Abstract
Non-alcoholic steatohepatitis (NASH) is the most common etiology of chronic liver dysfunction in the United States and can progress to cirrhosis and liver failure. Inflammatory insult resulting from fatty infiltration of the liver is central to disease pathogenesis. Dendritic cells (DC) are antigen presenting cells with an emerging role in hepatic inflammation. We postulated that DC are important in the progression of NASH. We found that intrahepatic DC expand and mature in NASH liver and assume an activated immune-phenotype. However, rather than mitigating the severity of NASH, DC depletion markedly exacerbated intrahepatic fibro-inflammation. Our mechanistic studies support a regulatory role for DC in NASH by limiting sterile inflammation via their role in clearance of apoptotic cells and necrotic debris. We found that DC limit CD8+ T cell expansion and restrict Toll-like receptor expression and cytokine production in innate immune effector cells in NASH, including Kupffer cells, neutrophils, and inflammatory monocytes. Consistent with their regulatory role in NASH, during the recovery phase of disease, ablation of DC populations results in delayed resolution of intrahepatic inflammation and fibroplasia.
Conclusion
Our findings support a role for DC in modulating NASH. Targeting DC functional properties may hold promise for therapeutic intervention in NASH.
Keywords: Liver, Kupffer cells, Neutrophils, Monocytes, Fibrosis
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the hepatic consequence of the metabolic syndrome, which includes insulin resistance, hypertension, hyperlipidemia, and visceral adiposity. Obesity itself is an independent risk factor for NAFLD which is now recognized as the most common cause of liver dysfunction in the United States, representing 75% of all cases of chronic liver disease.1 Moreover, future projections estimate that 50% of all Americans will have elements characteristic of NAFLD by 2030.1 In most cases of NAFLD, liver steatosis is mild and reversible; however, 10–20% of cases progress to non-alcoholic steatohepatitis (NASH), characterized by intense intrahepatic inflammation, exacerbated steatosis, hepatocellular injury, and incipient fibrosis.2 Further, NASH can progress to cirrhosis, liver failure, and hepatocellular carcinoma. Between 2000 and 2010, the percentage of orthotopic liver transplants performed for NASH in the United States increased from 1.2% to 7.4%.3
The precise cellular and biochemical pathogeneses of NASH are incompletely understood. However, a “two-hit” hypothesis has been gaining experimental traction. In general terms, hepatic lipid accumulation, the “first hit,” is thought to induce oxidative stress and hepatocyte damage which subjects the liver to inflammatory cell infiltration - the “second hit” - leading to the cyclical development of further inflammatory injury and eventual fibrosis. A number of inflammatory mediators have been implicated. Kupffer cells (KC) reside in liver sinusoids and contribute to hepatocyte cell death via Toll-like receptor (TLR) 9-mediated production of IL-1β.4 TNF-α production by activated KC is essential for fibrosis development in NASH.5 Moreover, NASH is mitigated in mice fed a methionine-choline deficient diet (MCD) in the absence of KC.6 Neutrophils are also important mediators of hepatocellular damage in NASH. Neutrophils are activated by necrotic hepatocytes and perpetuate hepatitis through release of pro-inflammatory cytokines and secretion of myeloperoxidase (MPO), an abundant source of free radicals which contributes to disease progression by increasing oxidative hepatocyte damage.7 An increased liver neutrophil to lymphocyte ratio has been shown to increase the likelihood of progression of steatosis to steatohepatitis and ultimately fibrosis in patients with NASH.8
Dendritic cells (DC) are professional antigen presenting cells (APC) that initiate potent adaptive immune responses. DC have also recently emerged as important mediators in non-infectious chronic fibro-inflammatory conditions. For example, DC modulate the severity of inflammation during exacerbations of asthma and are necessary for bleomycin-mediated pulmonary fibrosis.9 Mucosal DC in the small and large intestine are thought to be responsible for triggering deleterious T cell responses to the endogenous microflora in inflammatory bowel disease.10 We recently showed that, despite their activated phenotype, DC can have a protective role in acute pancreatitis by limiting sterile inflammation.11 The role of DC in chronic liver disease is incompletely defined. We reported that DC become highly pro-inflammatory in thioacetamide-induced chronic liver fibrosis.12 However, the resolution of murine liver fibrosis was recently found to be accelerated by the recruitment of DC.13 In NASH liver, our initial investigations uncovered a robust recruitment of phenotypically activated DC early in disease. Based on these data, we postulated that DC augment the cycle of inflammation in NASH. However, our investigations utilizing continuous in vivo depletion of DC populations revealed a more complex relationship, as DC limit fibro-inflammation in NASH by curtailing the destructive effects of KC and neutrophils. Further, during the recovery phase of disease, DC depletion delays resolution of intrahepatic inflammation and fibroplasia. This work offers novel insight to the pathogenesis of and resolution of NASH and has potential implications for targeting DC in experimental therapeutics.
Materials and Methods
Animals and model of NASH
Six week old male C57BL/6 (H-2kb), BALB/c (H-2kd), OT-I (B6.Cg-RAG2tm1Fwa-TgN), OT-II (B6.Cg-RAG2tm1Alt-TgN), CD45.1 (B6.SJL-Ptprca/BoyAiTac) and CD11c-DTR (B6.FVB-Tg[Itgax-DTR/EGFP]57Lan/J) mice were purchased from Jackson (Bar Harbor, ME). NASH was induced by administration of a methionine-choline deficient diet (MP Biomedicals, Solon, OH) for 6 weeks. Bone marrow chimeric mice were generated as described11. Briefly, C57BL/6 mice were anesthetized and irradiated (1200 Rads), followed by i.v. transfer with 1×107 bone marrow cells from CD11c.DTR mice or C57BL/6 controls. Chimeric mice were used in experiments seven weeks later. DC depletion was achieved with serial i.p. injections of diphtheria toxin (4ng/g; Sigma, Saint Louis, MO) beginning one day prior to initiation of MCD diet. Serum ALT was measured using the Olympus AU400 Chemistry Analyzer. Control mice were aged-matched, made chimeric using bone marrow from WT mice, fed standard chow, and also received diphtheria toxin injections. In recovery experiments, mice were returned to standard chow and DC depletion was initiated at the time of reintroduction of a normal diet. In selected experiments, mice were treated with LPS (300µg, i.p.; Invivogen, Carlsbad, CA) and sacrificed at 12h. All procedures were approved by the New York University School of Medicine IACUC.
Cellular Isolation and Culture
Hepatic non-parenchymal cells (NPC) were collected as previously described.14 Briefly, the portal vein was cannulated and infused with 1% Collagenase IV (Sigma). The liver was then removed and minced. Hepatocytes were excluded with serial low speed (300 RPM) centrifugation followed by high speed (1500 RPM) centrifugation to isolate the NPC which were then further enriched over a 40% Optiprep (Sigma) gradient. For DC isolation, CD11c+MHCII+ hepatic NPC were selected by FACS. Splenocytes were isolated by mechanical disruption of the spleen and splenic T cells were purified using immunomagnetic beads and positive selection columns (Miltenyi Biotec, Bergisch-Gladbach, Germany). “NASH DC” is defined as liver DC harvested from mice at 6 weeks after initiation of an MCD diet. Cellular suspensions were cultured in complete media (RPMI 1640 with 10% heat-inactivated FBS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.05 mM 2-ME). In selected experiments, DC were stimulated with TLR9 ligand CpG ODN1826 (5uM; Invivogen).
Results
Hepatic DC populations expand in NASH
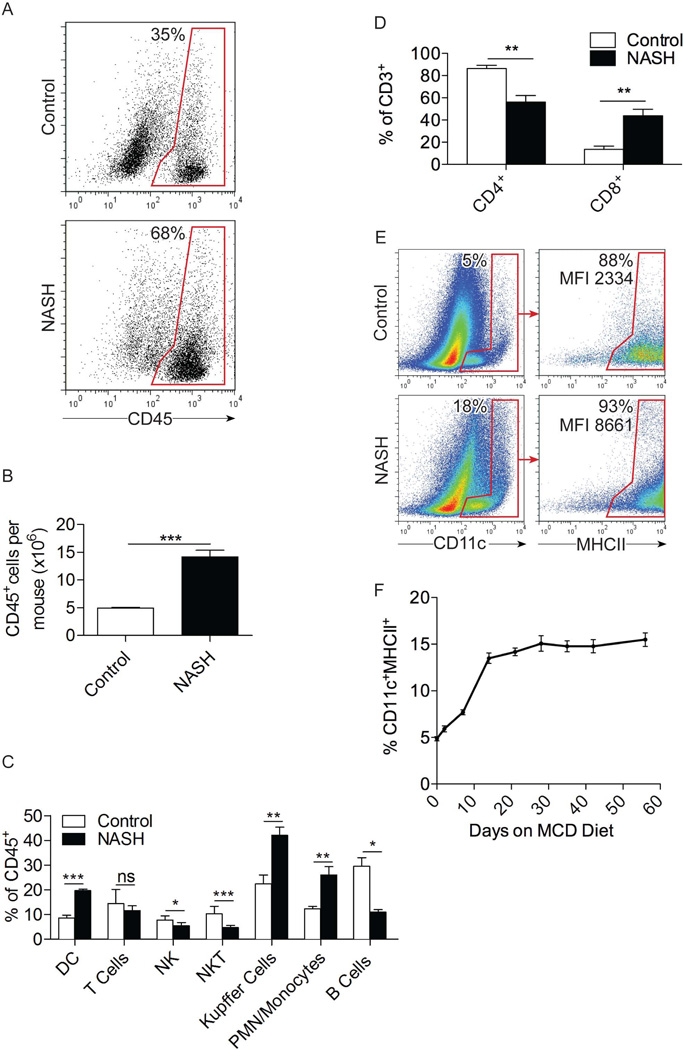
The number of CD45+ hepatic leukocytes increased by approximately 3-fold in NASH (Figure 1a, b). Further, the composition of hepatic NPC in NASH was markedly different from control liver (Figures 1c; S1a). F4/80+ Kupffer cells expanded from a baseline of 20–25% in control liver to 40–50% in NASH. Gr1+ neutrophils and inflammatory monocytes expanded from ~10% in controls to ~25% in NASH whereas both NKT cells and B cells decreased as a fraction of total NPC (Figure 1c). The fraction of hepatic CD3+ T cells remained fairly stable in NASH; however, we observed marked upward skewing of the CD8+:CD4+ ratio (Figure 1d). Moreover, CD11c+MHCII+ DC expanded from a baseline of ~5% of liver leukocytes in control liver to 15–18% in NASH (Figure 1c, e). Expansion of CD11c+MHCII+ DC began within days of initiating an MCD diet, plateaued by 2 weeks, and remained stably elevated for the duration of disease (Figure 1f). By contrast, there was no change in splenocyte composition, splenomegaly, or evident expansion of splenic DC in NASH, implying that the effects of NASH on DC are specific to the liver (Figure S1b, c).
Figure 1. DC expand in NASH liver.
(a) The fraction and (b) total number of CD45+ leukocytes in control and NASH liver (c) as well as the fraction of specific hepatic leukocyte subsets were determined by flow cytometry at 6 weeks after beginning an MCD diet in C57BL/6 mice. (d) The fraction of CD4+ and CD8+ T cells in control and NASH liver was determined by flow cytometry. (e) Co-expression of the DC markers CD11c and MHCII in control and NASH liver at 6 weeks and (f) the time course of hepatic DC recruitment in mice fed an MCD diet were determined by flow cytometry. Experiments were repeated more than 5 times with similar results using 3–5 mice per data point (*p<0.05, **p<0.01, ***p<0.001).
DC exhibit a mature phenotype in NASH liver
Besides expanding in number, hepatic DC underwent phenotypic maturation in NASH. MHCII and CD40, both essential for antigen presentation, were upregulated on NASH DC, as was expression of co-stimulatory molecules CD54, CD80, and CD86 (Figures 1e, S2a). CD1d, necessary for DC induction of NKT cells, was expressed at lower levels on NASH DC (Figure S2a), which correlates with the observed diminution in the fraction of NKT cells in NASH liver (Figure 1c). The increased maturation of NASH DC compared with controls was also evident after 24h of in vitro culture (Figure S2b). Besides phenotypic maturation, the fractional subsets of liver DC were markedly altered in NASH. The B220+ plasmacytoid DC population was decreased in NASH. Conversely, the CD11b+CD8− myeloid DC population expanded by approximately 20–30%, while the fraction of CD11b−CD8a+ lymphoid DC decreased proportionately (Figure S2c). In contrast to liver DC, spleen DC phenotype was unaltered in NASH (Figure S2d).
NASH DC produce elevated immune-modulatory cytokines
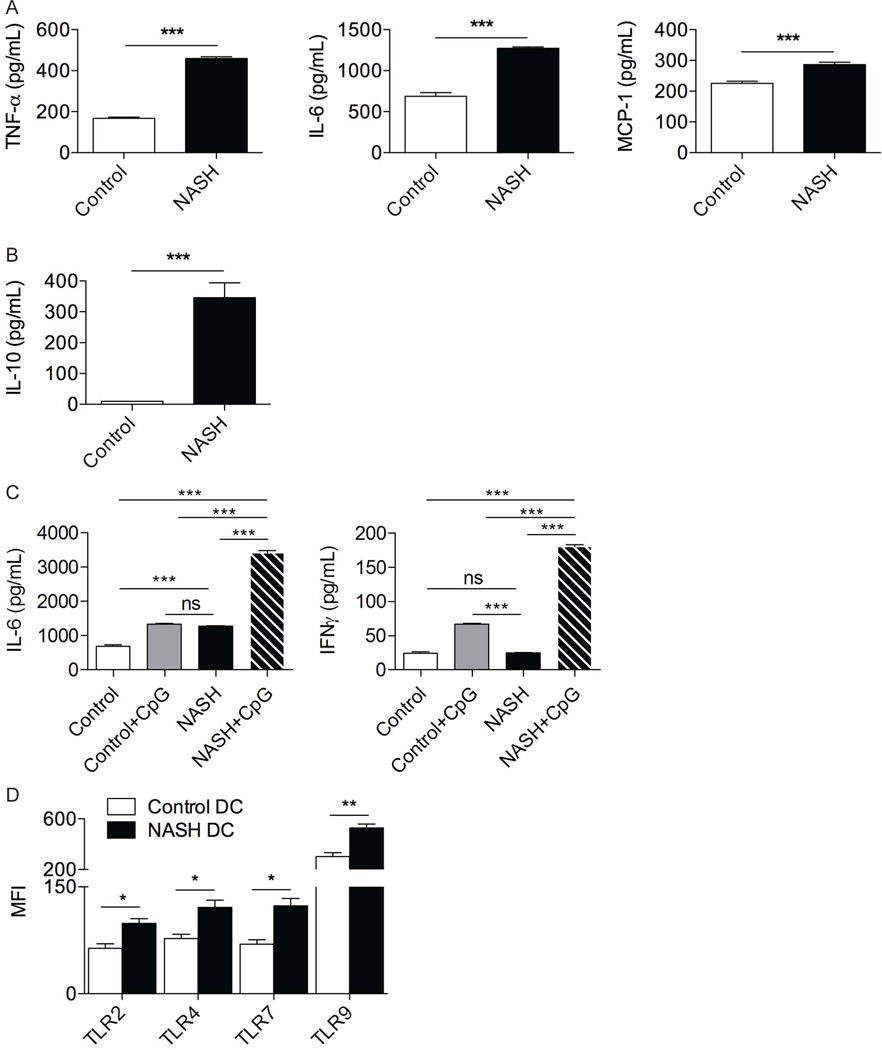
Since secreted cytokines are critical in NASH pathogenesis and DC can regulate inflammation via production of soluble inflammatory mediators, we tested cytokine production from DC isolated from NASH liver. NASH DC produced increased levels of TNFα, IL-6, MCP-1 and IL-10 compared with normal liver DC (Figure 2a, b). NASH DC also exhibited increased cytokine responses to TLR9 ligation (Figure 2c). Consistent with these observations, hepatic DC increased their expression of TLRs in NASH (Figure 2d).
Figure 2. NASH DC are pro-inflammatory.
DC derived from control and NASH liver in C57BL/6 mice at 6 weeks after beginning an MCD diet were tested for production of (a) TNF-α, IL-6, MCP-1, and (b) IL-10 in cell culture supernatant. (c) DC production of IL-6 and IFN-γ in cell culture supernatant were analyzed after stimulation with TLR9 ligand CpG ODN1826. (d) DC from NASH and control liver were analyzed for surface expression of TLR2 and TLR4 and intracellular expression of TLR7 and TLR9. MFIs are shown for each respective TLR. Experiments were repeated at least 3 times with similar results (*p<0.05, **p<0.01, ***p<0.001).
NASH DC differentially activate CD4+ T cells
Liver DC have the capacity to induce either immunogenic responses or tolerance depending on physiologic circumstance.15 NASH liver DC exhibited an increased ability to induce allogeneic T cell stimulation (Figure S3a). Similarly, liver DC capacity to induce antigen-restricted CD4+ T cell proliferation (Figure S3b) as well as CD4+ T cell production of Th1, Th2, and Th17 cytokines was increased in NASH (Figure S3c). NASH DC also downregulated expression of the CD25+FoxP3+ Treg phenotype in DC-T cell co-culture experiments to a greater extent than control DC (Figure S3d). However, DC activation of antigen-restricted CD8+ T cells was unchanged in NASH. In particular, peptide-pulsed control and NASH DC induced comparable antigen-restricted CD8+ T cell proliferation (Figure S3e) and cytokine production (Figure S3f). Similarly, the antigen-specific lytic capacity of hepatic CD8+ T cells against Ova-expressing targets was equivalent after in vivo adoptive transfer immunization using Ova-pulsed control or NASH DC (Figure S3g). Taken together, these data suggest that, in NASH, hepatic DC gain enhanced capacity to activate CD4+ T cells but not CD8+ T cells.
DC modulate hepatitis and fibrosis in NASH
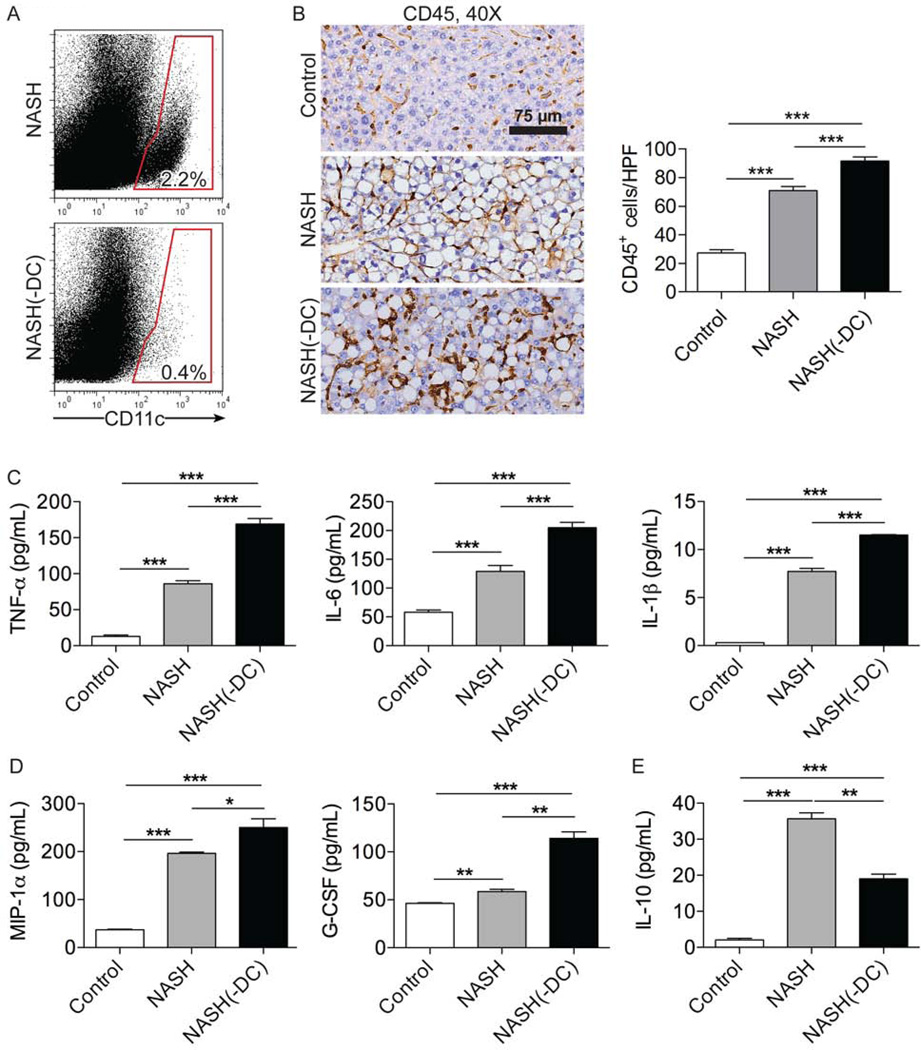
Since DC expand, mature, and gain enhanced capacity to produce inflammatory mediators in NASH, we postulated that DC may contribute to exacerbation of disease. To test this, we employed bone marrow chimeric CD11c.DTR mice in which continuous DC depletion could be accomplished (Figures 3a, S4). Control mice were made chimeric using bone marrow from WT mice. Surprisingly, ablation of DC populations - rather than mitigating hepatic insult - worsened disease. In particular, NASH(-DC) mice experienced more precipitous weight loss compared with NASH mice with intact DC populations (Figure S5a). Further, DC depletion in NASH resulted in a larger intrahepatic inflammatory cell infiltrate compared with controls (Figure 3b). In addition, analysis of cytokines produced by liver NPC revealed that DC depletion resulted in increased NPC production of numerous cytokines linked to hepatic injury in NASH, including TNF-α, IL-6, and IL-1β (Figure 3c), as well as chemokines critical for hepatic leukocyte recruitment, including MIP-1α and G-CSF (Figure 3d). Conversely, IL-10, a regulatory cytokine, had decreased expression in NASH liver in the context of DC depletion (Figure 3e). ALT levels were similarly elevated in NASH and NASH(-DC) liver (Figure S5b). DC depletion did not alter hepatic NPC composition (Figure S6a–e) or production of inflammatory mediators (Figure S6f) in mice on control diet. DC depletion similarly had no effect on NPC composition in LPS treated mice on a normal diet (Figure S7).
Figure 3. DC depletion exacerbates inflammation in NASH liver.
(a) Splenocytes from NASH and NASH(-DC) mice at 6 weeks after beginning an MCD diet were tested for expression of CD11c. (b) Paraffin-embedded hepatic sections were stained using an mAb against CD45. The number of CD45+ cells per HPF was calculated. NPC production of (c) TNF-α, IL-6, IL-1β, (d) MIP-1α, G-CSF, and (e) IL-10 in cell culture supernatant was determined in control, NASH, and NASH(-DC) liver. Experiments were repeated at least 3 times using 3–5 mice per group (*p<0.05, **p<0.01, ***p<0.001).
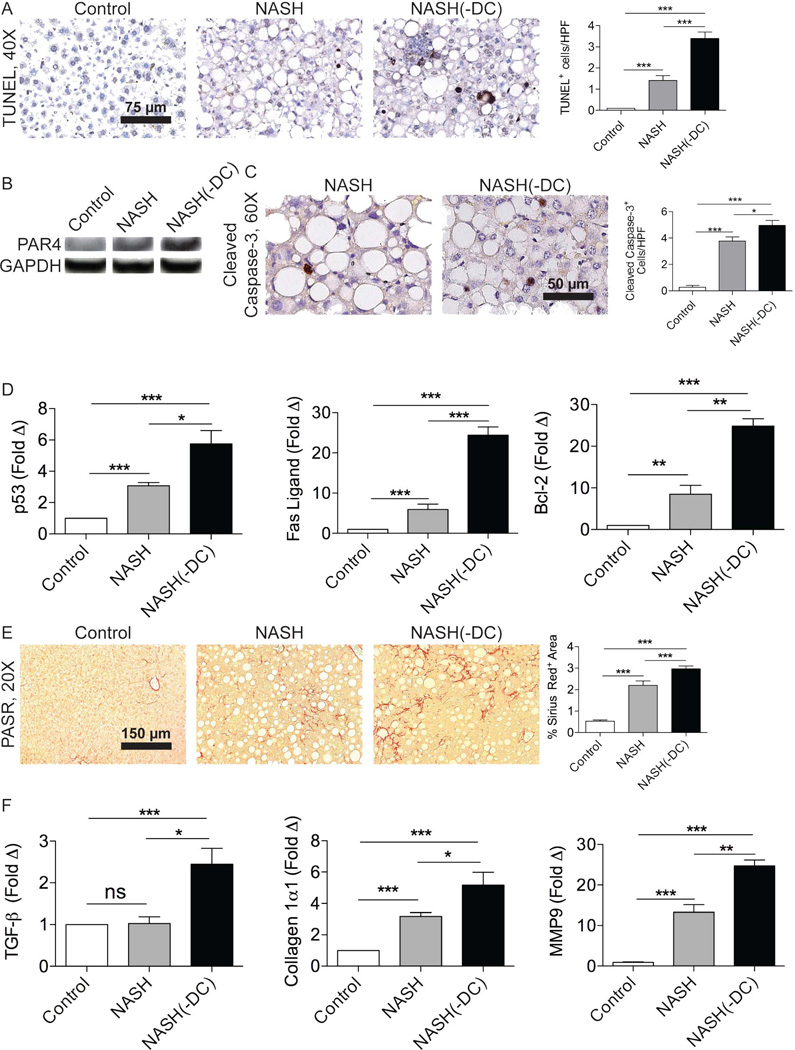
Intrahepatic inflammation has a reciprocal pathogenic relationship with cellular apoptosis in NASH liver.16 Consistent with elevated intrahepatic inflammation, NASH(-DC) liver exhibited the increased presence of apoptotic bodies (Figure 4a). Accordingly, expression of PAR4, a marker of apoptosis, was increased in NASH liver in the context of DC depletion (Figure 4b). Cleaved caspase-3 was also more prevalent in NASH(-DC) liver compared with controls (Figure 4c). Further, p53, Fas ligand, and Bcl-2, well-described mediators of apoptosis in NASH,17–18 exhibited markedly elevated expression in NASH(-DC) liver (Figure 4d).
Figure 4. DC depletion exacerbates apoptosis and fibrosis in NASH liver.
(a) TUNEL staining was performed in control, NASH, and NASH(-DC) liver from mice sacrificed 6 weeks after beginning an MCD diet. The number of apoptotic bodies per HPF was quantified. (b) Lysates from control, NASH, and NASH (-DC) liver were probed for expression of PAR4. (c) Cleaved Caspase-3 staining was performed on paraffin sections from control, NASH, NASH(-DC) liver and results quantified. (d) mRNA from each group was tested for p53, Fas ligand, and Bcl-2 by PCR. (e) Picric Acid Sirius Red (PASR) staining was performed in control, NASH, and NASH (-DC) liver. Fibrosis was quantified by examining 10 HPFs per liver. (f) TGF-β, Collagen Iα1, and MMP9 expression were determined by PCR. Experiments were repeated at least 3 times with similar results (*p<0.05, **p<0.01, ***p<0.001).
Besides augmented intrahepatic inflammation and an increased prevalence of apoptosis, NASH(-DC) mice exhibited accelerated hepatic fibrosis (Figure 4e). Accordingly, TGF-β and Collagen Iα1 (Figure 4f), and TIMP-1 (not shown) were more highly expressed in NASH(-DC) liver compared with controls. MMP9, which is associated with extracellular matrix remodeling, was similarly increased in NASH(-DC) liver (Figure 4f). Taken together, these data imply that the absence of DC in NASH leads to exacerbated intrahepatic fibro-inflammation.
Ablation of DC populations augments effector cell expansion and activation in NASH
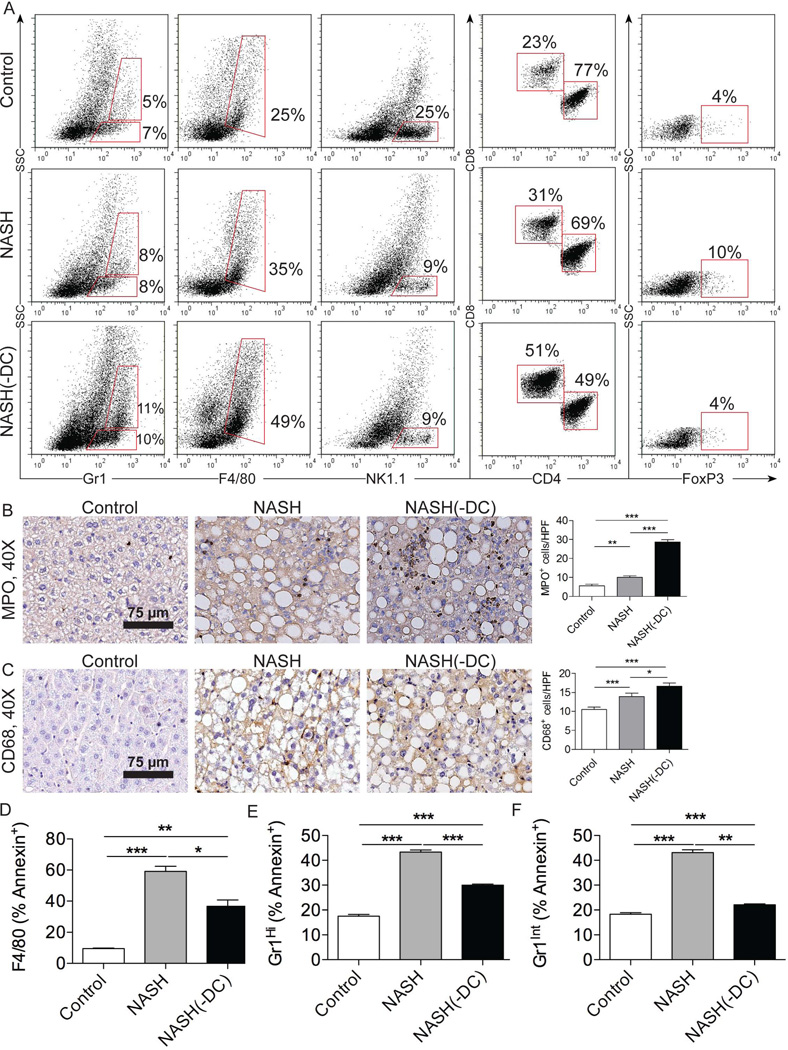
To better understand the mechanism for exacerbated hepatitis in NASH(-DC) liver, we investigated whether ablation of DC populations was associated with a compensatory expansion or activation of specific effector cell subsets linked to disease pathogenesis. We found that there was a large fractional increase in neutrophils, inflammatory monocytes, and KC upon DC depletion in NASH (Figure 5a). Immunohistochemical staining confirmed an increase in total number of neutrophils (Figure 5b) and KC (Figure 5c) in NASH(-DC) liver. Conversely, the fractional decrease in NK1.1+ cells in NASH was unchanged upon DC depletion (Figure 5a). CD8+ T cells have also been implicated in intrahepatic inflammation, whereas the expansion of FoxP3+ Tregs has been associated with mitigation of hepatic injury.19–20 We found that DC depletion resulted in markedly greater skewing of the intrahepatic CD8:CD4 ratio and diminished accumulation of Tregs in NASH (Figure 5a). Similar observations were made when examining the total numbers of leukocyte subsets in NASH(-DC) compared with NASH liver (Figure S8). Taken together, these data imply that DC may limit hepatic injury in NASH by regulating expansion of innate and adaptive immune cellular subsets. Consistent with these observations, we further found that there was a decrease in Annexin V+ apoptotic KC, neutrophils, and monocytes in NASH(-DC) liver (Figure 5d–f), suggesting that DC may limit effector cell expansion in NASH by inducing apoptosis of innate effector cells, as we have previously described in acute liver injury.21 DC depletion in CD11c.DTR chimeric mice did not appreciably alter splenocyte composition in NASH or in inflammation induced by LPS suggesting the effects are specific to the role of DC in NASH liver (Figure S9a, b).
Figure 5. DC depletion increases Kupffer cell and granulocyte proliferation in NASH liver.
(a) Gr1, F4/80, and NK1.1 expression in bulk liver NPC, co-expression of CD4 and CD8 on hepatic CD3+ T cells, and expression of FoxP3 on CD4+CD25+ T cells were analyzed in control, NASH, and NASH(-DC) liver from mice sacrificed 6 weeks after initiating an MCD diet. (b) MPO and (c) CD68 IHC were performed on paraffin-embedded liver sections. The number of positive cells per HPF was quantified. (d–f) The fraction of Annexin V+ apoptotic (d) Kupffer cells, (e) neutrophils and (f) inflammatory monocytes in control, NASH, and NASH (-DC) liver were determined by flow cytometry. Experiments were repeated 3 times with similar results (*p<0.05, **p<0.01, ***p<0.001).
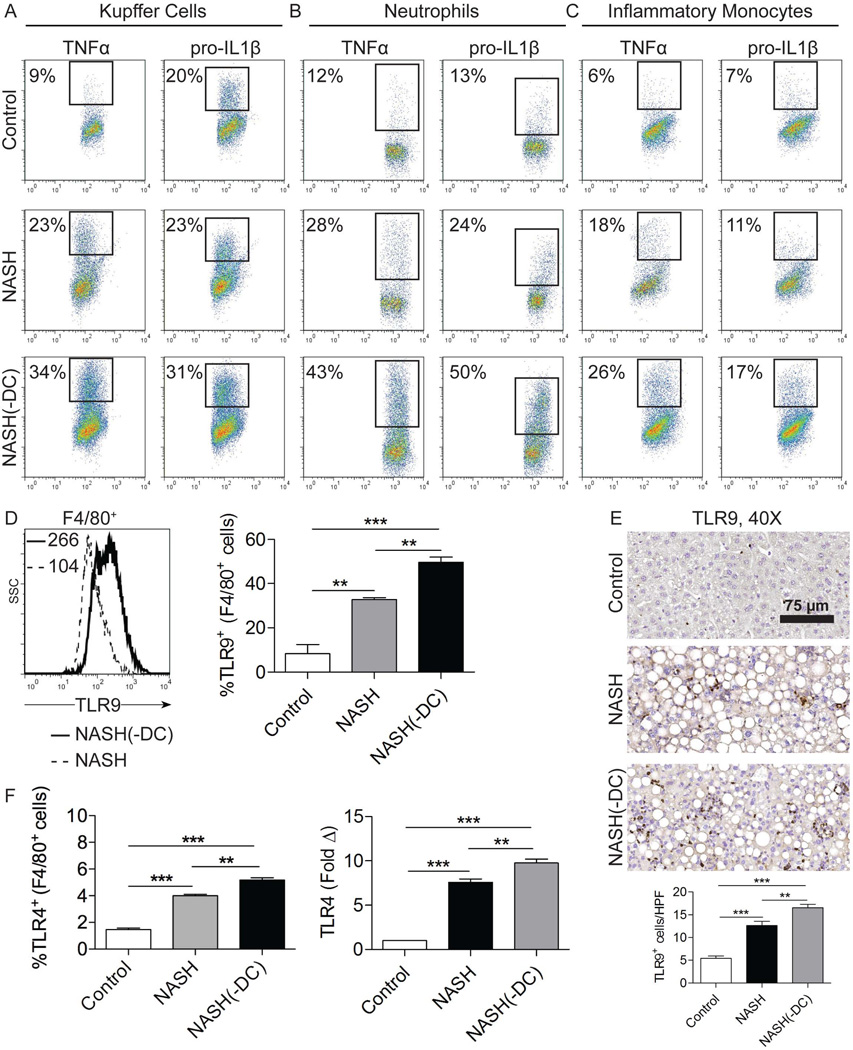
To investigate whether DC regulate effector cell activation - in addition to expansion - in NASH, we harvested KC, neutrophils, and inflammatory monocytes from NASH(-DC) mice and controls and measured their expression of intracellular cytokines implicated in disease pathogenesis.4–5 We found that the absence of DC resulted in markedly higher production of TNF-α and IL-1β by KC, neutrophils, and inflammatory monocytes in NASH liver (Figure 6a–c). IL-6 was also upregulated by these cellular subsets in NASH(-DC) liver (not shown). Further, since the pathogenesis and severity of NASH has been linked to TLR4 and TLR9 activation of KC,23–24 we tested whether ablation of DC populations results in upregulation of KC expression of TLRs. We found that KC from NASH(-DC) liver exhibited markedly elevated TLR9 expression (Figure 6d). Immunohistochemical staining confirmed increased TLR9 expression in NASH(-DC) liver (Figure 6e). TLR4 was similarly upregulated on KC and liver tissues in NASH(-DC) mice (Figure 6f). Taken together, these data imply that DC depletion results in activation of innate immune cells in NASH.
Figure 6. DC depletion increases activation of Kupffer cells, neutrophils, and inflammatory monocytes in NASH liver.
(a–c) Intracellular expression of TNF-α and pro-IL-1β in freshly isolated (a) F4/80+ Kupffer cells, (b) Gr1HiCD11b+ neutrophils, and (c) Gr1IntCD11b+ inflammatory monocytes from control, NASH, and NASH(-DC) liver is shown. (d) Expression of TLR9 in Kupffer cells was determined by flow cytometry. MFI is indicated and the fraction TLR9+ Kupffer cells was quantified for each group. (e) Paraffin-embedded hepatic sections were stained using an mAb directed against TLR9. The number of positive cells per HPF was quantified. (f) F4/80+ Kupffer cell expression of TLR4 on was determined by flow cytometry and whole liver tissue expression level of TLR4 was determined by PCR in control, NASH, and NASH(-DC) groups. In all experiments, mice were sacrificed 6 weeks after initiating an MCD diet. Experiments were repeated at least three times with similar results (**p<0.01, ***p<0.001).
DC limit sterile inflammation in NASH
Since DC have recently been implicated in the clearance of dead cells in other contexts11, 22, and a pathogenic role for sterile inflammation is emerging in NASH23–25, we postulated that - in the absence of DC - delayed clearance of apoptotic cells and necrotic debris results in augmentation of sterile inflammation within the liver, precipitating effector cell proliferation and activation. Augmented sterile inflammation in the hepatic microenvironment is supported by our observation of increased apoptotic bodies and mediators of apoptosis in NASH(-DC) liver (Figure 4a–d). Additionally, levels of HMGB1, a marker of sterile inflammation, were elevated in NASH(-DC) liver compared with controls (Figure S10a). We also found that - compared with other hepatic APC - liver DC express high levels of CLEC9A (Figure S10b), a type II membrane protein with an extracellular C-type lectin domain, which is essential for DC recognition and clearance of necrotic cells.26,27 To directly test whether hepatic DC are vital to the clearance of necrotic debris in NASH liver, we compared in vivo uptake of exogenously administered 7AAD+ necrotic cells by CD11c+MHCII+ liver DC compared with other MHCII+ APC subsets. We found that DC achieved greater capture of necrotic elements in vivo (Figure S10c). Consistent with these observations, DC from NASH liver also captured necrotic debris in vitro at a higher rate than other APC subsets (Figure S10d). Further, in NASH, DC acquired greater capacity for necrotic cellular clearance compared to DC from control liver (Figure S10e). We also tested DC capacity to clear apoptotic bodies in NASH. We found that NASH DC captured Annexin V+ apoptotic cells in vivo at higher rates compared to other MHCII+ APC subsets (Figure S10f). Further, NASH DC captured apoptotic bodies at modestly higher rates than DC from control liver (Figure S10f). Taken together, these data suggest that DC may limit sterile inflammation in NASH via their clearance of necrotic cellular debris and apoptotic bodies whereas absence of DC leaves the diseased liver with APC less equipped for this task. Notably, uptake of apoptotic or necrotic cellular debris by KC was unchanged in NASH(-DC) liver compared with NASH (Figure S10g).
DC depletion delays recovery from NASH
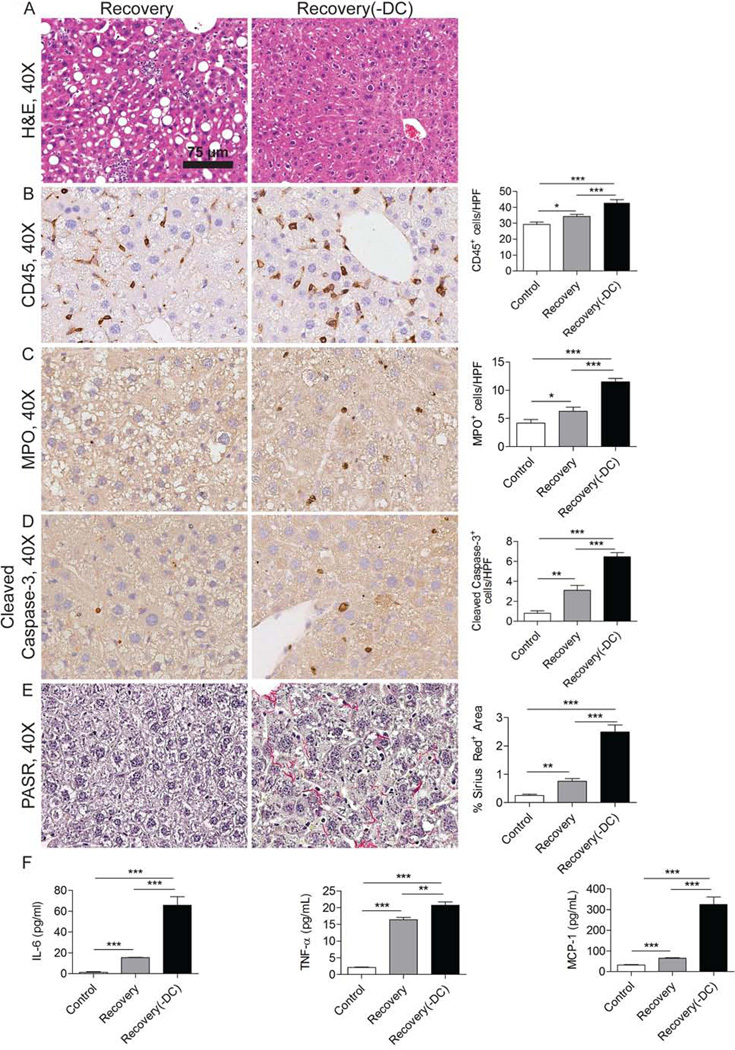
NASH is reversible in its early stages. However, the role of DC in the recovery phase of disease is unknown. To investigate this, WT chimeric or CD11c-DTR chimeric mice fed an MCD diet for 6 weeks were abruptly transitioned to normal chow. In selected cohorts, DC were depleted beginning at the time of cessation of the MCD diet. Consistent with our data implicating a protective role for DC in NASH, DC depletion delayed the resolution of NASH (Figure 7a). In particular, absence of DC on day 3 of normal diet resumption markedly delayed clearance of the intrahepatic CD45+ leukocytic infiltrate (Figure 7b), neutrophilic infiltrate (Figure 7c), and apoptotic bodies (Figure 7d). Residual fibroplasia was also conspicuously more pronounced in mice depleted of DC during the recovery period (Figure 7e). Further, DC depletion delayed resolution of the elevated cytokine and chemokine secretion by NASH NPC (Figure 7f). Similar differences between control and DC depleted mice were seen on day 7 of NASH recovery. However, by 14 days there was complete resolution of NASH even in mice depleted of DC (not shown). Taken together, these data imply that DC facilitate the recovery from NASH.
Figure 7. DC depletion delays resolution of fibro-inflammation during recovery from NASH.
(a) Representative H&E staining is shown in livers of mice sacrificed three days after of cessation of an MCD diet (Recovery) and in mice where DC were depleted coincident with MCD diet cessation (Recovery(-DC)). (b) CD45, (c) MPO, and (d) Cleaved Caspase-3 expression were tested by IHC in Recovery and Recovery(-DC) liver. (e) Picric Acid Sirius Red (PASR) staining was performed in Recovery, and Recovery(-DC) liver. Data were quantified by examining 10 HPFs per mouse. (f) NPC production of IL-6, TNF-α, and MCP-1 in cell culture supernatant were measured in each group (n=3–5 mice/group; *p<0.05, ** p<0.01, ***p<0.001).
Discussion
This is the first investigation to report a significant role for hepatic DC in NASH. We demonstrated that DC are recruited to the liver soon after MCD diet initiation, plateau at 3–4 times normal levels by two weeks, and remain at an elevated level unless there is disease resolution. NASH DC exhibit an activated surface phenotype and increase their production of both pro-inflammatory cytokines. Consistent with their mature phenotype, our in vitro experimentation shows that NASH DC potently induce proliferation of both allogeneic T cells and antigen restricted CD4+ T cells while reducing CD4+ T cell expression of the CD25+FoxP3+ Treg phenotype. The finding of intrahepatic DC activation after hepatic insult is consistent with our previous reports showing immunogenic transformation of liver DC in thioacetamide-induced liver fibrosis and acute hepatic injury induced by acetaminophen overdose.12, 21 However, despite their phenotypic and functional activation, ablation of DC in NASH results increased hepatic inflammation, diminished numbers of Tregs, expansion of CD8+ T cells, enhanced viability and production of pro-inflammatory cytokines by immune effector cells, increased hepatocyte apoptosis, and, ultimately, accelerated liver fibrosis. These ostensibly paradoxical findings are not entirely unprecedented. Recent studies have shown that, despite adopting a pro-inflammatory phenotype, hepatic DC can accelerate the regression of hepatic fibrosis and ameliorate hepatic ischemia-reperfusion injury.13, 28 For example, exogenous expansion of hepatic DC populations via Flt3 ligand administration accelerates regression of CCl4-induced liver fibrosis despite phenotypic activation of DC.13
Our findings show that effects of DC contrast sharply with role of KC, whose expansion have been strongly linked to worsening intrahepatic fibro-inflammation in NASH.5 Our investigations suggest multiple parallel mechanisms by which DC may regulate hepatitis. Importantly, we found that DC in NASH liver are differentially capable of activating CD4+ T cells in comparison to CD8+ T cells. Further, upon DC depletion, the CD8:CD4 T cell ratio is skewed markedly upwards with associated diminution of Tregs. The protective role of Tregs in chronic liver disease is well established.29–30 Further, relative suppression of CD8+ T cell expansion may be protective, as CD8+ T cells have recently been shown to drive adipose tissue inflammation and have an emerging role in NASH pathogenesis.31–32
Additionally, the exacerbated hepatic insult associated with ablation of DC populations may be mechanistically related to the DC’s role in limiting sterile inflammation through clearance of apoptotic bodies and necrotic debris. Sterile inflammation in the liver increases recruitment, viability, and activation of innate immune cells.33 We show that liver DC express high CLEC9A, which recognizes and binds death signals on necrotic cells and is primary in DC capacity to clear necrotic products.26,27 Accordingly, we found that NASH liver DC have remarkable capacity to capture necrotic cellular debris and apoptotic targets when compared to other hepatic APC subsets and DC from control liver. Further, we found that DC depletion leads to an accentuation of sterile inflammation within the liver, as NASH(-DC) liver contains modestly higher HMGB1, elevated markers of apoptosis including p53, which has been demonstrated to play a pivotal role as a mediator of apoptosis in experimental NASH.17 This also results in augmented production of pro-inflammatory cytokines - including IL-1β, TNF-α, and IL-6 - and enhanced viability and expression of TLR4 and TLR9 in innate effector cells. Miura et al. demonstrated that signaling through TLR9 leads to progression of NASH via KC production of IL-1β.4 TLR4 signaling in KC has also been linked to the severity of steatohepatitis.6
DC production of IL-10 may also have an important role in limiting hepatic damage in NASH. Bamboat et al. recently showed that DNA released from apoptotic hepatocytes stimulates liver DC to secrete IL-10 in a TLR9 dependent manner.28 Further, IL-10 derived from hepatic DC can ameliorate liver injury through suppression of inflammatory monocyte function.28 Additional studies in contexts such as allergen-induced asthma and cisplatin-induced nephrotoxicity have shown that DC attenuate sterile inflammation via release of IL-10.34–35 We found that NASH DC exhibited markedly elevated IL-10 production compared to normal liver DC. Moreover, IL-10 production by NASH NPC was reduced by 40–60% in the absence of DC, suggesting that DC production of IL-10 may have an important regulatory role in NASH.
Interestingly, despite showing varied evidence of increased inflammation, fibrosis, hepatocyte apoptosis, and delayed recovery from NASH upon DC depletion, we did not find significant elevations in serum ALT in NASH(-DC) compared with NASH mice with intact DC populations. However, this is consistent with previous reports showing that the severity of NASH may not correlate with serum ALT levels.36–37 Further, clinical severe NASH can exist without overt elevations in serum ALT.38 These studies suggest that ALT alone cannot be used as a ‘hard endpoint’ in NASH.
Numerous studies have used the CD11c.DTR model to investigate the role of DC in diverse inflammatory conditions within the liver including ischemia-reperfusion injury and acute acetaminophen hepatotoxicity.21, 28 Similarly, the CD11c.DTR model has been useful in determining the role of DC in many extrahepatic diseases including allergic asthma, acute lung injury, pancreatitis, and renal ischemia-reperfusion injury.11, 28, 39–41 However, a sobering report by Tittel et al. recently showed that DC depletion in CD11c.DTR mice is associated with an early non-specific neutrophilia in multiple organs including a modest neutrophilia within the liver, implying that conclusions drawn using the CD11c.DTR model may be confounded by non-specific effects.42 The mechanism for the reported neutrophilia in CD11c.DTR mice depleted of DC remains uncertain. However, we did not observe unintended changes in leukocyte composition in bone marrow chimeric CD11c.DTR mice upon DC depletion that were independent of NASH. Possible explanations for our disparate results may be that the bone marrow chimeric CD11c.DTR model is not affected by the neutrophilia associated with the endogenous model. Endogenous CD11c.DTR mice are distinct from the chimeric model in that repeated administration of diphtheria toxin is lethal. Further, chronic DC depletion as in NASH may not cause the neutrophilia associated with acute single dose depletion. Nevertheless, the CD11c.DTR model, whilst it is the best available tool to study the role of DC in vivo in mice, is not the perfect model as the effects of DC depletion may not necessarily faithfully mimic the role of DC in situ. Thus, additional insight on the role of DC in NASH and other inflammatory diseases may be forthcoming pending the advent of additional experimental tools to study DC effects in vivo.
In summary, our data suggest that DC have complex influences on both the pathogenesis and resolution of steatohepatitis which may have implications to human disease. A limitation of our study, however, is that there is no perfect murine model of NASH mimicking human disease. Additionally, direct comparison of the current data even to other murine studies of NASH employing a MCD diet may confounded by alternate durations of treatment between studies. That is, since the development of NASH as well as its resolution is a dynamic process, examining the intrahepatic phenotype and immune milieu after varied durations of feeding mice an MCD diet may yield inconsistent findings. Further, interrupting of the pathogenesis of NASH by targeting DC in experimental therapeutics may prove challenging given the technical limitations in modulating human DC function in vivo. Thus, additional investigations are needed to evaluate the clinical utility of these findings in treating patients with NASH or preventing disease onset.
Supplementary Material
Acknowledgments
Grant Support: This work was supported in-part by a Liver Scholar Award from the American Liver Foundation (GM) and National Institute of Health Awards 1UL1RR029893 (JRH), DK085278 (GM), and CA155649 (GM).
List of abbreviations
- ALT
Alanine aminotransferase
- APC
Antigen presenting cell
- DC
Dendritic cells
- KC
Kupffer cell
- MCD
Methionine-choline deficient
- MPO
Myeloperoxidase
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- NASH(-DC)
Non-alcoholic steatohepatitis with depletion of dendritic cells
- NPC
Non-parenchymal cells
- i.p.
intraperitoneal
- Treg
Regulatory T cell
- TLR
Toll-like receptor
- 7-AAD
7- amino-actinomycin
Reference List
- 1.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the Prevalence of the Most Common Causes of Chronic Liver Diseases in the United States From 1988 to 2008. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2011;9:524.e1–530.e1. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Marrero J, Fontana R, Su G, et al. NAFLD May Be a Common Underlying Liver Disease in Patients with Hepatocellular Carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 3.Afzali A, Berry K, Ioannou GN. Excellent posttransplant survival for patients with nonalcoholic steatohepatitis in the United States. Liver Transplantation. 2012;18:29–37. doi: 10.1002/lt.22435. [DOI] [PubMed] [Google Scholar]
- 4.Miura K, Kodama Y, Inokuchi S, et al. Toll-Like Receptor 9 Promotes Steatohepatitis by Induction of Interleukin-1β in Mice. Gastroenterology. 2010;139:323.e7–334.e7. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomita K, Tamiya G, Ando S, et al. Tumour necrosis factor α signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rivera CA, Adegboyega P, van Rooijen N, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. Journal of Hepatology. 2007;47:571–579. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rensen SS, Slaats Y, Nijhuis J, et al. Increased Hepatic Myeloperoxidase Activity in Obese Subjects with Nonalcoholic Steatohepatitis. The American Journal of Pathology. 2009;175:1473–1482. doi: 10.2353/ajpath.2009.080999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkhouri N, Morris-Stiff G, Campbell C, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver International. 2012;32:297–302. doi: 10.1111/j.1478-3231.2011.02639.x. [DOI] [PubMed] [Google Scholar]
- 9.Bantsimba-Malanda C, Marchal-Sommé J, Goven D, et al. A Role for Dendritic Cells in Bleomycin-induced Pulmonary Fibrosis in Mice? American Journal of Respiratory and Critical Care Medicine. 2010;182:385–395. doi: 10.1164/rccm.200907-1164OC. [DOI] [PubMed] [Google Scholar]
- 10.Niess JH. Role of mucosal dendritic cells in inflammatory bowel disease. World Journal of Gastroenterology. 2008;14:5138. doi: 10.3748/wjg.14.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedrosian AS, Nguyen AH, Hackman M, et al. Dendritic Cells Promote Pancreatic Viability in Mice With Acute Pancreatitis. Gastroenterology. 2011;141:1915.e14–1926.e14. doi: 10.1053/j.gastro.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connolly MBA, Mallen-St. Clair J, Mitchell A, Ibrahim J, Stroud A, Pachter HL, Bar-Sagi D, Frey A, Miller G. In liver fibrosis, dendritic cells govern hepatic inflammation via TNF-a. J. Clin. Invest. 2009;119:3213–3225. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiao J, Sastre D, Fiel MI, et al. Dendritic cell regulation of carbon tetrachloride-induced murine liver fibrosis regression. Hepatology. 2012;55:244–255. doi: 10.1002/hep.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller G, Lahrs S, Pillarisetty V, Shah AB, DeMatteo RP. Adenovirus infection enhances dendritic cell immunostimulatory properties and induces natural killer and Tcell-mediated tumor protection. Cancer Res. 2002;62:5260–5266. [PubMed] [Google Scholar]
- 15.Jomantaitė I, Dikopoulos N, Kröger A, et al. Hepatic dendritic cell subsets in the mouse. European Journal of Immunology. 2004;34:355–365. doi: 10.1002/eji.200324336. [DOI] [PubMed] [Google Scholar]
- 16.Syn WK, Choi SS, Diehl AM. Apoptosis and cytokines in non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:565–580. doi: 10.1016/j.cld.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrell GC, Larter CZ, Hou JY, et al. Apoptosis in experimental NASH is associated with p53 activation and TRAIL receptor expression. Journal of Gastroenterology and Hepatology. 2009;24:443–452. doi: 10.1111/j.1440-1746.2009.05785.x. [DOI] [PubMed] [Google Scholar]
- 18.Bechmann LP, Gieseler RK, Sowa J-P, et al. Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver International. 2010;30:850–859. doi: 10.1111/j.1478-3231.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 20.Ma X, Hua J, Mohamood AR, et al. A high-fat diet and regulatory T cells influence susceptibility to endotoxin-induced liver injury. Hepatology. 2007;46:1519–1529. doi: 10.1002/hep.21823. [DOI] [PubMed] [Google Scholar]
- 21.Connolly MK, Ayo D, Malhotra A, et al. Dendritic cell depletion exacerbates acetaminophen hepatotoxicity. Hepatology. 2011;54:959–968. doi: 10.1002/hep.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rovere P, Peri G, Fazzini F, et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood. 2000;96:4300–4306. [PubMed] [Google Scholar]
- 23.Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis & Tissue Repair. 2010;3 doi: 10.1186/1755-1536-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: Update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Chen L, Hu L, et al. Nuclear factor high-mobility group box1 mediating the activation of toll-like receptor 4 signaling in hepatocytes in the early stage of nonalcoholic fatty liver disease in mice. Hepatology. 2011;54:1620–1630. doi: 10.1002/hep.24552. [DOI] [PubMed] [Google Scholar]
- 26.Sancho D, Joffre OP, Keller AM, et al. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JG, Czabotar PE, Policheni AN, et al. The Dendritic Cell Receptor Clec9A Binds Damaged Cells via Exposed Actin Filaments. Immunity. 2012;36:646–657. doi: 10.1016/j.immuni.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Bamboat ZM, Ocuin LM, Balachandran VP, et al. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. The Journal of Clinical Investigation. 2010;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katz SC, Ryan K, Ahmed N, et al. Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. J Immunol. 2011;187:1150–1156. doi: 10.4049/jimmunol.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang Y, Bian Z, Zhao L, et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol. 2011;166:281–290. doi: 10.1111/j.1365-2249.2011.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 32.Popov Y, Schuppan D. CD8+ T cells drive adipose tissue inflammation--a novel clue for NASH pathogenesis? J Hepatol. 2010;52:130–132. doi: 10.1016/j.jhep.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nature immunology. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 35.Tadagavadi RK, Reeves WB. Endogenous IL-10 Attenuates Cisplatin Nephrotoxicity: Role of Dendritic Cells. The Journal of Immunology. 2010;185:4904–4911. doi: 10.4049/jimmunol.1000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell EE, Cooksley WG, Hanson R, et al. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 37.Fishbein MH, Miner M, Mogren C, et al. The spectrum of fatty liver in obese children and the relationship of serum aminotransferases to severity of steatosis. J Pediatr Gastroenterol Nutr. 2003;36:54–61. doi: 10.1097/00005176-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 39.Fei M, Bhatia S, Oriss TB, et al. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc Natl Acad Sci U S A. 2011;108:5360–5365. doi: 10.1073/pnas.1015476108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu L, Faubel S, He Z, et al. Depletion of macrophages and dendritic cells in ischemic acute kidney injury. Am J Nephrol. 2012;35:181–190. doi: 10.1159/000335582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venet F, Huang X, Chung CS, et al. Plasmacytoid dendritic cells control lung inflammation and monocyte recruitment in indirect acute lung injury in mice. Am J Pathol. 2010;176:764–773. doi: 10.2353/ajpath.2010.090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tittel AP, Heuser C, Ohliger C, et al. Functionally relevant neutrophilia in CD11c diphtheria toxin receptor transgenic mice. Nat Methods. 2012;9:385–390. doi: 10.1038/nmeth.1905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.