Abstract
Hemangioblastoma (HB), a rare neoplasm of uncertain histogenesis, is characterized histologically by the presence of vacuolated; lipid-containing cells ‘stromal cells’ and a well developed, fine capillary network. Stromal cells are the neoplastic component of this tumor. Five-um sections were stained using streptavidin- biotin immunoperoxidase and immunofluorescent techniques. The stromal cells were uniformly “HIF-1α, Galectin-3, VEGF, VEGFR, WT-1, and bcl2,” positive. Endothelial cells but not stromal cells were uniformly immunoreactive to CD31. Co-localization of HIF-1α with galectin-3 and VEGF as well as galectin-3 with VEGF in stromal cells is confirmed by immunofluorescent technique. In conclusion, the development of HB is multi-factorial and the expression of galectin-3 correlates with the expression of HIF-1α and VEGF. Galectin-3 can be used as a marker for the diagnosis of HB as well as it can be a valuable candidate for future targeting immunotherapy.
Keywords: CNS neoplasms, hemangioblastoma, HIF, galectin-3
Introduction
Haemangioblastoma (HB) is a rare neoplasm of uncertain histogenesis. HB represents 1.5-2.5% of all intracranial neoplasms and 7-12% of posterior fossa neoplasms. They are usually infratentorial, with a majority of them occurring over the cerebellum (76%) around the fourth ventricle and less commonly in the cerebral hemispheres (9%), spinal cord (7%) and brain-stem (5%) [1]. HB is characterized histologically by the presence of vacuolated; lipid-containing cells ‘stromal cells’ and a well developed, fine capillary network. Stromal cells represent the neoplastic component of the neoplasm [1-3].
HB is subdivided into two variants: a reticular variant, where stromal cells are evenly distributed among the capillary meshwork; and a cellular variant in which stromal cells are arranged in larger nests or sheets [1-3]. Although it has been the subject of long-term controversy, the histogenesis of stromal cells is still unresolved [2]. Hence, hemangioblastoma constitutes a single entity under the designation ‘tumors of uncertain histogenesis’ in the WHO 2000 classification [3] and ‘other neoplasms related to meninges’ in the WHO 2007 classification [4]. Galectin-3, a glycoprotein with a 31-kDa molecular weight, is a member of the beta-galactoside binding family of lectins [5]. It has been suggested to play a role in a variety of biological processes such as cell growth, cellular adhesion, cell cycle regulation, neoplastic transformation and metastasis [5]. Galectin-3 is a proangiogenic molecule that plays an important role in vascular endothelial proliferation [6].
Hypoxia-inducible factor (HIF) is a set of transcription factors that regulate the cellular response to hypoxia [7]. HIF is a heterodimeric DNA-binding complex composed of two basic helix-loop-helix proteins, the constitutive expressed HIF-β or aryl hydrocarbon nuclear translocator [8] and the oxygen sensitive hypoxia-inducible HIF-α [9]. HIF heterodimers recognize and bind to hypoxia response elements (HREs) in the genes that have the consensus sequence G/ACGTG [8]. Under normoxic conditions, hydroxylation of two prolyl residues by prolyl hydroxylases in the oxygen dependent degradation domain of the α-subunits occurs [10]. This causes the von Hippel-Lindau tumor suppressor protein (pVHL) to interact with the α-subunit, targeting it for proteolysis by the ubiquitin-proteasome pathway [11]. Therefore, in normoxia HIF-1α subunit has a very short half-life [12] and cells continuously synthesize and degrade HIF-1α protein. In hypoxia, prolyl hydroxylation is inhibited, HIF-1α protein escapes proteasomal degradation, translocates to the nucleus and dimerizes with HIF-1β. This complex then binds to the HRE in promoter or enhancer sequences of target genes [13] and results in their transcription. In the current monograph we show the expression of galectin-3 correlates with the expression of HIF-1α and VEGF in HB.
Materials and methods
Tissue sections
Five-μm sections were prepared from Paraffin blocks of four cases of HBs which were retrieved from the surgical pathology archive in the pathology department at Tawam hospital in AlAin city in the United Arab Emirates (UAE). All Sections were stained with hematoxylin and eosin.
Immunohistochemistry
Five-μm sections were used for immunohistochemical staining which was performed by standard techniques using the following antibodies: galectin-3 (mouse monoclonal, clone NCL, 1:100, Novocastra, UK); HIF-1α (rabbit polyclonal antibody, 1:250, Davids Biotechnologie GmbH, Germany), WT-1 (mouse monoclonal, clone 6FH2, 1:50, LAB VISSION, USA), CD31 (mouse monoclonal, clone JC70, 1:100 Cell Marque, USA), bcl2 (mouse monoclonal, clone 124, 1:150 Cell Marque, USA), VEGF (rabbit polyclonal A20, 1:100, Santa Cruz biotechnology, USA), VEGFR (rabbit polyclonal C20, 1:100, Santa Cruz biotechnology, USA). Sections from benign prostatic hyperplasia tissue were used as positive controls for galectin-3. Placental tissue was used as a positive control for VEGF, VEGFR, CD31 and HIF 1α. Sections from lymph node were used as positive control for bcl2. Sections from ovarian serous carcinoma were used as positive control for WT-1. For negative controls, primary antibody was replaced with normal goat serum and carried out the whole procedure.
Immunofluorescent double labeling
Double-immunofluorescence analysis was done on 5-μm sections to show co-localization of our proteins of interest in various parts of the tissue sections. Five-μm sections were deparaffinized with xylene and rehydrated with descending concentrations of ethanol. The sections were later treated with HIF-1α (rabbit polyclonal antibody, 1:50, Davids Biotechnologie GmbH, Germany) overnight at 4°C. Similar sections were also treated separately with VEGF (rabbit polyclonal A20, 1:50, Santa Cruz biotechnology, USA). All sections were subsequently incubated in donkey Ig conjugated-Rodamine (1:100, Santa Cruz biotechnology, USA) for 2 hours at room temperature. After washing several times in PBS, the same sections were later incubated overnight at 4°C with galectin-3 (rabbit polyclonal, 1:50, H160, Santa Cruz biotechnology, USA). After washing several times in PBS, the sections were incubated for 2 hours at room temperature in donkey Ig conjugated-FITC (1:50). The sections were mounted in water-soluble mounting media and viewed with Olympus Fluorescent microscope.
Interpretation of results
Two pathologists reviewed the slides independently. For IHC, cells were labeled as positive for HIF 1α, WT-1 expression if the neoplastic cells were reactive in a nuclear staining pattern. Cells were labeled positive for galectin-3 if they were reactive in a cytoplasmic and/or nuclear staining pattern. Cells were labeled as positive for CD31, VEGF, VEGF-R, and bcl2 expression if the neoplastic cells were reactive in a cytoplasmic and or membranous staining pattern. For immunofluorescent double labeling, cells were counted positive when the show combined expression of galectin-3 with HIF 1α and galectin-3 with VEGF.
Stromal cells and endothelial cells expressing Galectin-3, HIF 1α and VEGF were counted per 100 cells of hemangioblastoma using image J software (http://nih.gov/ij/).
Statistical analysis
Data were analyzed using Statistical Package for Social Sciences (SPSS) (version 20) statistical program (SPSS, Chicago, IL). P value≤0.05 considered significant. Pearson’s R was used to measure the correlation between the expression of galectin-3 and the expression of HIF 1α and VEGF.
Results
Light microscopic examination
The tumor sections exhibited numerous arborizing capillary-size blood vessels lined by endothelial cells with intervening clear stromal cells. The stromal cells have round to elongated hyperchromatic nuclei with inconspicuous nucleoli and vacuolated clear cytoplasm (Figure 1A).
Figure 1.
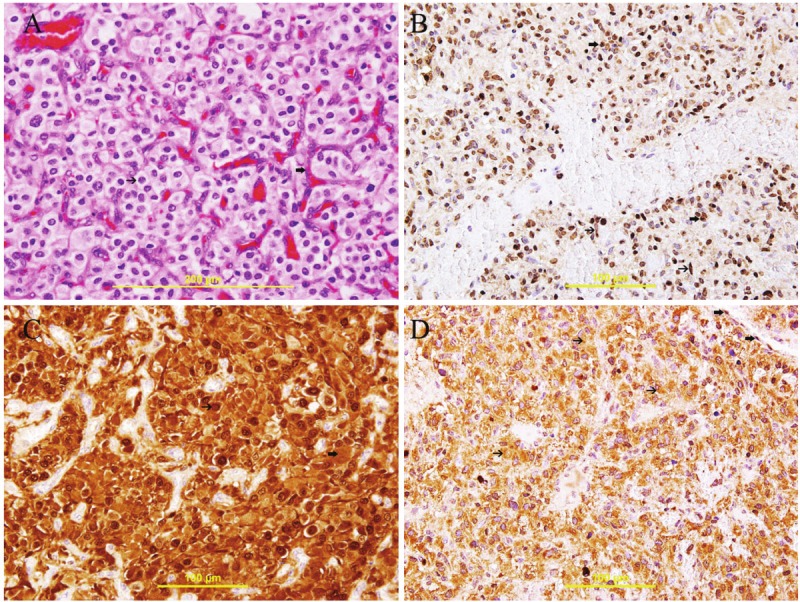
Cerebellar Hemangioblastoma. A: Clusters and thick trabeculae of cells with clear cytoplasm and uniform round nuclei (thin arrow) surrounded by rich capillary net work (thick arrow), X400, H&E. B: Nuclear expression of HIF 1α in all stromal cells (thick arrow) and some endothelial cells (thin arrow), streptavidin-biotin immunoperoxidase method, X400. C: Nuclear (thin arrow) and cytoplasmic (thick arrow) expression of galectin-3 in stromal cells and some endothelial cells, streptavidin-biotin immunoperoxidase method, X400. D: Cytoplasmic expression of VEGF in stromal cells (thin arrow and some endothelial cells (thick arrow), streptavidin-biotin immunoperoxidase method, X400.
Immunohistochemical study
Expression of HIF 1α
There is nuclear expression of HIF 1α by all stromal cells and many endothelial cells in 94.1% and 93.8% respectively (Table 1) and (Figure 1B).
Table 1.
Frequency of expression of galectin-3, HIF 1α and VEGF in HB
| Cell Type | Galectin-3 | HIF 1α | VEGF |
|---|---|---|---|
| Stromal cells | 92.80% | 94.1% | 94.80% |
| Endothelial cells | 89.4% | 93.8% | 93.4% |
Expression of VEGF
There is cytoplasmic expression of VEGF by 94.8% of stromal cells and 93.4% of endothelial cells (Table 1) and (Figure 1D).
Expression of VEGFR
The stromal cells show diffuse cytoplasmic expression of VEGFR in all sections (Figure 2A).
Figure 2.
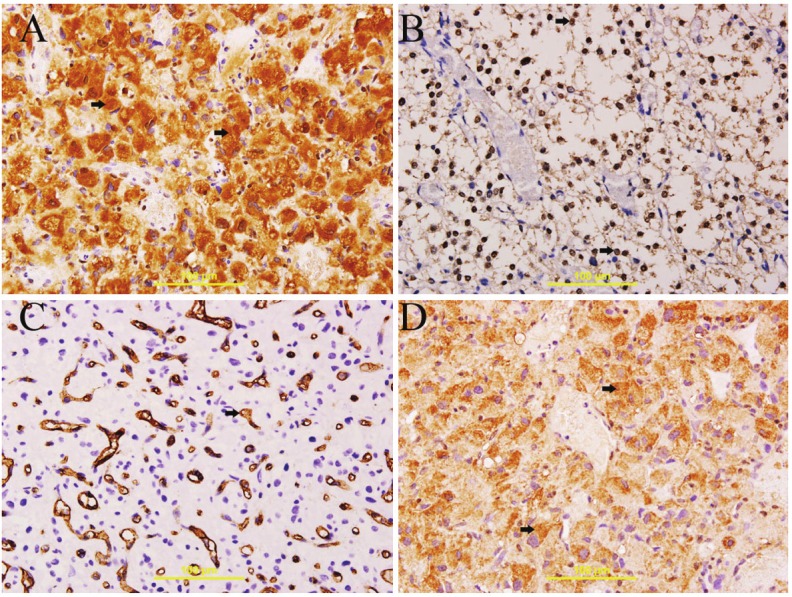
Expression of VEGFR, WT-1, CD31 and bcl2 in HB. A: Cytoplasmic expression of VEGFR in stromal cells (thick arrow), streptavidin-biotin immunoperoxidase method, X400. B: Nuclear expression of WT-1 protein in all stromal cells (thick arrow), streptavidin-biotin immunoperoxidase method, X400. C: cytoplasmic and membranous expression of CD31 in endothelial cells, streptavidin-biotin immunoperoxidase method, X400. D: cytoplasmic expression of bcl2 in stromal cells (thick arrow), streptavidin-biotin immunoperoxidase method, X400.
Expression of galectin-3
There is cytoplasmic and nuclear expression of galectin-3 by 92.8% of stromal cells and 89.4% of endothelial cells (Table 1) and (Figure 1C).
Expression of WT-1 protein
There is diffuse nuclear expression of WT-1 protein by all stromal cells in all sections (Figure 2B).
Expression of bcl2
There is diffuse cytoplasmic expression of bcl2 by all stromal cells in all sections (Figure 2D).
Expression of CD31
There is diffuse cytoplasmic and membranous expression of CD31 by all endothelial cells while stromal cells show no expression in all sections (Figure 2C).
Immunofluorescent double labeling Study
HIF 1α and Galectin-3 double labeling
There is co-localization of HIF 1α and Galectin-3 in stromal cells (Figure 3).
Figure 3.
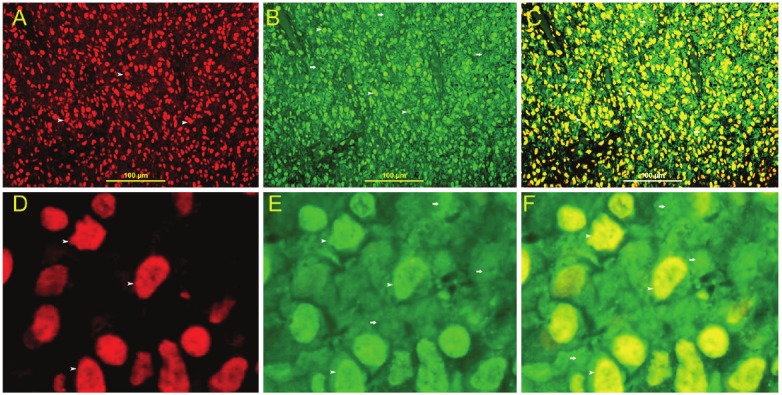
HIF 1α and Galectin-3 double labeling. A: Nuclear expression of HIF 1α in stromal cells (arrow head), Rhodamine, IF, X400. B: Nuclear (arrow head) and cytoplasmic (thick arrow) expression of galectin-3 in stromal cells, FITC, IF, X400. C: Co-expression of HIF 1α and galectin-3 in all stromal cells. There is nuclear co-localization of HIF 1α and galectin-3 giving a yellow color (arrow head). There is cytoplasmic expression of galectin-3 (thick arrow), IF, Rhodamine-FITC double labeling, X400. D, Nuclear expression of HIF 1α in stromal cells (arrow head), Rhodamine, IF, X1000. E. Nuclear (arrow head) and cytoplasmic (thick arrow) expression of galectin-3 in stromal cells, FITC, IF, X1000. F. Co-expression of HIF 1α and galectin-3 in all stromal cells. There is nuclear co-localization of HIF 1α and galectin-3 giving a yellow color ( arrow head). There is cytoplasmic expression of galectin-3 (thick arrow), IF, Rhodamine-FITC double labeling, X1000.
HIF 1α and VEGF double labeling
There is co-localization of HIF 1α and VEGF in all stromal cells (Figure 4).
Figure 4.
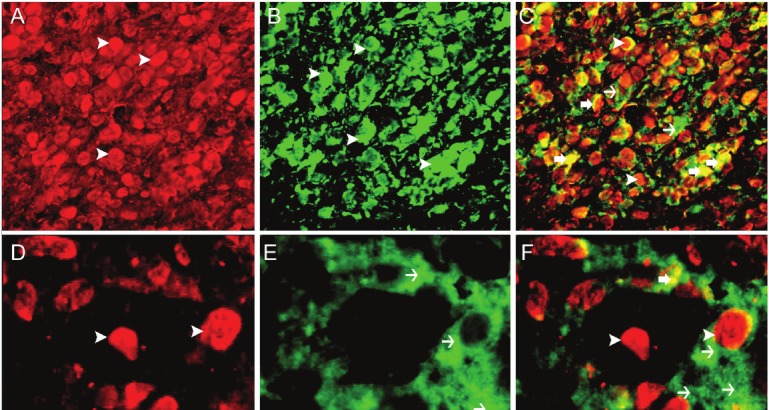
HIF 1α and VEGF double labeling. A: Nuclear expression of HIF 1α in stromal cells (arrow head), IF, Rhodamine, X600. B: cytoplasmic (arrow head) expression of VEGF in stromal cells, IF, FITC, X600. C: Co-expression of HIF 1α and VEGF in all stromal cells. There is nuclear expression of HIF 1α (arrow head). There is cytoplasmic expression of VEGF (thin arrow). There is cytoplasmic and peri-nuclear co-localization of HIF 1α and VEGF (thick arrow) giving a yellow color, IF, Rhodamine-FITC double labeling, X600. D: Nuclear expression of HIF 1α in stromal cells (arrow head), IF, Rhodamine, X1000. E: Cytoplasmic (arrow head) expression of VEGF in stromal cells, IF, FITC, X1000. F: Co-expression of HIF 1α and VEGF in all stromal cells. There is nuclear expression of HIF 1α (arrow head). There is cytoplasmic expression of VEGF (thin arrow). There is cytoplasmic and peri-nuclear co-localization of HIF 1α and VEGF (thick arrow) giving a yellow color, IF, Rhodamine-FITC double labeling, X1000.
Galectin-3 and VEGF double labeling
There is co-localization of galectin-3 and VEGF in all stromal cells (Figure 5).
Figure 5.
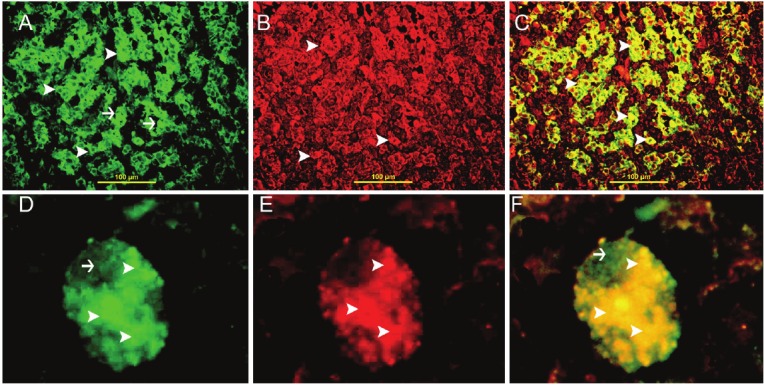
Galectin-3 and VEGF double labeling. A: Cytoplasmic (arrow head) and nuclear (thin arrow) expression of Galectin-3 in stromal IF, FITC, X400. B: Cytoplasmic expression of VEGF (arrow head) by all stromal cells, IF, Rhodamine, X400. C: Co-expression of galectin-3 and VEGF in all stromal cells. There is cytoplasmic co-localization of galectin-3 and VEGF (arrow head) in stromal cells giving a yellow color, IF, Rhodamine-FITC double labeling, X400. D: Cytoplasmic (arrow head) and nuclear (thin arrow) expression of Galectin-3 in one stromal cell, IF, FITC, X10000. E: Cytoplasmic expression of VEGF (arrow head) in one stromal cell, IF, Rhodamine X10000. F: Co-expression of galectin-3 and VEGF in one stromal cell. There is cytoplasmic co-localization of galectin-3 and VEGF (arrow head) giving a yellow color, IF, Rhodamine-FITC double labeling, X10000.
Correlation between the expression of Galectin-3, HIF 1α and VEGF
The expression of galectin-3 is significantly correlated with the expression of HIF 1α in stromal cells (P=0.04) and endothelial cells (P=0.019). The expression of galectin-3 is significantly correlated with the expression of VEGF in stromal cells (P=001) and endothelial cells (P=0.05) (Table 2).
Table 2.
Correlation between the expression of galectin-3, HIF 1α and VEGF in HB
| Galectin-3 | Cell Type | HIF 1α | VEGF |
|---|---|---|---|
| Stromal cells | P=0.04 | P=001 | |
| Endothelial cells | P=0.019 | P=0.05 |
Discussion
HB is a rare low grade neoplasm that occurs either sporadically in 75% of cases or as a manifestation of von Hippel-Lindau (VHL) disease in 25% of cases, with VHL alleles reported to be inactivated in up to 50% of sporadic and 100% in VHL associated.
HB [1-3]. Although the embryological origin of the stromal cells in HB is debated, it is clear from laser capture microdissection and immunohistochemical studies that stromal cells are the cells in which von Hippel-Lindau protein (pVHL) function has been compromised [2,3]. VHL mutations in HB invariably lead to HIF dysregulation [14]. HIF is not only induced in hypoxia but also other factors such as insulin-like growth factor [15], epidermal growth factor [16], interleukin-1 [17], Ras [18] and Src [19] oncogenes are also known to regulate it.
Loss or inactivation of VHL leads to accumulation of HIF in pVHL-defective HB stromal cells and drives the production of various hypoxia inducible mRNAs including the mRNAs encoding vascular endothelial growth factor (VEGF), platelet-derived growth factor B, erythropoietin, Galectin-3, WT-1 protein, and transforming growth factor alpha. VEGF is a paracrine growth factor that stimulates endothelial cells and leads to vascular proliferation [20].
In the present study, we show stromal cells with strong and diffuse nuclear and cytoplasmic staining of galectin-3 suggesting over expression of galectin-3 gene (Figure 1C). We show diffuse and strong nuclear staining of HIF-1α in stromal cells and many endothelial cells (Figure 1B). The diffuse and intense staining of galectin-3 by stromal cells can be used as a histologic marker to diagnose HB. In addition, it highlights a possible role of galectin-3 in the development of HB; however, this is just speculative and needs further studies for confirmation.
We also show co-localization of HIF-1α and galectin-3 in stromal cells (Figure 3) and show a significant correlation between the expression of galectin-3 and HIF-1α in stromal cells suggesting that up-regulation of galectin-3 gene might be mediated by HIF-1α. This is a novel finding and has not been previously reported in HB. This finding is supported by Greijer et al. which have shown galectin-3 up-regulation to be mediated mainly by HIF-1α [20]. Zeng et al. also demonstrates that HIF-1α regulates galectin-3 expression by interacting with hypoxia regulatory elements in the promoter region [21]. Koch et al, have shown secreted galectin-3 may lead to proliferation of endothelial cells by inducing chemotaxis and facilitating their motility during the initial phase of tube formation, which might be an essential step in the development of HB [22].
We also show diffuse expression of VEGF (Figure 1D) and VEGFR (Figure 2A) by stromal cells as well as we were able to co-localize HIF-1α and VEGF in stromal cells (Figure 4), suggesting that VEGF expression in HB might be mediated by HIF-1α. Calvani et al. have also shown that up-regulation of both VEGF & bFGF is mediated mainly by HIF-1α [23]. In addition, Galectin-3 is an important pro-angiogenic molecule that plays an important role in vascular endothelial proliferation through up-regulation of VEGF and bFGF [6]. We also show co-localization of galectin-3 and VEGF in stromal cells and a significant correlation between the expression of galectin-3 and VEGF in stromal cells and endothelial cells, which supports their role in the proliferation of stromal cells and endothelial cells. This finding is supported by Koch et al. which have also shown a significant up-regulation of αvß3 integrins by treating the endothelial cells with exogenous galectin-3 and the formation of capillary tubes induced by galectin-3 was inhibited by the neutralization of the action of galectin-3 by its specific carbohydrate inhibitors or the specific antibodies [22]. Overexpresion of integrin αvß3 will lead to endothelial cell migration, attachment and vascular proliferation [24].
The high and specific expression of galectin-3 by stromal cells in HB can be a useful marker for the diagnosis of HB as well as it can be a valuable candidate for future targeting immunotherapy.
We also show expression of WT-1 by stromal cells (Figure 2B). WT-1 gene is an HIF-1α target and over expression of HIF-1α by stromal cells mediates expression of WT-1 protein. WT-1 protein is a transcription factor and can mediate transcription of factors involved in angiogenesis [25]. We also show expression of Bcl2 by stromal cell. Bcl2 is an antiapoptotic protein; hence its expression by stromal cells might be an essential step in tumor formation [26]. Bcl2 can stabilize HIF-1α through the impairment of ubiquitin dependent HIF-1α degradation with the involvement of the β isoform of the molecular chaperone HSP90 and leading to over expression of HIF-1α [27]. On the other hand, up-regulation of HIF-1α can induce over expression of bcl2 [26].
We believe that there is a synergistic action between HIF-1α and galectin-3 in the development of HB, since galectin-3 up-regulation is possibly HIF-1α-mediated, and both VEGF and bFGF-up-regulation are probably mediated by both galectin-3 and HIF-1α, however; functional studies are needed to confirm the mechanistic relationship between the expression of galectin-3, HIF-1α and VEGF in HB, as immunohistochemical analysis is not enough to establish a mechanistic relationship.
In conclusion, the development of HB is multi-factorial and the expression of galectin-3 correlates with the expression of HIF-1α and VEGF. Galectin-3 can be used as a marker for the diagnosis of HB as well as it can be a valuable candidate for future targeting immunotherapy.
Acknowledgement
We would like to Acknowledge Ms Manjusha Sudhadevi from department of Pathology, Faculty of Medicine & Health Sciences, UAE University and Mr. Aktham Awwad from department of Laboratory Medicine, Tawam Hospital, AlAin, UAE, for their technical efforts.
Declaration
Authors declare that there is No conflict of interest.
References
- 1.Hussein MR. Central nervous system capillary hemangioblastoma: the pathologist’s view-point. Int J Exp Pathol. 2007;88:311–324. doi: 10.1111/j.1365-2613.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishizawa K, Komori T, Hirose T. Stromal cells in hemangioblastoma: neuroectodermal differentiation and morphological similarities to ependymoma. Pathol Int. 2005;55:377–385. doi: 10.1111/j.1440-1827.2005.01841.x. [DOI] [PubMed] [Google Scholar]
- 3.Lantos PL, Louis DN, Rosenblum MK, Kleihues P. Tumours of the nervous system. In: Graham DI, Lantos PL, editors. Greenfield’s Neuropathology. 7th edition. London: Arnold; 2002. pp. 767–1052. [Google Scholar]
- 4.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins: structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- 6.Markowska AI, Liu FT, Panjwani N. Galectin-3 is an important mediator of VEGF- and bFGF-mediated angiogenic response. J Exp Med. 2010;207:1981–1993. doi: 10.1084/jem.20090121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 8.Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J, Ratcliffe PJ. Genome-wide Association of Hypoxia-inducible Factor (HIF)-1 and HIF-2 DNA Binding with Expression Profiling of Hypoxia-inducible Transcripts. J Biol Chem. 2009;284:16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helixloophelix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIF alpha targeted for VHL mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 11.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 12.Jewell UR, Kvietikova I, Scheid A, Bauer C, Wenger RH, Gassmann M. Induction of HIF-1alpha in response to hypoxia is instantaneous. FASEB J. 2001;15:1312–1314. [PubMed] [Google Scholar]
- 13.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 14.Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- 15.Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- 16.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 17.Stiehl DP, Jelkmann W, Wenger RH, Hellwig-Bürgel T. Normoxic induction of the hypoxia-inducible factor 1alpha by insulin and interleukin-1beta involves the phosphatidylinositol 3-kinase pathway. FEBS Lett. 2002;512:157–162. doi: 10.1016/s0014-5793(02)02247-0. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001;276:9519–9525. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- 19.Karni R, Dor Y, Keshet E, Meyuhas O, Levitzki A. Activated pp 60c-Src leads to elevated hypoxia-inducible factor (HIF)-1alpha expression under normoxia. J Biol Chem. 2002;277:42919–42925. doi: 10.1074/jbc.M206141200. [DOI] [PubMed] [Google Scholar]
- 20.Greijer AE, van der Groep P, Kemming D, Shvarts A, Semenza GL, Meijer GA, van de Wiel MA, Belien JA, van Diest PJ, van der Wall E. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) J Pathol. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Y, Danielson KG, Albert TJ, Shapiro IM, Risbud MV. HIF-1alpha is a Regulator of Galectin-3 Expression in the Intervertebral Disc. J Bone Miner Res. 2007;22:1851–61. doi: 10.1359/jbmr.070620. [DOI] [PubMed] [Google Scholar]
- 22.Koch AE, Halloran MM, Haskel CJ, Shah NR, Polverini PJ. Angiogenesis mediated by soluble forms of E-selectin and vascular cell adhesion molecule 1. Nature. 1995;376:517–519. doi: 10.1038/376517a0. [DOI] [PubMed] [Google Scholar]
- 23.Calvani M, Rapisarda A, Uranchimeg B, Shoemaker RH, Melillo G. Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood. 2006;107:2705–2712. doi: 10.1182/blood-2005-09-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nangia-Makker P, Honjo Y, Sarvis R, Akahani S, Hogan V, Pienta KJ, Raz A. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000;156:899–909. doi: 10.1016/S0002-9440(10)64959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarty G, Awad O, Loeb DM. WT1 protein directly regulates expression of vascular endothelial growth factor and is a mediator of tumor response to hypoxia. J Biol Chem. 2011;286:43634–43. doi: 10.1074/jbc.M111.310128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Pakunlu RI, Tsao W, Pozharov V, Minko T. Bimodal effect of hypoxia in cancer: role of hypoxia inducible factor in apoptosis. Mol Pharm. 2004;1:156–165. doi: 10.1021/mp034031n. [DOI] [PubMed] [Google Scholar]
- 27.Trisciuoglio D, Gabellini C, Desideri M, Ziparo E, Zupi G, Del Bufalo D. Bcl-2 regulates HIF-1alpha protein stabilization in hypoxic melanoma cells via the molecular chaperone HSP90. PLoS One. 2010;5:e11772. doi: 10.1371/journal.pone.0011772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


