual Opioid Dependence Counseling (SMM+ODC; Pantalon et al., 1999). Participants were stratified in Phase 1 by 1) presence of current chronic pain, and 2) a lifetime history of heroin use. In Phase 2, participants were stratified by the condition to which they were assigned in Phase 1.
Figure 1. Study Design.
1Stratified by presence or absence of a history of heroin use and current chronic pain
2Standard Medical Management; phase 1, week 1: 2 visits; weeks 2 to 4: 1 visit/wk; and weeks 5 to 8: biweekly visits
3Opioid dependence counseling (ODC); phase 1, Weeks 1 to 4: 2 visits/wk; Weeks 5 to 8: biweekly visits
4Buprenorphine-naloxone (bup/nx) dose: 8 to 32 mg/d
5Phase 1 primary endpoint: completion of week 12 with self-reported opioid use on no more than 4 days in a month; absence of 2 consecutive opioid-positive urine test results; no additional substance use disorder treatment (other than self-help); and no more than 1 missing urine sample
6Stratified by phase 1 counseling condition, that is, SMM or SMM+ODC
7SMM; phase 2, week 1: 2 visits; and weeks 2 to 16: 1 visit/wk
8ODC; phase 2, Weeks 1 to 6: 2 visits/wk; and weeks 7 to 12: 1 visit/wk
9Phase 2 primary endpoint: abstinent from opioids during week 12 (the final week of bup/nx stabilization) and during at least 2 of the previous 3 weeks (weeks 9-11)
10Phase 2 secondary endpoint: abstinent from opioids during week 24 and during at least 2 of the previous 3 weeks (weeks 21-23)
This figure is reprinted with permission of the American Medical Association (license number 3011491044721) from “Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence,” Arch. Gen. Psychiatry. 2011;68(12):1238-1246. Weiss, Potter, Fiellin, Byrne, Connery, Dickinson, et al.
2.2. Study Population
Participants in Phase 1 met DSM-IV (American Psychiatric Association, 2000) criteria for current opioid dependence and were at least 18 years old. Key exclusion criteria included any of the following: heroin use on ≥ 4 days in the past month; a lifetime diagnosis of opioid dependence due to heroin alone; a history of ever injecting heroin; or concurrent formal ongoing substance abuse treatment (see Weiss et al. (2010b) for details). Patients who were currently prescribed opioids for pain needed permission from their prescribing physician to enter the study. Of the 653 participants enrolled in Phase 1, 360 went on to Phase 2. The current study population includes only the 360 participants who were unsuccessful in Phase 1 and subsequently enrolled in Phase 2.
2.3.Treatments
Participants in each phase were inducted onto sublingual buprenorphine/naloxone, receiving 4-12 mg on the day of induction, and once-daily doses ranging from 8-32 mg per day for the subsequent duration of stabilization treatment (2 weeks in Phase 1, 12 weeks in Phase 2). At each SMM visit, the study physician could adjust the dose by increments up to 8 mg/wk. All participants received manual-based SMM, which has previously demonstrated efficacy when used in a primary care setting (O’Connor et al., 1998), in a 30-60 minute initial session and subsequent 15-20 minute sessions. In addition to SMM, half of the participants were randomly assigned to receive manual-based ODC (Pantalon et al., 1999), administered in 45-60 minute sessions by trained substance abuse or mental health professionals. In Phase 1, ODC occurred twice per week. In Phase 2, ODC was delivered twice per week for the first six weeks and weekly for the next six weeks; see Weiss et al. (2010b) for further details.
2.4. Measures
A series of standardized assessments was administered to all participants. The Composite International Diagnostic Interview (World Health Organization, January 1997) was used to diagnose substance use disorders, major depressive disorder, and posttraumatic stress disorder. The Pain And Opiate Analgesic Use History (Weiss et al., 2010b) was administered at baseline to assess opioid use history. The Substance Use Report (Weiss et al., 2010b), corroborated by weekly urine drug screens, was administered weekly during treatment and every two weeks during follow-up, and was used as the primary measure to determine “successful outcome” in Phase 2: abstinence from opioids during the final week of buprenorphine/naloxone treatment (week 12) and during ≥2 of the 3 weeks prior (weeks 9-11; Weiss et al., 2011).
2.5. Main Study Results
In the main POATS trial, 610 (93.4%) of the 653 participants had unsuccessful outcomes in Phase 1 (i.e., after receiving two weeks of buprenorphine/naloxone treatment, followed by a two-week taper). Among this group, 360 participants entered Phase 2, of whom approximately half (N=177; 49.2%) were successful at the end of 12 weeks of buprenorphine/naloxone stabilization. At week 24 of Phase 2, eight weeks following completion of the taper during weeks 9-12, only 31 patients (8.6%) had successful opioid use outcomes (i.e., abstinent during week 24 and ≥ 2 of the previous 3 weeks) (Weiss et al., 2011). The current study explored baseline characteristics that distinguished patients with successful outcomes at week 12, while taking buprenorphine/naloxone (our primary outcome measure), from those who were unsuccessful.
2.6.Statistical analysis
Bivariate analyses compared patients who were successful at the end of buprenorphine/naloxone treatment (Phase 2, week 12) with those who were not successful. Continuous variables were assessed with independent t-tests, and dichotomous variables with chi-square tests. Multivariate logistic regression models assessed the relative contribution of the baseline predictors when examined in combination with other variables. As a preliminary step, predictor variables from the bivariate analyses were sorted according to content into the following categories: (1) sociodemographic variables, (2) lifetime substance dependence diagnoses other than opioid dependence, (3) opioid use history, (4) opioid use treatment, and (5) other clinical characteristics (see Table 1 for variables assessed by category). Each category was examined in a separate logistic regression analysis, with variables entered simultaneously. Setting a fairly lenient criterion of P ≤ 0.10 significance so as not to exclude any potentially relevant variables, variables significant in each preliminary regression analysis were then combined and entered simultaneously into a final logistic regression model. All models were adjusted for treatment condition (SMM or SMM+ODC).
Table 1.
Baseline predictors of successful outcome at the end of treatment (week 12)
| Patient Characteristics | Success (n=177) |
Failure (n=183) |
|
|---|---|---|---|
| Sociodemographics | |||
| Female, No. (%) | 78(44.1) | 73(39.9) | |
| Age, mean (SD) | 33.9(10.0) | 31.2(9.1)** | |
| White race, No. (%) | 159 (89.8) | 167(91.3) | |
| Education, mean years (SD) | 12.8(2.3) | 13.0(2.1) | |
| Never married, No. (%) | 83(46.9) | 97(53.0) | |
| Employed full-time, No. (%) | 104(58.8) | 113(61.7) | |
| Clinical | |||
| Substance dependence diagnoses, other than opioid, No. (%) | |||
| Any | Past year | 36(20.3) | 31(16.9) |
| Lifetime | 92(52.0) | 90(49.2) | |
| Opioid use history | |||
| Ever used route other than swallowing/sublingually, No. (%) | 138(78.0) | 166(90.7)** | |
| Years of opioid use1, mean (SD) | 4.5(1.4) | 4.6(1.3) | |
| First source of opioids, No. (%)* | |||
| Medical prescription | 109(61.6) | 90(49.2) | |
| Given by someone | 34(19.2) | 47(25.7) | |
| Dealer | 11(6.2) | 25(13.7) | |
| Reason for first opioid use, No. (%)* | |||
| To relieve pain | 124(70.1) | 109(59.6) | |
| To “get high”/For euphoria | 42(23.7) | 61(33.3) | |
| Used extended-release oxycodone most in past 30 days, No. (%) | 49(27.7) | 77(42.1)** | |
| Used heroin prior to baseline, No. (%) | 36(20.3) | 58(31.7)* | |
| Craving score for opioids, 0-10 scale, mean (SD) | 7.8(2.2) | 8.1(2.1) | |
| Prior opioid use disorder treatment, No. (%) | 52(29.4) | 74(40.4)* | |
| Goal of total opioid abstinence, No. (%) | 112(63.3) | 105(57.4) | |
| Other | |||
| Days of non-opioid substance use, past 30 days2, mean (SD) | |||
| Marijuana | 4.8(9.5) | 4.5(9.1) | |
| Sedatives other than barbiturates | 3.7(7.7) | 3.8(7.9) | |
| Alcohol to intoxication | 1.2(4.1) | 0.8(2.6) | |
| Cocaine | 0.3(1.4) | 0.6(2.0) | |
| Depression | Past year3, No. (%) | 46(26.0) | 26(14.2)* |
| Lifetime3, No. (%) | 73(41.2) | 50(27.3)* | |
| Score4, 0-33 scale, mean (SD) | 24.0(11.8) | 22.2(12.1) | |
| Post-traumatic stress disorder | Lifetime, No. (%) | 32(18.3) | 34(18.7) |
| Current chronic pain, No. (%) | 79(44.6) | 70(38.3) | |
| Nicotine dependence score, 0-10 scale, mean (SD) | 3.2(2.9) | 3.6(2.9) | |
p < 0.05
p < 0.01
Ordinal variable, with 4 = 2 to < 4 years; 5 = 4 to < 6 years
Some drug categories are not shown because use was negligible: amphetamines (n = 23), prescribed methadone (n = 7), barbiturates (n = 7), hallucinogens (n = 2), and inhalants (n = 2).
Major Depressive Disorder diagnosis calculated from the CIDI-E subsection
Depression score calculated from the Beck Depression Inventory
3. RESULTS
3.1.Sample description
Just over half (59.0%, n = 360) of patients unsuccessful in Phase 1 (e.g., those who used opioids for more than four days per month) were randomized into Phase 2. Of the Phase 2 participants, most (91.0%) were white and just under half (41.9%) were female. The mean age was 32.5 (SD = 9.7); the mean years of education were 12.9 (SD = 2.2). Half (50.0%) were never married, and most (60.3%) were employed full-time. About a quarter (26.1%) reported lifetime heroin use, and 41.4% endorsed current chronic pain at baseline. The mean drug craving score at baseline was 7.8 (SD = 2.2) on a 0-10 scale. In other clinical history, 34.2% of patients were diagnosed with lifetime major depressive disorder, 20.0% with past-year major depressive disorder, and 18.5% with lifetime posttraumatic stress disorder. Most participants (64.7%) initially used opioids to relieve physical pain, whereas 28.6% first used to get high. Approximately half (55.3%) first obtained opioids via a legitimate prescription, while 22.5% were given their first opioids by a family member or friend and 10.0% initially bought them from a drug dealer.
Patients who were unsuccessful in Phase 1 and then dropped out of the study (n = 250) differed slightly from those who continued into Phase 2 (n = 360). Those entering Phase 2 were more likely than dropouts to have taken opioids by routes of administration other than swallowing or sublingual (84.4% vs. 76.8%; χ2 = 5.67, p = 0.02) and to be dependent on another substance in addition to opioids in the past year (18.6% vs. 12.0%; χ2 = 4.82, = 1, p = 0.03). Dropouts were similar to continuing patients in sociodemographic characteristics, other opioid use history, and other clinical diagnoses.
3.2. Predictors of Successful Outcome: Bivariate analysis
As reported previously (Weiss et al., 2011), at the end of buprenorphine/naloxone treatment (Phase 2, week 12), 49.2% (n = 177) of patients had a successful outcome, as defined above; there were no differences in success rates between SMM and SMM+ODC. We examined the relation between these outcomes and sociodemographic characteristics, other substance dependence diagnoses, opioid use and treatment history, and other clinical characteristics. As shown in Table 1, older patients were more successful; the remaining sociodemographic factors (gender, education, race, employment, and marital status) were unrelated to outcome. No differences between successful and unsuccessful patients were found in past-year or lifetime dependence on any non-opioid substance, both overall and when examining each substance specifically. Successful patients were less likely to have ever used opioids by non-recommended routes of administration (i.e., chewing, snorting, smoking, or injection use instead of swallowing or sublingual administration), to report extended-release oxycodone as the opioid used most often in the 30 days prior to baseline, and to have ever used heroin. Having a legitimate prescription as the first source of opioids was associated with successful treatment, whereas obtaining opioids from a drug dealer or another non-medical source was associated with unsuccessful outcome. Similarly, patients who first used opioids to relieve physical pain were more likely to succeed, while those who had first used to get high were less likely to do so. Prior opioid use disorder treatment was associated with unsuccessful outcome. Duration of opioid use, severity of craving, and having a goal of abstinence were unrelated to success. Participants with past-year or lifetime major depressive disorder were more likely to succeed while on buprenorphine/naloxone, while the remaining clinical characteristics were unrelated to outcome.
3.3. Predictors of Successful Outcome: Multivariate analysis
Predictor variables were sorted into categories by content, and each category was examined in a separate logistic regression analysis. Those meeting the significance criterion (p ≤ 0.10) were included in the final logistic regression model (see Table 2). Results of the final logistic regression model indicated that participants with successful outcomes at the end of buprenorphine/naloxone treatment were older, more likely to have a lifetime major depressive disorder diagnosis, and less likely to have had previous opioid dependence treatment or to have used opioids by a route other than swallowing or sublingually. In these adjusted analyses, only alcohol dependence was unrelated to treatment outcome.
Table 2.
Final logistic regression model for predictors of successful outcome at the end of treatment (week 12)
| Baseline variables | Odds ratio | 95% CIs | p-value |
|---|---|---|---|
| Treatment condition | 1.34 | 0.87-2.07 | 0.18 |
| Age@ | 1.28 | 1.00-1.64 | 0.05 |
| Lifetime depression | 1.82 | 1.15-2.89 | 0.01 |
| Prior treatment | 0.62 | 0.39-0.99 | 0.04 |
| Lifetime route of use other than oral or sublingual |
0.51 | 0.26-1.01 | 0.05 |
| Lifetime alcohol dependence | 1.16 | 0.71-1.89 | 0.55 |
The OR for age is adjusted to show the increased likelihood of success for every 10 years older.
It is notable that lifetime heroin use, which was significantly associated with poorer outcomes in the primary outcome paper (Weiss et al., 2011; in which a history of heroin use and the presence of chronic pain were included as outcome predictors in a priori analyses), was not included in the final logistic regression, as it was not significant when the model was adjusted for other opioid use characteristics. Specifically, heroin users were more likely to report use of prescription opioids by a non-recommended route (94.7% vs. 80.8%; χ2 = 10.15, p < 0.001), to use extended-release oxycodone more than any other opioid analgesic in the past 30 days (52.1% vs. 28.9%; χ2 = 16.41, p < 0.001), and to report receiving prior treatment (48.9% vs. 30.1%; χ2 = 10.86, p < 0.001), when compared to patients who had never used heroin.
4. DISCUSSION
Our study employed bivariate and multivariate analyses to examine patient characteristics associated with outcome of 12-week buprenorphine/naloxone treatment plus medical management, with or without adjunctive drug counseling, in a large national sample of patients dependent on prescription opioids. In bivariate analyses, successful patients were 1) older than unsuccessful patients, and 2) less likely to a) have ever used opioids via a route other than swallowing or sublingual administration, b) have ever used heroin, c) report using extended-release oxycodone most frequently, or d) have received prior treatment for opioid dependence. Those who succeeded while on buprenorphine/naloxone were more likely to 1) have initially used opioids to relieve physical pain, 2) have first obtained opioids with a medical prescription, and 3) have a diagnosis of major depressive disorder. In multivariate analysis, older age, lifetime major depressive disorder, absence of prior opioid dependence treatment, and absence of a history of having used opioids via a non-recommended route remained as significant characteristics associated with successful outcome.
In the present study, the only sociodemographic characteristic related to treatment outcome was age. Successful patients were older than those who were unsuccessful, consistent with studies of primarily heroin-dependent patients (Backmund et al., 2001; Saxon et al., 1996). Although the difference in mean age of successful and unsuccessful participants was relatively small, the logistic regression model and odds ratio demonstrates that a middle-aged patient would be considerably more likely to succeed than one in his or her early 20s, as there was more than a 25% increase in likelihood of success with every 10-year increase in patient age.
Aspects of opioid use history that were found to be related to treatment outcome include some that are, by definition, specific to prescription opioid users and thus have not been examined in previous research. For example, patients who reported having ever used opioids via routes other than swallowing and sublingual administration were less likely to be successful than were those who had never used opioids via non-recommended routes. Notably, though, while recommended routes are specific to prescription opioids, existing research has demonstrated that more frequent intravenous use (considered to be a more dangerous route than intranasal use or inhalation) predicts poor outcome in opioid-dependent individuals in methadone maintenance treatment (Darke et al., 2005; Simpson et al., 1997), underlining the likely implications of type of route of administration for prescription opioid-dependent patients. Having first acquired opioids through medical prescription and having first used opioids to relieve pain were associated with treatment success in the current study; these have not been examined in prior research and are only germane in those dependent upon opioid analgesics. Also specific to prescription opioid users, reporting previous use of heroin and having used extended-release oxycodone most frequently in the past 30 days were associated with unsuccessful treatment outcome in our study. The association between extended-release oxycodone and poor outcome may be pharmacological (i.e., oxycodone may have greater abuse potential than some other prescription opioids (Zacny and Gutierrez, 2009; Zacny and Lichtor, 2008)); may be due to higher doses, which were not measured in the current study due to the unreliability of those data; or may be due to oxycodone’s reputation, with this drug attracting more substance abusers (Goodnough, 2011; Meier, 2003).
Although having first used opioids to relieve physical pain was associated with successful treatment outcome, the presence of current chronic pain was unrelated to outcome. This could be due to the fact that patients in the study, on average, reported only moderate pain intensity; patients whose physicians determined their pain severe enough to require ongoing opioid therapy were excluded from the study. The absence of a relationship in our study between non-opioid substance use disorder diagnoses and treatment outcome differs from results of previous research (Chatham et al., 1997; Peles et al., 2010), which has consistently shown the presence of other substance use disorders to be associated with poorer treatment outcome. This difference may be due to the relatively low rates of non-opioid substance use disorder diagnoses in our population. Prior treatment for opioid dependence was associated with a lower likelihood of succeeding in treatment in our study, although it is noteworthy that only 35% of our patients had previously sought opioid dependence treatment. This finding corroborates results from methadone-treated heroin users (McLellan et al., 1994; Teesson et al., 2008), despite the higher number of prior treatment episodes typically found in the latter group as compared to our population (McLellan et al., 1994).
Depression was another feature associated with successful outcome of buprenorphine/naloxone maintenance in the current study. Although this result may appear contradictory to some previous research finding more severe overall psychiatric problems to predict poorer outcome of opioid dependence treatment (e.g., Peles et al., 2010; Strain, 1998), it is consistent with results of some (Gerra et al., 2004; Rao et al., 2004) but not all (Teesson et al., 2008) prior treatment studies examining depression specifically as a predictor of outcome in opioid-dependent individuals. Depression has been associated with greater motivation to change drinking behavior (Blume et al., 2001; Holt et al., 2009) and higher receptivity to alcohol abuse treatment (Wells-Parker et al., 2006; Wells-Parker and Williams, 2002) in individuals with alcohol abuse and dependence, and depression could have functioned similarly in prescription opioid-dependent patients in the present study. Another possible contributing factor to the association between depression and better outcome may be the putative antidepressant properties of buprenorphine (Bodkin et al., 1995; Emrich et al., 1982), although not all studies have found greater improvement in depression with buprenorphine treatment than with methadone treatment (Dean et al., 2004).
In this initial study of characteristics associated with treatment outcome in a large sample of patients dependent on prescription opioids, patient characteristics associated with successful outcome of 12-week buprenorphine/naloxone treatment included some previously established as predictors of heroin users’ success in opioid maintenance therapy as well as characteristics that are specific to prescription opioid dependence. In totality, our findings reflect the prognostic importance of two major components of the addictive process (Marlatt, 1997): the attempt to achieve euphoria (Dackis and Gold, 1985; Newton et al., 2009) and the desire to relieve pain (George and Koob, 2010; Khantzian, 1985, 1997). In the current study, patients who initially used prescription opioids in the ways these drugs are intended to be used, including first using to alleviate pain and via routes by which these opioids are intended, to be administered fared better in treatment. Conversely, patients who used these drugs in non-intended ways more reflective of attempts to achieve euphoria, such as first using prescription opioids to “get high” and administering them via non-recommended routes, performed more poorly in treatment.
Limitations of the current study include the fact that POATS was a relatively short-term study of prescription opioid dependence treatment, allowing us to examine only those patient characteristics associated with early success in buprenorphine/naloxone treatment. Moreover, all of the Phase 2 patients in this study had, by definition, failed the short-term taper in Phase 1, potentially limiting the generalizability of the results to the larger population of treatment-seeking prescription opioid-dependent individuals; generalizability may be further affected by the high number of the unsuccessful Phase 1 patients who dropped out of the study before Phase 2. Additionally, because this study intentionally excluded individuals with an extensive history of heroin use so as to examine a distinct population of individuals dependent on prescription opioids (Weiss et al., 2010a), generalization to treatment seekers dependent on both prescription opioids and heroin and to those with injection heroin use may be reduced. Characteristics predictive of treatment success in the current study are not necessarily generalizable to other forms of treatment, such as longer-term buprenorphine/naloxone maintenance, treatment with methadone or naltrexone, or other forms of behavioral treatment. Only half of POATS patients were able to achieve success while maintained on buprenorphine/naloxone, and few of these successful patients were able to sustain this success at follow-up after tapering off their medication (Weiss et al., 2011). Other predictors of treatment outcome may also exist that were not examined in this trial, including genetic predictors, which have been found to be associated with pharmacotherapeutic treatment outcome in other populations of substance abusers (Anton et al., 2008; Heinzerling et al., 2012) It is hoped that knowledge about patient characteristics associated with successful (and unsuccessful) outcome from the current study can be used to help develop more effective treatments for this patient population.
Acknowledgments
Role of Funding Sources
This work was supported by a series of grants from NIDA as part of the Cooperative Agreement on the Clinical Trials Network (CTN): grants U10 DA015831, U10 DA020024, U10 DA13035, U10 DA013714, and U10 DA013727; NIDA grants K24 DA022288, K23 DA022297, R01 DA019511, R01 DA020576, R01 DA025991, and RC1 DA028245; and NIAAA grant U01 AA020795. NIDA and NIAAA had no further role in study design; in the collection, analysis and interpretation of data; or in the writing of the report. The NIDA CTN Publication Committee has reviewed a draft of this manuscript and approved it for submission for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors have contributed substantively to the research and manuscript preparation and have approved the final manuscript.
Conflict of Interest
Dr. Weiss has served as a consultant to Titan Pharmaceuticals and Reckitt Benckiser Pharmaceuticals. Dr. Fiellin has received honoraria from Pinney Associates and ParagonRx for serving on external advisory boards monitoring the diversion and abuse of buprenorphine. All other authors declare that they have no conflicts of interest.
Clinical Trial Registration
clinicaltrials.gov identifier: NCT00316277
REFERENCES
- Alterman AI, Rutherford MJ, Cacciola JS, McKay JR, Boardman CR. Prediction of 7 months methadone maintenance treatment response by four measures of antisociality. Drug Alcohol Depend. 1998;49:217–223. doi: 10.1016/s0376-8716(98)00015-5. [DOI] [PubMed] [Google Scholar]
- Alterman AI, Rutherford MJ, Cacciola JS, McKay JR, Woody GE. Response to methadone maintenance and counseling in antisocial patients with and without major depression. J. Nerv. Ment. Dis. 1996;184:695–702. doi: 10.1097/00005053-199611000-00007. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch. Gen. Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfken CL, Johanson CE, di Menza S, Schuster CR. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: national surveys of physicians. J. Subst. Abuse Treat. 2010;39:96–104. doi: 10.1016/j.jsat.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Backmund M, Meyer K, Eichenlaub D, Schutz CG. Predictors for completing an inpatient detoxification program among intravenous heroin users, methadone substituted and codeine substituted patients. Drug Alcohol Depend. 2001;64:173–180. doi: 10.1016/s0376-8716(01)00122-3. [DOI] [PubMed] [Google Scholar]
- Blume AW, Schmaling KB, Marlat GA. Motivating drinking behavior change depressive symptoms may not be noxious. Addict. Behav. 2001;26:267–272. doi: 10.1016/s0306-4603(00)00087-3. [DOI] [PubMed] [Google Scholar]
- Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J. Clin. Psychopharmacol. 1995;15:49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Cacciola JS, Alterman AI, Rutherford MJ, McKay JR, Mulvaney FD. The relationship of psychiatric comorbidity to treatment outcomes in methadone maintained patients. Drug Alcohol Depend. 2001;61:271–280. doi: 10.1016/s0376-8716(00)00148-4. [DOI] [PubMed] [Google Scholar]
- Chatham LR, Rowan-Szal GA, Joe GW, Simpson DD. Heavy drinking, alcohol-dependent vs. nondependent methadone-maintenance clients: a follow-up study. Addict. Behav. 1997;22:69–80. doi: 10.1016/s0306-4603(96)00005-6. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Gold MS. Pharmacological approaches to cocaine addiction. J. Subst. Abuse Treat. 1985;2:139–145. doi: 10.1016/0740-5472(85)90043-1. [DOI] [PubMed] [Google Scholar]
- Darke S, Ross J, Teesson M, Ali R, Cooke R, Ritter A, Lynskey M. Factors associated with 12 months continuous heroin abstinence: findings from the Australian Treatment Outcome Study (ATOS) J. Subst. Abuse Treat. 2005;28:255–263. doi: 10.1016/j.jsat.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Dean AJ, Bell J, Christie MJ, Mattick RP. Depressive symptoms during buprenorphine vs. methadone maintenance: findings from a randomised, controlled trial in opioid dependence. Eur. Psychiatry. 2004;19:510–513. doi: 10.1016/j.eurpsy.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Emrich HM, Vogt P, Herz A. Possible antidepressive effects of opioids: action of buprenorphine. Ann. N. Y. Acad. Sci. 1982;398:108–112. doi: 10.1111/j.1749-6632.1982.tb39483.x. [DOI] [PubMed] [Google Scholar]
- Favrat B, Rao S, O’Connor PG, Schottenfeld R. A staging system to predict prognosis among methadone maintenance patients, based on admission characteristics. Subst. Abuse. 2002;23:233–244. doi: 10.1080/08897070209511496. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Pantalon MV, Schottenfeld RS, Gordon L, O’Connor PG. Manual for Standard Medical Management of Opioid Dependence with Buprenorphine. Yale University; New Haven, CT: 1999. [Google Scholar]
- Flynn PM, Joe GW, Broome KM, Simpson DD, Brown BS. Recovery from opioid addiction in DATOS. J. Subst. Abuse Treat. 2003;25:177–186. doi: 10.1016/s0740-5472(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Gelkopf M, Weizman T, Melamed Y, Adelson M, Bleich A. Does psychiatric comorbidity affect drug abuse treatment outcome? A prospective assessment of drug abuse, treatment tenure and infectious diseases in an Israeli methadone maintenance clinic. Isr. J. Psychiatry Relat. Sci. 2006;43:126–136. [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci. Biobehav. Rev. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Borella F, Zaimovic A, Moi G, Bussandri M, Bubici C, Bertacca S. Buprenorphine versus methadone for opioid dependence: predictor variables for treatment outcome. Drug Alcohol Depend. 2004;75:37–45. doi: 10.1016/j.drugalcdep.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Goodnough A. Pharmacies besieged by addicted thieves. The New York Times; New York, NY: 2011. p. A14. [Google Scholar]
- Heinzerling KG, McCracken JT, Swanson AN, Ray LA, Shoptaw SJ. COMT Val158Met, BDNF Val66Met, and OPRM1 Asn40Asp and methamphetamine dependence treatment response: preliminary investigation. J. Clin. Psychopharmacol. 2012;32:135–137. doi: 10.1097/JCP.0b013e318240a48e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt LJ, O’Malley SS, Rounsaville BJ, Ball SA. Depressive symptoms, drinking consequences, and motivation to change in first time DWI offenders. Am. J. Drug Alcohol Abuse. 2009;35:117–122. doi: 10.1080/00952990802585398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Grella CE, Hsieh SC, Anglin MD, Brown BS. Prior treatment experience related to process and outcomes in DATOS. Drug Alcohol Depend. 1999;57:137–150. doi: 10.1016/s0376-8716(99)00081-2. [DOI] [PubMed] [Google Scholar]
- Iguchi MY, Stitzer ML. Predictors of opiate drug abuse during a 90-day methadone detoxification. Am. J. Drug Alcohol Abuse. 1991;17:279–294. doi: 10.3109/00952999109027552. [DOI] [PubMed] [Google Scholar]
- Joe GW, Simpson DD, Broome KM. Retention and patient engagement models for different treatment modalities in DATOS. Drug Alcohol Depend. 1999;57:113–125. doi: 10.1016/s0376-8716(99)00088-5. [DOI] [PubMed] [Google Scholar]
- Joe GW, Simpson DD, Sells SB. Treatment process and relapse to opioid use during methadone maintenance. Am. J. Drug Alcohol Abuse. 1994;20:173–197. doi: 10.3109/00952999409106781. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N. Engl. J. Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am. J. Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv. Rev. Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Mancino M, Curran G, Han X, Allee E, Humphreys K, Booth BM. Predictors of attrition from a national sample of methadone maintenance patients. Am. J. Drug Alcohol Abuse. 2010;36:155–160. doi: 10.3109/00952991003736389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA. Introduction. In: Marlatt GA, VandenBos GR, editors. Addictive Behaviors. American Psychological Association; Washington, DC: 1997. pp. xi–xxv. [Google Scholar]
- Marsch LA, Stephens MA, Mudric T, Strain EC, Bigelow GE, Johnson RE. Predictors of outcome in LAAM, buprenorphine, and methadone treatment for opioid dependence. Exp. Clin. Psychopharmacol. 2005;13:293–302. doi: 10.1037/1064-1297.13.4.293. [DOI] [PubMed] [Google Scholar]
- McLellan AT. Patient characteristics associated with outcome. In: Cooper JR, Altman F, Brown BS, Czechowicz D, editors. Research on the Treatment of Drug Addiction: State of the Art. US Government Printing Office; Washington, DC: 1983. pp. 500–529. [Google Scholar]
- McLellan AT, Alterman AI, Metzger DS, Grissom GR, Woody GE, Luborsky L, O’Brien CP. Similarity of outcome predictors across opiate, cocaine, and alcohol treatments: role of treatment services. J. Consult. Clin. Psychol. 1994;62:1141–1158. doi: 10.1037//0022-006x.62.6.1141. [DOI] [PubMed] [Google Scholar]
- Meier B. Pain Killer: A “Wonder” Drug’s Trail of Addiction and Death. Rodale; Emmaus, PA: 2003. [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O’Connor PG, Schottenfeld RS. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J. Gen. Intern. Med. 2007;22:527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SA, Lynch KG, Oslin D, McKay JR, TenHave T. Developing adaptive treatment strategies in substance abuse research. Drug Alcohol Depend. 2007;88(Suppl. 2):S24–30. doi: 10.1016/j.drugalcdep.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, 2nd, Kalechstein AD, Tziortzis D, Jacobsen CA. Theories of addiction: methamphetamine users’ explanations for continuing drug use and relapse. Am. J. Addict. 2009;18:294–300. doi: 10.1080/10550490902925920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor PG, Oliveto AH, Shi JM, Triffleman EG, Carroll KM, Kosten TR, Rounsaville BJ, Pakes JA, Schottenfeld RS. A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. Am. J. Med. 1998;105:100–105. doi: 10.1016/s0002-9343(98)00194-6. [DOI] [PubMed] [Google Scholar]
- Pani PP, Maremmani I, Pirastu R, Tagliamonte A, Gessa GL. Buprenorphine: a controlled clinical trial in the treatment of opioid dependence. Drug Alcohol Depend. 2000;60:39–50. doi: 10.1016/s0376-8716(99)00140-4. [DOI] [PubMed] [Google Scholar]
- Pantalon MV, Fiellin DA, Schottenfeld RS, Gordon L, O’Connor PG. Manual for Enhanced Medical Management of Opioid Dependence with Buprenorphine. Yale University; New Haven, CT: 1999. [Google Scholar]
- Peles E, Schreiber S, Adelson M. 15-Year survival and retention of patients in a general hospital-affiliated methadone maintenance treatment (MMT) center in Israel. Drug Alcohol Depend. 2010;107:141–148. doi: 10.1016/j.drugalcdep.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK. Therapeutic alliance and psychiatric severity as predictors of completion of treatment for opioid dependence. Psychiatr. Serv. 1999;50:219–227. doi: 10.1176/ps.50.2.219. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK. Gender differences in hostility of opioid-dependent outpatients: role in early treatment termination. Drug Alcohol Depend. 2000;58:27–33. doi: 10.1016/s0376-8716(99)00058-7. [DOI] [PubMed] [Google Scholar]
- Rao SR, Broome KM, Simpson DD. Depression and hostility as predictors of long-term outcomes among opiate users. Addiction. 2004;99:579–589. doi: 10.1111/j.1360-0443.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- Resnick RB, Resnick E, Galanter M. Buprenorphine responders: a diagnostic subgroup of heroin addicts? Prog. Neuropsychopharmacol. Biol. Psychiatry. 1991;15:531–538. doi: 10.1016/0278-5846(91)90028-y. [DOI] [PubMed] [Google Scholar]
- Saxon AJ, Wells EA, Fleming C, Jackson TR, Calsyn DA. Pre-treatment characteristics, program philosophy and level of ancillary services as predictors of methadone maintenance treatment outcome. Addiction. 1996;91:1197–1209. doi: 10.1046/j.1360-0443.1996.918119711.x. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes JR, Kosten TR. Prognostic factors in Buprenorphine-versus methadone-maintained patients. J. Nerv. Ment. Dis. 1998;186:35–43. doi: 10.1097/00005053-199801000-00006. [DOI] [PubMed] [Google Scholar]
- Sigmon SC. Characterizing the emerging population of prescription opioid abusers. Am. J. Addict. 2006;15:208–212. doi: 10.1080/10550490600625624. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Rowan-Szal GA. Drug abuse treatment retention and process effects on follow-up outcomes. Drug Alcohol Depend. 1997;47:227–235. doi: 10.1016/s0376-8716(97)00099-9. [DOI] [PubMed] [Google Scholar]
- Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int. J. Neuropsychopharmacol. 2008;11:641–653. doi: 10.1017/S146114570700836X. [DOI] [PubMed] [Google Scholar]
- Stenbacka M, Beck O, Leifman A, Romelsjo A, Helander A. Problem drinking in relation to treatment outcome among opiate addicts in methadone maintenance treatment. Drug Alcohol Rev. 2007;26:55–63. doi: 10.1080/09595230601036994. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Useful predictors of outcome in methadone-treated patients: results from a controlled clinical trial with three doses of methadone. J. Maint. Addict. 1998;1:15–28. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . The TEDS Report: Substance Abuse Treatment Admissions Involving Abuse of Pain Relievers: 1998 and 2008. Office of Applied Studies; Rockville, MD: 2010. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2010 National Survey on Drug Use and Health: Volume I. Summary of National Findings. NSDUH Series H-41. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2011. [Google Scholar]
- Sullivan LE, Moore BA, O’Connor PG, Barry DT, Chawarski MC, Schottenfeld RS, Fiellin DA. The association between cocaine use and treatment outcomes in patients receiving office-based buprenorphine/naloxone for the treatment of opioid dependence. Am. J. Addict. 2010;19:53–58. doi: 10.1111/j.1521-0391.2009.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teesson M, Mills K, Ross J, Darke S, Williamson A, Havard A. The impact of treatment on 3 years’ outcome for heroin dependence: findings from the Australian Treatment Outcome Study (ATOS) Addiction. 2008;103:80–88. doi: 10.1111/j.1360-0443.2007.02029.x. [DOI] [PubMed] [Google Scholar]
- Torrens M, Castillo C, Perez-Sola V. Retention in a low-threshold methadone maintenance program. Drug Alcohol Depend. 1996;41:55–59. doi: 10.1016/0376-8716(96)01230-6. [DOI] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Copersino ML, Prather K, Jacobs P, Provost S, Chim D, Selzer J, Ling W. Conducting clinical research with prescription opioid dependence: defining the population. Am J Addict. 2010a;19:141–146. doi: 10.1111/j.1521-0391.2009.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, Gardin J, Griffin ML, Gourevitch MN, Haller DL, Hasson AL, Huang Z, Jacobs P, Kosinski AS, Lindblad R, McCance-Katz EF, Provost SE, Selzer J, Somoza EC, Sonne SC, Ling W. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch. Gen. Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Provost SE, Huang Z, Jacobs P, Hasson A, Lindblad R, Connery HS, Prather K, Ling W. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): rationale, design, and methodology. Contemp. Clin. Trials. 2010b;31:189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells-Parker E, Dill P, Williams M, Stoduto G. Are depressed drinking/driving offenders more receptive to brief intervention? Addict. Behav. 2006;31:339–350. doi: 10.1016/j.addbeh.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Wells-Parker E, Williams M. Enhancing the effectiveness of traditional interventions with drinking drivers by adding brief individual intervention components. J. Stud. Alcohol. 2002;63:655–664. doi: 10.15288/jsa.2002.63.655. [DOI] [PubMed] [Google Scholar]
- Williamson A, Darke S, Ross J, Teesson M. The effect of baseline cocaine use on treatment outcomes for heroin dependence over 24 months: findings from the Australian Treatment Outcome Study. J. Subst. Abuse Treat. 2007;33:287–293. doi: 10.1016/j.jsat.2006.12.009. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Composite International Diagnostic Interview (CIDI): Core Version 2.1. World Health Organization; Geneva, Switzerland: Jan, 1997. [Google Scholar]
- Zacny JP, Gutierrez S. Within-subject comparison of the psychopharmacological profiles of oral hydrocodone and oxycodone combination products in non-drug-abusing volunteers. Drug Alcohol Depend. 2009;101:107–114. doi: 10.1016/j.drugalcdep.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Zacny JP, Lichtor SA. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacol. (Berl.) 2008;196:105–116. doi: 10.1007/s00213-007-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]