Abstract
Introduction
Epicardial fat volume (EFV) is linked to cardiovascular event risk. We aimed to investigate the relationships between EFV and weight change.
Methods
From the EISNER (Early Identification of Subclinical Atherosclerosis using Non-invasive Imaging Research) Registry with baseline and follow-up coronary calcium scans (1248 subjects), we selected a cohort of 374 asymptomatic subjects matched using age decade, gender and coronary calcium score (CCS) as a measure of subclinical cardiovascular risk, who underwent 2 scans at an interval of 4.1±0.4 years. Using semi-automated validated software, pericardial contours were generated on all slices by spline interpolation from 5–10 control points. EFV was computed as fat volume within the pericardial contours. Weight gain/loss was defined as >5% change.
Results
At baseline, EFV was moderately correlated to weight, body mass index (BMI) and waist circumference (r=0.51, 0.41 and 0.50, p<0.0001). EFV change was weakly correlated to change in weight (r=0.37, p<0.0001), BMI (r=0.39, p<0.0001) and waist circumference (r=0.21, p=0.002). On multivariable linear regression analysis, weight change [β=1.2, 95% confidence interval (CI) 0.9–1.5, p<0.001], BMI change (β=1.2, 95% CI 0.9–1.5, p<0.001), gender (β=−6.4, 95% CI −10.9 −1.8, p=0.006) and hypertension (β=4.7, 95% CI 0.5–9.0, p=0.03) predicted EFV change. EFV decreased in 54 subjects with weight loss and increased in 71 subjects with weight gain (−2.3±21.1% vs. 23.3±24.4%, p<0.001).
Conclusions
EFV is related to body weight, BMI and waist circumference. Reduction in weight may stabilize or reduce EFV, while weight gain may promote EFV increase.
Keywords: weight, weight loss, adipose tissue, epicardial fat volume
Introduction
Epicardial fat volume (EFV) describes the volume of adipose tissue around the heart that is constrained by the visceral pericardium. Recent studies have demonstrated that EFV measured on non-contrast computed tomography (CT) may be an important marker of coronary artery disease (CAD), with concurrent associations to coronary atherosclerosis and inflammatory cytokines (1,2). EFV has been shown to be related to not only the coronary calcium score (CCS) and the development of coronary plaque (3–6) but also to myocardial ischemia (7) and adverse clinical outcomes (8,9).
Preliminary studies have indicated that substantial weight loss in severely obese patients following bariatric surgery or marked calorie intake reduction may be accompanied by a corresponding decrease in EFV (10,11). While this observation suggests that EFV may be modifiable, it is unknown whether weight fluctuations have a similar effect in subjects who are not enrolled in an intensive weight-loss program. The aims of the current study were to assess the relationship of EFV to weight, body mass index (BMI) and waist circumference, and to evaluate whether changes in these parameters over a four-year period influence EFV measured by non-contrast CT.
Methods
Study population
Subjects for this study were selected from the EISNER (Early Identification of Subclinical Atherosclerosis using Non-invasive Imaging Research) Registry, which consists of asymptomatic healthy volunteers and patients who underwent coronary artery calcium (CAC) scanning (12). From within this registry, we identified 1248 subjects who both had a serial CAC scan performed 3–5 years after their index scan (mean duration 4.1±0.4 years) and who had none of the following exclusion criteria: history of chest pain, myocardial infarction, coronary revascularization, cardiomyopathy, peripheral artery disease, stroke, history of having a prior CAC scan or invasive coronary angiogram, active pregnancy, clinical instability, or significant medical co-morbidity. Since quantification of EFV for this entire cohort would have been prohibitively time and labor intensive, we further narrowed our sample to include equal representative samples of subjects, by matching using age decade, gender and CCS as a measure of subclinical cardiovascular risk. First, we identified 106 random subjects who had conversion from a normal to abnormal CAC scan during serial scanning. These subjects were paired with 106 age and gender-matched subjects who had normal scans (no coronary calcium) both at baseline and follow-up scan. In the matching process, when there was more than one available candidate, pseudo-random numbers were generated to sort and randomly select the matched candidate. Second, we randomly identified a second group of 375 subjects with a baseline CCS of 50–399 and then divided this group into tertiles of CCS progression (n=81 in each tertile) at follow-up. The tertile of 81 subjects with the lowest progression of CCS (low progressors) and the tertile of 81 subjects with the highest progression of CCS (high progressors) were included in this study, whereas the middle tertile group was not considered. Hence, our final sample included 374 subjects (106+106+81+81), now including 212 with an initially normal CAC scan and 162 with an initially abnormal CAC scan.
Clinical assessment
All subjects underwent a comprehensive clinical evaluation and assessment of biophysical parameters (weight, height, BMI and waist circumference) at the time of each non-contrast CT scan. Prior to CT imaging, a medical history was collected to assess prior cardiac disease, cardiovascular risk factors, and medication use. Hypertension was defined as history of physician-diagnosed high blood pressure, or blood pressure medication usage or blood pressure ≥140/90 mm Hg (systolic/diastolic). Diabetes was defined as a history of physician diagnosed diabetes or by having a fasting glucose ≥126 mg/dl. Smoking was defined as self-reported history of current smoking. Three hundred forty two subjects out of 374 had a fasting lipid profile [total cholesterol (TC), high density lipoprotein (HDL) cholesterol, triglycerides (TG) and low density lipoprotein (LDL) levels] using a Cholestech (Alere Inc. Waltham, MA) desktop chemical analyzer. Hypercholesterolemia was defined as history of physician-diagnosed high cholesterol, or cholesterol medication usage or LDL ≥140 mg/dl or TC ≥240 mg/dl. Waist circumference was measured to the nearest 0.1 cm using a tape measure. Weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively. BMI was calculated as the quotient of the weight in kilograms (kg) to the height in meters (m) squared. Percentage changes in these biophysical parameters were calculated as the difference between the values at follow-up and baseline, divided by the value at baseline, multiplied by 100. Blood serum was stored and analyzed for 321 (86%) of the 374 subjects. For these subjects, C-reactive protein (CRP), adiponectin and interleukin-6 (IL-6) were measured by Alere San Diego Inc. (San Diego, CA).
CT data acquisition
Non-contrast CT was acquired using either an electron-beam CT (EBCT) scanner (e-Speed, GE Healthcare, Milwaukee, WI), a 4-slice multi-detector CT (MDCT) scanner (Somatom Volumezoom, Siemens Medical Solutions, Forchheim, Germany), or a dual-source CT scanner (Somatom Definition, Siemens Medical Solutions, Forchheim, Germany). All scanners were calibrated daily using air and water phantoms. Each scan extended from the aortic arch to the diaphragm and was obtained during a single breath-hold. The following scan parameters were used: Heart-rate dependent ECG-triggering (typically 45–60% of the R-R interval), 35 cm field-of-view, and 512×512 matrix size. Tube voltage was 120 kVp with MDCT scanning. Slice thickness was 3 mm for EBCT and 2.5 mm for MDCT. All coronary artery calcium scan images were initially reviewed by an expert reader, using semi-automatic commercially available software (ScImage, Los Altos, CA) to quantify coronary calcification in the manner described by Agatston et al (13). All images were also transferred to a research workstation for epicardial fat quantification. This study was conducted according to guidelines of the Cedars-Sinai Medical Center Institutional Review Board. All subjects provided written informed consent for participation in the study and the use of their data.
Epicardial fat quantification
Epicardial fat quantification was performed by software (QFAT) developed at Cedars-Sinai Medical Center, as previously described (6,9,14). Epicardial fat was defined as adipose tissue enclosed by the visceral pericardium, including fat directly surrounding the coronary arteries. For defining epicardial contours, the upper slice limit, marked by bifurcation of the pulmonary artery trunk, and lower slice limit, identified as the slice just below the posterior descending artery, were chosen, as previously described (7). This lower limit was chosen to better distinguish epicardial fat from fat around the diaphragm. As in our previous work, 5–10 control points on the pericardium in each transverse view were assigned by an experienced imaging cardiologist blinded to subject status and clinical non-contrast CT interpretation. From these control points, piecewise cubic Catmull-Rom spline functions were automatically generated to create a smooth closed epicardial contour (15). EFV was then automatically calculated by identifying contiguous 3D voxels displaying Hounsfield Units (HU) between −190 and −30 (16). EFV was reported in cubic centimeters (cm3). Percent (%) EFV change was calculated using baseline EFV as reference [(follow-up EFV – baseline EFV)/baseline EFV)].
Statistical analysis
All continuous variables included in the analysis are presented as mean ± SD. Non-parametric variables are presented as median with range. Univariate analyses were performed on continuous variables using the two sample t-test for normally distributed variables, and the Mann-Whitney U test for non-normally distributed data. Pearson’s correlation coefficient was used to assess the relationship between continuous variables. For more than 2 groups, the one-way ANOVA test with Bonferroni multiple comparison was used. Weight gain and loss were defined as an increase or decrease >5% of baseline value. This threshold was defined based on distribution of percent weight change [(follow-up weight – baseline weight)/baseline weight] of the population. Significant change in EFV (gain and loss) was defined as an increase or decrease ≥10% of baseline value, this threshold was derived from recently reported inter-scan reproducibility of EFV measurement from our group (17). Multivariable linear regression was sequentially used to assess whether weight change and BMI change were predictive of absolute and percentage EFV change after adjusting for age, gender and conventional cardiovascular risk factors. Multivariable conditional logistic regression analyses adjusting for conventional cardiovascular risk factors were performed to determine whether weight change and BMI change were independently related to significant change in EFV. Statistical significance for all analyses was set at the 5% level. All statistical calculations were performed using STATA (Version 11, StataCorp LP, College Station, Texas, USA) for Windows.
Results
Of the 374 study subjects, 222 (59%) were men, and mean age was 58±8 (range 36–79) years. Table 1a shows the baseline and follow-up demographics of the study population. Prevalence of diabetes, hypertension, and cigarette smoking increased at follow-up (Table 1a). Serum TC, LDL and TG levels were lower at follow-up compared to baseline (p<0.0001 for all). Serum HDL level was similar between baseline and follow-up (p=0.4). CCS increased significantly from baseline [median 0 (0–399)] to follow-up [median 19.5 (0–1051)] (p<0.0001).
Table 1.
| a Clinical characteristics | |||
|---|---|---|---|
| Baseline | Follow up | p value | |
| Age (yrs) | 58±8 | - | - |
| Male (%) | 222 (59) | - | - |
| Family history of CAD, n (%) | 119 (32%) | - | - |
| Hypertension, n (%) | 216 (58%) | 249, (67%) | <0.0001 |
| Systolic BP (mmHg) | 133 ± 18 | 126 ± 16 | <0.0001 |
| Diastolic BP (mmHg) | 82±11 | 78±11 | <0.0001 |
| Hypertension medication, n (%) | 114 (30%) | 161 (43%) | <0.0001 |
| Diabetes, n (%) | 11 (3%) | 21 (6%) | <0.0001 |
| Current smoking, n (%) | 20 (5%) | 21 (6%) | <0.0001 |
| Hypercholesterolemia, n (%) | 248 (66%) | 296 (79%) | <0.0001 |
| TC (mg/dl) | 216±40 | 189±38 | <0.0001 |
| HDL (mg/dl) | 53±17 | 53±16 | 0.4 |
| LDL (mg/dl) | 137±38 | 144±33 | <0.0001 |
| Hypercholesterolemia medication, n (%) | 86 (23%) | 176 (47%) | <0.0001 |
| TG (mg/dl) | 133±80 | 113±62 | <0.0001 |
| CCS* | 0 (0–399) | 19.5 (0–1051) | <0.0001 |
| b Biophysical parameters | ||||
|---|---|---|---|---|
| Baseline | Follow-up | Absolute change (%) | p value | |
| EFV (cm3) | 84±38 | 93±40 | 9±19 (13±22%) | <0.0001 |
| Weight (kg) | 81.4±18.3 | 81.7±19.1 | 0.3±6.7 (0.5±7.1%) | 0.4 |
| BMI (kg/m2) | 27.8±5.4 | 28.0±5.5 | 0.2±2.3 (0.9±7.2%) | 0.1 |
| Waist (cm) | 93.0±13.7 | 95.3±14.2 | 2.3±10.7 (1.6±12.0 %) | <0.0001 |
Median and range reported for CCS
CAD, coronary artery disease; BP, blood pressure; TC, total cholesterol; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; TG, triglycerides; CCS, Coronary calcium score.
EFV, epicardial fat volume; BMI, body mass index.
Relationship of EFV to biophysical parameters
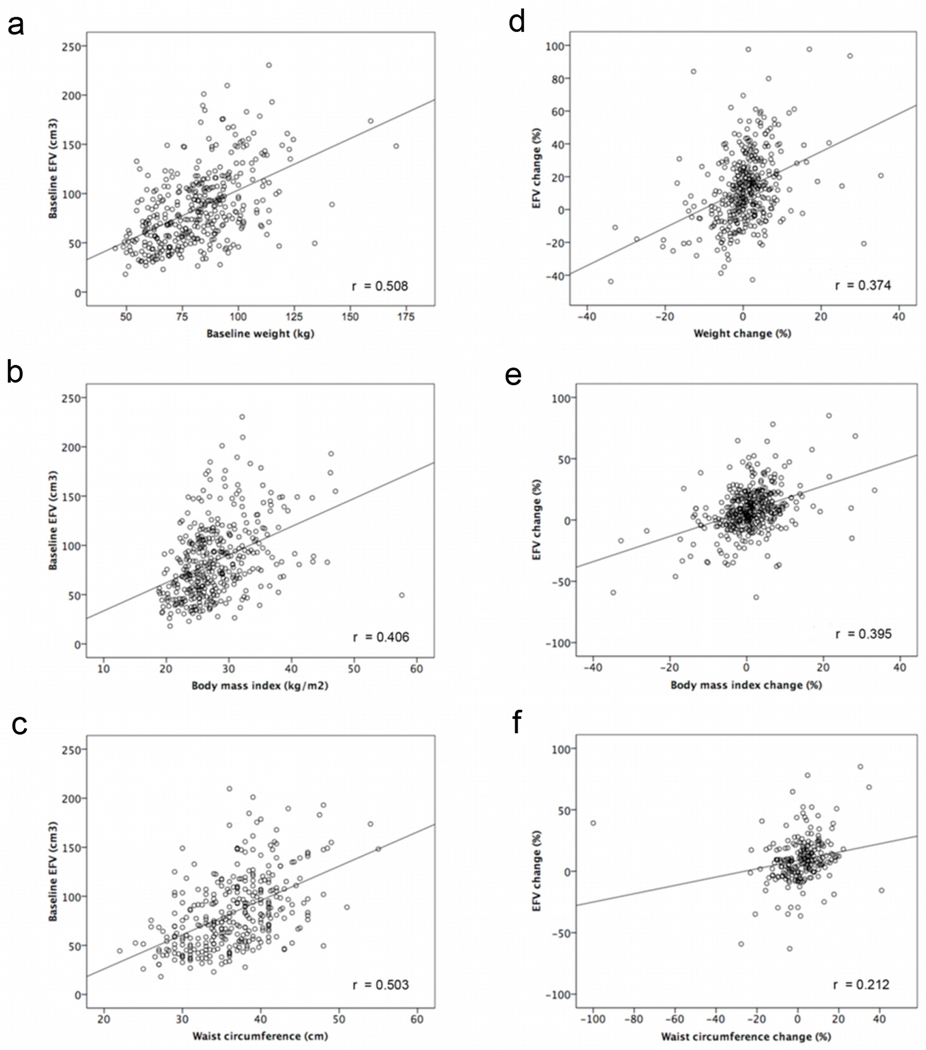
Baseline EFV was moderately correlated to baseline weight (r=0.51, p<0.0001), BMI (r=0.41, p<0.0001) and waist circumference (r=0.50, p<0.0001) measurements (Figure 1 a, b, c). At follow up EFV increased compared to baseline (93±40 vs. 84±38, p<0.0001). This was accompanied by an increase in waist circumference (95.3±14.2 vs. 93.0±13.7, p<0.0001) but not weight (81.7±19.1 vs. 81.4±18.3, p=0.4) or BMI (28.0±5.5 vs. 27.8±5.4, p=0.1) (Table 1b).
Figure 1.
Relationship between baseline EFV and baseline weight (a), BMI (b) and waist circumference (c). Relationship between percentage EFV change and percentage weight (d), BMI (e) and waist circumference change (f). EFV, epicardial fat volume.
Relationship between EFV change and biophysical parameter changes
EFV change was weakly correlated to weight change (r=0.37, p<0.0001), BMI change (r=0.39, p<0.0001) and waist circumference change (r=0.21, p=0.002) (Figure 1 d, e, f). Using multivariable linear regression analysis, weight change (%) (β= 1.2, 95% CI 0.9–1.5, p<0.001), BMI change (%) (β=1.2, 95% CI 0.9–1.5, p<0.001), and hypertension (β=4.7, 95% CI 0.5–9.0, p=0.03) were significantly associated with EFV increase (%) (Table 2a). Male sex (β=−6.4, 95% CI −10.9–−1.8, p=0.006) was significantly associated with EFV decrease. Multivariable logistic regression analysis showed that hypertension (OR=1.6, 95% CI 1.0–2.6, p=0.03), weight change (%) (OR=1.1, 95% CI 1.1–1.2, p<0.001) and BMI change (%) (OR=1.1, 95% CI 1.1–1.2, p<0.001) predicted significant change (10% increase) in EFV (Table 2b).
Table 2.
| a Multivariable linear regression analysis for the prediction %EFV change | |||
|---|---|---|---|
| Beta | 95% C.I | p value | |
| Age | 0.07 | −0.2 to 0.3 | 0.6 |
| Gender | −6.4 | −10.9 to −1.8 | 0.006 |
| Hypercholesterolemia | 1.0 | −3.4 to 5.4 | 0.7 |
| Hypertension | 4.7 | 0.5 to 9.0 | 0.03 |
| Diabetes | 2.2 | −7.1 to 11.5 | 0.6 |
| Smoking | 5.7 | −3.8 to 15.1 | 0.2 |
| Family History of CAD | −1.8 | −6.4 to 2.7 | 0.4 |
| %weight change | 1.2 | 0.9 to 1.5 | <0.001 |
| %BMI change | 1.2 | 0.9 to 1.5 | <0.001 |
| b Multivariable logistic regression analysis for the prediction of significant EFV change | |||
|---|---|---|---|
| Odds Ratio | 95% C.I | p value | |
| Hypercholesterolemia | 1.0 | 0.6 to 1.6 | 0.9 |
| Hypertension | 1.6 | 1.0 to 2.6 | 0.03 |
| Diabetes | 1.3 | 0.5 to 3.5 | 0.6 |
| Smoking | 2.3 | 0.8 to 6.5 | 0.1 |
| Family History of CAD | 0.9 | 0.6 to 1.5 | 0.7 |
| %weight change | 1.1 | 1.1 to 1.2 | <0.001 |
| %BMI change | 1.1 | 1.1 to 1.2 | <0.001 |
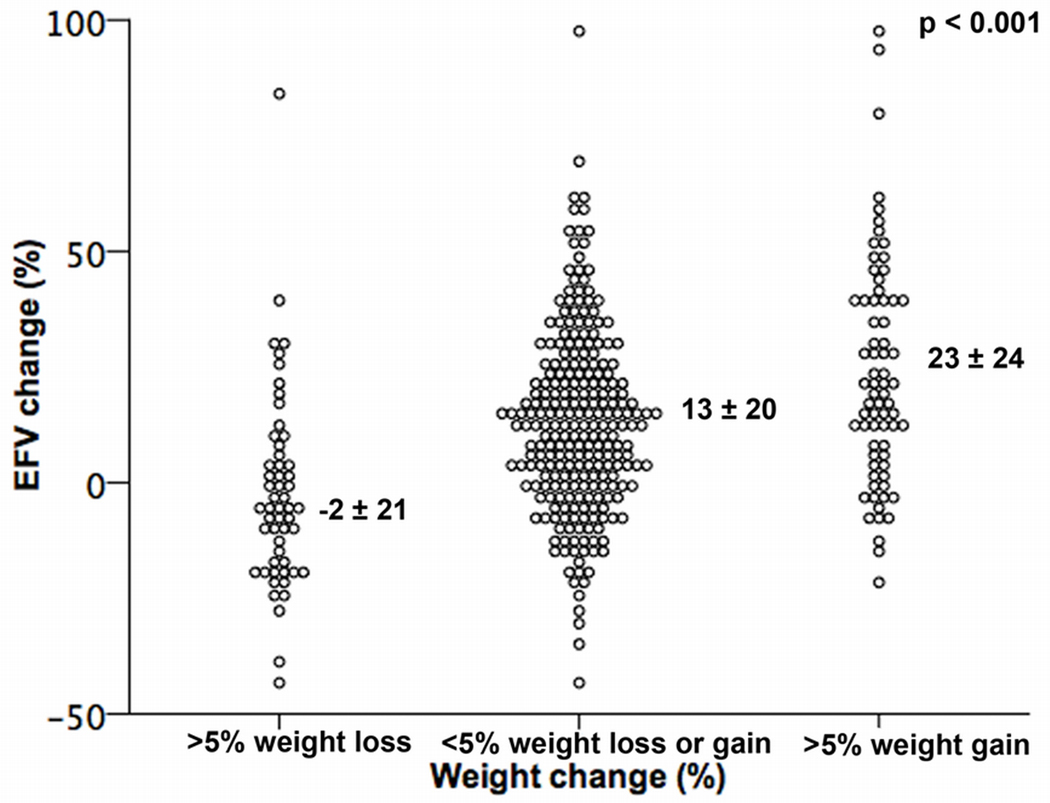
Of the 374 study subjects, 71 subjects showed a >5% weight gain, 54 subjects >5% weight loss, and 249 subjects had a weight change of less than 5%. EFV change in these three groups is shown in Figure 2. EFV change was lowest (−2.3±21.1%) in the subjects with weight loss, and increased progressively with weight change (p<0.001, Figure 2).
Figure 2.
Scatter plot comparing the percentage EFV change among subjects with >5% weight loss, maintained weight and >5% weight gain.
Subjects with weight loss also showed lower frequencies of EFV gain (20%, compared to 54% in subjects with no significant weight change and 70% in subjects with weight gain, p<0.001) and higher frequencies of EFV loss (35%, compared to 10% in subjects with no significant weight change and 4% in subjects with weight gain, p<0.0001).
We also assessed change in EFV according to change in CCS upon follow-up scan. The mean %EFV change was significantly higher in high progressors compared to low progressors (14.4±22.5 vs. 7.3±20.7, p=0.04) but the mean %EFV change was similar in converters and non-converters (15.3±23.4 vs. 13.1±20.7, p=0.46). Table 3 shows the correlation of baseline EFV with blood serum biomarkers; EFV correlated with CRP, TG, and glucose and showed inverse correlation with adiponectin and HDL. However, after adjusting waist circumference, BMI, age and gender, the remaining significant associations were only with HDL (coefficient −0.05, standardized coefficient −0.12, p=0.03) and TG (coefficient 0.72, standardized coefficient 0.33, p<0.001).
Table 3.
Spearman rank correlation of baseline EFV with serum biomarkers
| EFV rank correlation | p value | |
|---|---|---|
| HDL | −0.33 | <0.0001 |
| LDL | 0.09 | 0.08 |
| TG | 0.42 | <0.0001 |
| Glucose | 0.13 | 0.01 |
| Adiponectin | −0.24 | <0.0001 |
| CRP | 0.23 | <0.0001 |
| IL-6 | −0.10 | 0.07 |
CRP, C-reactive protein; IL-6 – interleukin-6.
Discussion
There are three main findings of the current study. First, in an asymptomatic population, weight, BMI and waist circumference demonstrated moderate cross-sectional relationships to EFV. Second, changes in these parameters were related to EFV change. Finally, subjects with >5% weight loss demonstrated lower frequencies of EFV gain and higher frequencies of EFV loss, whereas subjects with >5% weight gain showed the opposite trend.
The relationship between EFV and biophysical measures of body mass is known to be weak. Our findings support those from previous study, which have consistently demonstrated less impressive associations between body mass measures and EFV than visceral adipose tissue (6).
Two prior studies using transthoracic echocardiography provided initial evidence that marked weight loss may reduce epicardial fat burden. Iacobellis et al. showed a decrease in epicardial fat thickness in obese subjects who underwent an aggressive 6-month long weight loss program (mean 20 kg) by adhering to a very low-calorie diet (900 kcal/day) (11). Similarly, in severely obese patients, Willens et al. reported that weight loss after bariatric surgery (average weight loss of 40 kg) was associated with a decrease in epicardial adipose tissue thickness (10).
Our work differs from these prior studies in substantive ways. We evaluated a relatively healthy asymptomatic population, assessed subjects with far more modes weight changes than in previous studies (10,11), and employed a three-dimensional volume measurement of epicardial fat that is less variable and clinically more significant than one-dimensional thickness measurements. Prior study has demonstrated a correlation between volumetric measurement of EFV and coronary artery disease (18) and has established that these measurements are highly reproducible among different observers (17). Importantly, whereas the prior studies of Iacobellis et al. and Willens et al. found reduction in EFV associated with major weight loss, our study establishes that EFV decrease can also accompany relatively minor weight reduction (<5% change in body weight). We also observed that hypertension was a predictor of EFV change. It may be that in future, larger studies, hypertension could emerge as a predictor of increase in EFV.
In our study, baseline EFV correlated with low-adiponectin and low-HDL levels and with high CRP, glucose and triglyceride levels. However, after adjusting waist circumference, BMI, age and gender, the association was remained only in HDL and TG. It has been shown that human adipose tissue expresses adiponectin and that adiponectin expression is significantly higher in epicardial fat from subjects with normal coronary arteries than in patients with severe coronary artery disease (19). Additionally, baseline EFV correlated with high CRP, a plasma inflammatory biomarker. It is believed that epicardial fat would have a more paracrine than endocrine association with atherosclerosis. Therefore our new results support that the contribution of epicardial fat for the concentration of systemic inflammatory biomarkers would be negligible and probably more an epiphenomenon.
Limitations
There were several limitations to this study. First, our method of EFV quantification required operator assistance in determining heart limits and pericardial contour and was thus subject to observer bias. However, we have shown good inter-observer and cross-observer reproducibility of this measurement, even when this is measured from 2 scans (17). In addition, perivascular or pericoronary adipose tissue around coronary arteries is part of the epicardial adipose tissue compartment. Prior studies have shown that pericoronary fat is associated with the presence of any plaque in the corresponding segment as seen by contrast-enhanced CT (20). However, quantification of pericoronary fat requires segmentation of the coronary arteries from non-contrast CT using arbitrary lines, which has not yet been validated to our knowledge. Therefore, we did not segment epicardial fat volume into locality. Our previous work indicates, however, that although local perivascular fat may have a more pronounced effect on the coronary arteries than total epicardial fat volume, the technique is sensitive enough to detect a true pathogenic effect. Second, information regarding lifestyle or treatment modification that may affect EFV was not available in the current study. Third, matching on the basis of weight change may have provided a more optimized population for studying our hypothesis; while this was challenging in the EISNER population with serial CT scans (n=1248), this warrants further study. Finally, serum biomarkers (CRP, adiponectin and IL-6) were only available for the baseline scan and changes in these biomarkers data were not available in this study.
Conclusions
In conclusion, our study indicates that reduction in total body weight (>5%) is associated with stabilization or reduction in epicardial fat burden, whereas weight gain is associated with greater epicardial fat progression. Thus, further study to assess the relationship between weight change and epicardial fat formation in broader spectrum of subjects may now be warranted. In addition, future study should more broadly assess the extent to which EFV is influenced by other risk-reducing interventions, such as exercise (21), and whether dynamic positive or negative changes in EFV, as noted in this study, is a mediator of clinical outcomes.
Acknowledgments
This work was partly supported by a grant from the Eisner, Glazer, and Lincy Foundations (to Dr. Berman), the National Institute of Biomedical Imaging and Bioengineering (R21EB006829 to Dr. Dey). The EISNER study was partially supported by the NIH NCRR GCRC grant (M01-RR00425). Dr. Ronak Rajani was an advanced imaging fellow and was supported by the British Cardiac Society and the American College of Cardiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All authors disclose no conflict of interest.
REFERENCES
- 1.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 2.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 3.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 4.Greif M, Becker A, von Ziegler F, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:781–786. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 5.Ding J, Kritchevsky SB, Harris TB, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914–1919. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dey D, Wong N, Tamarappoo B, et al. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and Metabolic Syndrome. Atherosclerosis. 2010;209:136–141. doi: 10.1016/j.atherosclerosis.2009.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamarappoo B, Dey D, Shmilovich H, et al. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging. 2010;3:1104–1112. doi: 10.1016/j.jcmg.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahabadi A, Massaro J, Rosito G, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J. 2009;30:850–856. doi: 10.1093/eurheartj/ehn573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng V, Dey D, Tamarappoo B, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–360. doi: 10.1016/j.jcmg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willens HJ, Byers P, Chirinos JA, Labrador E, Hare JM, de Marchena E. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol. 2007;99:1242–1245. doi: 10.1016/j.amjcard.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 11.Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) 2008;16:1693–1697. doi: 10.1038/oby.2008.251. [DOI] [PubMed] [Google Scholar]
- 12.Rozanski A, Gransar H, Shaw LJ, et al. Impact of coronary artery calcium scanning on coronary risk factors and downstream testing the EISNER (Early Identification of Subclinical Atherosclerosis by Noninvasive Imaging Research) prospective randomized trial. J Am Coll Cardiol. 2011;57:1622–1632. doi: 10.1016/j.jacc.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agatston A, Janowitz W, Hildner F, Zusmer N, Viamonte MJ, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Dey D, Suzuki Y, Suzuki S, et al. Automated quantitation of pericardiac fat from noncontrast CT. Invest Radiol. 2008;43:145–153. doi: 10.1097/RLI.0b013e31815a054a. [DOI] [PubMed] [Google Scholar]
- 15.Karthikeyan G, Teo K, Islam S, et al. Lipid profile, plasma apolipoproteins, and risk of a first myocardial infarction among Asians: an analysis from the INTERHEART Study. J Am Coll Cardiol. 2009;53:244–253. doi: 10.1016/j.jacc.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler G, Shi R, Beck S, et al. Pericardial and visceral adipose tissues measured volumetrically with computed tomography are highly associated in type 2 diabetic families. Invest Radiol. 2005;40:97–101. doi: 10.1097/00004424-200502000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Nakazato R, Shmilovich H, Tamarappoo BK, et al. Interscan reproducibility of computer-aided epicardial and thoracic fat measurement from noncontrast cardiac CT. J Cardiovasc Comput Tomogr. 2011;5:172–179. doi: 10.1016/j.jcct.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastarrika G, Broncano J, Schoepf UJ, et al. Relationship between coronary artery disease and epicardial adipose tissue quantification at cardiac CT: comparison between automatic volumetric measurement and manual bidimensional estimation. Acad Radiol. 2010;17:727–734. doi: 10.1016/j.acra.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Iacobellis G, Pistilli D, Gucciardo M, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Mahabadi AA, Reinsch N, Lehmann N, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis. 2010;211:195–199. doi: 10.1016/j.atherosclerosis.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, Tanaka K. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol. 2009;106:5–11. doi: 10.1152/japplphysiol.90756.2008. [DOI] [PubMed] [Google Scholar]