Abstract
Background and Purpose
In healthy humans, observation of another individual performing a motor training task (action observation, AO) facilitates, in the observer, the effects of physical training (PT) on motor memory formation. It is not known if this facilitatory process, of potential value for neurorehabilitation, occurs after stroke.
Methods
Eight chronic stroke patients completed this crossover-randomized investigation. A transcranial magnetic stimulation protocol that tests formation of motor memories was used to determine the effects of PT alone and in combination with AO in two different forms: congruent (PT+AOCONGRUENT) and incongruent (PT+AOINCONGRUENT) to the practiced task.
Results
The magnitude of motor memory formation was larger with PT+AOCONGRUENT than with PT alone or PT+AOINCONGRUENT. This effect was associated with a differential corticomotor excitability change in the muscles acting as agonist and antagonist of the trained/observed movements.
Conclusions
These results indicate that congruent AO in association with physical training can enhance the effects of motor training after stroke.
Keywords: stroke, action observation, mirror neurons system, rehabilitation
Introduction
Performing a motor task or observing another individual performing the same motor actions (Action Observation, AO) activates “mirror neurons” in the premotor and parietal cortex of macaque monkeys 1, 2. In humans, AO results in increased cortical excitability of the primary motor cortex (M1) 3, 4, and has been implicated in cognitive processes like understanding the actions and intentions of others 5, imitation learning 6, motor learning 7, and motor memory formation 8. Recently, we have shown that action observation combined with physical practice results in more prominent training effects relative to plain training in healthy volunteers, as reflected by formation of simple motor memories 9. These findings suggested that AO could be a valuable strategy to improve motor rehabilitation following brain lesions like stroke 10, 11. Additionally, recent evidence support the view that action observation could facilitate training of activities of daily living after stroke 12. Here, we tested specifically the hypothesis that AO could enhance the beneficial effects of physical training on motor memory formation in patients with chronic stroke.
Methods
Nine chronic stroke patients with single unilateral cortical or subcortical lesions (5 women, age range 40-74 years; online only Table) gave written informed consent to participate in the study. Eight of them completed the experimental protocol. One patient could not complete the protocol due to TMS-related headache. The National Institute of Neurological Disorders and Stroke, and Johns Hopkins School of Medicine Institutional Review Boards approved the protocol.
Experimental Design
Formation of a motor memory
The experimental design has been previously described in detail 9. Transcranial magnetic stimulation (TMS, Magstim 200 (Jali Medical, Woburn, MA) was delivered through a figure-eight coil applied over the primary motor cortex (M1) to evoke contralateral thumb movements. Each TMS-evoked thumb movement direction was determined from the first-peak acceleration vector recorded using a small 2-dimensional accelerometer mounted on the thumb (Kistler Instrument, Amherst, NY; Fig. 1). Electromyographic (EMG) activity was recorded from surface electrodes placed over the extensor (EPB) and flexor (FPB) pollicis brevis muscles of the arm contralateral to the stimulated M1. EMG signals were digitized (sampling rate 4,000Hz) and fed into a computer for later analysis. Under this protocol, motor training consisting of voluntary thumb movements performed in a specific direction modifies the TMS-evoked movement directions in a way that indicates encoding of the kinematic details of the practiced movements8, 13, 14.
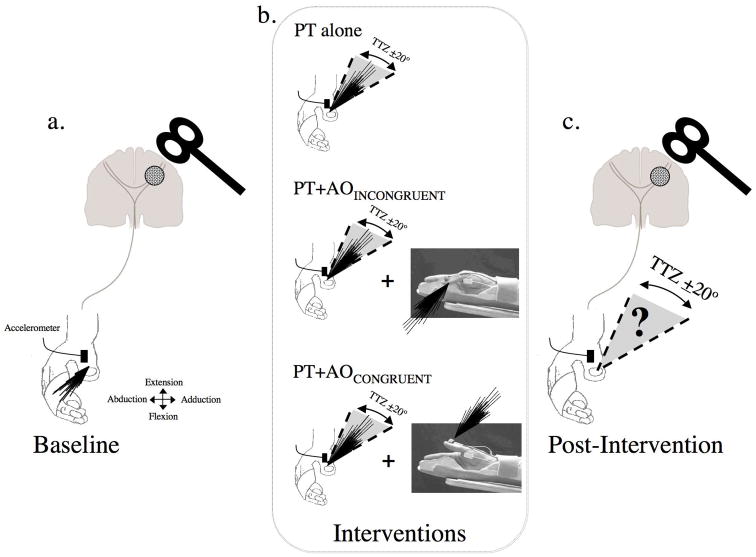
Fig. 1.
Graphic representation of the experimental set up. a) Baseline. Black lines depict the direction of individual TMS-evoked thumb movements, in this example a combination of flexion and abduction. b) Interventions. Patients perform: (1) physical training (PT) alone (represented by a drawing of a thumb and black lines showing the direction of the practice movements), (2) PT+AOINCONGRUENT (same drawing as in PT plus a still picture obtained from the video presented during the experiments showing the thumb in flexion position and black lines depicting the observed movements direction, opposite to the physically trained motions), and (3) PT+AOCONGRUENT (shown by the same drawing as in PT plus a still picture with the thumb in extension position and black lines depicting the direction of the observed movements). In the 3 experimental sessions, the physical training component consisted of thumb movements practiced in a direction opposite to the baseline TMS-evoked movement direction (in the sketch this is represented by the TTZ, a training target zone defined as a window of ± 20° centered on the mean training direction. c) Post-intervention. The percent of TMS-evoked thumb movements falling within the TTZ, the primary outcome measure, was calculated.
Experimental protocol
Each patient participated in three testing sessions separated by at least 7 days in a crossover design. The order of the sessions was counterbalanced. Each session started by recording the direction of 60 TMS-evoked thumb movements (approximately a 10min period, baseline, Fig. 1a). Immediately after baseline determinations, subjects underwent one of the following 30mins interventions in each separate session (Session 1, 2 or 3 in a random order, Fig. 1b): Physical Training (PT, n=8), consisting of performance of voluntary thumb movements, visually paced at 1Hz, performed in a direction opposite to the Baseline TMS-evoked movement direction (3 blocks of 10 min each separated by 2 min rest); Physical Training and Congruent Action Observation (PT+AOCONGRUENT, n=8). In this session, PT was carried out as in the previous session simultaneously with observation of a video displaying the hand of a healthy volunteer performing the training task in the same direction to that physically practiced. Patients were instructed to perform the thumb training motions simultaneously with the observed thumb movements, both in the same direction for 30 min. This training mode is referred to as PT+AOCONGRUENT to reflect the fact that trained and observed thumb movements were in the same direction. Physical Training and Incongruent Action Observation (PT+AOINCONGRUENT, n=8). Motor training in this session was performed in the same way as in the previous two. The only difference with the previous session was that patients observed a video-displaying thumb training motions in a direction opposite to that physically trained. We called this training type PT+AOINCONGRUENT to reflect the fact that trained and observed movements were in approximately opposite directions. The hand orientation in the video of both sessions was as if the observer was looking at their own hand (first person observation), since it has been shown that the degree of corticomotor excitability modulation is maximal when the action is observed from the prospective of the observer 4. To ensure proper attentional focus on the video observation component of the training, patients were instructed to count silently the number of rare movements (6% of the total) that occurred in the direction opposite to the majority of observed motions (94%) in each of the training sessions containing action observation. When physical practice was performed in combination with action observation, patients were instructed to perform the movements at the same time as in the video. Relaxation of uninvolved muscles was monitored on-line by EMG. Verbal feedback was provided along the training to ensure training consistency and synchronization to the observed movements, and relaxation of the uninvolved muscles or in between thumb motions. After each intervention, we determined the direction of 60 TMS-evoked thumb movements (post-intervention), as previously done during baseline (Fig. 1c).
The primary endpoint measure was the percentage of TMS-evoked movements that fell within the training target zone (TTZ), defined as a window of ± 20° centered on the mean training direction 8, 9 (see Fig. 1b). The training direction was determined using data originated in the accelerometer attached to the finger, as done also for determination of pre and post intervention TMS-evoked movement directions (see Formation of a motor memory).
Consistency of motor training performance was monitored in all sessions by measuring three kinematic parameters: (1) the angular difference between TMS-evoked movement directions at baseline and during training, (2) the angular dispersion of training movement directions, and (3) the magnitude of the first peak acceleration of the trained movements. All patients reported their level of attention and fatigue during the interventions using visual analogue scales (range 1–7; 1=worst possible response, 7=best response). Motor cortical excitability was measured recording motor evoked potentials (MEP) amplitudes from muscles mediating movements in the trained (MEPAGONIST) and baseline (MEPANTAGONIST) directions. In this setting, agonist refers to the muscle agonistic to the physically trained motions, while antagonist refers to muscles antagonistic to the physically trained motions. To calculate the effects of each training intervention (sessions 1, 2 and 3) on the motor cortical excitability of both muscle groups (agonist and antagonist), we calculated the post/pre (baseline) MEP amplitude ratio, referred to along the manuscript as MEPPOST-/PRE-INTERVENTION. This measure provides information on the effects of each intervention on the relative weight of corticospinal influences on muscles agonistic and antagonistic to the TMS-evoked movement directions.
Data analysis
An investigator blinded to the intervention type performed data analysis. The primary end point measure of the study, the percent of TMS-evoked movement falling in the TTZ before and after each session training type (dependent variable, a measure of motor memory formation14, 15), was analyzed using repeated measures analysis of variance (ANOVARM) with independent factors TIME (BASELINE, POST-INTERVENTION) and SESSION (MT, MT+AOcongruent, MT+AOincongruent). Separate ANOVAs were used to evaluate changes in MEP amplitudes in each muscle group, attention, fatigue, each of the motor training kinematic parameters and MEPPOST-/PRE-INTERVENTION ratio. Post hoc analysis was done when appropriate using Fisher's PLSD. All data is presented as mean ± standard error of the mean (SEM) unless otherwise stated.
Results
Patients reported comparable levels of attention and fatigue across sessions (Table 1). Kinematic monitoring showed comparable angular difference between TMS-evoked movement directions at baseline and during training and angular dispersion of training movement directions across interventions (Table 2). First peak acceleration of the trained movements was slightly higher in PT+AOCONGRUENT than in PT+AOINCONGRUENT (ANOVARM Intervention: F[2,8]= 4.02, p< 0.05; Fisher's PLSD Post Hoc p<0.02, an effect more prominent in 3 subjects that may reflect a relatively higher difficulty in training in one direction while observing movements in the opposite direction).
Table 1.
Subjective reports in visual analogue scales.
| Pt1 | Pt2 | Pt3 | Pt4 | Pt5 | Pt6 | Pt7 | Pt8 | Mean±SEM | Stats | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatigue | PT | 5 | 5 | 6 | 6 | 5 | 7 | 5 | 5 | 5.5±0.3 | F=0.97 p=0.40 |
| PT+AOINCONGRUENT | 6 | 5 | 4 | 5 | 5 | 6 | 5 | 5 | 5.2±0.3 | ||
| PT+AOCONGRUENT | 5 | 5 | 5 | 3 | 5 | 6 | 5 | 2 | 4.5±0.4 | ||
| Attention | PT | 5 | 4 | 6 | 5 | 5 | 5 | 5 | 6 | 5.2±0.3 | F=2.01 p=0.17 |
| PT+AOINCONGRUENT | 4.5 | 5 | 5 | 5 | 5 | 5 | 5 | 3 | 4.7±0.3 | ||
| PT+AOCONGRUENT | 5 | 5 | 6 | 5 | 5 | 2 | 5 | 3 | 4.5±0.4 |
Subjective reports of fatigue and attention as rated by the participants in a visual analogue scale (fatigue and attention: 1= worst, 7= least fatigue or best attention). Data for individual patients (Pt1, Pt2, …Pt8) and the group mean±SEM is presented. p and F values were calculated from independent ANOVARM.
Table 2.
Physical training kinematics.
| Pt1 | Pt2 | Pt3 | Pt4 | Pt5 | Pt6 | Pt7 | Pt8 | Avg±SEM | Stats | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak Acceleration (m/s2) | PT | 3.5 | 0.2 | 2.2 | 4.3 | 0.9 | 0.1 | 10.3 | 1.6 | 2.9±0.6 | F=4.02 p< 0.05 |
| PT+AOINCONGRUENT | 2.8 | 0.2 | 2.0 | 3.9 | 0.9 | 0.1 | 8.2 | 1.4 | 2.4±0.6 | ||
| PT+AOCONGRUENT | 3.9 | 0.1 | 3.0 | 5.9 | 0.8 | 0.1 | 12.2 | 1.5 | 3.4±0.7 | ||
| Angular Difference (°) | PT | 6.3 | 183.5 | 105.7 | 116.4 | 24.2 | 91.9 | 236.4 | 180.6 | 118.1±28 | F=0.43 p= 0.65 |
| PT+AOINCONGRUENT | 46.5 | 201.0 | 19.4 | 127.1 | 75.0 | 111.6 | 147.9 | 203.0 | 116.5±23 | ||
| PT+AOCONGRUENT | 16.6 | 237.6 | 76.5 | 82.1 | 52.5 | 126.0 | 231.5 | 216.8 | 129.9±30 | ||
| Angular Dispersion | PT | 0.8 | 0.9 | 0.5 | 0.8 | 0.7 | 1.0 | 0.9 | 0.9 | 0.8±0.1 | F=1.63 p= 0.23 |
| PT+AOINCONGRUENT | 0.7 | 0.9 | 0.5 | 0.7 | 0.8 | 0.9 | 0.9 | 0.8 | 0.8±0.1 | ||
| PT+AOCONGRUENT | 0.8 | 0.9 | 0.8 | 0.7 | 0.9 | 1.0 | 1.0 | 0.8 | 0.9±0.1 |
The magnitude of the first peak acceleration during training movements is presented in m/s2, degrees for angular difference between the mean training angle and the mean baseline angle, and length of unit vector for angular dispersion. Data for individual patients (Pt1, Pt2, …Pt8) and the group mean±SEM is presented. p and F values originate from separate ANOVARM.
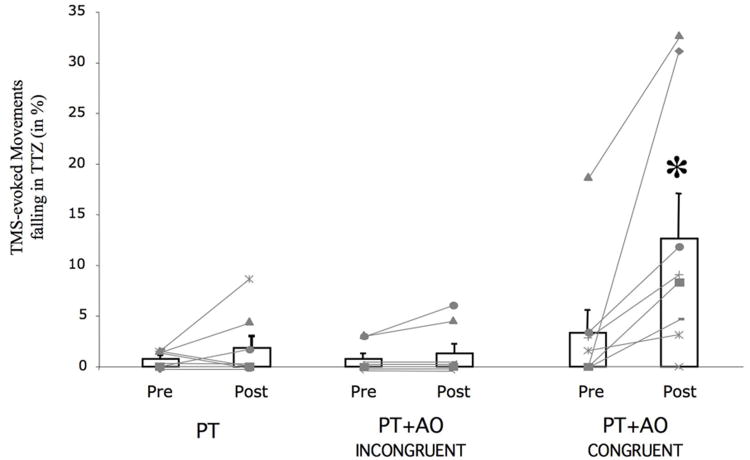
Baseline determination of TMS-evoked thumb movements in the TTZ were comparable across sessions (ANOVARM Session: F[2,7]= 1.5, p=0.26). PT, and PT+AOINCONGRUENT training sessions did not elicit increases in the percentage of TMS-evoked thumb movements in the TTZ (Fig 2). On the other hand, the PT+AOCONGRUENT training type resulted in a marked increase in the percentage of TMS-evoked thumb movements falling in the TTZ (Paired t-Test: t[7]=-2.7, p<0.04). This change in the PT+AOCONGRUENT condition was larger than those observed with PT+AOINCONGRUENT or PT alone (ANOVARM Time: F[1,7]=9.5, p< 0.02; Session: F[2,7]=5.4, p< 0.02; Time by Session interaction: F[2,14]=5.2, p<0.02; Fisher's PLSD Post Hoc for PT+AOINCONGRUENT, PT+AOCONGRUENT: p<0.02; Post Hoc for PT alone, PT+AOCONGRUENT: <0.02). The PT+AOCONGRUENT effect was present in 7 of the 8 patients that completed the experimental protocol (Fig. 2, light gray lines). Of note, two participants had a dramatic effect after PT+AOCONGRUENT. However, removing these subjects from the main statistical analysis did not modify the significance (ANOVARM Time F[1,5]=10.7, p< 0.03; Session: F[2,5]=3.8, p= 0.05; Time by Session interaction: F[2,10]=4.1, p=0.05; Fisher's PLSD Post Hoc for PT+AOINCONGRUENT, PT+AOCONGRUENT: p<0.03; Post Hoc for PT alone, PT+AOCONGRUENT: p=0.05).
Fig 2.
Percent of intervention-dependent TMS-evoked thumb movements falling in the TTZ (bar graph, n=8; mean ± SEM). Note the increase in the percentage of TMS-evoked thumb movements falling in the TTZ when MT+AOCONGRUENT is performed. *, p< 0.02. In light gray, the percent of movements in TTZ for each subject is shown. Seven of eight subjects experience an increased in TMS-evoked motions following the trained and observed directions in the PT+AOCONGRUENT condition.
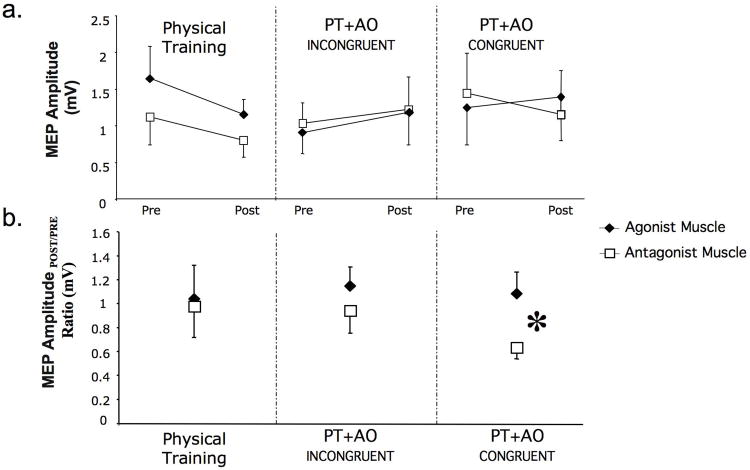
At baseline, MEP amplitudes were comparable across sessions in both muscle groups (ANOVARM Muscle F[1,7]=0.005 p= 0.94, Intervention F[1,7]=1.85 p=0.19, Muscle by Session Interaction F[1,7]=1.02 p=0.38; Table 3). At post-intervention, MEPAGONIST and MEPANTAGONIST amplitudes did not change significantly (ANOVARM MUSCLE F[1,7]=0.06 p= 0.80, Intervention F[1,7]=0.49 p=0.62, Muscle by Intervention Interaction F[1,7]=1.89 p=0.83, Fig 3a). Both muscles MEP amplitudes slightly decreased in PT and increased in the PT+AOINCONGRUENT sessions. However, in the PT+AOCONGRUENT session MEPANTAGONIST had a slight increase while MEPANTAGONIST decreased. This differential change in excitability is reflected by a statistically significant change in the MEPPOST-/PRE-INTERVENTION ratio (ANOVARM Muscle: F[1,7]= 8.71, p= 0.03; Session: F[2,7]= 0.24, p= 0.79; Muscle by Session Interaction: F[2,14]= 5.73, p< 0.02; Fig. 3b).
Table 3.
Baseline corticomotor excitability and stimulation parameters.
| Pt1 | Pt2 | Pt3 | Pt4 | Pt5 | Pt6 | Pt7 | Pt8 | Avg±SEM | Stats | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Motor Threshold (Agonist muscle; % of stimulator output) | PT | 34.0 | 69.0 | 56.0 | 60.0 | 55.0 | 49.0 | 58.0 | 79.0 | 57.5±1.3 | F= 1.47 p= 0.27 |
| PT+TOINCONGRUENT | 35.0 | 63.0 | 55.0 | 61.0 | 53.0 | 56.0 | 57.0 | 74.0 | 56.8±1.2 | ||
| PT+TOCONGRUENT | 34.0 | 61.0 | 52.0 | 61.0 | 52.0 | 48.0 | 59.0 | 75.0 | 55.3±1.2 | ||
| Stimulation Intensity (used to elicit TMS- movements; % of stimulator output) | PT | 43.0 | 74.0 | 68.0 | 70.0 | 78.0 | 74.0 | 74.0 | 85.0 | 70.8±1.2 | F= 1.23 p= 0.32 |
| PT+TOINCONGRUENT | 42.0 | 74.0 | 68.0 | 72.0 | 84.0 | 80.0 | 72.0 | 85.0 | 72.1±1.3 | ||
| PT+TOCONGRUENT | 42.0 | 74.0 | 68.0 | 68.0 | 85.0 | 72.0 | 74.0 | 80.0 | 70.4±1.3 | ||
| Agonist MEP (mV) | PT | 2.0 | 0.1 | 3.2 | 1.0 | 3.2 | 0.4 | 1.2 | 0.6 | 1.5±0.4 | Muscle F= 0.005 p= 0.94 Intervention F=1.85 p=0.19 Interaction F=1.02 p=0.38 |
| PT+TOINCONGRUENT | 1.0 | 0.2 | 0.9 | 0.2 | 2.4 | 0.2 | 1.4 | 0.2 | 0.8±.03 | ||
| PT+TOCONGRUENT | 1.3 | 0.4 | 1.0 | 0.1 | 4.5 | 0.2 | 1.2 | 0.3 | 1.1±0.4 | ||
| Antagonist MEP (mV) | PT | 3.5 | 0.1 | 1.2 | 0.3 | 1.2 | 0.6 | 0.7 | 0.7 | 1.0±0.4 | |
| PT+TOINCONGRUENT | 1.8 | 0.2 | 2.4 | 0.9 | 0.9 | 0.3 | 0.6 | 0.4 | 0.9±0.3 | ||
| PT+TOcongruent | 2.9 | 0.2 | 4.5 | 0.5 | 1.0 | 0.5 | 0.7 | 0.3 | 1.3±0.4 |
Data for individual patients (Pt1, Pt2, …Pt8) and the group mean±SEM is presented. p and F values originate from independent ANOVARM.
Fig. 3.
Corticomotor excitability changes as measured by motor evoked potential amplitudes (MEP). In 3a, absolute MEP amplitudes for the agonist and antagonist muscles to the physically practice direction is shown at baseline (pre) and after (post) each intervention in the 3 sessions. After PT alone MEP amplitude decreased similarly for both muscles and have minimal changes after PT+AOINCONGRUENT. In the PT+AOCONGRUENT condition, MEP amplitude for the agonist muscle had a slight increased whereas the antagonist muscle decreased. This differential modulation of excitability is evidenced in the MEPPOST-/PRE- INTERVENTION ratio (3b). Here, only PT+AOCONGRUENT elicited a significant different ratio. *, p< 0.03.
Discussion
This study shows that action observation can enhance the beneficial effects of motor training on motor memory formation in patients with chronic stroke. Interestingly, the kinematic details of the observed action influence these modulatory effects: they are present when the observed action matches the direction of the physical training, and absent when they do not match. This effect was associated with an increase in corticomotor excitability of the muscle representations mediating movements in the trained and observed direction, whereas the excitability of the antagonist muscles decreased.
Previous investigations in the macaque monkey brain demonstrated the existence of “mirror neurons” that discharge both, with performance of a motor action and with observation of another individual performing similar motor actions1, 2. Human studies have described a “mirror neuron system” with similar characteristics 16, involved in action understanding17, imitation 6, motor learning 7, socialization 18, and capable of modulating training effects in healthy individuals 9. Given these properties and the capacity to engage the motor execution network it has been proposed that action observation could contribute to enhance the effects of motor rehabilitation after stroke 10, 11. A recent small clinical trial in fifteen stroke patients investigating this strategy reported beneficial effects of observation of other individuals performing tasks involving activities of daily living on recovery of the ability to perform certain motor tasks. These observational training elicited fMRI activation of areas in which mirror neurons have been found 12. However, performance of action observation and training exercises were not done simultaneously, which may have reduced the effects of action observation, as it is known that modulation of action observation on corticomotor excitability is stronger when high degree of specificity between phase 3 and direction is present 4.
In the present study we found that observation of another subject performing training motions in the same direction and in phase with those physically trained enhanced motor memory formation relative to physical training alone. This effect cannot be explained by differences in baseline corticomotor excitability, motor training kinematics, attention or fatigue during the different interventional sessions (tables 1, 2 and 3).
The finding that 30 minutes physical training alone under our experimental conditions was not enough to encode a motor memory is consistent with prior studies in chronic stroke patients 19, 20. This relative inability of 30 min training to elicit the desired effect on motor memory formation represents an excellent model against which to compare various strategies designed to boost training effects. It has been shown that dopaminergic agents could enhance training effects on motor memory formation in older adults 21 and in patients with stroke 19. Interestingly, action observation in older healthy volunteers can also enhance training effects to elicit motor memory encoding similar to that induced in younger healthy volunteers by physical training alone 9. Action observation enhanced training effects to a similar extent in elderly healthy volunteers 9 and in our present results in stroke patients.
Changes in cortical excitability identified here provide information on the underlying mechanisms associated to these behavioral effects. The differential modulation of corticomotor excitability of the agonist and antagonist muscles involved in the performed and observed movements suggests a change in the balance of inhibition and excitation within the cortical representation of the thumb. It is likely that Hebbian-like confluence of inputs arriving to the corticospinal neurons within the hand representation of M1 from the ventral premotor cortex 22, 23, where mirror-like activity is found 5, 24, and non-primary motor regions 25, 26, associated to performance of motor tasks, is the mechanism underlying the corticomotor excitability change. Interestingly, similar brain regions activated by hand movements after stroke may contribute to recovery of motor function 27-29. Therefore, it is possible that using action observation to activate premotor areas and in turn modulate motor neuronal output may be particularly suited in stroke patients.
In summary, our results indicate that action observation could contribute to neurorehabilitation by enhancing the beneficial effects of training on motor function in a partially paralyzed hand, an issue of relevance for approximately 50 to 70% of patients post stroke 30. The influence of AO in patients with more severe motor impairment has not been investigated. These preliminary results support a role for action observation in neurorehabilitative treatments after stroke and suggest that it would be worthwhile to investigate this hypothesis in double-blind, controlled multicenter clinical trials.
Summary
This preliminary study shows that simultaneous observation of another individual performing the same action as that physically trained can enhance the effects of motor training on motor memory formation. This effect, accompanied by specific and differential changes in corticomotor excitability within the hand motor representation of the primary motor cortex, suggests the potential use of action observation as a strategy to enhance motor rehabilitation in patients with chronic stroke.
Acknowledgments
Pablo Celnik is supported by the American Heart Association (0665347U), NCMRR, NICHD, NIH (R01HD053793-01A1) and the Rehabilitation Medicine Scientist Training Program (RMSTP; 5K12HD001097). This research was also supported in part by the Intramural Research Program of the NINDS, NIH.
Footnotes
Conflicts of Interest Disclosures: None of the authors have any conflicts of interest to disclose.
All authors have read and approved submission of the manuscript. The materials in this manuscript have not been published and are not being considered for publication elsewhere in whole or in part in any language except as an abstract. All persons mentioned in the acknowledgment have seen and approved mention of their names in the article.
References
- 1.Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 2.Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: From action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- 3.Gangitano M, Mottaghy FM, Pascual-Leone A. Phase-specific modulation of cortical motor output during movement observation. Neuroreport. 2001;12:1489–1492. doi: 10.1097/00001756-200105250-00038. [DOI] [PubMed] [Google Scholar]
- 4.Maeda F, Kleiner-Fisman G, Pascual-Leone A. Motor facilitation while observing hand actions: Specificity of the effect and role of observer's orientation. J Neurophysiol. 2002;87:1329–1335. doi: 10.1152/jn.00773.2000. [DOI] [PubMed] [Google Scholar]
- 5.Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 2005;3:e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 7.Mattar AA, Gribble PL. Motor learning by observing. Neuron. 2005;46:153–160. doi: 10.1016/j.neuron.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, Ungerleider L, Classen J. Formation of a motor memory by action observation. J Neurosci. 2005;25:9339–9346. doi: 10.1523/JNEUROSCI.2282-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celnik P, Stefan K, Hummel F, Duque J, Classen J, Cohen LG. Encoding a motor memory in the older adult by action observation. Neuroimage. 2006;29:677–684. doi: 10.1016/j.neuroimage.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 10.Pomeroy VM, Clark CA, Miller JS, Baron JC, Markus HS, Tallis RC. The potential for utilizing the “Mirror neurone system” To enhance recovery of the severely affected upper limb early after stroke: A review and hypothesis. Neurorehabil Neural Repair. 2005;19:4–13. doi: 10.1177/1545968304274351. [DOI] [PubMed] [Google Scholar]
- 11.Buccino G, Solodkin A, Small SL. Functions of the mirror neuron system: Implications for neurorehabilitation. Cogn Behav Neurol. 2006;19:55–63. doi: 10.1097/00146965-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Ertelt D, Small S, Solodkin A, Dettmers C, McNamara A, Binkofski F, Buccino G. Action observation has a positive impact on rehabilitation of motor deficits after stroke. Neuroimage. 2007;36(2):T164–173. doi: 10.1016/j.neuroimage.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 13.Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci U S A. 2000;97:3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Classen J, Liepert J, Wise SP, Hallett M, Cohen L. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- 15.Butefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91:2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- 16.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 17.Flanagan JR, Johansson RS. Action plans used in action observation. Nature. 2003;424:769–771. doi: 10.1038/nature01861. [DOI] [PubMed] [Google Scholar]
- 18.Wolpert DM, Doya K, Kawato M. A unifying computational framework for motor control and social interaction. Philos Trans R Soc Lond B Biol Sci. 2003;358:593–602. doi: 10.1098/rstb.2002.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Floel A, Hummel F, Breitenstein C, Knecht S, Cohen LG. Dopaminergic effects on encoding of a motor memory in chronic stroke. Neurology. 2005;65:472–474. doi: 10.1212/01.wnl.0000172340.56307.5e. [DOI] [PubMed] [Google Scholar]
- 20.Sawaki L, Wu CW, Kaelin-Lang A, Cohen LG. Effects of somatosensory stimulation on use-dependent plasticity in chronic stroke. Stroke. 2006;37:246–247. doi: 10.1161/01.STR.0000195130.16843.ac. [DOI] [PubMed] [Google Scholar]
- 21.Floel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG. Dopaminergic influences on formation of a motor memory. Ann Neurol. 2005;58:121–130. doi: 10.1002/ana.20536. [DOI] [PubMed] [Google Scholar]
- 22.Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci. 2004;24:1200–1211. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerri G, Shimazu H, Maier MA, Lemon RN. Facilitation from ventral premotor cortex of primary motor cortex outputs to macaque hand muscles. J Neurophysiol. 2003;90:832–842. doi: 10.1152/jn.01026.2002. [DOI] [PubMed] [Google Scholar]
- 24.Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: An fmri study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- 25.Dum RP, Strick PL. Motor areas in the frontal lobe of the primate. Physiol Behav. 2002;77:677–682. doi: 10.1016/s0031-9384(02)00929-0. [DOI] [PubMed] [Google Scholar]
- 26.Mima T, Sadato N, Yazawa S, Hanakawa T, Fukuyama H, Yonekura Y, Shibasaki H. Brain structures related to active and passive finger movements in man. Brain. 1999;122(Pt 10):1989–1997. doi: 10.1093/brain/122.10.1989. [DOI] [PubMed] [Google Scholar]
- 27.Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: A review. Stroke. 2003;34:1553–1566. doi: 10.1161/01.STR.0000071761.36075.A6. [DOI] [PubMed] [Google Scholar]
- 28.Ward NS. The neural substrates of motor recovery after focal damage to the central nervous system. Arch Phys Med Rehabil. 2006;87:30–35. doi: 10.1016/j.apmr.2006.08.334. [DOI] [PubMed] [Google Scholar]
- 29.Yozbatiran N, Cramer SC. Imaging motor recovery after stroke. NeuroRx. 2006;3:482–488. doi: 10.1016/j.nurx.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]