Abstract
The author has studied the organogenesis of human intrahepatic bile duct in fetal livers. The developmental studies of the liver have focused mainly on the development of intrahepatic bile ducts including the ductal plate (DP). The DP is a single or double layered structures composed of small cuboidal cells and located in the interface between the hepatoblasts and portal mesenchyme. Herein, the author discovered the DP within the parenchymal hepatocytes. The DP-like structures within the hepatoblasts were found in 17 human fetal livers out of the 32 human fetal livers. The gestational ages of the 17 fetal livers were as follows: 7, 8, 9, 10 (n=2), 11, 12, 13, 14 (n=2), 15, 16, 17, 18, 19, 21, and 23 weeks. The presence of intraparenchymal DP-like structures were mainly found in the fetus of early gestational ages. Morphologically, the DP-like structures within the hepatoblasts were composed of small cuboidal epithelial cells with normal chromatin patterns. The cytoplasm was scant and relatively basophilic. The nuclei were small and round, and had no nucleoli. These cells formed the DP-like structures. The DP-like structures frequently formed cords, tubules, and duplicating patterns. These DP-like structures were scattered and the remaining hepatoblasts are normal hepatoblasts. The density of these DP-structures was low (one or two per 5 low power fields), but varied from case to case and area to area in the same case. The overall appearances were very similar to the true DP. Comparative observations of HE and CK immunostaining were performed. The DP-like structures within the hepatoblasts were positive for biliary-type CK7 and CK19. They were also positive for CK8 and CK18 that are expressed in both hepatocyte and biliary lineages. The true DP was positive for biliary-type CK7 and CK19. They were also positive for CK8 and CK18. Thus, the intraparenchymal DP-like structures were the same as the true DP located in the interface. Thus, the author discovered the intraparenchymal DP in the human fetal livers. This discovery should be confirmed by other researchers. If it is true, many studies of the functions of these intraparenchymal DPs are need.
Keywords: Ductal plate, liver development, hepatoblasts, fetal liver, immunohistochemistry
Introduction
The author has investigated the fetal development of intrahepatic bile ducts (IBDs) in humans [1-16]. The similar studies of fetal development of IBDs in humans have been reported by Desmet’s group [17-20] and Gerber’s group [21-23]. The author’s studies [1-16] and other studies [17-23] have revealed that the IBDs are derived from fetal ductal plate (DP) which is a double layered cylindrical structures located in the interface between hepatoblasts and portal mesenchyme [1-23]. The DP undergoes remodeling, giving rise to the emergence of future IBDs. The remnants of DP disappear by apoptosis [7]. The remodeled DP further give rise to mature IBDs resembling adult IBDs [1-23]. The author demonstrated that intrahepatic peribiliary glands, which were discovered by the author [24-36], are also derived from DP in human livers [1,5]. The author also proved that pancreatic acinar cells clusters may be derived from remodeling DP and remodeled DP in human fetal livers [1,5,6]. The author demonstrated that the normal development of the human fetal IBDs involves many molecules and molecular mechanisms including apoptosis, apoptosis-related proteins, DP cell proliferation, pancreatic digestive enzymes such as α-amylase, trypsinogen and lipase, some proteinases including matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases, peribiliary vascular plexus, carbohydrate structures of many glycoproteins, MUC apomucin expression, expression of cytokeratin (CK), E-cadherin-catenin systems, double-stranded RNA-activated protein kinase, midkine, truncated midkine, type IV collagen, laminin, tenascin, trypsin, chymotrypsin, transforming growth factor-α and its receptor, and pancreatic amylase mRNA [1-16].
The developmental failures of these human fetal IBDs of DP give rise to the persistence of biliary structures in the postnatal human livers. Such structures are called DP malformations (DPM) or hepatobiliary fibropolycystic disease, which is seen in congenital hepatic fibrosis, polycystic diseases (adult and infantile) of the liver and kidneys, congenital biliary atresia, von-Meyenburg complex, and Caroli’s disease [17-20,37-42]. DP is also seen in ductular reactions [43-45] and DPM in some liver hamartoma [44] and cholangiocarcinoma [46]. The DP in ductular reaction of focal nodular hyperplasia interestingly may express KIT [45], a receptor of stem cell factor [47].
However, little attention has been given in the primitive hepatocytes (hepatoblasts). The ductal plate (DP) is a single or double layered structure of the human fetal livers and is present in the interface between hepatoblasts and portal mesenchyme. The mesenchymal and stromal interactions may play an important role in the formation of DP. DP is progenital cells of IBDs; it becomes mature IBD cells via remodeling and remodeled processes [1-23]. The DP give rise to intrahepatic peribiliary glands [24-36] and pancreatic acinar cells [1,5,6,31] in human fetal livers [1-16]. Recently, Carpentier et al [48] have suggested, in mouse, that mouse embryonic DP cells give rise to cholangiocytes, periportal hepatocytes (hepatoblasts), and adult liver progenitor cells. However, this hypothesis has not been investigated in humans.
During the author’s observations of human fetal livers for more than 20 years, the author recently noticed the presence of DP-like structures in the parenchymal hepatoblasts, not in the portal interface and not in the periportal areas. This prompted the author to examine the DP-like structures within the parenchymal hepatoblasts in the human’s livers.
Herein, the author shows there are occasional DP-like structures within the hepatocytes. The DP-like structures in the parenchyma are morphologically very similar to the DP present in the interface of the parenchyma and portal mesenchyme. The author also demonstrates that DP-like structures in the hepatoblasts express biliary-type cytokeratin (CK) (CK7 and CK19).
Materials and methods
The author collected 32 human fetal livers at various hospitals. They were abortions (spontaneous and artificial), intrauterine fetal death, and autopsies. The gestational ages (weeks) of the 32 fetal livers were as follows: 7, 8, 9 (n=2), 10 (n=3), 11 (n=2), 12 (n=3), 13 (n=2), 14 (n=2), 15 (n=2), 16 (n=2), 17, 18, 19, 21, 23, 24, 26, 29, 30, 36, 38, and 40 week. The sex was unclear. Informed consent was obtained from each mother. The fetal liver specimens thus obtained were immediately fixed in formalin and embedded in paraffin. Many 3μm thin sections were cut, and they were subjected to hematoxylin and eosin (HE) stain and an immunohistochemical analysis for cytokeratin (CK) 7, CK8, CK18, and CK19.
An immunohistochemical study was performed with the use of Dako Envision method (Dako Corp, Glostrup, Denmark) as previously described [49,50]. The antibodies used were as follows CK7 (clone OV-TL, Dako Corp, dilution = 1:200), CK8 (Clone 35bH11, Dako Corp, dilution = 1:150), CK18 (clone DC10, Dako Corp, dilution = 1:200), and CK19 (clone BA17, Dako Corp, dilution = 1:100). Microwave pretreatment was performed by each immunohistochemical run.
The author observed all the HE slides of the 32 human fetuses and all the CK-stained slides. A competitive observation of HE and immunostaining was done. Morphologically, the processes of the human IBD development were categorized into four stages; DP, remodeling DP, remodeled DP, and mature IBDs. The DP was composed of single or double-layered cylindrical structures located in the interface between primitive hepatocytes (hepatoblasts) and mesenchyme of the portal tracts. The remodeling DP showed disappearance of some cells of the DP and development of tubular structures of future bile ducts, which was moving into the portal mesenchyme. The remodeled DP was characterized by almost disappearance of DP and the tubular structures moved into the portal mesenchyme. The mature IBDs were mature duct similar to adult IBDs. These presses progressed from the hilar IBDs to peripheral IBDs. The periods of these processes in the portal tracts near the hepatic hilus were as follows: the DP stages, 7-10 gestational weeks; the remodeling DP stage 11-15 gestational weeks; the remodeled DP stage, 16-24 gestational weeks; the mature IBDs stage, 26-40 weeks. However, these stage periods differed from case to case as well as from portal tract to another. Interestingly, differentiation of intrahepatic peribiliary glands and pancreatic acinar cells from the remodeling and remodeled DP were seen frequently and occasionally in the hilar areas, respectively.
Results
The DP-like structures were found in 17 fetal livers of the 32 human fetal livers. The gestational ages of the 17 fetal livers were as follows: 7, 8, 9, 10 (n=2), 11, 12, 13, 14 (n=2), 15, 16, 17, 18, 19, 21, and 23 weeks.
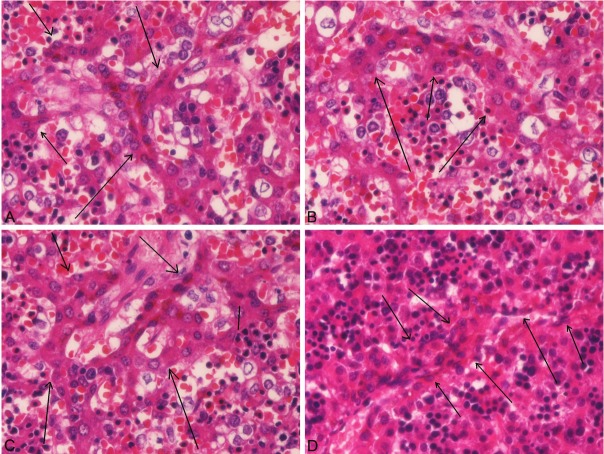
Morphologically, the DP-like structures within the hepatoblasts were composed of small cuboidal epithelial cells with normal chromatin patterns (Figure 1A-D). The cytoplasm was scant and relatively basophilic. The nuclei were small and round, and had no nucleoli. These cells formed the DP-like structures. The DP-like structures within the hepatoblasts frequently formed cords, tubules, and duplicating patterns (Figure 1A-D). The overall appearances were very similar to the true DP (Figure 2A and 2B). These DP-like structures were scattered and the remaining hepatoblasts are normal hepatoblasts. The density of these DP-structures was low (one or two per 5 low power fields), but varied from case to case and area to area in the same case.
Figure 1.
Histological features of intraparenchymal ductal plate within the hepatoblasts. Four examples are shown (arrows; A-D). The DP-like structures within the hepatoblasts were composed of small cuboidal epithelial cells with normal chromatin patterns. The cytoplasm was scant and relatively basophilic. The nuclei were small and round, and had no nucleoli. These cells formed the DP-like structures. DP-like structures frequently formed cords, tubules, and duplicating patterns. These DP-like structures were scattered with the background of normal hepatoblasts. The density of these DP-structures was low (one or two per 5 low power fields), but varied from case to case and area to area in the same case. The overall appearances were very similar to the true DP (Figure 2A and 2B). A-D: HE, x300.
Figure 2.
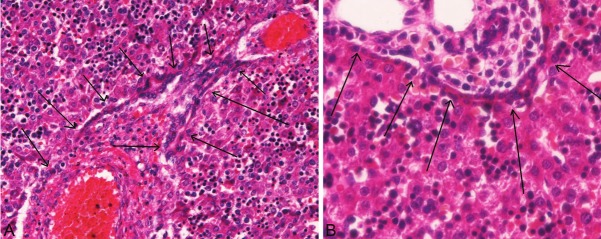
The ductal plated. Two examples are shown (arrows, A and B). The ductal plate is a single or double layered structure composed of cuboidal small cells, and is located exclusively in the interface between hepatoblasts and portal mesenchyma in the human fetal livers. The resemblance of these true DP (Figure 2) and intraparenchymal DP-like cells (Figure 1) is apparent. Compare Figures 1 and 2. A, B: HE, x300.
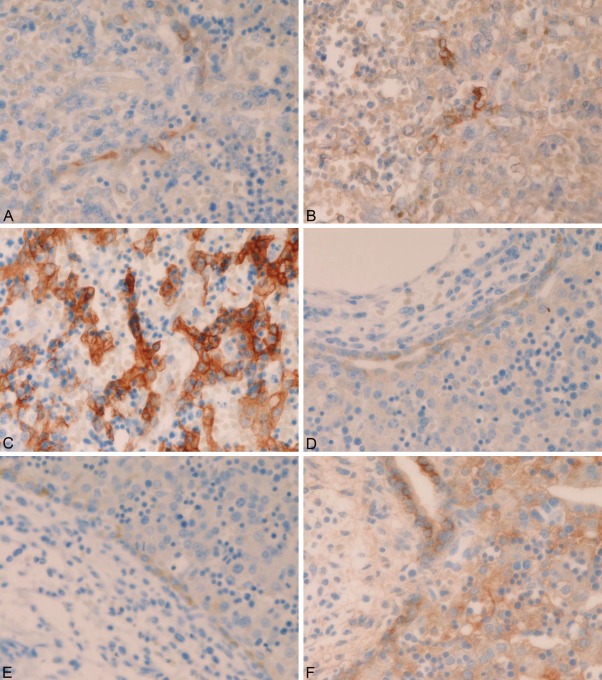
Comparative observations of HE and CK immunostaining were performed. The DP-like structures were positive for biliary-type CK7 (Figure 3A) and CK19 (Figure 3B). They were also positive for CK8 and CK18 (Figure 3C) that are expressed in both hepatocyte and biliary lineages. The true DP was positive for biliary-type CK7 (Figure 3D) and CK19 (Figure 3E). They were also positive for CK8 (Figure 3F) and CK18.
Figure 3.
Immunohistochemical findings of cytokeratins. The DP-like structures within the hepatoblasts were positive for biliary-type CK7 (A) and CK19 (B). They were also positive for CK8 and CK18 (FC) that are expressed in both hepatocyte and biliary lineages. The true DP was positive for biliary-type CK7 (D), CK19 (E), CK8 (F) and CK18. All, immunostaining, x300.
Discussion
The studies of human liver development have been focused on the fetal development of the IBD including DP [1-23], and little attention has been given to other cells types. In the present study, the author observed the primitive hepatocytes (hepatoblasts), the major component of the human fetal livers.
The author for the first time found that DP-like structures were present within the hepatoblasts in the human fetal livers. These DP-like structures were found in 17 (53%) out of the 32 human fetal livers. The gestational ages of the fetal livers containing the DP-like structures are 7-23 weeks, spearing fetuses of 24-40 weeks. Thus, the DP-like structures appears in the relatively early stages of hepatoblastic differentiation.
Morphologically, the DP-like structures within the hepatoblasts were composed of small cuboidal epithelial cells with normal chromatin patterns. The cytoplasm was scant and basophilic. The nuclei were small and round, and had no nucleoli. The DP-like structures frequently formed cords, tubules, and duplicating patterns. The DP-like structures were scattered among the remaining hepatoblasts. The density of the DP-structures was low, and varied from case to case and area to area in the same case. The overall appearances very resembled the true DP. The formations of tubular, cords and duplications are entirely almost the same as the true DP. This high resemblance can be confirmed by comparison as shown in the Figures. The DP-like structures are not the DP which is located in the interface between hepatoblasts and portal mesenchyme. The DP-like structures were shown to morphologically correspond to a kind of DP located within the hepatoblasts.
To confirm the nature of these DP-like structures, the author performed the immunohistochemistry of CK. As is well known, CK7 and CK19 are expressed only in the cholangiocytic element including DP in the human liver [1-23]. CK8 and CK18 label both biliary cells and hepatoblasts in human livers [1-23]. Therefore, CK7 and CK19 are markers of the biliary element including DP and IBDs in human livers.
In the present study, the DP-like structures were positive for biliary-type cytokeratins (CK7 and CK19), strongly suggesting that the DP-like structures in the hepatoblasts have biliary lineage. These findings strongly suggest that the DP-like structures within the hepatoblasts are true DP. They also showed hepatocyte-type cytokeratins (CK8 and CK18), suggesting that they also show hepatoblastic characteristics. In any way, the positive and strong expression of CK7 and CK19 in the DP-like structures almost indicates that they are true DPs located within the hepatoblasts. It can be concluded that the DP-like structures are true DPs located within the hepatoblasts.
The origin of the DP within the hepatoblasts is not known. It may be originated from hepatoblasts or DP located in the interfaces. Now, it is well known that DP can differentiated into peribiliary glands, pancreatic acinar cells, hepatic stem/progenitor cells, and periportal hepatocytes in addition to IBD in human and mouse fetal livers [1-23,48].
These observations of the presence of the DP within the hepatic parenchyma (hepatoblasts) in fetal livers are entirely new findings. The author wants that other researches will also examine these intraparenchymal DP in the fetal livers of humans and mouse. It is well known that the DP plays an important role in the morphogenesis of the liver. The DP may be the hepatic stem/progenitor cells and give rise to stem cells, cholangiocytes, hepatocytes, pancreatic acinar cells, peribiliary glands, and other elements [1-23,48]. Many studies await the functions of these intraparenchymal (hepatoblastic) DPs.
In summary, the author discovered intraparenchymal DP located within the hepatoblasts in human fetal livers.
Conflict of interest statement
The author has no conflict of interest.
References
- 1.Terada T, Nakanuma Y. Development of human intrahepatic peribiliary glands: Histological, keratin immunohistochemical and mucus histochemical analyses. Lab Invest. 1993;68:261–269. [PubMed] [Google Scholar]
- 2.Terada T, Nakanuma Y. Profiles of expression of carbohydrate chain structures during human intrahepatic bile duct development and maturation: a lectin-histochemical and immunohistochemical study. Hepatology. 1994;20:388–397. [PubMed] [Google Scholar]
- 3.Terada T, Kitamura Y, Nakanuma Y. Normal and abnormal development of the intrahepatic biliary system: A review. Tohoku J Exp Med. 1997;181:19–32. doi: 10.1620/tjem.181.19. [DOI] [PubMed] [Google Scholar]
- 4.Terada T, Ashida K, Kitamura Y, Matsunaga Y, Takashima K, Kato M, Ohta T. Expression of E-cadherin, alpha-catenin and beta-catenin during human intrahepatic bile duct development. J Hepatol. 1998;28:263–269. doi: 10.1016/0168-8278(88)80013-8. [DOI] [PubMed] [Google Scholar]
- 5.Terada T. Differentiation of intrahepatic peribiliary glands and pancreatic acinar cells from the remodeling ductal plate in human fetuses. Hepatology. 2012;56:2004–2005. doi: 10.1002/hep.25750. [DOI] [PubMed] [Google Scholar]
- 6.Terada T, Nakanuma Y. Expression of pancreatic enzymes (α-amylase, trypsinogen and lipase) during human liver development and maturation. Gastroenterology. 1995;108:1236–1245. doi: 10.1016/0016-5085(95)90225-2. [DOI] [PubMed] [Google Scholar]
- 7.Terada T, Nakanuma Y. Detection of apoptosis and expression of apoptosis-related proteins during human intrahepatic bile duct development. Am J Pathol. 1995;146:67–74. [PMC free article] [PubMed] [Google Scholar]
- 8.Terada T, Okada Y, Nakanuma Y. Expression of matrix proteinases during human intrahepatic bile duct development: A possible role in biliary cell migration. Am J Pathol. 1995;147:1207–1213. [PMC free article] [PubMed] [Google Scholar]
- 9.Kato M, Shinozawa T, Kato S, Terada T. Divergent expression of midkine in the human fetal liver and kidney: an immunohistochemical analysis of developmental changes in hilar primitive bile ducts and hepatocytes. Liver. 2000;20:475–481. doi: 10.1034/j.1600-0676.2000.020006475.x. [DOI] [PubMed] [Google Scholar]
- 10.Kato M, Shinozawa T, Kato S, Terada T. Immunohistochemical localization of truncated midkine in developing human bile ducts. Histol Histopathol. 2003;18:129–134. doi: 10.14670/HH-18.129. [DOI] [PubMed] [Google Scholar]
- 11.Terada T, Nakanuma Y. Development of human peribiliary capillary plexus: A lectin-histochemical and immunohistochemical study. Hepatology. 1993;18:529–536. [PubMed] [Google Scholar]
- 12.Terada T, Nakanuma Y. Expression of tenascin, type IV collagen and laminin during human intrahepatic bile duct development and in intrahepatic cholangiocarcinoma. Histopathology. 1994;25:143–150. doi: 10.1111/j.1365-2559.1994.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki M, Nakanuma Y, Terada T, Kim YS. Biliary epithelial expression of MUC1, MUC2, MUC3, and MUC5/6 apomucins during human intrahepatic bile duct development and maturation: An immunohistochemical study. Am J Pathol. 1995;147:574–579. [PMC free article] [PubMed] [Google Scholar]
- 14.Terada T, Kato M, Horie S, Endo K, Kitamura Y. Expression of pancreatic alpha-amylase protein and messenger RNA on hilar primitive bile ducts and hepatocytes during human fetal liver organogenesis: an immunohistochemical and in situ hybridization study. Liver. 1998;18:313–319. doi: 10.1111/j.1600-0676.1998.tb00811.x. [DOI] [PubMed] [Google Scholar]
- 15.Terada T, Ukita Y, Ueyama J, Ohta T. Protein expression of double-stranded RNA-activated protein kinase (PKR) in intrahepatic bile ducts in normal adult livers, fetal livers, primary biliary cirrhosis, hepatolithiasis and intrahepatic cholangiocarcinoma. Liver. 2000;20:450–457. doi: 10.1034/j.1600-0676.2000.020006450.x. [DOI] [PubMed] [Google Scholar]
- 16.Terada T, Ohta T, Nakanuma Y. Expression of transforming growth factor-α and its receptor during human liver development and maturation. Virchows Archiv. 1994;424:669–675. doi: 10.1007/BF00195783. [DOI] [PubMed] [Google Scholar]
- 17.Van Eyken P, Sciot R, Callea F, Van der Steen K, Moerman P, Desmet VJ. The development of the intrahepatic bile ducts in man: a keratin immunohistochemical study. Hepatology. 1988;8:1586–1595. doi: 10.1002/hep.1840080619. [DOI] [PubMed] [Google Scholar]
- 18.Desmet VJ. Congenital disease of intrahepatic bile ducts: variations on the theme “ductal plate malformation”. Hepatology. 1992;16:1069–1083. doi: 10.1002/hep.1840160434. [DOI] [PubMed] [Google Scholar]
- 19.Desmet VJ. Intrahepatic bile duct under the lens. J Hepatol. 1985;1:545–559. doi: 10.1016/s0168-8278(85)80752-2. [DOI] [PubMed] [Google Scholar]
- 20.Roskams T, Desmet V. Embryology of extra- and intrahepatic bile ducts, the ductal plate. Anat Rec (Hoboken) 2008;291:628–635. doi: 10.1002/ar.20710. [DOI] [PubMed] [Google Scholar]
- 21.Shah KD, Gerber MA. Development of intrahepatic bile ducts in humans: immunohistochemical study using monoclonal cytokeratin antibodies. Arch Pathol Lab Med. 1989;113:1135–138. [PubMed] [Google Scholar]
- 22.Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. Identification of bipotential progenitor cells in human liver development. Hepatology. 1996;23:476–481. doi: 10.1002/hep.510230312. [DOI] [PubMed] [Google Scholar]
- 23.Shar KD, Gerber MA. Development of intrahepatic bile ducts in humans: possible role of laminin. Arch Pathol Lab Med. 1990;114:597–600. [PubMed] [Google Scholar]
- 24.Terada T, Nakanuma Y, Ohta G. Glandular elements around the intrahepatic bile ducts in man: their morphology and distribution in normal livers. Liver. 1987;7:1–8. doi: 10.1111/j.1600-0676.1987.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 25.Terada T, Nakanuma Y. Solitary cystic dilation of the intrahepatic bile duct: Morphology of two autopsy cases and a review of the literature. Am J Gastroenterol. 1987;82:1301–1305. [PubMed] [Google Scholar]
- 26.Terada T, Takegoshi T, Doishita K, Nakanuma Y. Histological study of intrahepatic cavernous transformation in a patient with primary myelofibrosis and portal venous thrombosis. Virchows Arch A Pathol Anat Histopathol. 1988;412:339–345. doi: 10.1007/BF00750260. [DOI] [PubMed] [Google Scholar]
- 27.Terada T, Nakanuma Y. Morphological examination of intrahepatic bile ducts in hepatolithiasis. Virchows Arch A Pathol Anat Histopathol. 1988;413:167–176. doi: 10.1007/BF00749679. [DOI] [PubMed] [Google Scholar]
- 28.Terada T, Ishida F, Nakanuma Y. Vascular plexus around intrahepatic bile ducts in normal livers and portal hypertension. J Hepatol. 1989;8:139–149. doi: 10.1016/0168-8278(89)90001-9. [DOI] [PubMed] [Google Scholar]
- 29.Terada T, Nakanuma Y. Pathologic observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: IV. Hyperplasia of intramural and extramural glands. Hum Pathol. 1992;23:483–490. doi: 10.1016/0046-8177(92)90124-l. [DOI] [PubMed] [Google Scholar]
- 30.Terada T, Nakanuma Y. Innervation of intrahepatic bile ducts and peribiliary glands in normal livers, extrahepatic biliary obsrtruction and hepatolithiasis: an immunohistochemical study. J Hepatol. 1989;9:141–148. doi: 10.1016/0168-8278(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 31.Terada T, Nakanuma Y, Kakita A. Pathologic observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: Heterotopic pancreas in the liver. Gastroenterology. 1990;98:1333–1337. doi: 10.1016/s0016-5085(12)90353-4. [DOI] [PubMed] [Google Scholar]
- 32.Terada T, Nakanuma Y. Pathological observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: II. A possible source of cholangiocarcinoma. Hepatology. 1990;12:92–97. doi: 10.1002/hep.1840120115. [DOI] [PubMed] [Google Scholar]
- 33.Terada T, Nakanuma Y. Pathological observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: III. Survey of necroinflammation and cystic dilatation. Hepatology. 1990;12:1229–1233. doi: 10.1002/hep.1840120525. [DOI] [PubMed] [Google Scholar]
- 34.Terada T, Kono N, Nakanuma Y. Immunohistochemical and immunoelectron microscopical analyses of α-amylase isozymes in the intrahepatic biliary epithelium and hepatocytes. J Histochem Cytochem. 1992;40:1627–1635. doi: 10.1177/40.11.1431051. [DOI] [PubMed] [Google Scholar]
- 35.Terada T, Nakanuma Y. Intrahepatic cholangiographic appearance simulating primary sclerosing cholangitis in hepatobiliary diseases: A postmortem cholangiographic and histolopathological study in 154 autopsy livers. Hepatology. 1995;22:75–81. [PubMed] [Google Scholar]
- 36.Terada T, Kida T, Nakanuma Y. Extrahepatic peribiliary glands express α-amylase isozymes, trypsin and pancreatic lipase: An immunohistochemical analysis. Hepatology. 1993;18:803–808. doi: 10.1002/hep.1840180409. [DOI] [PubMed] [Google Scholar]
- 37.Terada T, Nakanuma Y. Congenital biliary dilatation in autosomal dominant adult polycystic disease of the liver and kidneys. Arch Pathol Lab Med. 1988;112:1113–1116. [PubMed] [Google Scholar]
- 38.Jorgensen MJ. The ductal plate malformation. Acta Pathol Microbiol Scand Suppl. 1977;257:1–87. [PubMed] [Google Scholar]
- 39.Summerfield JA, Nagafuchi Y, Sherlock S, Cadafalch J, Scheuer PJ. Hepatobiliary fibropolycystic diseases: a clinical and histological review of 51 patients. J Hepatol. 1986;2:141–156. doi: 10.1016/s0168-8278(86)80073-3. [DOI] [PubMed] [Google Scholar]
- 40.Terada T, Moriki T. Monolobar ductal plate malformation disease of the liver. Pathol Int. 2010;60:407–12. doi: 10.1111/j.1440-1827.2010.02535.x. [DOI] [PubMed] [Google Scholar]
- 41.Terada T, Moriki T. Monolobar hepatobiliary fibropolycystic disease. Pathol Oncol Res. 2011;17:159–165. doi: 10.1007/s12253-010-9285-3. [DOI] [PubMed] [Google Scholar]
- 42.Nakanuma Y, Terada T, Ohta G, Matsubara F, Kurachi M. Caroli’s disease in congenital hepatic fibrosis and infantile polycystic disease. Liver. 1982;2:346–354. doi: 10.1111/j.1600-0676.1982.tb00833.x. [DOI] [PubMed] [Google Scholar]
- 43.Desmet VJ. Ductal plates in hepatic ductular reactions: hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011;458:251–259. doi: 10.1007/s00428-011-1048-3. [DOI] [PubMed] [Google Scholar]
- 44.Terada T. Hepatic nodular hamartoma containing liver cysts, ductal plate malformations and peribiliary glands. Hepatol Res. 2011;41:93–98. doi: 10.1111/j.1872-034X.2010.00746.x. [DOI] [PubMed] [Google Scholar]
- 45.Terada T. Projected focal nodular hyperplasia (FNH) of the liver with pronounced atypical ductular reaction resembling ductal plate and expressing KIT. Hepatol Res. 2012;42:721–726. doi: 10.1111/j.1872-034X.2012.00967.x. [DOI] [PubMed] [Google Scholar]
- 46.Nakanuma Y, Sato Y, Ikeda H, Harada K, Kobayashi M, Sano K, Uehara T, Yamamoto M, Arrizumi S, Park YN, Choi JH, Yu E. Intrahepatic cholangiocarcinoma with predominant “ductal plate malformation” pattern: a new subtype. Am J Surg Pathol. 2012;36:1629–1635. doi: 10.1097/PAS.0b013e31826e0249. [DOI] [PubMed] [Google Scholar]
- 47.Terada T. Mutations and protein expression of KIT and PDGFRA genes in ipsilateral testicular seminomas: an immunohistochemical and molecular genetic study. Appl Immunohistochem Mol Morphol. 2011;19:450–453. doi: 10.1097/PAI.0b013e31820d2872. [DOI] [PubMed] [Google Scholar]
- 48.Carpentier R, Suner RS, Van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, Leclercq IA, Sander M, Lemaigre FP. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–1438. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 50.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;271:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]