Abstract
The aim of this study was to investigate the role of Mesenchymal Stem Cell (MSC) conditioned medium (CMMSC) on apoptosis of cultured mouse primary hepatocytes after in vivo carbon tetrachloride (CCl4)-induced acute liver injury. The acute liver injury was induced by injecting CCl4 intraperitoneally in C57/BL6 mice. Hepatocytes were isolated by liver perfusion, cultured in a defined medium to maintain their differentiation and characterized by reverse transcriptase polymerase chain reaction (RT-PCR) using the hepatic cell specific genes albumin, hepatocyte nuclear factor 4 (HNF4) and cytokeratin 18 (CK18). CMMSC was generated from cultured bone marrow-derived MSCs (BM-MSCs). BM-MSCs were positive for CD73, CD90, CD44 by flow cytometry and able to differentiate into chondrocytes, adipocytes and osteocytes. Apoptosis was evaluated by both annexin V. CMMSC were examined by flow cytometry to detect MSC-derived annexin V- and CD54/CD44-positive microparticles (MPs). In the CCl4-CMMSC treated hepatocytes, interleukin-6 (IL-6) was increased on the first day of culture compared to control and CCl4 and was followed by upregulation of fibroblast-like-protein (FGL1) expression after 48 hrs. This was associated with a significant decrease of annexin V positive CCl4-CMMSC treated hepatocytes at day 3 post plating. Recombinant IL-6 was induced FGL1 expression in hepatocytes derived from CCl4-treated mice suggesting that CMMSC, which is enriched also in microparticles, attenuates CCl4-induced early apoptosis in hepatocytes through activation of FGL1.
Keywords: Hepatic cell apoptosis, mesenchymal stem cell-conditioned medium, FGL1, IL-6, microparticles
Introduction
Bone-marrow derived mesenchymal stem cells (MSCs) have been shown to promote repair of damaged tissues by inhibiting apoptosis and fibrosis [1-3]. MSCs also secrete a number of bioactive factors, a number of which mediate tissue repair [1,4,5]. Recently MSC-derived molecules were shown to exert a therapeutic effect in fulminant hepatic failure [6].
In previous studies we have demonstrated that granulocyte colony-stimulating factor (GCSF) either on its own or by mobilizing HSCs promotes endogenous repair programs in acute and chronic liver injury [7]. Acute liver injury represents a serious condition with high mortality rate. Liver is the only organ with regenerative capacity although injury can progress to irreversible stages [8,9]. CCl4-induced acute liver injury is a well-known model in rodents. Hepatotoxicity is histopathologically characterized by hepatocyte necrosis as well as apoptosis [10]. Apoptosis is a process by which cells undergo nonnecrotic cellular suicide. Phosphatidylserine, one component of cell membrane phospholipids is confined to the inner leaflet of the plasma membrane. Annexin V-binding to phosphatidylserine in the outer leaflet is an early apoptotic event [11]. In an acute liver injury model, increased hepatocyte cell protection is associated with downregulation of proapoptotic signaling [12].
MSC therapy might offer a promising strategy for acute liver injury treatment, determination of treatment protocol for MSC transplantation requires improvement. Transdifferentiation of MSCs into hepatocytes seems also to be a rare event [6] while the usage of secreted factors from MSC could exert a real beneficial effect on hepatocytes. One of the MSC derived factors is the hepatoprotective cytokine IL6 [13,14], which regulates the acute phase response and plays a pivotal role in liver regeneration as well as the down-regulation of anti-apoptotic genes [15]. Additionally, IL-6 induces the expression of fibroblast-like-protein 1 gene (FGL1) [16] also called as hepassocin (HPS) [17] and MFREP-1 (hepatocyte-derived fibrinogen-related protein) [18] after hepatectomy or turbentine oil-induced acute phase response [19]. Therefore it has been suggested that FGL1 may be involved in the liver regeneration process [17,20] and that the FGL1 gene is a key gene involved in repair following CCl4-hepatotoxicity [16]. Recently several studies reported that MSC-derived microparticles, which are plasma membrane-derived vesicles participate in physiological and pathological processes [21]. Furthermore, MPs facilitate intercellular communication [22] and confers protective effect against tissue injury [21] as well as accelerates hepatic regeneration [23].
We hypothesized that the CMMSC, a rich source of growth factors and paracrine mediators secreted by MSCs such as IL6, can protect hepatocytes from acute injury. FGL1, which has been shown to be induced by IL6 [17], could also play a role in hepatocyte apoptosis. Therefore, we aimed to evaluate the effect of CMMSC on hepatoprotection and modulation of apoptosis of primary hepatocytes in an acute model of CCl4 induced-liver injury. Furthermore, we showed that CMMSC can increase IL6 production that may contribute to the elevated expression of FGL1 in primary hepatocytes derived from CCl4-treated mice.
Materials and methods
Animals and treatment
Eight-week old C57BL/6 mice, maintained in 12-hour light/12-hour dark cycles in a conventional clean facility, were used in these experiments. All experiments were performed in an accredited animal facility (number EL 54 BIO 14) and complied with the Federation of European Laboratory Animal Science Associations guidelines. Acute liver damage was induced by injecting mice with 2 ml/kg CCl4 (dissolved in corn oil in a ratio 1:1) intraperitoneally [24].
Mouse primary hepatocyte cultures
The liver was perfused in situ through the portal vein using Ca++-free HBSS (pH 7.4) based on a modified protocol published by Klaunig [25]. Perfusion was continued with Waymouth’s 752/1 medium (Gibco, USA) supplemented with 0.57 mg/ml Collagenase (type IV, Sigma-Aldrich, USA). Immediately after perfusion the liver was removed and hepatocytes were cultured in fibronectin coated culture plates at a density 1×106 cells/ml in serum free Waymouth’s 752/1 supplemented with10 μg/ml insulin, 25 μg/ml transferrin, 100 μg/ml bovine serum albumin, 50 ng/ml EGF, 10 μg/ml glucagon and 10-7 M dexamethasone (Sigma, USA). This medium was found to promote maintenance of hepatocyte differentiation. At the indicated time points (1, 3, 4 and 6 days) the supernatants were harvested and, after cell debris removal, stored at -20°C until used.
Culture of MSCs and preparation of MSC-conditioned medium
Bone marrow cells from femurs and tibiae of C57/BL6 mice were plated at a density of 106 cells per cm2 in a-MEM medium containing 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco, USA) [26,27]. The cells were then incubated at 37°C in a humified atmosphere containing 5% CO2 and non adherent cells were removed by changing the culture medium. The medium was changed every 4 days while adherent cells were harvested by trypsinization and re-plated. After 4-5 passages culture supernatants (CMMSC) were collected, centrifuged for 10 min at 1000 g to remove cell debris and stored at -80°C until used.
Characterization of MSC
Flow cytometry
Samples were analyzed by a flowcytometer (BD FACSCalibur 4 Color). Cells were washed, pelleted, resuspended in PBS and then incubated with anti-CD45-PE, anti-CD73-PE, anti-CD90-FITC, anti-CD44-PE antibodies (BD, USA). Specific isotype controls were used for background staining. All monoclonal antibodies (mAbs) used for flow cytometry in this study were purchased from BD Pharmingen.
Differentiation assays
For adipogenic differentiation, cells were seeded at a concentration of 2,5×104/cm2 in a 6-well plate. After 24 hrs of culture, adipogenic medium containing a-MEM, 10% FBS, (Gibco, USA) 10 ng/ml insulin and 1×10-8 M dexamethasone (Sigma, USA) was added and cells were grown for 3 weeks with medium replacement twice per week. Adipogenesis was detected by Oil Red O staining.
For osteogenic differentiation, cells were seeded at a concentration of 2,5×104/cm2 in a 6-well plate. After 24 hrs of culture, osteogenic medium containing a-MEM, 50 μg/ml L-ascorbic acid-2 phosphate, 10 mM glycerol 2-phosphate disodium salt, 1×10-8 M dexamethasone (Sigma, USA) and 200 μg/ml rBMP-2 (R&D, MN, USA) was added and cells were grown for 3 weeks with medium replacement twice per week. Osteogenic differentiation was detected by Alizarin red staining.
For chondrogenic differentiation, cells were grown in a micromass culture supplied with chondrogenic medium (0,2×106/tube). Chondrocytes were grown in a-MEM supplemented with, 1% FBS, 6,25 μg/ml insulin, 50 nM L-ascorbic acid 2-phosphate and 10 ng/ml TGF-β3 (R&D, MN, USA). Medium was replaced twice a week and chondrogenic differentiation was detected by Alcian blue staining [23].
Annexin V assay
Viability of hepatocyte cultures was monitored by FACS analysis using annexin V-propidium iodide staining. Cultured hepatocytes were detached, centrifuged, suspended in PBS and stained with annexin V-FITC and propidium iodide (BD Pharmigen, CA, USA). Apoptotic cells were identified as an annexin V-positive/propidium iodide-negative population. Analysis was performed using the FACScalibur cytometer (BD, USA) using FACs Diva 6 software.
Microparticle analysis
Microparticles were identified based on size determined by the 0.9 μm beads (BIOCYTEX, France) in a plot side scatter. The scatter characteristics based in 0.9 μm beads size was assessed by the logarithmic amplification of FCS and SSC signals. MSC supernatant was incubated with Annexin-FITC, anti-CD54-PE and anti-CD44-PE antibodies (BD, USA). The number of positive events for anti-CD54-PE, anti-CD44-PE antibodies and Annexin-FITC was calculated after accumulation of one hundred five thousands and seven hundred beads (105700) from Cytocount (ZEBRA BIOSCIENCE, NL). A 530/30 band pass filter was used for FL1 fluorescence to measure binding of Annexin V FITC. A 585/42 band pass filter was used for FL2 fluorescence to measure binding of PE-labeled antibodies. Data were analyzed using FACS Diva 6.1.2 software (BD, USA).
RT-PCR
Total RNA was isolated using Trisure (Bioline, UK) according to the manufacturer’s instructions. For cDNA synthesis 3 μg RNA were used as template in RT-PCR. The following primer sequences were used: albumin, 5’-GCATGAAGTTGCCAGAAGACATCC-3’ (forward) and 5’-TCTGCAGTTTGCTGGAGATAGTCG-3’ (reverse); HNF4, 5’-AAATGTGCAGGTGTTGACCA-3’ (forward) and 5’-GCTGTCCTCGTAGCTTGACC-3’ (reverse); cytokeratin, 18 5’-CGATACAAGGCACAGATGGA-3’ (forward) and 5’-CTTCTCCATCCTCCAGCAAG-3’ (reverse); β-actin 5’-TGGAATCCTGTGGCATCCATGAAAC-3’ (forward) and 5’-TAAAACGCAGCTCAGTAACAGTCCG-3’ (reverse).
Gene expression using the comparative method (ΔΔCT)
Total RNA was extracted using Qiagen RNeasy Mini kits (Qiagen, Germany) from the hepatocyte culture. Quantitative real-time PCR (qRT-PCR) was used to determine the FGL1 gene expression at different days. For gene expression analysis, pre-designed gene-specific primer pairs were used (Primer Express 3.0 program, Applied Biosystems, Foster City, USA). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene served as endogenous control. The reverse transcription reaction was performed with 2 μg of total RNA, random hexamer primers and the AMV reverse transcriptase (Promega, Madison, WI) using SYBR-Green PCR master mix (SA Biosciences, Foster City, USA), the manufacturer’s instructions and a 7500 Fast Real-Time PCR System (ABI, USA). Each PCR reaction was performed in triplicate and the average threshold cycle (ct) was used for the relative quantity (RQ) calculation after normalization to GAPDH. For qRT-PCR analysis of the mRNA populations the following primers were used FGL1-F (5’-CTCAGGTGCACCAGCTTGAG-3’), FGL1-R (5’-TCCGATCCTTTATCCAGAAACTG-3’) and GAPDH-F (5’-TGTGTCCGTCGTGGATCT-3’); GAPDH-R (5’-TTGCTGTTGAAGTCGCAGGAG-3’).
Total RNA was extracted from IL6-stimulated hepatocytes (100 ng/ml) (R&D, USA). Each sample was analysed using one-step qRT-PCR protocol according to the manufacturer’s instructions (Kappa Biosystems, USA). FGL1 and GAPDH reference gene sequence has been amplified in triplicate wells for 45 cycles on the same plate.
Determination of IL6
Interleukin 6 (IL-6) concentration in hepatocyte culture supernatants was determined by enzyme-linked immunosorbent assay using the Quantikine mouse IL-6 kit (R&D, MN, USA) in accordance to manufacturer’s instructions.
Statistical analysis
Results are reported as the mean ± SEM. Multiple comparisons were performed by the two-way analysis of variance (ANOVA). P values < 0.05 were considered significantly different.
Results
Gene expression of liver specific markers in hepatocytes
RT-PCR analysis of RNA isolated from cultured primary hepatocytes derived from untreated animals (control) or animals treated with CCl4, revealed strong expression of the hepatocyte specific genes cytokeratin 18, HNF4 and albumin at day 1 of culture (Figure 1A). Furthermore, time course experiments revealed stable albumin gene expression up to six days post culture initiation (Figure 1B). In addition, Northern analysis revealed stable levels of albumin or a1-antitrypsin mRNA levels in mouse primary hepatocytes cultured for 7 days that were reduced by day 9 (data not show). These data indicate that mouse primary hepatocytes maintain their differentiation under the culture conditions used. CMMSC from bone marrow-derived cells, exhibiting characteristic MSCs immunophenotype and differentiation capacity (Figure 2A and B), did not affect cytokeratin 18, albumin and HNF4 gene expression (Figure 1A).
Figure 1.
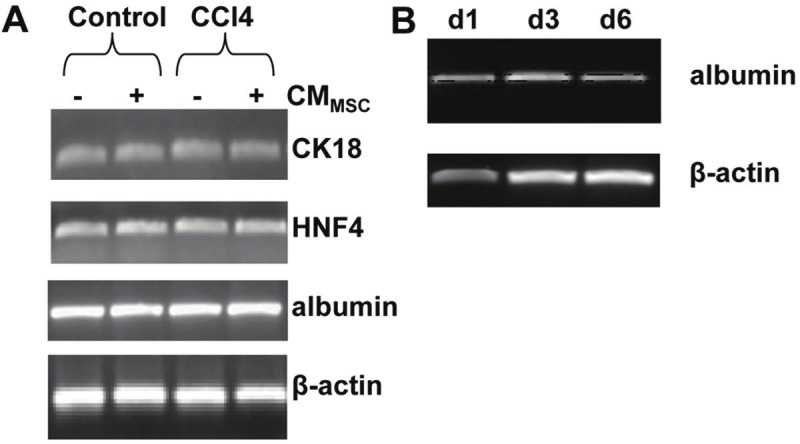
Analysis of hepatocyte marker gene expression in hepatocyte cultures. A: RT-PCR analysis of CK18, HNF4, albumin, and β-actin gene expression in hepatocyte cell culture derived from untreated (control) and CCl4-treated mouse livers at day 1 of hepatocyte culture. RT-PCR amplified RNA from hepatocyte cultures derived from control, and CCl4-treated mouse supplemented with CMMSC (+), or not supplemented (-). B: Expression of albumin was monitored by RT-PCR analysis on various days from control hepatocyte cell culture derived from day 1 (d1), day 3 (d3) and 6 (d6) respectively using β-actin gene as internal control.
Figure 2.
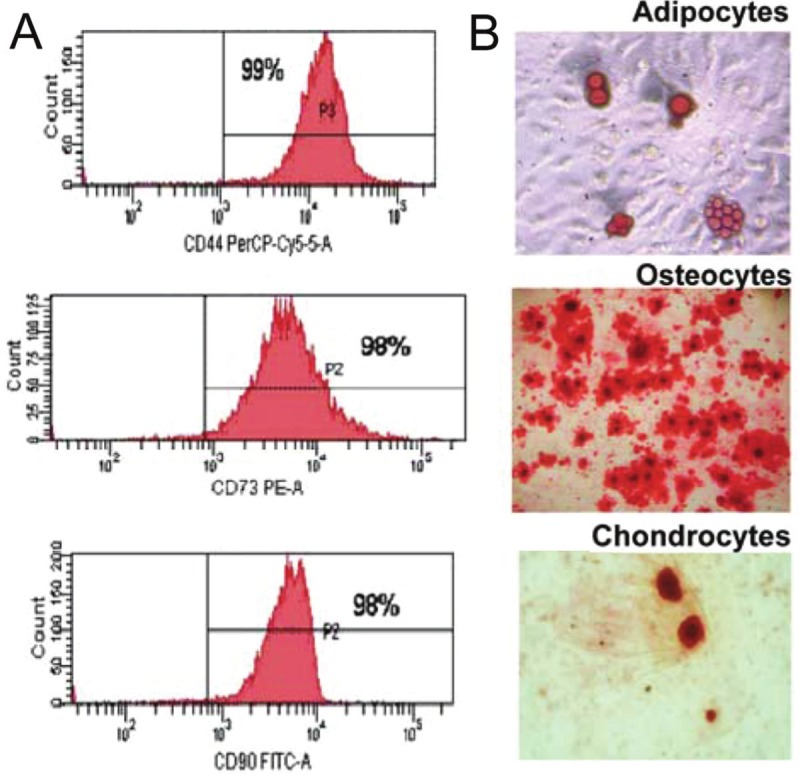
MSC characterization. A: Flow cytometric analysis of MSC cells. The cells at passage 4 were labeled with the CD44-Cy5 antibody, CD73-PE and CD90-FITC against the CD45-PE. Values show the percentage of positive cells. B: Assays for MSC differentiation. MSCs were cultured with lineage-specific media for 3 to 4 weeks to induce cell differentiation. Differentiation into adipocytes, osteocytes and chondrocytes was detected by oil Red O, alizarin red and alcian blue, respectively. Original magnification x 20 for adipocytes and chondrocytes and x 10 for osteocytes.
Effect of CMMSC on hepatocyte apoptosis
MSCs secrete a variety of growth factors and cytokines that inhibit hepatocellular death. To examine whether CMMSC affect early apoptosis of hepatocytes, annexin V levels were determined in cultures of control or CCl4 treated hepatocytes. To discriminate between apoptotic and necrotic cells the relative amount of the annexin V and propidium iodide (PI)-stained cells in the population were determined. Cultured hepatocytes derived from mice following a 3-day CCl4 treatment and addition of CMMSC in culture medium resulted in significant decrease of Annexin V positive cells/PI(-) (p<0.01) compared to CCl4 treated hepatocytes suggesting that CMMSC prevented activation of early apoptosis at days 3 (p<0.001) (Figure 3A and B). At day 3, the percentage of dead cells in CCl4 and CCl4-CMMSC annexin V (+)/PI(+) was higher compared to control cells. At day 4, an increase of annexin V(+)/PI(-) was observed in CCl4-CMMSC and control groups. CCl4 and CCl4-CMMSC annexin (+)/PI(+) cells were significantly higher compared to the control cells in contrast to CCl4 and CCl4-CMMSC early apoptotic cells (Figure 3A). These results indicate that CMMSC decreases early apoptosis rate in hepatocytes derived from CCl4 treated mice.
Figure 3.
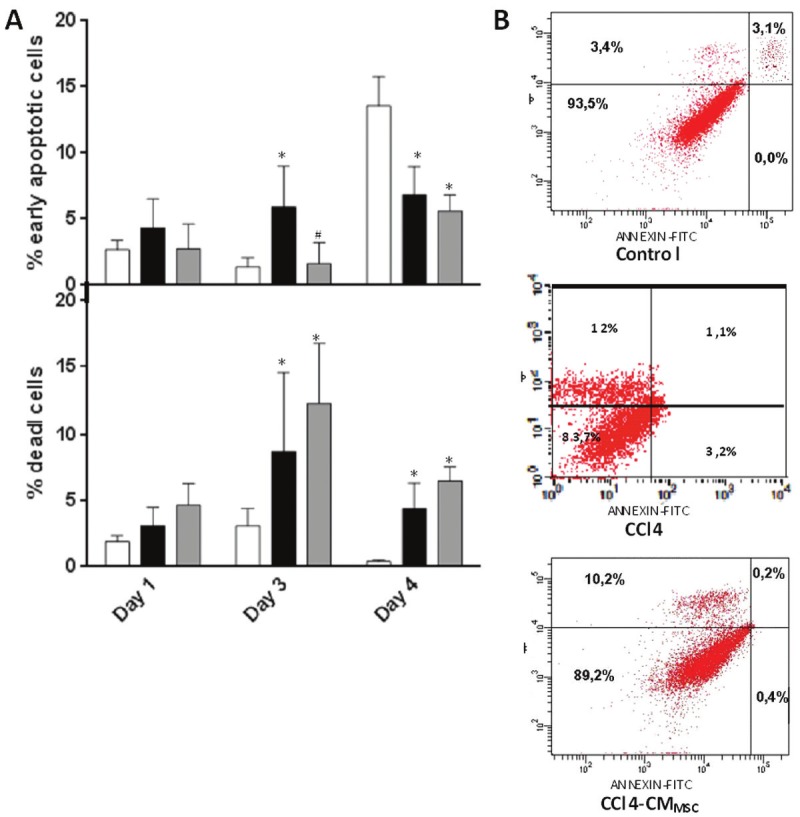
A: Analysis of apoptosis of hepatocytes by Annexin FITC staining. B: Flow cytometric analysis using Annexin and PI in primary hepatocytes derived from CCl4 treated mice with and without addition of CMMSC at day 3. Results are representative of 4 independent experiments. Control, CCl4 and CCl4-CMMSC represented by a white box, a black and a grey box, respectively. *P<0.05 compared with the control group and #P<0.05 compared with the CCl4 group.
Characterization of microparticles formed by MSC
MPs are membrane vesicles originating from blebbing membranes of apoptotic cells and can be identified by annexin V staining. These microparticles may contain surface glycoproteins and other molecules. The identification of MPs was assessed by flow cytometry based on size (scatter characteristics) and membrane marker expression. Small percentage of CMMSC-MPs expressed phoshatidylserine as demonstrated by binding of Annexin V localized within (R3) region (Figure 4A). Flow cytometry analysis showed 7,46% of annexin V-positive MPs reacted with Ab against CD44 and CD54 localized within the (R4) region (Figure 4B). These data demonstrate that MPs are generated by MSC and express membrane antigen CD54 and CD44.
Figure 4.
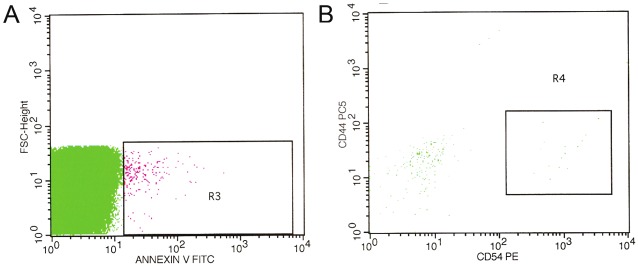
Detection of microparticles from CMMSC. A: Microparticles isolated from MSC supernatants were stained using CD54-PE and CD44-Cy5 antibodies and (B) microparticles were stained with annexin V and detected by flow cytometry.
IL-6 is highly secreted in the CCl4-CMMSC of primary hepatocytes
Under stress conditions in the liver, IL-6 is a major regulator of the acute phase response participating also in liver regeneration [28]. Under similar conditions, MSCs also produce IL-6 [5,6]. To determine IL-6 production in supernatants of hepatocyte cultures, we performed enzyme-linked immunosorbent assay. In MSC-conditioned medium, the IL-6 concentration was 319,8 ± 40,95 pg/ml. The data presented in Figure 6 show that IL-6 production is increased in hepatocytes treated with CCl4 and CCl4-CMMSC compared to control hepatocytes on days 1, 3 in culture (p<0.001). IL-6 production was significantly higher in CCl4-CMMSC compared to CCl4 cells (day 1 vs day 6 p<0.001, day6 vs day 3 p<0.01) (Figure 5). This finding indicates that CMMSC augmented IL6 production.
Figure 6.
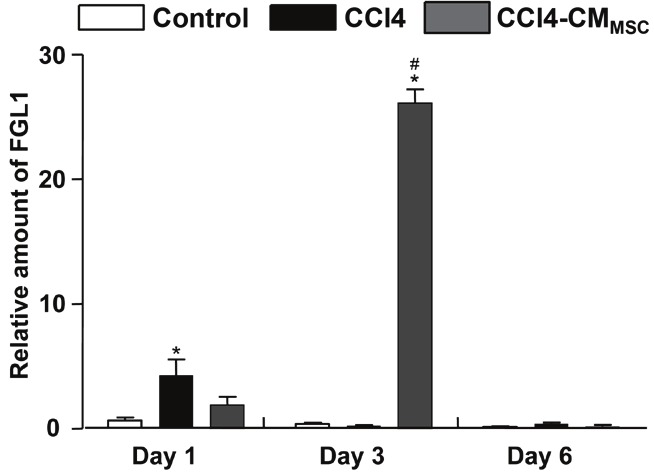
Relative mRNA expression of FGL1 in primary hepatocytes derived from untreated (control), CCl4-treated mice and CCl4-treated mice supplemented with CMMSC and was analysed by real time PCR. Values are expressed relative to GAPDH expression. Total RNA was extracted at days 1, 3 and 6 from primary hepatocytes and was analysed by real time PCR. Values are the mean ± SD of triplicate measurements *, #P<0.05 compared with the control and CCl4 groups respectively.
Figure 5.
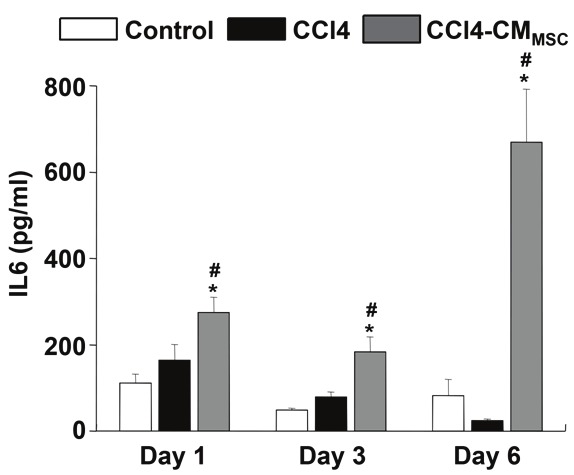
CMMSC induced IL6 secretion in hepatocytes. Cell supernatant was collected and analyzed by ELISA for IL6. Values are means ± SEM. At least 3 animals were examined in each group. Control, CCl4 and CCl4-CMMSC represented by a white box, a black and a grey box, respectively. *, #P<0.05 compared with the control and CCl4 groups respectively.
FGL1 expression in primary hepatocytes
It was recently reported that IL-6 induces expression of the mitogenic factor FGL1 in vitro, which is produced by parenchymal hepatocytes [15,17]. Relative quantification by real time PCR of FGL1 showed a marked increase by day 3 in hepatocytes derived from CCl4-treated mice and exposed to CMMSC, followed by a significant decline on culture day 6 (Figure 6). In hepatocytes derived from control livers, levels of FGL1 expression were relatively low.
IL6 induces FGL1 expression in primary hepatocytes
In order to assess the effect of IL6 in FGL1 expression in mouse primary hepatocytes, cells exposed to recombinant IL-6 at the concentration of 100 ng/ml for 24 hrs after dose response analysis (data not shown). The experiment demonstrate that there is an induction of FGL1 mRNA by IL-6 within 24 hours compared to cells derived from control mice but there is stronger induction in cells derived from CCl4-treated mice (Figure 7). These results indicate that FGL1 is activated at early stages of acute hepatocyte injury.
Figure 7.
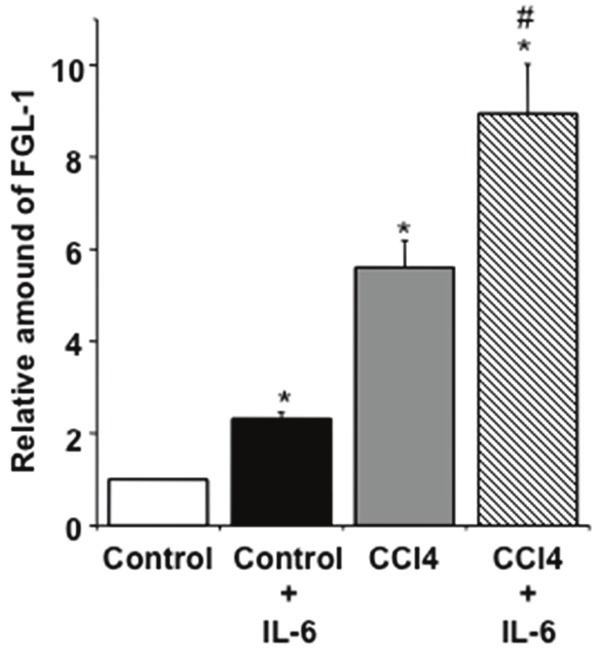
Total RNA was extracted from primary hepatocytes after 24 hrs treatment with recombinant IL6 at the concentration of 100 ng/ml for 24 hrs. Control, CCl4 and CCl4-CMMSC represented by a white box, a black and a grey box, respectively. Values are the mean ± SD of triplicate measurements *, #P<0.05 compared with the control and CCl4 groups respectively.
Discussion
This study demonstrates the early antiapoptotic activity of BM-MSC conditioned medium (CMMSC) on apoptosis induced in mouse primary hepatocytes by CCl4 and indicates that this activity is mediated by IL-6 and FGL1 overproduction. Our findings suggest that CMMSC contains factors that might be used as a therapeutic tool in acute liver injury.
There are reports in the literature showing that paracrine factors released by MSCs exhibit protective organ-actions [29,30]. The present work explored putative factors in a mouse model of CCl4-induced acute liver injury. Hepatocytes were isolated and cultured in the presence and absence of CMMSC. CMMSC significantly decreased the number of cultured hepatocytes undergoing early apoptosis following in vivo exposure to CCl4 with no significant difference at the percentage of dead cells. This indicates that CMMSC delays CCl4-induced apoptosis.
MSC-derived molecules modulate hepatocellular death and regeneration in a D-galactosamine-induced rat model [2]. Further evidence suggests that CMMSC-derived microparticles induces proliferation and apoptosis resistance in hepatocytes [23]. In our study, we detected few annexin V-positive microparticles released by MSCs. Flow cytometry analysis showed that 7,8% of annexin V-positive binding MPs coexpressed CD54/CD44 meaning that MP may contain signaling molecules which can regulate apoptosis. In parallel, there are annexin V-negative MPs, which may activate cell proliferation and tissue regeneration [21]. Further studies will be necessary to clarify the precise mechanism of action of microparticles.
MSCs as well as hepatocytes produce IL6 [13,14]. IL6 has a hepatoprotective function by preventing CCl4 toxicity [14,15]. In our experiments, IL6 levels were increased in CCl4-CMMSC hepatocytes. Hepatocytes are triggered to release IL6 especially in CCl4-CMMSC cells through autocrine or paracrine function of IL6. The significantly higher IL6 levels in CCl4-CMMSC hepatocytes compared to control and CCl4 hepatocytes on the first day of culture indicate that IL6 is derived from CMMSC. Subsequently, IL6 was increased simultaneously with a decrease of early apoptosis suggesting that it might promote survival. However, the increased IL6 levels on the last day of culture contributes to the progression of apoptosis. Therefore IL6 regulates the apoptosis of hepatocytes derived from CCl4-treated mice.
Our study reinforces previous observation that IL6 induces FGL1 expression [17]. IL6 induces FGL1 expression in hepatocytes derived from control and CCl4-treated mice. The FGL1 induction was higher in hepatocytes from CCl4 treated mice compared to control. FGL1 has been characterised mainly as a mitogenic and survival factor [19,31] although there are indications that the human FGL1 suppress proliferation in hepatocellular carcinoma cells [17]. Our data indicate that FGL1 gene expression was upregulated when early apoptosis (culture day 3) was inhibited in CCl4-CMMSC hepatocytes with a subsequent decrease on the last day (culture day 6). Thus, it would be reasonable to postulate that IL6 and FGL1 contribute to the antiapoptotic effect of CMMSC treatment noticed on culture day 3. Taken together this data suggest that IL6 contained in CMMSC delays apoptosis.
The downstream factors in IL-6 signal transduction pathways, nuclear factor for IL6 (NF-IL6) as well as signal transducer and activator of transcription 3 (STAT-3) show conserved DNA binding sites at the 5′ flanking region of FGL1 in rats [19]. The upregulation of FGL1, which occurred when early apoptosis (culture day 3) was inhibited, may suggest that IL6 modulates the apoptotic process in acute liver injury.
Taken together, our observations indicate that BM-MSC conditioned medium contains factors that could exert a potential therapeutic benefit in CCl4-induced acute hepatocyte injury disease.
Acknowledgement
The authors gratefully acknowledge the helpful comments of Professor E.Lianos in reviewing this manuscript, Dr E.Yiannaki and Dr E. Chlichlia for helpful suggestions and Dr Y. Dalezios for assistance in statistical analysis. They also thank members of Cell and Gene Therapy lab for their assistance with animal injections.
Declairation
All authors declare no conflict of interest.
References
- 1.Caplan A, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 2.van Poll D, Parekkadan B, Cho CH, Berthiaume F, Nahmias Y, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2004;47:1634–43. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 3.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–7. doi: 10.1073/pnas.0704421104. Epub 2007 Jun 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salazar KD, Lankford SM, Brody AR. Mesenchymal stem cells produce Wnt isoforms and TGF-beta1 that mediate proliferation and procollagen expression by lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1002–11. doi: 10.1152/ajplung.90347.2008. Epub 2009 Sep 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, Proya E, Anagnostopoulos A, Fassas A. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108–19. doi: 10.1016/j.exphem.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Bucher NL. Experimental aspects of hepatic regeneration. N Engl J Med. 1967;277:738–46. doi: 10.1056/NEJM196710052771405. [DOI] [PubMed] [Google Scholar]
- 9.Michalopoulos G. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi J, Aisaki K, Ikawa Y, Wake K. Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am J Pathol. 1998;153:515–25. doi: 10.1016/S0002-9440(10)65594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leu JI, Crissey MA, Taub R. Massive hepatic apoptosis associated with TGF-beta1 activation after Fas ligand treatment of IGF binding protein-1-deficient mice. J Clin Invest. 2003;111:129–39. doi: 10.1172/JCI16712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weil BR, Markel TA, Herrmann JL, Abarbanell AM, Meldrum DR. Mesenchymal stem cells enhance the viability and proliferation of human fetal intestinal epithelial cells following hypoxic injury via paracrine mechanisms. Surgery. 2009;146:190–197. doi: 10.1016/j.surg.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 14.Bansal MB, Kovalovich K, Gupta R, Li W, Agarwal A, Radbill B, Alvarez CE, Safadi R, Fiel MI, Friedman SL, Taub RA. Interleukin-6 protects hepatocytes from CCl4-mediated necrosis and apoptosis in mice by reducing MMP-2 expression. J Hepatol. 2005;42:548–556. doi: 10.1016/j.jhep.2004.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovalovich K, Li W, DeAngelis R, Greenbaum L, Cilberto G, Taub R. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-XL. J Biol Chem. 2001;276:26605–26613. doi: 10.1074/jbc.M100740200. [DOI] [PubMed] [Google Scholar]
- 16.Yun JW, Kim CW, Bae IH, Park YH, Chung JH, Lim KM, Kang KS. Determination of the key innate genes related to individual variation in carbon tetrachloride-induced hepatotoxicity using a pre-biopsy procedure. Toxicol Appl Pharmacol. 2009;239:55–63. doi: 10.1016/j.taap.2009.05.018. Epub 2009 May 27. [DOI] [PubMed] [Google Scholar]
- 17.Yu HT, Yu M, Li CY, Zhan YQ, Xu WX, Li YH, Li W, Wang ZD, Ge CH, Yang XM. Specific expression and regulation of hepassocin in the liver and down-regulation of the correlation of HNF1a with decreased levels of hepassocin in human hepatocellular carcinoma. J Biol Chem. 2009;284:13335–13347. doi: 10.1074/jbc.M806393200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan J, Ying H, Gu F, He J, Li YL, Liu HM, Xu YH. Cloning and characterization of a mouse liver-specific gene mfrep-1, upregulated in liver regeneration. Cell Res. 2002;12:353–361. doi: 10.1038/sj.cr.7290137. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Ukomadu C. Fibrinogen-like-protein 1, a hepatocyte derived protein is an acute phase reactant. Biochem Biophys Res Commun. 2008;365:729–734. doi: 10.1016/j.bbrc.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara H, Uchida S, Yoshimura H, Aoki M, Toyoda Y, Sakai Y, Morimoto S, Fukamachi H, Shiokawa K, Hanada K. Isolation and characterization of a novel liver-specific gene, hepassocin, up-regulated during liver regeneration. Biochim Biophys Acta. 2000;1492:31–44. doi: 10.1016/s0167-4781(00)00056-7. [DOI] [PubMed] [Google Scholar]
- 21.Tetta C, Fonsato V, Deregibus MC, Camussi G. The role of microvesicles in tissue repair. Organogenesis. 2001;7:105–115. doi: 10.4161/org.7.2.15782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–24. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C, Camussi G. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–1618. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otsuka T, Takagi H, Horiguchi N, Toyoda M, Sato K, Takayama H, Mori M. CCl4-induced acute liver injury in mice is inhibited by hepatocyte growth factor overexpression but stimulated by NK2 overexpression. FEBS Lett. 2002;532:391–5. doi: 10.1016/s0014-5793(02)03714-6. [DOI] [PubMed] [Google Scholar]
- 25.Klaunig JE, Goldblatt PJ, Hinton DE, Lipsky MM, Chacko J, Trump BF. Mouse liver culture. In vitro. 1981;17:913–925. doi: 10.1007/BF02618288. [DOI] [PubMed] [Google Scholar]
- 26.Sun S, Guo Z, Xiao X, Liu B, Liu X, Tang PH, Mao N. Isolation of Mouse Marrow Mesenchymal Progenitors by a Novel and Reliable Method. Stem Cells. 2003;21:527–535. doi: 10.1634/stemcells.21-5-527. [DOI] [PubMed] [Google Scholar]
- 27.Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–71. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 28.Wuestefeld T, Klein C, Streetz KL, Betz U, Lauber J, Buer J, Manns MP, Müller W, Trautwein C. Interleukin-6/Glycoprotein 130-dependent pathways are protective during liver regeneration. J Biol Chem. 2003;278:11281–11288. doi: 10.1074/jbc.M208470200. [DOI] [PubMed] [Google Scholar]
- 29.Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, Zhang L, Pratt RE, Dzau VJ, Ingwall JS. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009;27:971–9. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tögel FE, Westenfelder C. Mesenchymal stem cells: a new therapeutic tool for AKI. Nat Rev Nephrol. 2010;6:179–83. doi: 10.1038/nrneph.2009.229. [DOI] [PubMed] [Google Scholar]
- 31.Li CY, Cao CZ, Xu WX, Cao MM, Yang F, Dong L, Yu M, Gao YB, Li W, Wang ZD, Ge CH, Wang QM, Peng RY, Yang XM. Recombinant human hepasoccin stimulates proliferation of hepatocytes in vivo and improves survival in rats with fulminant hepatic failure. Gut. 2010;59:817–826. doi: 10.1136/gut.2008.171124. [DOI] [PubMed] [Google Scholar]


