Abstract
Glutamine decreases myocardial damage in ischemia/reperfusion injury. However, the cardioprotective effect of glutamine after burn injury remains unclear. Present study was to explore the protective effect of glycyl-glutamine dipeptide on myocardial damage in severe burn rats. Seventy-two Wistar rats were randomly divided into three groups: normal control (C), burned control (B) and glycyl-glutamine dipeptide-treated (GG) groups. B and GG groups were inflicted with 30% total body surface area of full thickness burn. The GG group was given 1.5 g/kg glycyl-glutamine dipeptide per day and the B group was given the same dose of alanine via intraperitoneal injection for 3 days. The serum CK, LDH, AST, and, blood lactic acid levels, as well as the myocardium ATP and GSH contents, were measured. The indices of cardiac contractile function and histopathological change were analyzed at 12, 24, 48, and 72 post-burn hours (PBH). The serum CK, LDH, AST and blood lactic acid levels increased, and the myocardium ATP and GSH content decreased in both burned groups. Compared with B group, the CK, LDH, AST and blood lactic acid levels reduced, myocardium ATP and GSH content increased in GG group. Moreover, the inhibition of cardiac contractile function and myocardial histopathological damage were reduced significantly in GG group. We conclude that myocardial histological structure and function were damaged significantly after burn injury, glycyl-glutamine dipeptide supplementation is beneficial to myocardial preservation by improving cardiocyte energy metabolism, increasing ATP and glutathione synthesis.
Keywords: Glycyl-glutamine dipeptide, myocardial damage, energy metabolism, GSH, burns, rat
Introduction
Ischemic/hypoxic damage is the major cause of tissue and organ damage after burn injury. Traditional opinion suggests that the heart does not manifest ischemic and hypoxic damage [1]. However, we showed in our previous study that the myocardial regional blood flow declined significantly 1 hour after severe burn, still considerably lower than that in the control group at 24 h post-burn with myocardium ATP synthesis decreasing simultaneously [2,3]. These results indicate that ischemia/hypoxia and energy metabolism disorder on cardiocyte are the primary factors that cause cardiac dysfunction after severe burn injury. Therefore, amelioration of myocardium hemoperfusion and improvement of cardiocyte energy metabolism, as well as protection of myocardium functions and reduction of cardiac damage, are very important topics in burn therapy.
Recent studies have shown that several special amino acids, such as glutamine, glycine, arginine and taurine, exhibit cytoprotective effect on the cardiocyte [4-7], and have established the cardioprotective properties of glutamine [8,9]. Glutamine is one of the principal free intracellular amino acids in mammalian cardiocyte, and its function as energy and nitrogen donor is important in the biosynthesis of nucleotides and protein [10-12]. A previous study has shown that glutamine administration can improve cardiac myocyte energy metabolism, promote ATP biosynthesis, increase energy reserve, and accelerate cardiac muscle functional recovery in an ischemia/reperfusion (I/R) injury model [13-15] However, glutamine is not administered intravenously because of its low solubility and low thermostability. Glycyl-glutamine (Gly-Gln) dipeptide, synthesised from glutamine and glycine, has higher solubility, and thermostability. This compound can hydrolyze quickly into glutamine and glycine after intravenous infusion. Glycine can also protect myocardial cells from ischemic damage by preventing cellular membrane leakage [16]. In addition, it can protect myocardial cells by enabling ATP-depleted cells to maintain their structural integrity of ion homeostasis [17]. Gly-Gln dipeptide is currently used in treating intestinal diseases, such as intestinal fistula, short-bowel syndrome, and so on [18]. To the present, there is no study has demonstrated the cytoprotective effect of Gly-Gln dipeptide on cardiocyte after burn injury. In the present study, we demonstrate the therapeutic effects and several possible mechanisms of Gly-Gln dipeptide on rat cardiac damage induced by severe burn injury.
Materials and methods
Drugs and reagents
Standard ATP preparation was obtained from Sigma Chemical Co. (St. Louis, MO). Blood lactic acid and glutathione detection kits were obtained from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Gly-Gln dipeptide (Sanction Number: 050401) was provided by the Beibei Institute of Pharmaceutical Research (Chongqing, China). Alanine injection was provided by Fujian Haiwang Pharmaceutical Ltd. (Fuzhou, China). Other chemicals and reagents were of analytical grade.
Experimental animals
Seventy-two male adult Wistar rats (weighing 205 g to 255 g) were obtained from the Laboratory Animal Centre, Third Military Medical University. The rats were placed in individual wire-bottomed cages under controlled temperature and humidity with a 12 h light-dark cycle. The rats were acclimatised to the environment with a diet of standard rat pellets for 7 d prior to the experiment. The rats were then randomly divided into three groups, namely, normal control (C), burned control (B), and Gly-Gln dipeptide-treated (GG) groups. Eight rats from the C group were shaved and received anaesthesia, but were not burned. Sixty-four burned rats from the B and GG groups were inflicted with 30% total body surface area of full thickness burn under general anaesthesia (pentobarbital, 40 mg/kg of body weight) and analgesia (buprenorphine, 1 mg/kg of body weight) following a modified procedure as previously described [17]. The rats were anaesthetised, shaved, and napalm-burned for 18 s. The rats were quickly injected intraperitoneally with a lactated ringer’s solution (1.5 ml/kg per 1% body surface area burned) for resuscitation. Four observed phases, namely, at 12, 24, 48, and 72 post-burn hours (PBH), were established with eight rats from each group at each phase.
The investigation process conforms to the regulations stipulated by the Third Military Medical University Animal Care Committee, according to the protocol outlined in the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication no. 85-23, revised 1996).
Treatment procedure
The GG group was supplemented with 1.5 g/kg of Gly-Gln dipeptide per day and the B group was supplemented with the same dose of alanine via intraperitoneal injections for 3 days. All medicines were administered twice per day. The rats were housed in solitary cages and had free access to food and water. At each observed phase, the cardiac contractile function index was measured, and the myocardium tissue, as well as blood plasma, was harvested.
Histological and water content determine examination
The harvested cardiac apex was fixed in formalin. The tissues were dehydrated, mounted with paraffin wax, and cut into 5 μm sections. The sliced tissues were then pasted. Histological sections were stained with haematoxylin and eosin after the tissues were removed from the paraffin. At the end of each experiment, a sample of the cardiac apex (0.1~0.2 g) was dissected, weighed and stored in separate glass containers. After it was dried in an oven at 100°C for 48 hours, each sample was reweighed on the same scale. Water content was calculated as (wet weight - dry weight)/wet weight x 100%.
Myocardial zymogram
The blood samples were centrifuged at 1700 g at 4°C for 10 min, and the serum fraction was transferred into another clean tube. The myocardial injury was assessed by determining the serum creatine kinase (CK), lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) levels using an autoanalyzer AU-800 (Olympus, Japan). The results were expressed in international units per litre.
Lactic acid content in blood and glutathione content in tissues
The lactic acid content in blood was measured using a blood lactic acid detection kit and a 721 spectrophotometer (Beckman, US). Blood (0.1 ml) was processed with protein precipitant (0.6 ml). The mixture was centrifuged at 1700 g at 4°C for 8 min. The procedure was performed according to the kit guidelines. Colour reaction was monitored by measuring the absorbance at 530 nm. The results were expressed as millimole lactic acid per litre of whole blood.
The glutathione (GSH) content in the tissue was determined using a GSH detection kit and a 721 spectrophotometer. Approximately 100 mg of myocardium tissue was homogenated with 5% sulphosalicylic acid and 1 ml of PBS solution. The homogenate was kept on ice for 30 min and then centrifuged at 34300 g at 4°C for 20 min. Total GSH concentrations were determined by adding 2-nitrobenzoic acid into appropriate aliquots of the supernate. The procedure was performed according to the kit guidelines. Colour reaction was monitored by measuring the absorbance at 412 nm. Protein concentration of the supernate was determined using a BCA reagent (Pierce, US). The results were expressed as micromole GSH per gram protein.
ATP content in tissues
The heart was arrested in vivo with a cold cardioplegic solution, and the myocardial tissue of the cardiac apex was immediately frozen using a metal splint precooled by liquid nitrogen. The frozen myocardial tissue was mulled into a fine powder using a mortar and pestle. The powder was transferred into a test tube containing 0.6 N cold perchloric acid. The metabolites were extracted, and the extract was neutralised with a mixture of KOH and K2CO3. The extract was then centrifuged at 6800 g at 4°C for 15 min. The supernate (10 μl) was subjected to high performance liquid chromatography UV/VIS-152 (Gilson, France). The results were expressed as micromole ATP per milligram protein.
Assay of cardiac contractile function
Pentobarbital sodium (40 mg/kg) was used as anaesthesia in exposing the right common carotid artery. A polyethylene catheter filled with 100 U/ml of heparin saline was inserted into the left cardiac ventricle, and the other end of the catheter was connected to the press transducer of a four-channel physiological recorder (Nihon Kohden, Japan). After 5 min of stability, aortic systolic pressure (AOSP), aortic diastolic pressure (AODP) were measured. Left ventricular systolic peak pressure (LVSP), left ventricular developed pressure (LVDP) and ±dp/dtmax were recorded continuously through aside arm in the aortic cannula using the Millar MIKROTIP catheter pressure transducer system.
Statistical analysis
All values were expressed as means ± the standard deviation ( x̅ ± S.D.). In order to analyze the correlation between variables, the Pearson's correlation coefficients were calculated and tested. Since our experimental design is a repeated measures experiment, based on the guidance of Ludbrook J’s article [19], repeated measures analysis of variance was performed to test the significance of the two variables (variable 1: treatment; variable 2: time) and their interaction simultaneously. In order to avoid excessive type I error, the Greenhouse-Geisser adjustment for multisample asphericity and Dunn-Šidàk adjustment for pairwise contrasts are preferred in the tests of within-subjects effects and multiple pairwise comparisons of repeated measurements respectively. All statistical analyses were done using the statistical software program SPSS, version 13.0. A two-sided probability value less than 0.05 were considered significant.
Results
Myocardium histology and water content
Changes in the pathomorphological features of the myocardium appeared after burn injury. Cardiac interstitial oedema, fibre engorgement, transverse striation derangement, cell boundary unsharpness, and cytoplasm destruction were observed at 12 PBH. Inflammatory appearances, such as blood capillary engorgement and erythrocyte exudation, were also observed in the myocardial tissues at 24 PBH. The GG group exhibited lesser structural damage compared with the B group. Major pathomorphological changes include cardiac interstitial engorgement and oedema (Figure 1). Water content in the myocardial tissue was significantly higher in B and GG group compared with the C group (P<0.01). The maximum water content in B group was observed at 48 PBH compared with the normal control group. By contrast, Gly-Gln dipeptide supplements can reverse these changes, and the water content in GG group was lower than that in B group (P < 0.05, P<0.01) (Figure 2).
Figure 1.
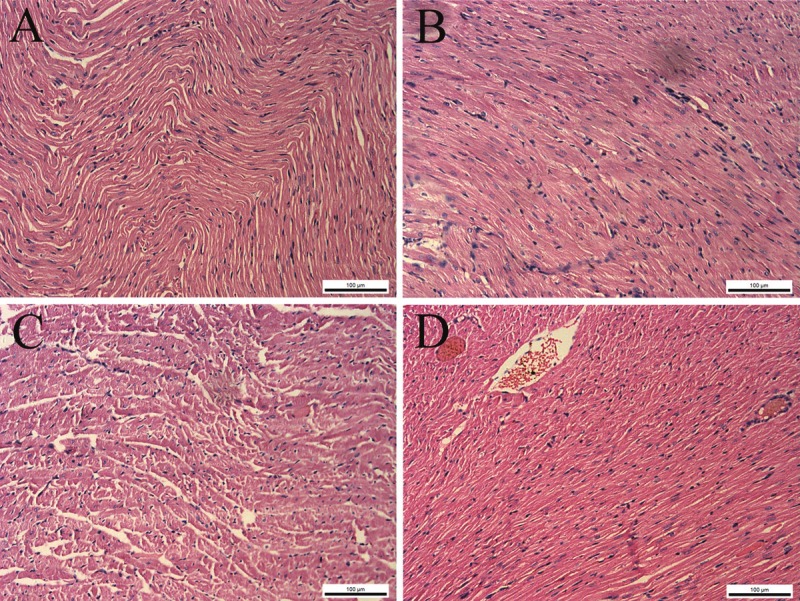
Cardiac pathological change with Gly-Gln dipeptide treatment after burn injury (HE stain, 200x). A. Cardiac apex of the B group at 12 PBH; B. cardiac apex of the GG group at 12 PBH; C. cardiac apex of the B group at 24 PBH; D. cardiac apex of the GG group at 24 PBH.
Figure 2.

Water content changes of myocardial tissue at 12, 24, 48 and 72 PBH. Data are mean±S.D. Repeated measures analysis of variance was performed and Dunn-Šidàk adjustment for pairwise contrasts are preferred in the tests of multiple pairwise comparisons of repeated measurements , **P < 0.01 compared with the C group; #P < 0.05, ##P < 0.01 compared with the B group.
Myocardial zymogram
Low serum AST, CK, and LDH levels were found in the C group initially, but all increased evidently after burn injury. Maximum serum myocardial zymogram activities were observed at 12 PBH (CK and LDH) and at 24 PBH (AST). Then, serum myocardial zymogram activities continuously decreased until minimum values were observed at 72 PBH. Serum myocardial zymogram activities in the GG group decreased compared with those in the B group (P<0.05, P<0.01) (Figure 3).
Figure 3.
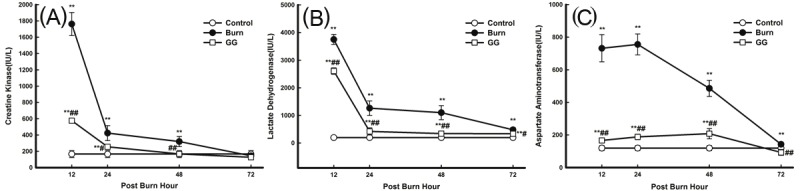
Effect of Gly-Gln dipeptide treatment on myocardial zymogram activities at 12, 24, 48 and 72 PBH. Changes in the activities of various indices: A. CK; B. LDH; and C. AST. Data are mean±S.D. (n = 6 per group). Repeated measures analysis of variance was performed and Dunn-Šidàk adjustment for pairwise contrasts are preferred in the tests of multiple pairwise comparisons of repeated measurements **P < 0.01 compared with the C group; #P < 0.05, ##P < 0.01 compared with the B group.
Blood lactic acid content
The C group exhibited low blood lactic acid content, which increased evidently after burn injury. The lactic acid content reached its maximum value at 24 PBH, and then it decreased continuously until the minimum value was observed at 72 PBH. Although blood lactic acid content in the GG group was lower than that in the B group (P<0.05, P<0.01), this parameter remained higher than that in the C group (P<0.05, P<0.01) (Figure 4).
Figure 4.
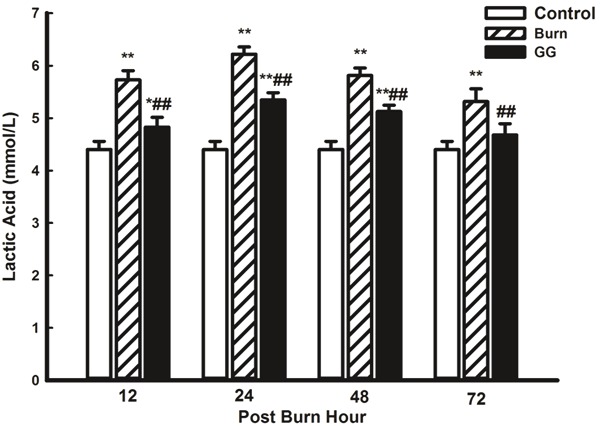
Effect of Gly-Gln dipeptide treatment on lactic acid content in blood at 12, 24, 48 and 72 PBH. Data are mean±S.D. (n = 6 per group). Repeated measures analysis of variance was performed and Dunn-Šidàk adjustment for pairwise contrasts are preferred in the tests of multiple pairwise comparisons of repeated measurements, *P < 0.05, **P < 0.01 compared with the C group; ##P < 0.01 compared with the B group.
ATP content in myocardial tissue
ATP content in the myocardial tissue continuously decreased in the B group compared with that in C group (P<0.05, P<0.01). The minimum ATP content in the B group was observed at 72 PBH. However, Gly-Gln dipeptide supplementation for 3 days reversed the changes, and the ATP content in the GG group was higher than that in the B group (P<0.05, P<0.01) (Figure 5).
Figure 5.

Effect of Gly-Gln dipeptide treatment on myocardial ATP content at 12, 24, 48 and 72 PBH. Data are mean±S.D. (n = 6 per group). Repeated measures analysis of variance was performed and Dunn-Šidàk adjustment for pairwise contrasts are preferred in the tests of multiple pairwise comparisons of repeated measurements *P < 0.05, **P < 0.01 compared with the C group; #P < 0.05, ##P < 0.01 compared with the B group.
GSH content in myocardial tissue
The C group exhibited a high GSH level in the myocardial tissue, which evidently decreased after burn injury in the B group (P<0.01). Gly-Gln dipeptide administration improved GSH synthesis and increased GSH content (P<0.01 vs. the B group) (Figure 6).
Figure 6.
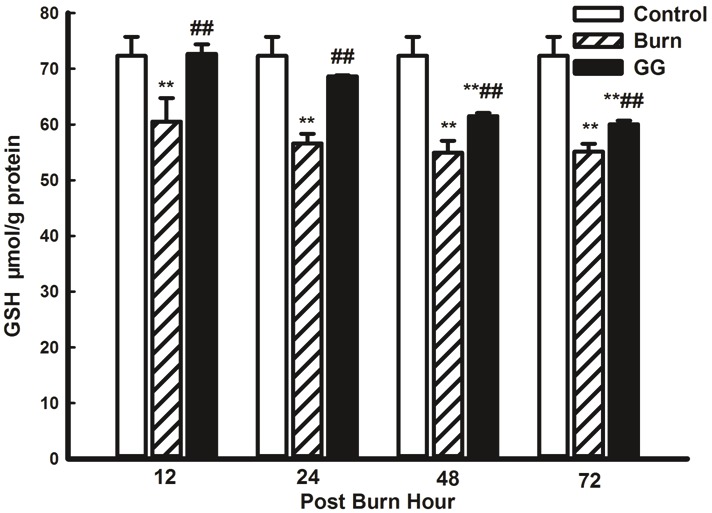
Effect of Gly-Gln dipeptide treatment on myocardial GSH content at 12, 24, 48 and 72 PBH. Data are mean±S.D. (n = 6 per group). Repeated measures analysis of variance was performed and Dunn-Šidàk adjustment for pairwise contrasts are preferred in the tests of multiple pairwise comparisons of repeated measurements **P < 0.01 compared with the C group; ##P < 0.01 compared with the B group.
Assay of cardiac contractile function
Cardiac contractile dysfunction was observed at the early stage of burn injury. The AOSP, AODP, LVSP, and +dp/dtmax of the B and GG group decreased compared with those in the C group. The minimum values of the parameters used in myocardiac mechanics were observed at 48 PBH (AODP) and 72 PBH (AOSP, LVSP, and +dp/dtmax). The cardiac function indices in the GG group improved significantly compared with the B group (P<0.05, P<0.01) (Figure 7). In addition, Pearson correlation analysis showed that synthesis of ATP and GSH were closely related to the improvement of myocardial contractile function in the GG group (P<0.05, P<0.01) (Table 1).
Figure 7.
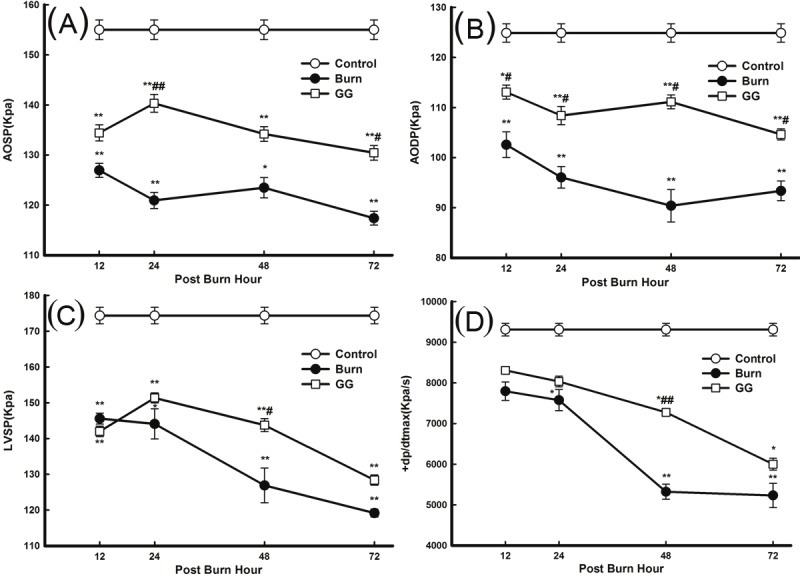
Effect of Gly-Gln dipeptide treatment on cardiac function at 12, 24, 48 and 72 PBH. Changes in the various cardiac function indices: (A) AOSP; (B) AODP; (C) LVSP; and (D) +dp/dtmax. Data are mean±S.D. (n = 6 per group). Repeated measures analysis of variance was performed and Dunn-Šidàk adjustment for pairwise contrasts are preferred in the tests of multiple pairwise comparisons of repeated measurements *P < 0.05,**P < 0.01 compared with the C group; #P < 0.05,##P < 0.01 compared with the B group.
Table 1.
ATP, GSH and cardiac contractile function correlation analysis
| Group | Index | AOSP | AODP | LVSP | +dp/dtmax | |
|---|---|---|---|---|---|---|
| B | ATP | Pearson | 0.2088 | 0.3306 | 0.6236** | 0.6496** |
| Sig. (2-tailed) | 0.2515 | 0.0646 | 0.0001 | 0.0001 | ||
| GSH | Pearson | 0.0911 | 0.1295 | 0.4197* | 0.5091** | |
| Sig. (2-tailed) | 0.6199 | 0.4800 | 0.0168 | 0.0029 | ||
| GG | ATP | Pearson | 0.3438 | 0.1655 | 0.5999** | 0.4191* |
| Sig. (2-tailed) | 0.0540 | 0.3655 | 0.0003 | 0.0170 | ||
| GSH | Pearson | 0.4320* | 0.4549** | 0.4306* | 0.8600** | |
| Sig. (2-tailed) | 0.0136 | 0.0089 | 0.0139 | 0.0000 |
Data are mean±S.D. (n = 6 per group). Pearson correlation coefficients of ATP, GSH and myocardial contractile function in the GG group (* P<0.05, ** P<0.01).
Correlation is significant at the 0.05 level (2-tailed),
Correlation is significant at the 0.01 level (2-tailed).
Discussion
Gly-Gln dipeptide is synthesised from glutamine and glycine. Both substances are beneficial to protect myocardial cells in I/R injury [13-17]. However, almost no study has been done to demonstrate the cytoprotective effect of Gly-Gln dipeptide on cardiocyte after burn injury. The present study shows that myocardial histological structure and function are damaged significantly after burn injury. Glycyl-glutamine dipeptide supplementation is beneficial to myocardial preservation via improved cardiocyte energy metabolism, increased ATP level and glutathione synthesis.
Myocardial energy metabolism improvement using Gly-Gln dipeptide after burn injury
Although the main energy sources of the cardiac myocyte are glucose and fatty acid, several studies have shown that glutamine can improve energy synthesis in the pathological state [12,13,15,20]. The results from the present study indicate an obvious abnormality in energy synthesis and energy reserve in cardiac tissues after burn injury. ATP content in the myocardial tissue decreased remarkably in both GG and B groups compared with C group from 12 PBH to 72 PBH. However, the ATP content in the GG group was higher than that in the B group from 24 PBH to 72 PBH.
Simultaneously, the blood lactic acid content also increased significantly, suggesting that aerobic oxidation was blocked and anaerobic glycolysis increased after the early stages in burn injury. Gly-Gln dipeptide administration improved aerobic oxidation, inhibited anaerobic glycolysis, and reduced lactic acid accumulation. These results demonstrate that burn injury can induce myocardial ischemia, oxygen uptake insufficiency, and oxidative metabolism blockage. Gly-Gln dipeptide supplementation significantly improved myocardial tissue energy metabolism and promoted ATP synthesis.
Gly-Gln dipeptide is rapidly hydrolysed by dipeptidase and its half-life in plasma is less than 10 min. These characteristics make Gly-Gln dipeptide an efficient source of glutamine and glycine [21]. Glutamine is one of the principal free intracellular amino acids in mammalian cardiac myocyte that maintains its normal energy metabolism and influences cellular activities [22]. Recent studies have shown that glycine can protect cells against ischemic injury by preventing cellular membrane leakage [16]. Glycine also protects myocardial cells by enabling ATP-depleted cells to maintain their structural integrity through ion homeostasis [17].
Both glutamine and glycine are highly utilized by myocardial cells. Glutamine is quickly transported to the cardiac myocytes via high-capacity, saturable, stereospecific, and sodium-dependent transporter in the cardiocyte membrane [12,20,23,24]. A previous study has shown that cardiac glutamine catabolism rate is at least four-times higher than that in the skeletal muscle [22]. Glycine receptor has been found in the myocardial cell membrane, and myocardial cells utilize glycine effectively [16]. Thus, Gly-Gln dipeptide is more suited to be used in myocardial cells.
Glutathione synthesis promotion using Gly-Gln dipeptide after burn injury
During reperfusion after myocardial ischemia, molecular oxygen is reintroduced to the ischemic myocardium and is converted to oxygen free radicals. These radicals are toxic and can oxidise protein sulfhydryl groups as well as promote further tissue injury by inducing lipid peroxidation of cell membranes [25], Furthermore, a huge amount of free radicals, produced by the post-burn outburst inflammatory reaction, can result in the damage of cardiac myocyte [26,27]. Nabavi, SM et al. find many antioxidants, such as quercetin and curcumin, have protective effects against oxidative stress in the tissue and cells [28,29]. GSH is a major antioxidative substance, it can protect mammalian cells against ischemic injury induced by oxidative stress and maintain the structural integrity of cells [17]. The results in the present study indicate that the myocardial GSH content was reduced significantly at the early stage of burn injury, and higher in GG group than that in B group from 24 PBH to 72 PBH. These results demonstrate that Gly-Gln dipeptide supplement can significantly improve myocardial tissue GSH synthesis, abate cardiac myocyte lipid peroxidation injury, protect mitochondrial respiratory function, and ameliorate cardiac energy metabolism.
Myocardial damage alleviation and cardiac blood-pumping function improvement using Gly-Gln dipeptide after burn injury
LDH, CK, and AST are the major metabolic enzymes in the heart and can be released into the serum during cardiac damage. Our results show that the serum AST, CK, and LDH levels increased quickly after burn injury and reached maximum values at 12 PBH. These values in the B and GG groups were evidently higher than those in the C group from 12 PBH to 48 PBH. Gly-Gln dipeptide supplement reduced the increase in serum AST, CK, and LDH levels compared with that in the B group. After severe burn injury, the myocardial zymogram changed significantly, and histopathological changes were also observed. At 12 PBH, cardiac interstitial oedema, fibre engorgement, transverse striation derangement, cell boundary unsharpness, and cytoplasm destruction were observed. Inflammatory appearances, such as blood capillary engorgement and erythrocyte exudation, were also observed in the myocardial tissues at 24 PBH. During ischemia and hypoxia, the pathomorphological changes, such as punctiform and lamellar necroses, were more apparent at 48 PBH. Gly-Gln dipeptide supplement reduced significantly the structural damage, compared with the B group. The major pathomorphological changes include cardiac interstitial engorgement and oedema.
We have shown in our previous study that the myocardial regional blood flow after severe burn declined significantly at 1 PBH and it still markedly lower than that of the control group at 24 PBH [2]. The structural damage induced by ischemia/hypoxia in the myocardium tissue caused cardiac blood-pumping dysfunction. The results in the present study show that some parameters of myocardiac mechanics were altered significantly. The AOSP, AODP, LVSP, and +dp/dtmax decreased with different levels from 12 PBH to 72 PBH. This suggests that at the early stage of burn injury, cardiac contractile function, cardiac afterload, peripheral vascular resistance, and maximal isolength tension of the myocardial fibres all decreased significantly. The results further suggest that the velocity of myocardial contractile element also decreased. Gly-Gln dipeptide supplement can significantly increased the AOSP, AODP, LVDP, and +dp/dtmax levels and improve cardiac contractile function compared with the B group. Moreover, the changes in the contractibility parameters closely correlated with myocardial energy metabolism. Gly-Gln treatment after burn injury increased the GSH level and improved energy metabolism as well as cardiac contractile function. High-energy phosphate compounds were used by cardiac myocyte as an energy source, and affecting directly cardiac myocyte activity and systolic function. Therefore, we infer that the possible mechanism of Gly-Gln dipeptide on cardiac protection function after burn injury is due to the improved GSH synthesis and cellular energy metabolism, as well as the reduced lipid peroxidation damage, in cardiac myocyte.
Acknowledgments
This work was supported by Natural Science Foundation of Chongqing (Grant No. cstc2013jjB0140), the State Key Laboratory of independent subject (No. SKLZZ201010 and No. SKLZZ201111), and the Clinical Research Foundation of TMMU (Grant No. SWH2012LC01).
Declaration
The authors report no conflict of interest.
References
- 1.Latenser BA. Critical care of the burn patient: the first 48 hours. Crit Care Med. 2009;37:2819–2826. doi: 10.1097/CCM.0b013e3181b3a08f. [DOI] [PubMed] [Google Scholar]
- 2.Huang Y, Li Z, Yang Z. Roles of ischemia and hypoxia and the molecular pathogenesis of post-burn cardiac shock. Burns. 2003;29:828–833. doi: 10.1016/s0305-4179(03)00204-3. [DOI] [PubMed] [Google Scholar]
- 3.Huang YS, Yang ZC, Yan BG, Yang JM, Chen FM, Crowther RS, Li A. Pathogenesis of early cardiac myocyte damage after severe burns. J Trauma. 1999;46:428–432. doi: 10.1097/00005373-199903000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Jiang L, Qin X, Zhong X, Liu L, Lu Y, Fan L, He Z, Chen Q. Glycine-induced cytoprotection is mediated by ERK1/2 and AKT in renal cells with ATP depletion. Eur J Cell Biol. 2011;90:333–341. doi: 10.1016/j.ejcb.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Sahin MA, Yucel O, Guler A, Doganci S, Jahollari A, Cingoz F, Arslan S, Gamsizkan M, Yaman H, Demirkilic U. Is there any cardioprotective role of Taurine during cold ischemic period following global myocardial ischemia? J Cardiothorac Surg. 2011;6:31. doi: 10.1186/1749-8090-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg S, Chernin G, Shapira I, George J, Wollman Y, Laniado S, Keren G. Captopril and L-arginine have a synergistic cardioprotective effect in ischemic-reperfusion injury in the isolated rat heart. J Cardiovasc Pharmacol Ther. 2000;5:281–290. doi: 10.1054/JCPT.2000.18021. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda Y, Young LH, Scalia R, Ross CR, Lefer AM. PR-39, a proline/arginine-rich antimicrobial peptide, exerts cardioprotective effects in myocardial ischemia-reperfusion. Cardiovasc Res. 2001;49:69–77. doi: 10.1016/s0008-6363(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 8.Lomivorotov VV, Efremov SM, Shmirev VA, Ponomarev DN, Lomivorotov VN, Karaskov AM. Glutamine Is Cardioprotective in Patients with Ischemic Heart Disease following Cardiopulmonary Bypass. Heart Surg Forum. 2011;14:E384–388. doi: 10.1532/HSF98.20111074. [DOI] [PubMed] [Google Scholar]
- 9.Ugurlucan M, Erer D, Karatepe O, Ziyade S, Haholu A, Gungor Ugurlucan F, Filizcan U, Tireli E, Dayioglu E, Alpagut U. Glutamine enhances the heat shock protein 70 expression as a cardioprotective mechanism in left heart tissues in the presence of diabetes mellitus. Expert Opin Ther Targets. 2010;14:1143–1156. doi: 10.1517/14728222.2010.521500. [DOI] [PubMed] [Google Scholar]
- 10.Venturini A, Ascione R, Lin H, Polesel E, Angelini GD, Suleiman MS. The importance of myocardial amino acids during ischemia and reperfusion in dilated left ventricle of patients with degenerative mitral valve disease. Mol Cell Biochem. 2009;330:63–70. doi: 10.1007/s11010-009-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeulen MA, van de Poll MC, Ligthart-Melis GC, Dejong CH, van den Tol MP, Boelens PG, van Leeuwen PA. Specific amino acids in the critically ill patient--exogenous glutamine/arginine: a common denominator? Crit Care Med. 2007;35:S568–576. doi: 10.1097/01.CCM.0000278600.14265.95. [DOI] [PubMed] [Google Scholar]
- 12.Bolotin G, Raman J, Williams U, Bacha E, Kocherginsky M, Jeevanandam V. Glutamine improves myocardial function following ischemia-reperfusion injury. Asian Cardiovasc Thorac Ann. 2007;15:463–467. doi: 10.1177/021849230701500603. [DOI] [PubMed] [Google Scholar]
- 13.McGuinness J, Neilan TG, Cummins R, Sharkasi A, Bouchier-Hayes D, Redmond JM. Intravenous glutamine enhances COX-2 activity giving cardioprotection. J Surg Res. 2009;152:140–147. doi: 10.1016/j.jss.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol. 2007;42:177–185. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stottrup NB, Kristiansen SB, Lofgren B, Hansen BF, Kimose HH, Botker HE, Nielsen TT. L-glutamate and glutamine improve haemodynamic function and restore myocardial glycogen content during postischaemic reperfusion: A radioactive tracer study in the rat isolated heart. Clin Exp Pharmacol Physiol. 2006;33:1099–1103. doi: 10.1111/j.1440-1681.2006.04497.x. [DOI] [PubMed] [Google Scholar]
- 16.Pan C, Bai X, Fan L, Ji Y, Li X, Chen Q. Cytoprotection by glycine against ATP-depletion-induced injury is mediated by glycine receptor in renal cells. Biochem J. 2005;390:447–453. doi: 10.1042/BJ20050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberg JM, Davis JA, Venkatachalam MA. Cytosolic-free calcium increases to greater than 100 micromolar in ATP-depleted proximal tubules. J Clin Invest. 1997;100:713–722. doi: 10.1172/JCI119584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song JX, Tu XH, Wang L, Li CJ. Glutamine dipeptide-supplemented parenteral nutrition in patients with colorectal cancer. Clinical Nutrition Supplements. 2004;1:49–53. [Google Scholar]
- 19.Ludbrook J. Repeated measurements and multiple comparisons in cardiovascular research. Cardiovasc Res. 1994;28:303–311. doi: 10.1093/cvr/28.3.303. [DOI] [PubMed] [Google Scholar]
- 20.Khogali SE, Pringle SD, Weryk BV, Rennie MJ. Is glutamine beneficial in ischemic heart disease? Nutrition. 2002;18:123–126. doi: 10.1016/s0899-9007(01)00768-7. [DOI] [PubMed] [Google Scholar]
- 21.Jiang JW, Zheng SS, Xue F, Gao LH, Jiang GP, Xie HY. Enteral feeding of glycyl-glutamine dipeptide improves the structure and absorptive function of the small intestine after allogenetic liver transplantation in rats. Hepatobiliary Pancreat Dis Int. 2006;5:199–204. [PubMed] [Google Scholar]
- 22.Boelens PG, Nijveldt RJ, Houdijk AP, Meijer S, van Leeuwen PA. Glutamine alimentation in catabolic state. J Nutr. 2001;131:2569S–2577S. doi: 10.1093/jn/131.9.2569S. discussion 2590S. [DOI] [PubMed] [Google Scholar]
- 23.Rennie MJ, Khogali SE, Low SY, McDowell HE, Hundal HS, Ahmed A, Taylor PM. Amino acid transport in heart and skeletal muscle and the functional consequences. Biochem Soc Trans. 1996;24:869–873. doi: 10.1042/bst0240869. [DOI] [PubMed] [Google Scholar]
- 24.Schachter D. L-glutamine in vitro regulates rat aortic glutamate content and modulates nitric oxide formation and contractility responses. Am J Physiol Cell Physiol. 2007;293:C142–151. doi: 10.1152/ajpcell.00589.2006. [DOI] [PubMed] [Google Scholar]
- 25.Tsai MS, Sun S, Tang W, Ristagno G, Chen WJ, Weil MH. Free radicals mediate postshock contractile impairment in cardiomyocytes. Crit Care Med. 2008;36:3213–3219. doi: 10.1097/CCM.0b013e31818f23f0. [DOI] [PubMed] [Google Scholar]
- 26.Ren J, Privratsky JR, Yang X, Dong F, Carlson EC. Metallothionein alleviates glutathione depletion-induced oxidative cardiomyopathy in murine hearts. Crit Care Med. 2008;36:2106–2116. doi: 10.1097/CCM.0b013e31817bf925. [DOI] [PubMed] [Google Scholar]
- 27.Todorova V, Vanderpool D, Blossom S, Nwokedi E, Hennings L, Mrak R, Klimberg VS. Oral glutamine protects against cyclophosphamide-induced cardiotoxicity in experimental rats through increase of cardiac glutathione. Nutrition. 2009;25:812–817. doi: 10.1016/j.nut.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Nabavi SF, Nabavi SM, Abolhasani F, Moghaddam AH, Eslami S. Cytoprotective effects of curcumin on sodium fluoride-induced intoxication in rat erythrocytes. Bull Environ Contam Toxicol. 2012;88:486–490. doi: 10.1007/s00128-011-0495-5. [DOI] [PubMed] [Google Scholar]
- 29.Nabavi SM, Nabavi SF, Eslami S, Moghaddam AH. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chemistry. 2012;132:931–935. [Google Scholar]


