Abstract
Increasing treatment intensity has improved outcomes for children with neuroblastoma. We performed a pilot study in the Children’s Oncology Group (COG) to assess feasibility and toxicity of a tandem myeloablative regimen without total body irradiation (TBI) supported by autologous CD34 selected peripheral blood stem cells. Forty-one patients with high-risk neuroblastoma were enrolled; eight patients did not receive any myeloablative consolidation procedure, and seven received only one. Two patients out of 41 (4.9%) experienced transplant-related mortality. CD34 selection was discontinued after subjects were enrolled due to serious viral illness. From the time of study enrollment, the overall 3-year event-free survival (EFS) and overall survival (OS) were 44.8±9.6% and 59.2±9.2% (N=41). These results demonstrate that tandem transplantation in the cooperative group setting is feasible and support a randomized comparison of single versus tandem myeloablative consolidation with PBSC support for high-risk neuroblastoma.
Keywords: pediatric, neuroblastoma, tandem transplant, hematopoietic stem cell transplant
INTRODUCTION
High-risk neuroblastoma remains among the most challenging of pediatric malignancies, with limited progress in clinical trials over the past three decades(ref. 1). Pending development of more targeted approaches, one of the principal approaches to improve OS in this disease is intensification of cytotoxic therapies, and improvements in survival probabilities have been associated with increasing dose intensity(ref. 2). While differing induction regimens have resulted in comparable disease response rates, use of myeloablative therapy in the consolidation phase of neuroblastoma treatment has improved outcomes in large phase III randomized studies(ref. 3, 4). Based on improvements in EFS after myeloablative consolidation, single and limited institution clinical protocols have tested varying approaches to intensifying consolidation therapy(ref. 5, 6). Among these approaches is the use of tandem transplantation using non-overlapping myeloablative conditioning regimens. The largest of these studies was a multi-institutional phase II study, using both unprocessed and CD34 selected peripheral blood stem cells (PBSC)(ref. 7, 8, 9) as autologous stem cell support, and employing total body irradiation (TBI) in the conditioning for the second transplant(ref. 10). This study enrolled 97 patients in 4 institutions and demonstrated a 3-year EFS of 55% from diagnosis(ref. 11). While the EFS observed in this study was promising, the use of TBI in conditioning clearly results in an increased risk of treatment-related long term side effects(ref. 12). As a result, there was interest in designing a tandem transplant regimen without TBI that could be brought forward into a phase III cooperative group trial.
For the first consolidation regimen, the myeloablative combination of thiotepa and cytoxan was chosen as a non-overlapping regimen with carboplatin/etoposide phosphate (etopophos)/melphalan (CEM), which has been the effective standard of care for neuroblastoma transplant in the Children’s Oncology Group (COG).(ref. 4) This regimen was selected as the first HDC/SCR cycle because it was expected to have less toxicity than CEM based on POG 9640.(ref. 13) 2) CEM was selected as the second regimen in order to facilitate the most straightforward evaluation of consolidation regimens in a subsequent single vs. tandem transplant cooperative group trial.
PATIENTS AND METHODS
Patient Selection and Evaluation
Patients with high-risk neuroblastoma who had received no prior systemic therapy, or who had only received localized emergency radiation to sites of life- or function-threatening disease and/or no more than one cycle of chemotherapy for apparent intermediate-risk disease prior to determination of MYCN amplification and Shimada histology, were enrolled on this protocol through the COG. Newly diagnosed patients with high-risk neuroblastoma aged 30 years or under at initial diagnosis were eligible if they were: 1) International Neuroblastoma Staging System (INSS) stage 2A or 2B, 365 days of age or older with MYCN amplification and unfavorable histology; 2) INSS stage 3, 365 days or older with MYCN amplified or unfavorable histology; 3) INSS stage 3, 4 or 4S, less than 365 days old with MYCN amplification; 4) INSS stage 4, 365 days or older. Patients aged 365 days or older who were initially INSS stage 1, 2, or 4S but had progressed without interval chemotherapy were also eligible. The protocol was activated on 2/1/2001 and closed to accrual on 2/20/2004. Response to therapy was evaluated by the National Cancer Institute’s Response Evaluation Criteria in Solid Tumors (RECIST), modified for INSS criteria.(ref. 14) Institutional Review Board approval for treatment on this study at each center was obtained.
Treatments
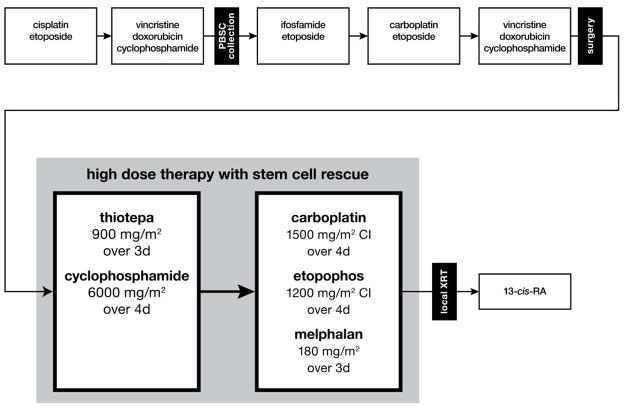
After diagnosis and enrollment on the COG biology protocol to determine MYCN, ploidy, and Shimada histology status, patients consented and enrolled on this study underwent five cycles of induction therapy, summarized in Figure 1. All patients underwent PBSC collection after recovery from Cycle 2, irrespective of interim bone marrow evaluation results. Resection of the primary tumor, if not completed at diagnosis, occurred after completion of cycle 4 or 5 and prior to high dose chemotherapy with stem cell rescue (HDC/SCR). Criteria for proceeding to transplant included absence of progressive disease, no uncontrolled infection, marrow involvement of <1% at the end of induction, and acceptable organ function. The conditioning regimens included thiotepa 900 mg/m2 over 3 days and cyclophosphamide 6000 mg/m2 over 4 days (HDC #1), and continuous infusions of carboplatin 1500 mg/m2 and etopophos1 1200 mg/m2 over 4 days plus melphalan 180 mg/m2 over 3 days (HDC #2) (Figure 1). Patients were eligible to proceed to HDC/SCR #2 if they met organ criteria, had not experienced severe VOD (grade 3) in HDC/SCR #1, had sufficient autologous stem cells available, had no evidence of progressive disease, had ANC recovery to >750/mcL, platelets >20,000/mcL, and were within 56 days of the start of HDC/SCR cycle #1. The protocol recommended local radiotherapy (2100–2160 cGy) after recovery from HDC/SCR #2. At day 90 after the second HDC/SCR, patients received 13-cis-retinoic acid 160 mg/m2, 14 days on and 14 days off, for a total of six months. The protocol required adverse event reporting within 10 business days. Patients who were off-protocol therapy were followed until they met the criteria for off-study (death, loss to follow-up, or entry into another COG therapeutic study).
Figure 1. Schema of induction, consolidation and post-consolidation therapy.
Cycle 1 included cisplatin 40 mg/m2 over 5 days plus etoposide 200 mg/m2 on days 2–4. Cycles 2 and 5 included vincristine 1.5 mg/m2 plus doxorubicin 60 mg/m2 on day 1 and cyclophosphamide 2,000 mg/m2 on days 1 and 2. Cycle 3 included ifosfamide 1.8 g/m2 plus etoposide 100 mg/m2 over 5 days. Cycle 4 included carboplatin 500 mg/m2 over 2 days plus etoposide 150 mg/m2 over 3 days. Abbreviations: CI, continuous infusion over 24 hours; PBSC, peripheral blood stem cell; XRT, radiotherapy; 13-cis-RA, 13-cis-retinoic acid.
Stem cell collection and processing
PBSC underwent CD34 positive selection of hematopoietic stem and progenitor cells to achieve potential tumor cell purging, either using Isolex or the CliniMACS devices according to the manufacturers’ protocols and based on institutional availability. PBSC collection was undertaken with a goal of 20×106 CD34+ cells/kg prior to CD34 selection, with the intent that at least 2×106 CD34+ cells/kg would be available for each of the 2 planned HDC/SCR procedures. Prior to selection, an aliquot of 2×106 CD34+ cells/kg was cryopreserved as a backup stem cell product. CD34 selection causes substantial T cell depletion in addition to tumor cell purging and thus could slow immune recovery after autologous transplantation, potentially increasing the risk of serious viral infection after stem cell transplant.(ref. 15, 16) Therefore, we included a stopping rule for viral infections, detailed in the next section.
Statistical analysis and stopping rules
The primary questions of this study were feasibility and toxicity of a tandem transplant regimen with CD34 selection. EFS and OS were calculated using the method of Kaplan and Meier(ref. 17) with standard errors per Peto(ref. 18). For EFS, time to event was calculated from the time of enrollment on the study until the first occurrence of relapse, progressive disease, secondary malignancy, or death, or until the time of last contact. For OS, time to event was calculated from the time of enrollment on the study until the time of death or until the time of last contact if the patient did not die. Survival estimates are expressed as the point estimate ± the standard error.
The intent-to-treat analysis from the time of study enrollment provides results of the intent to deliver two transplants and thus includes poor responders as well as those who survived to the second transplant. The 3-year point estimates of EFS and OS for patients who received at least one transplant (starting from the time of the first transplant) and for patients who received two transplants (starting from the time of the second transplant) have been presented to demonstrate that there does not appear to be a clinically meaningful drop-off or increase in survival in the subgroups who received one or two transplants relative to the group as a whole. Direct comparisons of the subgroups (i.e. of patients who received one HDC/SCR cycle versus those who received two) are not appropriate due to selection bias. Survival analyses from time of enrollment were not performed for any subgroups of patients, and specifically not for the subgroup that received two transplants, due to potential attrition bias, in which patients who survive long enough to receive two transplants comprise a more selected cohort whose outcome may not be representative of the general population.
Transplant related mortality (TRM) was defined as death from any cause occurring within 30 days after either the first or second transplant. We monitored for an infeasible/unsafe TRM rate significantly higher than 7.5% using the following early stopping rule: if three TRMs were observed within the first 17 patients who received HDC/SCR, then the trial would be stopped, and we would conclude that the regimen was unsafe. This rule has a significance level of 13.0%, power of 92.3%, and a TRM rate under the alternative hypothesis of 30%. If the TRM early stopping rule was not met, then EFS would become the primary endpoint. A secondary EFS analysis was performed on the subset of patients who underwent at least one HDC/SCR.
Toxicities were graded according to the Common Toxicity Criteria for Adverse Events, version 2.0 (CTCAE-2.0) and tabulated after the end of induction, between HDC/SCR #1 and #2, and after HDC/SCR #2. The proportion of patients with a complete response (CR), per metaiodobenzylguanidine (MIBG) scan, after HDC/SCR #2 was performed. CD34 selection was to be discontinued if a specific, severe viral syndrome (symptomatic cytomegalovirus, disseminated adenovirus, or Epstein-Barr virus lymphoproliferative disorder [EBV LPD]) occurred in one of the first 14 patients who received both HDC/SCR procedures supported by CD34 selected PBSC, or two incidents in the total cohort.
RESULTS
Patient Characteristics
Forty-two patients were enrolled on trial, and 41 patients with high-risk neuroblastoma meeting eligibility requirements were treated according to this protocol (one patient was declared ineligible due to wrong diagnosis) (Table 1). The majority of patients (38 of 41, or 93%) were enrolled within 4 weeks of diagnosis; however, one stage 3 patient was enrolled at 8 weeks, one stage 4 patient at 5 weeks, and one patient was initially stage 2a then progressed to stage 4 and was enrolled at 41 weeks from the original neuroblastoma diagnosis. Median time from study enrollment to HDC/SCR #1 was 148 days (range: 115–197 days; N=33), from study enrollment to HDC/SCR #2 was 203 days (range: 157–267 days; N=26), and median time from first to second transplant: 52 days (range: 18–77 days; N=26). Median age at diagnosis was 35 months (range: 10 months to 20 years). There was one patient under 12 months, and five between 12–18 months old. Most patients were either white (59%) or black (24%), and there were more males (24) than females (17). There was one patient with stage 2B and three with stage 3 disease, while the rest had stage 4 neuroblastoma. MYCN amplification was observed in 24% of tumors analyzed. Ploidy was fairly evenly divided between diploid (44%) and hyperdiploid (49%), and most patients had unfavorable Shimada histology (61%).
Table 1.
Characteristics of high-risk neuroblastoma patients enrolled on COG study ANBL00P1 (n = 41)
| Characteristic | Number | Percent (%) |
|---|---|---|
|
| ||
| Stratum | ||
| ISOLEX - Processed PBSC | 13 | 31.7 |
| MACS - Processed PBSC | 1 | 2.4 |
| Unselected PBSC | 27 | 65.9 |
|
| ||
| Age | ||
| < 12 months | 1 | 2.4 |
| 12 –<18 months | 5 | 12.2 |
| ≥ 18 months | 35 | 85.4 |
|
| ||
| Race | ||
| Black | 10 | 24.4 |
| White | 24 | 58.5 |
| Other | 4 | 9.8 |
| Unknown | 3 | 7.3 |
|
| ||
| Sex | ||
| Female | 17 | 41.5 |
| Male | 24 | 58.5 |
|
| ||
| INSS Stage | ||
| Stage 2B | 1 | 2.4 |
| Stage 3 | 3 | 7.3 |
| Stage 4 | 37 | 90.2 |
|
| ||
| MYCN | ||
| Amplified | 10 | 24.4 |
| Not Amplified | 24 | 58.5 |
| Unknown | 7 | 17.1 |
|
| ||
| Ploidy | ||
| Diploid | 18 | 43.9 |
| Hyperdiploid | 20 | 48.8 |
| Unknown | 3 | 7.3 |
|
| ||
| Shimada Histology | ||
| Favorable | 4 | 9.8 |
| Unfavorable | 25 | 61.0 |
| Unknown | 12 | 29.3 |
|
| ||
| Number of Transplants | ||
| None | 8 | 19.5 |
| Only one | 7 | 17.1 |
| Two | 26 | 63.4 |
The stopping rule for serious viral illness was met with a case of EBV LPD, after the first 8 patients had received two CD34 selected HDC/SCRs, and the use of CD34 selected PBSC was discontinued on subsequent patients. In all, 13 patients received Isolex-processed PBSC, one received MACS-processed PBSC, and 27 were transplanted with unselected PBSC (Table 1). The median CD34+ cells/kg pre-processing was 10.15 × 106 at collection episode 1, and 9.19 × 106 at collection episode 2 (N=7). Data on post-processing cell counts were available for 10 patients with a median 5.1 × 106 CD34+ cells/kg.
A major feasibility endpoint of this protocol was the ability of patients to receive both cycles of HDC/SCR. Eighty percent (33 of 41) of patients received the first transplant, and 63% (26 of 41) received both transplants. Eight of the 41 patients (20%) did not undergo any transplant on study due to progressive disease (N=2), toxic death on study (N=1), transfer of care to another institution (N=1), decision to seek alternative therapy (N=2), more than 2% marrow disease prior to cycle 4 (N=1), and insurance issues (N=1). Of the 33 patients who received the first HDC/SCR, seven (21%) did not complete the second HDC/SCR due to progressive disease (N=1), decreased glomerular filtration rate resulting in ineligibility to proceed to HDC/SCR #2 (N=1), TRM (N=1), parental/physician choice (N=3), and back up stem cells used in first transplant (N=1). Twenty-four patients were reported to have received radiation therapy, although data are not available regarding compliance with protocol specifications.
Toxicity, Feasibility of Tandem HDC/SCR, and Immune Recovery
As expected, most patients experienced grade 3–4 hematologic toxicities and mucositis during both HDC/SCR cycles. In addition, documented neutropenic infections were reported in 24% of patients after HDC/SCR #1 and 35% after HDC/SCR (Table 2). Grade 3–4 hypokalemia was reported in nearly half of patients after receiving CEM. There were two TRMs out of a total of 59 HDC/SCR cycles (3.4%) with an overall transplant-related mortality for the study of 4.9%. One patient died of respiratory arrest associated with Gram-negative rod sepsis five days following the first transplant, while the second died of veno-occlusive disease (VOD)-associated multi-organ failure 14 days after the second transplant. With two TRMs out of 33 patients who got at least one HDC/SCR, the study did not meet the stopping rule for TRM. Patients in the CD34-selected cohort had a significantly higher rate of catheter-associated infections in the induction period (p=0.04, Fisher’s exact test), but in the post-HDC/SCR periods, there were no significant differences in toxicity between CD34-selected and unselected stem cell product cohorts.
Table 2.
Toxicities (Grade 3, 4, or 5)2 reported by high-risk neuroblastoma patients enrolled on COG study ANBL00P1 (n = 41)
| Induction Phase | CD34 selected PBSC (N = 14) | Unselected PBSC (N = 27) | Total (N=41) | ||||
|---|---|---|---|---|---|---|---|
| Count | Incidence (%) | Count | Incidence (%) | Count | Incidence (%) | ||
| Hepatotoxicity | Ascites (non-malignant) | 0 | 0.0 | 2 | 7.4 | 2 | 4.9 |
| AST elevation | 2 | 14.3 | 2 | 7.4 | 4 | 9.8 | |
| Documented infection | Catheter-related infection | 6 | 42.9 | 3 | 11.1 | 9 | 22.0 |
| Infection with grade 3 or 4 neutropenia | 8 | 57.1 | 14 | 51.9 | 22 | 53.7 | |
| Non-neutropenic infection | 3 | 21.4 | 5 | 18.5 | 8 | 19.5 | |
| Lung toxicity | Acute respiratory distress syndrome | 1 | 7.1 | 1 | 3.7 | 2 | 4.9 |
| Nephrotoxicity | Hypokalemia | 5 | 35.7 | 9 | 33.3 | 14 | 34.1 |
| HDC/SCR #1 | CD34 selected PBSC (N = 11) | Unselected PBSC (N = 22) | Total (N=33) | ||||
| Hepatotoxicity | ALT elevation | 1 | 9.1 | 1 | 4.5 | 2 | 6.1 |
| Documented infection | Catheter-related infection | 2 | 18.2 | 2 | 9.1 | 4 | 12.1 |
| Infection with grade 3 or 4 neutropenia | 4 | 36.4 | 4 | 18.2 | 8 | 24.2 | |
| Non-neutropenic infection | 1 | 9.1 | 4 | 18.2 | 5 | 15.2 | |
| Infection/febrile neutropenia – Other | 1 | 9.1 | 0 | 0.0 | 1 | 3.0 | |
| Nephrotoxicity | Syndrome of inappropriate antidiuretic hormone | 1 | 9.1 | 0 | 0.0 | 1 | 3.0 |
| Hypokalemia | 1 | 9.1 | 6 | 27.3 | 7 | 21.2 | |
| HDC/SCR #2 | CD34 selected PBSC (N = 8) | Unselected PBSC (N = 18) | Total (N=26) | ||||
| Hepatotoxicity | Weight gain - veno-occlusive disease (VOD) | 0 | 0.0 | 1 | 5.6 | 1 | 3.8 |
| Ascites | 0 | 0.0 | 1 | 5.6 | 1 | 3.8 | |
| Bilirubin | 0 | 0.0 | 3 | 16.7 | 3 | 11.5 | |
| Hepatic enlargement | 0 | 0.0 | 3 | 16.7 | 3 | 11.5 | |
| Hypoalbuminemia | 0 | 0.0 | 2 | 11.1 | 2 | 7.7 | |
| Portal vein flow | 0 | 0.0 | 1 | 5.6 | 1 | 3.8 | |
| AST elevation | 2 | 25.0 | 2 | 11.1 | 4 | 15.4 | |
| ALT elevation | 1 | 12.5 | 4 | 22.2 | 5 | 19.2 | |
| Hepatic – Other | 0 | 0.0 | 2 | 11.1 | 2 | 7.7 | |
| Documented infection | Catheter-related infection | 0 | 0.0 | 2 | 11.1 | 2 | 7.7 |
| Infection with grade 3 or 4 neutropenia | 2 | 25.0 | 7 | 38.9 | 9 | 34.6 | |
| Non-neutropenic infection | 1 | 12.5 | 2 | 11.1 | 3 | 11.5 | |
| Nephrotoxicity | Renal failure | 0 | 0.0 | 1 | 5.6 | 1 | 3.8 |
| Hypokalemia | 5 | 62.5 | 6 | 33.3 | 11 | 42.3 | |
Grade 5 toxicities included one patient who died of sepsis on day 14 following HDC/SCR #1 and one patient who died of complications of VOD on day +14 of HDC/SCR #2.
Risk of Relapse and Survival Analysis
At last patient contact, 21 of 41 (51%) patients in this study remain alive. Eighteen of 41 patients were alive and in remission at last contact. Thirteen of 41 patients relapsed, of which two were alive at last contact. An additional six patients had disease progression, with one patient alive at last contact. Causes of death included disease (N=16), TRM (N=2), acute respiratory distress syndrome (ARDS) (N=1) after cycle 2 of induction chemotherapy, and multi-organ failure (N=1) nearly one year after second transplant.
The median follow-up time for patients not experiencing an event was 3.2 years (range: 0.3–6.3 years). The overall 1-year EFS was 75.1±6.8%, significantly higher than the 30% cutoff for feasibility (p<0.0001). The 3-year EFS and OS for the entire cohort were 44.8±9.6% and 59.2±9.2%, respectively (Figure 2). Among the 33 patients who received at least one transplant, 14 were alive and relapse-free at last contact. In patients who received at least one transplant, EFS at 3 years from first transplant was 39.8±12.6%, and OS was 49.8±12.5%. Four of the eight patients who did not receive at least one transplant on protocol have died. For patients receiving two transplants, the 3-year EFS from the time of second transplant was 46.2±16.9%, and OS was 50.2±15.8%.
Figure 2. Event-free survival (EFS) and overall survival (OS) for 41 patients with high-risk neuroblastoma on COG study ANBL00P1.
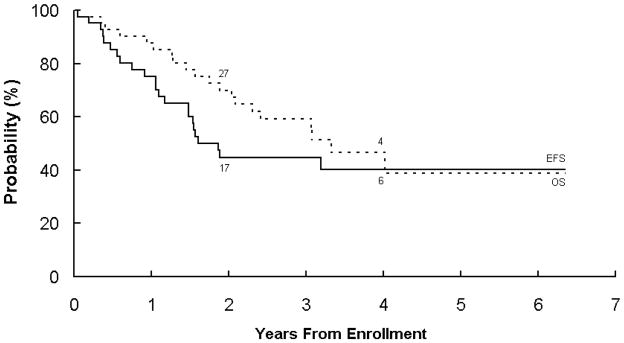
The number at risk for an event at the start of years 2 and 4 are given along the curves.
DISCUSSION
Despite recent advances in therapy, high-risk neuroblastoma remains a diagnosis with very poor prognosis. The current mainstay of therapy is a dose-intensive chemotherapy induction, followed by surgery (if not performed upfront), consolidation with HDC/SRC and local irradiation, and maintenance control of minimal residual disease with biologic therapies such as 13-cis-retinoic acid and immunotherapy directed against neuroblastoma surface antigens (ch14.18 plus adjuvant cytokines)(ref. 19). Past studies demonstrated improved efficacy with tandem transplantation over a single HDC/SCR consolidation cycle(ref. 6, 10, 11); however, the significant late effects of the TBI-containing regimens used(ref. 12, 20) prompted COG investigators to evaluate non-TBI-containing regimens in a limited-institution feasibility and safety trial. Retrospective analyses suggest no consistent differences in OS in TBI- and non-TBI-containing HDC/SCR regimens.(ref. 20, 21, 22) In addition to its feasibility and safety in a previous study [Pediatric Oncology Group (POG) 9640],(ref. 13) thiotepa has good central nervous system (CNS) penetration, which may be important given recent data suggesting an increased risk of CNS recurrence in some neuroblastoma patients.(ref. 23); however, the present study was neither powered nor designed to measure CNS-specific relapse rates, especially as head CTs were not in routine use for recurrence surveillance or staging at that time. The present study confirms the feasibility findings of Granger, et al., (ref. 13) and adds important data regarding the decreased safety of CD34 selection in this patient population; whereas, the POG trial used only unmanipulated peripheral stem cells. In addition, from a therapeutic development standpoint, while both studies demonstrated feasibility of tandem transplantation in children with high-risk neuroblastoma, the present manuscript demonstrates the feasibility of the exact HDC/SCR regimens subsequently used as the experimental arm of COG ANBL0532. The tandem regimen used in the POG study was not subsequently tested in this way.
Our study showed that tandem HDC/SCR with non-overlapping, non-TBI-containing, myeloablative regimens is feasible and tolerable in pediatric patients with high-risk neuroblastoma. Most patients (80%) went on to receive HDC/SCR, and 79% of patients who underwent a first HDC/SCR went on to receive a second transplant, comparable to previous trials.(ref. 10, 13) With the exception of one patient who required an additional stem cell rescue using the backup PBSCs following the first HDC/SCR, stem cell collection was not a limiting factor for proceeding to HDC/SCR, consistent with the feasibility of collecting stem cells from chemotherapy-treated patients in prior studies.(ref. 10, 24) Toxicities were as expected for the planned intensity of therapy, and TRM was below the predetermined safety threshold. Additional issues potentially impacting feasibility for individual patients included one patient requiring the stem cells banked for HDC/SCR #2 in order to engraft following the first cycle and a second patient experiencing toxicity preventing HDC/SCR #2 based on organ criteria. Expected grade 3–4 toxicities included hematopoietic suppression and mucositis, noted in a majority of patients in both the first and second HDC/SCR cycles and irrespective of CD34 selection. Neutropenic infection rates were consistent with previously reported studies.(ref. 3, 15, 22, 25) This study did not measure time to neutrophil engraftment; however, previous studies have demonstrated engraftment by day 11–12 without significant differences between CD34-selected versus unselected nor between HDC/SCR #1 versus #2.(ref. 10, 13) There was one death due to sepsis following the first transplant, and one patient out of 26 who received a second HDC/SCR died of VOD after the CEM cycle. The issue of VOD in the setting of a second myeloablative transplant procedure within 8 weeks of the first was a particular feasibility concern, but these results are not suggestive of increased risk of this complication.
CD34 selection was associated with a case of EBV LPD, triggering the stopping rule and necessitating subsequent HDC/SCR to be performed with unselected PBSC. While CD34 selection offers the potential advantage of purging circulating tumor cells by positively selecting CD34+ hematopoietic stem and progenitor cells(ref. 7), COG phase III data from the A3973 trial show no advantage in EFS or OS with a purged versus an unpurged PBSC product.(ref. 26),(ref. 27) EBV LPD has previously been reported by our group in patients receiving CD34 selected PBSC following HDC for neuroblastoma.(ref. 16) In addition, 21% of patients receiving CD34 selected autografts in a recent French series had varicella reactivation.(ref. 15) EBV LPD is an uncommon sequela of autologous transplant,(ref. 28) although it has been reported.(ref. 29, 30) This is generally a complication most often seen after allogeneic transplant, usually in the setting of some degree of T cell depletion. Data differ as to whether CD34 selection (and the accompanying T cell depletion) delays immune recovery after autologous PBSC transplant,(ref. 31, 32) but our experience in this trial and our prior tandem SCT experience(ref. 16) suggests that some combination of intensive therapy and a highly T cell depleted autologous graft can exceed a threshold of immunosuppression where significant viral infection/reactivation may occur. Given the potentially delayed immune recovery following CD34 selection, and with neuroblastoma-purging strategies not having demonstrated improvement in EFS,(ref. 26) unmodified autologous PBSC are the current COG standard.
In summary, the dose-intensification of a non-TBI-containing tandem transplant was shown to be feasible in a cooperative group trial with 3-year EFS and OS similar to previous trials.(ref. 27, 33) HDC/SCR has been shown to improve outcomes in high-risk neuroblastoma, and tandem transplant may add additional efficacy. Data from this phase II study were the foundation for the current COG ANBL0532 phase III study design. Using the transplant regimens outlined in this study, the ANBL0532 trial randomized patients to single HDC/SCR with CEM versus tandem transplant as in ANBL00P1 (the study presented here), in concert with a modified induction regimen and the option after completion of radiotherapy to enroll on ANBL0032, a phase III randomized trial of immunotherapy plus 13-cis-retinoic acid maintenance, which was amended to a single-arm study in April 2009 following interim analysis demonstrating improved efficacy on the combined therapy arm. Despite the proven efficacy of immunotherapy (ref. 34) and the promise of exciting new targeted agents, cytotoxic therapeutic intensity remains an important component of treatment for high-risk neuroblastoma. However, the long-term survival rates of less than 60% and the resultant risks and late effects of the current intensive multi-modality approach, underscore the urgent need for development of new strategies for this devastating disease.
Acknowledgments
Grant support: supported in part by the WW Smith Charitable Trust, and the Foerderer-Murray, Weinberg and Sanford Funds of the Children’s Hospital of Philadelphia (S.A. Grupp); the Robert A. Good/ASBMT New Investigator Award (A.E. Seif); NIH grants U10 CA98413 (COG SDC grant) and U10 CA98543 (COG Chair’s grant). The authors would also like to thank Danniel Gaidula, MFA, for assistance in preparing Figure 1.
Footnotes
Due to the high risk of infusional toxicity with myeloablative doses of etoposide, the etoposide phosphate (Etopophos) formulation was selected for this trial.
Disclosures: none
Conflicts of Interest
None.
References
- 1.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–20. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Cheung NV, Heller G. Chemotherapy dose intensity correlates strongly with response, median survival, and median progression-free survival in metastatic neuroblastoma. J Clin Oncol. 1991;9:1050–1058. doi: 10.1200/JCO.1991.9.6.1050. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341(16):1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 4.Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol. 2005;6(9):649–58. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 5.Kreissman SG, Rackoff W, Lee M, Breitfeld PP. High dose cyclophosphamide with carboplatin: a tolerable regimen suitable for dose intensification in children with solid tumors. J Pediatr Hematol Oncol. 1997;19:309–132. doi: 10.1097/00043426-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Kletzel M, Katzenstein HM, Haut PR, Yu AL, Morgan E, Reynolds M, et al. Treatment of high-risk neuroblastoma with triple-tandem high-dose therapy and stem-cell rescue: results of the Chicago Pilot II Study. J Clin Oncol. 2002;20:2284–2292. doi: 10.1200/JCO.2002.06.060. [DOI] [PubMed] [Google Scholar]
- 7.Donovan J, Temel J, Zuckerman A, Gribben J, Fang J, Pierson G, et al. CD34 selection as a stem cell purging strategy for neuroblastoma: preclinical and clinical studies. Med Pediatr Oncol. 2000;35(6):677–82. doi: 10.1002/1096-911x(20001201)35:6<677::aid-mpo42>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Kanold J, Berger M, Rapatel C, de Lumley L, Lutz P, Plantaz D, et al. CD34+ cell immunoselection from G-CSF-alone-primed peripheral blood in children with low body mass. Br J Haematol. 1995;91:431–433. doi: 10.1111/j.1365-2141.1995.tb05318.x. [DOI] [PubMed] [Google Scholar]
- 9.Kanold J, Yakouben K, Tchirkov A, Carret AS, Vannier JP, LeGall E, et al. Long-term results of CD34(+) cell transplantation in children with neuroblastoma. Med Pediatr Oncol. 2000;35(1):1–7. doi: 10.1002/1096-911x(200007)35:1<1::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Grupp SA, Stern JW, Bunin N, Nancarrow C, Ross AA, Mogul M, et al. Tandem high-dose therapy in rapid sequence for children with high-risk neuroblastoma. J Clin Oncol. 2000;18:2567–2575. doi: 10.1200/JCO.2000.18.13.2567. [DOI] [PubMed] [Google Scholar]
- 11.George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, Shamberger RC, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006;24(18):2891–6. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 12.Hobbie WL, Moshang T, Carlson CA, Goldmuntz E, Sacks N, Goldfarb SB, et al. Late effects in survivors of tandem peripheral blood stem cell transplant for high-risk neuroblastoma. Pediatr Blood Cancer. 2008;51(5):679–83. doi: 10.1002/pbc.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granger M, Grupp SA, Kletzel M, Kretschmar C, Naranjo A, London WB, et al. Feasibility of a tandem autologous peripheral blood stem cell transplant regimen for high risk neuroblastoma in a cooperative group setting: A Pediatric Oncology Group study: A Report from the Children’s Oncology Group. Pediatr Blood Cancer. 2012 doi: 10.1002/pbc.24207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–77. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 15.Marabelle A, Merlin E, Halle P, Paillard C, Berger M, Tchirkov A, et al. CD34+ immunoselection of autologous grafts for the treatment of high-risk neuroblastoma. Pediatr Blood Cancer. 2011;56(1):134–42. doi: 10.1002/pbc.22840. [DOI] [PubMed] [Google Scholar]
- 16.Powell JL, Bunin NJ, Callahan C, Aplenc R, Griffin G, Grupp SA. An unexpectedly high incidence of Epstein-Barr virus lymphoproliferative disease after CD34+ selected autologous peripheral blood stem cell transplant in neuroblastoma. Bone Marrow Transplant. 2004;33(6):651–7. doi: 10.1038/sj.bmt.1704402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–81. [Google Scholar]
- 18.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flandin I, Hartmann O, Michon J, Pinkerton R, Coze C, Stephan JL, et al. Impact of TBI on late effects in children treated by megatherapy for Stage IV neuroblastoma. A study of the French Society of Pediatric oncology. Int J Radiat Oncol Biol Phys. 2006;64(5):1424–31. doi: 10.1016/j.ijrobp.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Ladenstein R, Potschger U, Hartman O, Pearson AD, Klingebiel T, Castel V, et al. 28 years of high-dose therapy and SCT for neuroblastoma in Europe: lessons from more than 4000 procedures. Bone Marrow Transplant. 2008;41 (Suppl 2):S118–27. doi: 10.1038/bmt.2008.69. [DOI] [PubMed] [Google Scholar]
- 22.Qayed M, Chiang KY, Ricketts R, Alazraki A, Tahvildari A, Haight A, et al. Tandem stem cell rescue as consolidation therapy for high-risk neuroblastoma. Pediatr Blood Cancer. 2011 doi: 10.1002/pbc.23155. [DOI] [PubMed] [Google Scholar]
- 23.Kramer K, Kushner B, Heller G, Cheung NK. Neuroblastoma metastatic to the central nervous system. The Memorial Sloan-kettering Cancer Center Experience and A Literature Review. Cancer. 2001;91(8):1510–9. [PubMed] [Google Scholar]
- 24.Pradhan KR, Johnson CS, Vik TA, Sender LS, Kreissman SG. A novel intensive induction therapy for high-risk neuroblastoma utilizing sequential peripheral blood stem cell collection and infusion as hematopoietic support. Pediatr Blood Cancer. 2005 doi: 10.1002/pbc.20594. [DOI] [PubMed] [Google Scholar]
- 25.Zage PE, Kletzel M, Murray K, Marcus R, Castleberry R, Zhang Y, et al. Outcomes of the POG 9340/9341/9342 trials for children with high-risk neuroblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51(6):747–53. doi: 10.1002/pbc.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreissman SG, Villablanca JG, Seeger RC, Grupp SA, London WB, Maris JM, et al. A randomized phase III trial of myeloablative autologous peripheral blood stem cell (PBSC) transplant for high-risk neuroblastoma (HR-NB) employing immunomagnetic purged versus unpurged PBSC: A Children’s Oncology Group study. J Clin Oncol. 2008;26(suppl):abstr 10011. [Google Scholar]
- 27.Barrett D, Fish JD, Grupp SA. Autologous and allogeneic cellular therapies for high-risk pediatric solid tumors. Pediatr Clin North Am. 2010;57(1):47–66. doi: 10.1016/j.pcl.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross TG, Steinbuch M, DeFor T, Shapiro RS, McGlave P, Ramsay NK, et al. B cell lymphoproliferative disorders following hematopoietic stem cell transplantation: risk factors, treatment and outcome. Bone Marrow Transplant. 1999;23(3):251–8. doi: 10.1038/sj.bmt.1701554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peniket AJ, Perry AR, Williams CD, MacMillan A, Watts MJ, Isaacson PG, et al. A case of EBV-associated lymphoproliferative disease following high-dose therapy and CD34-purified autologous peripheral blood progenitor cell transplantation. Bone Marrow Transplant. 1998;22:307–309. doi: 10.1038/sj.bmt.1701335. [DOI] [PubMed] [Google Scholar]
- 30.Lones MA, Kirov I, Said JW, Shintaku IP, Neudorf S. Post-transplant lymphoproliferative disorder after autologous peripheral stem cell transplantation in a pediatric patient. Bone Marrow Transplant. 2000;26:1021–1024. doi: 10.1038/sj.bmt.1702593. [DOI] [PubMed] [Google Scholar]
- 31.Mackall CL, Fleisher TA, Brown MR, Magrath IT, Shad AT, Horowitz ME, et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood. 1994;84:2221–2228. [PubMed] [Google Scholar]
- 32.Mackall CL, Stein D, Fleisher TA, Brown MR, Hakim FT, Bare CV, et al. Prolonged CD4 depletion after sequential autologous peripheral blood progenitor cell infusions in children and young adults. Blood. 2000;96:754–762. [PubMed] [Google Scholar]
- 33.Fish JD, Grupp SA. Stem cell transplantation for neuroblastoma. Bone Marrow Transplant. 2008;41(2):159–65. doi: 10.1038/sj.bmt.1705929. [DOI] [PMC free article] [PubMed] [Google Scholar]