Abstract
Aim: Hepatic cirrhosis is a serious clinical problem caused by the accumulation of extracellular matrix, which can ultimately progress into hepatic failure. Transforming growth factor-beta1 (TGF-β1) plays a pivotal role in extracellular matrix production. Bone morphogenetic protein-7 (BMP-7), as a member of the TGF-β1 superfamily, has been well proved to be capable of reversing renal fibrosis in mice. In this study, we aim to investigate the potential effect of BMP-7 on hepatic fibrosis in rats. Methods: Sprague-Dawley rats were randomly divided into five groups. In the hepatic fibrosis model group (n=8), rats was treated with porcine serum at 0.5ml each time, twice a week. In the negative control group (n=10), rats were intraperitoneally injected with equal amount and frequency saline. Rats were injected with BMP-7 (100 μg/kg weight) before porcine serum intraperitoneal injection in the preventive group (n=9). For the early (n=10) and late (n=8) treatment group, rats were received with BMP-7 (100 μg/kg weight) every other day since the second and fourth week respectively after porcine serum injection. After eight weeks, the degree of liver fibrosis in rats was evaluated and the expression of TGF-β1 in liver tissues was detected by Western blot and immunohistochemistry. Results: The grade of hepatic fibrosis was significant attenuated by BMP-7 prevention and treatment compared with the rats in negative control group (P<0.05). In addition, the expression of TGF-β1 greatly decreased in the BMP-7 preventive and treatment groups detected by both Western blot and immunohistochemistry. Conclusions: BMP-7 can attenuate and even prevent the level of hepatic fibrosis in rats through inhibiting the expression of TGF-β1 in the liver fibrotic tissues. Therefore, it may be a potential clinical drug for the prevention and treatment of hepatic fibrosis.
Keywords: Hepatic fibrosis, bone morphogenetic protein-7, transforming growth factor-beta 1, extracellular matrix, hepatic stellate cell
Introduction
Hepatic fibrosis is a common pathological process of many chronic hepatic diseases which may result from the over-deposit of extracellular matrix (ECM) and its components changes [1]. ECM is mainly generated by hepatic stellate cell (HSC), of which the activation and proliferation is essential for the development of hepatic fibrosis [2,3]. Besides, transforming growth factor-beta1 (TGF-β1) can trigger the activation of HSC and further induce its differentiation into myofibroblast that is the main factor leading to hepatic fibrosis [4].
Bone morphogenetic protein-7 (BMP-7), as a member of TGF-β superfamily, has been reported to have the activity of anti-inflammatory and anti-fibrosis in the kidney fibrotic model. Besides, it is reported to own an antagonistic effect against TGF-β1 [5]. Our previous study has demonstrated that BMP-7 could reduce the collagen generation of hepatic cell and HSC in vitro. And the specific regulatory mechanism has been investigated in our preliminary study [6]. The study aimed to further discuss the effect of BMP-7 on hepatic fibrosis in rats and its possible mechanisms.
Methods and materials
Processes involved in the animal experiment have been approved by both Animal Care and Use Institutes and animal experimental ethics committee of Tongji hospital.
Hepatic fibrosis model
Sprague-Dawley (SD) rats were obtained from Shanghai SLAC laboratory Animal Co. LTD. The hepatic fibrosis models of 45 female rats (body weight 150 ± 10g) were established by repeated intraperitoneal injection of porcine serum.
Grouping and treatment
Rats were randomly divided into five groups. In the hepatic fibrosis (positive control) group (n=8), rats was treated with porcine serum at 0.5ml each time, twice a week. In the negative control group (n=10), rats were intraperitoneally injected with equal amount and frequency saline. Rats were received the injection of BMP-7 (Shanghai Medical Instruments Wholesale Department) at 100 μg/kg weight before porcine serum injection in the preventive group (n=9). For the early (n=10) and late (n=8) treatment group, rats were injected with BMP-7 (100 μg/kg weight) every other day since the second and fourth week respectively after porcine serum intraperitoneal injection. After eight weeks, liver tissues of rats in five groups were collected.
Liver damage classification
The degree of hepatic fibrosis in rats was assessed and the pathological sections were examined by three senior pathologists in our hospital. According to Metavir Scoring System, the liver damage was classified into the following 4 grades on the basis of inflammation, necrosis, fibrosis and structural changes; F0: normal hepatic structure; F1: portal (without septa) fibrosis; F2: portal (with few septa) fibrosis; F3: septa fibrosis; F4: cirrhosis.
Western blot
Proteins samples were extracted from rats’ livers with a subcellular proteome extraction kit (Merck kGaA, Darmstadt, Germany) and the concentration was examined by Lowry’s method. Samples were mixed with gel-loading buffer and boiled for 5min, separated in 12% poly-acrylamide SDS-gel under reducing conditions, and then transferred into a polyvinylidene difluoride membrane. Non-specific binding antibody was blocked by the pre-incubation in 1 x Tris buffer containing 5% skimmed milk for 1h at room temperature. Then, membranes were incubated with primary anti-TGF-β1 antibodies at a dilution of 1:2000 in 1 x TBS containing 2% skimmed milk overnight at 4°C. After washing three times, membranes were incubated with second anti-goat antibodies at 1:2000 dilutions for 1h at room temperature. Finally, bands were visualized on films using an enzyme-linked chemiluminescence kit.
Immunohistochemistry
Tissue samples were fixed with 10% formaldehyde in phosphate buffered saline, embedded in paraffin, and then cut into sections in 4mm thickness. Sections were heated for 45min at 60°C, processed in two changes of xylene, and rinsed in a decreasing ethanol series. Besides, samples were treated with 3% H2O2 for 30min in order to block endogenous peroxidase activity. Antigens were retrieved with 0.01M Na-citrate buffer (pH=6.0) in a microwave oven for 30min.
Sections were incubated with primary anti-TGF-β1 antibody overnight at 4°C. It was detected by incubation with biotinylated secondary antibody and then streptavidin peroxidase for 45min at room temperature. Finally, reaction products were visualized by immersing sections in diaminobenzidine tetrachloride and counterstained with haematoxylin. In addition, the immunoreactive score was calculated by the product of staining intensity multiplied positive cells percentage.
Statistical analysis
Experimental data were analyzed by Chi2-square test (X 2) in SPSS10.0. P<0.05 was considered as statistical significance.
Results
The effects of BMP-7 on the level of hepatic fibrosis
As shown in Table 1, the results of hepatic fibrosis classification suggested that BMP-7 had a visible preventive and therapeutic effect on the hepatic fibrosis in rats.
Table 1.
Classification of hepatic fibrosis in rats
| Group | Metavir Scoring System | Sample size (n) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| F0 | F1 | F2 | F3 | F4 | ||
| Negative control | 10 | 10 | ||||
| Positive control | 2 | 6 | 8 | |||
| Early treatment | 2 | 6 | 2 | 10 | ||
| Late treatment | 1 | 5 | 2 | 8 | ||
| Prevention | 2 | 3 | 3 | 1 | 9 | |
Chi-square test (X 2) showed that there were significant differences in each of the early treatment group, the late treatment group and the prevention group as compared with the model group (P<0.05). A significant difference was also found between the prevention group and the late treatment group (P<0.05), while no significant difference between the prevention group and the early treatment group (P>0.05).
The normal structure of liver tissues was observed in the negative control group (Figure 1A). The early treatment (Figure 1C), late treatment (Figure 1D) and prevention (Figure 1E) group showed a significant difference (P<0.05) of liver structure compared with the positive control group (Figure 1B). In addition, a statistical difference (P<0.05) in the prevention group was also observed compared with the late treatment group while no significant difference with the early treatment group (P>0.05).
Figure 1.
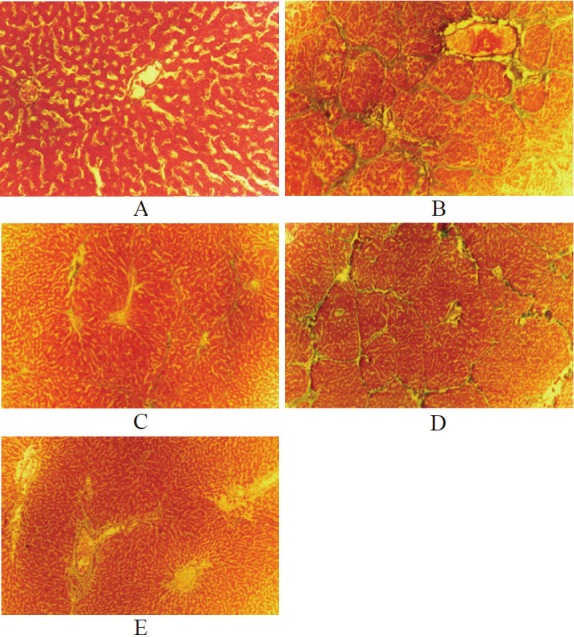
The prevention and treatment effects of BMP-7 on hepatic fibrosis in rats. The histopathological manifestations (Masson staining) of liver tissues in experimental rats: A. the negative control, x40; B. the positive control, x40; C. the early treatment, x10; D. the late treatment, x40; E. the prevention, x10.
The effects of BMP-7 on TGF-β1 detected by western blot
The results of Western blot showed that TGF-β1 expression in hepatic tissues of the preventive and treatment group was significantly reduced compared with the positive control group (Figure 2). It suggested that BMP-7 significantly inhibited the expression of TGF-β1 in the rats’ hepatic tissues.
Figure 2.
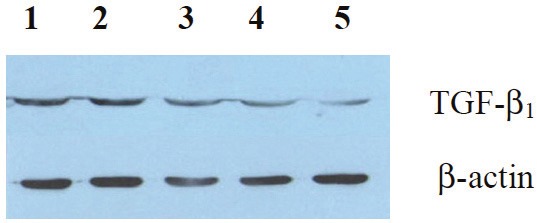
The effects of BMP-7 on TGF-β1 expression in liver tissues detected by Western blot: lane 1, the positive control; lane 2, the late treatment; lane 3, the early treatment; lane 4, the prevention; lane 5, the negative control.
The effects of BMP-7 on TGF-β1 detected by immunohistochemistry
According to the results of immunohistochem-istry, the expression of TGF-β1 in the early treatment (Figure 3C), late treatment (Figure 3D) and prevention group (Figure 3B) decreased significantly compared with that in positive control group (Figure 3A). It suggested that BMP-7 significantly inhibited TGF-β1 expression of hepatic tissues in rats.
Figure 3.
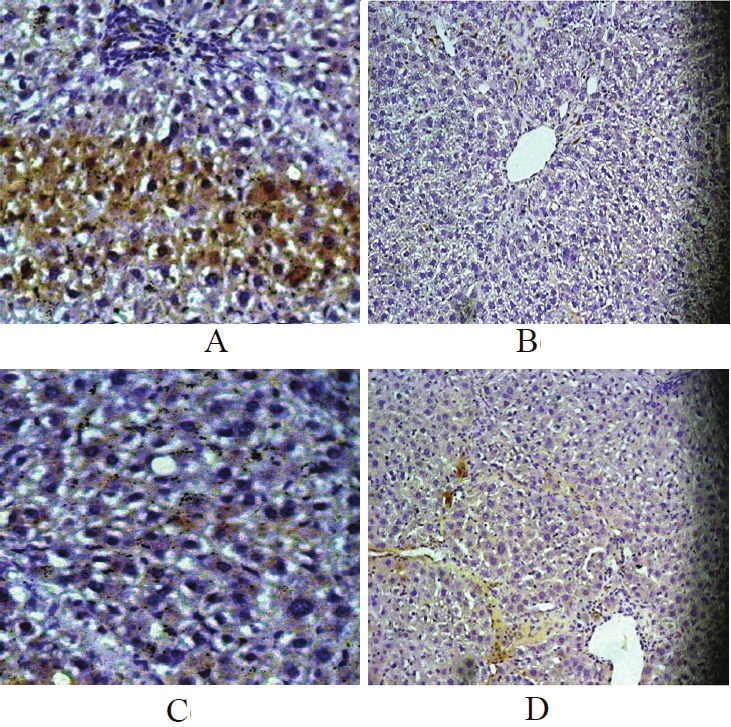
The effects of BMP-7 on TGF-β1 expression in liver tissues detected by immunohistochemistry: A. the positive control, x40; B. the prevention, x10; C. the early treatment, x40; D. the late treatment, x10.
Discussions
Marshall et al. proposed that bone ECM contained certain components contributing to the bone formation which was later confirmed to be BMP. BMP is essential in the process of embryonic development including the brain and bone formation [7-9]. The signal transduction of BMP is mediated by the transmembrane serine/threonine kinase receptors [10,11]. Recently, the cellular and molecular mechanisms of BMP-7 are the research focus around the world, however, the relationship between BMP-7 and hepatic fibrosis was rarely investigated. Recent studies have shown that the effect of BMP-7 was opposite to that of TGF-β1 which is one of the significant fibrogenic factors for the occurrence and development of hepatic fibrosis [12]. Therefore, this study investigated the preventive and therapeutic effects of BMP-7 in the hepatic fibrosis process.
In the study, varying levels of hepatic fibrosis in rats were found between the preventive and treatment group. In the preventive group where BMP-7 was administered at the beginning of the study, the number of rats with advanced-stage hepatic fibrosis (Grades III and IV) was significantly reduced. Besides, there were statistical differences (P<0.05) in the early treatment, late treatment and preventive group compared with the positive control. Significant difference (P<0.05) was also observed between the preventive group and late treatment group, which indicates that BMP-7 may have a preventive effect in the early stage of hepatic fibrosis. What’s more, the results of Western blot showed that TGF-β1 had a lower expression in normal liver tissues and a higher expression in hepatic fibrosis. After treating with intraperitoneal injection of BMP-7, liver tissues had a lower TGF-β1 expression than the positive control group. The expression level of TGF-β1 in the preventive group was lower than that in the treatment group. In addition, the results of immunohistochemistry showed that the level of TGF-β1 in the positive group was also higher than that of the treatment group. Therefore, it suggested that the mechanism of BMP-7 blocking the progress of hepatic fibrosis may be involved in the inhibition of TGF-β1 expression in liver tissues.
TGF-β1 is one of the most important fibrogenic growth factors and plays a vital role in the process of initiating and promoting HSC differentiation [13]. HSC is a kind of liver non-parenchymal cell and the main source of ECM, which is essential in the formation and development of hepatic fibrosis and cirrhosis. With the increasing activity of TGF-β1 in the hepatic fibrosis, the rising production and deposition of collagen leads to liver dysfunction.
HSC activation is featured by the enhanced expression and release of TGF-β1. It can inhibit the synthesis of matrix metalloproteinases and reduce the degradation of ECM by promoting the secretion of tissue inhibitors of metalloproteinases. Consequently, the activation of resting HSC and the up-regulated expression of type I, III and IV collagen result in the occurrence and development of hepatic fibrosis [14]. Studies showed that BMP-7 could antagonize the fibrogenic effects of TGF-β1 in liver tissues and was a significant peptide inhibiting fibrosis in vivo [15]. Recent data have shown that BMP-7 opposed the fibrogenic activity of TGF-β1 by suppressing the nuclear translocation of Smad3 in glomerular mesangial cells and pulmonary myofibroblasts [16,17]. In addition, BMP-7 antagonized the fibrogenic activity of myofibroblasts not only by inhibiting the TGF-β/Smad3 signal but also by inducing Id2 and Id3. Studies on renal injury found that the TGF-β1 induced signal transduction could be offset by BMP-7, leading to reverse of epithelial cells differentiation into mesenchymal cells [18]. Wang et al. found that BMP-7 stimulated or induced ECM degradation through matrix metalloproteinase-2, while this effect can be inhibited by TGF-β1 [19]. Another study carried by Saika et al. revealed that BMP-7 prevented the formation of lens fibrous tissues by inhibiting the epithelial to mesenchymal transition (EMT) in rats [20]. Similarity, BMP-7 could attenuate renal fibrosis by counteracting the TGF-β1 mediated EMT, reducing inflammatory cytokines release and maintaining the intracellular homoeostasis [21-23].
One of the mechanisms that BMP-7 antagonizes TGF-β1 is the interference with phospho-Smad1/5/8 by Smad3 for specific DNA binding and/or recruitment of co-factors [24]. Another possible mechanism is enhanced Smad7 expression via Smad1 signalling [25]. Moreover, BMP-7 can initiate intracellular pathways independently of Smad and activate Jun N-terminal kinase, p38 kinase and PI3 kinase/Akt [26,27]. However, BMP-7 may have no or opposite effect in other kinds of cells, such as hepatocytes or breast epithelial cells [28,29]. However, the specific fibrogenic effect of BMP-7 is still controversial. Recent clinical studies demonstrated that elevated levels of BMP-7 in patients with chronic liver disease may be related to the progression of liver fibrogenesis [29]. The significant difference of BMP-7 regulation is that the main pathways leading to fibrosis in HSC were not modulated by BMP-7, including TGF-β1 secretion and DNA binding activity of Smad3/4 [30]. Therefore, the anti-fibrogenic effect of BMP-7 in renal diseases models seems to be secondary to the anti-inflammatory and cytoprotective effect.
In the study, inhibitory effects of BMP-7 on hepatic fibrosis in rats were clearly observed. It suggested that BMP-7 had both preventive and therapeutic effects on hepatic fibrosis by inhibiting the over-expression of TGF-β1 in fibrotic liver tissues. According to the previous study in vitro, we have demonstrated that BMP-7 inhibited the activity of TGF-β1 signal channel protein Smad2/3 in HSC [6]. Therefore, we conclude that BMP-7 may be a potential clinical drug for the prevention and treatment of hepatic fibrosis.
Acknowledgements
This work was supported by National Natural Science Foundation of China (30570847); Shanghai Pujiang Program (05PJ14098); Shanghai Excellent Academic leaders Program (08×D14045) and Climbing Program (064119526). BMP-7 were obtained from Shanghai Medical Instruments Wholesale Department.
References
- 1.Wang JY, Guo JS, Yang CQ. Expression of exogenous rat collagenase in vitro and in a rat model of liver fibrosis. World J Gastroenterol. 2002;8:901–907. doi: 10.3748/wjg.v8.i5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gressner OA, Rizk MS, Kovalenko E, Weiskirchen R, Gressner AM. Changing the pathogenetic roadmap of liver fibrosis? Where did it start; where will it go? J Gastroenterol Hepatol. 2008;23:1024–1035. doi: 10.1111/j.1440-1746.2008.05345.x. [DOI] [PubMed] [Google Scholar]
- 3.Ueno T, Nakamura T, Torimura T, Sata M. Angiogenic cell therapy for hepatic fibrosis. Med Mol Morphol. 2006;39:16–21. doi: 10.1007/s00795-006-0311-1. [DOI] [PubMed] [Google Scholar]
- 4.Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER, Brenner DA. The role of tgfbeta1 in initiating hepatic stellate cell activation in vivo. J Hepatol. 1999;30:77–87. doi: 10.1016/s0168-8278(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 5.Zeisberg M, Shah AA, Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280:8094–8100. doi: 10.1074/jbc.M413102200. [DOI] [PubMed] [Google Scholar]
- 6.Yang L, Yang CQ, Yuan M, Wang SL, Chang YZ, Xu LJ, Zhang ZJ. [Effects of bone morphogenic proteins-7 on production of collagen from hepatocytes and hepatic stellate cells during liver fibrosis] . Zhonghua Yi Xue Za Zhi. 2009;89:419–422. [PubMed] [Google Scholar]
- 7.Adachi T, Takanaga H, Kunimoto M, Asou H. Influence of lif and bmp-2 on differentiation and development of glial cells in primary cultures of embryonic rat cerebral hemisphere. J Neurosci Res. 2005;79:608–615. doi: 10.1002/jnr.20373. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi A. [Application of bmp to bone repair] . Clin Calcium. 2007;17:263–269. [PubMed] [Google Scholar]
- 9.Zou H, Choe KM, Lu Y, Massague J, Niswander L. Bmp signaling and vertebrate limb development. Cold Spring Harb Symp Quant Biol. 1997;62:269–272. [PubMed] [Google Scholar]
- 10.Massague J, Weis-Garcia F. Serine/threonine kinase receptors: Mediators of transforming growth factor beta family signals. Cancer Surv. 1996;27:41–64. [PubMed] [Google Scholar]
- 11.Yamashita H, Ten Dijke P, Heldin CH, Miyazono K. Bone morphogenetic protein receptors. Bone. 1996;19:569–574. doi: 10.1016/s8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- 12.Reddi AH. Bone morphogenetic proteins: From basic science to clinical applications. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S1–6. doi: 10.2106/00004623-200100001-00001. [DOI] [PubMed] [Google Scholar]
- 13.Forino M, Torregrossa R, Ceol M, Murer L, Della Vella M, Del Prete D, D’Angelo A, Anglani F. TGFbeta1 induces epithelial-mesenchymal transition, but not myofibroblast transdifferentiation of human kidney tubular epithelial cells in primary culture. Int J Exp Pathol. 2006;87:197–208. doi: 10.1111/j.1365-2613.2006.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meindl-Beinker NM, Dooley S. Transforming growth factor-beta and hepatocyte transdifferentiation in liver fibrogenesis. J Gastroenterol Hepatol. 2008;23(Suppl 1):S122–127. doi: 10.1111/j.1440-1746.2007.05297.x. [DOI] [PubMed] [Google Scholar]
- 15.Hui AY, Friedman SL. Molecular basis of hepatic fibrosis. Expert Rev Mol Med. 2003;5:1–23. doi: 10.1017/S1462399403005684. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Hirschberg R. BMP7 antagonizes TGF-beta -dependent fibrogenesis in mesangial cells. Am J Physiol Renal Physiol. 2003;284:F1006–1013. doi: 10.1152/ajprenal.00382.2002. [DOI] [PubMed] [Google Scholar]
- 17.Izumi N, Mizuguchi S, Inagaki Y, Saika S, Kawada N, Nakajima Y, Inoue K, Suehiro S, Friedman SL, Ikeda K. BMP-7 opposes TGF-beta1-mediated collagen induction in mouse pulmonary myofibroblasts through id2. Am J Physiol Lung Cell Mol Physiol. 2006;290:L120–126. doi: 10.1152/ajplung.00171.2005. [DOI] [PubMed] [Google Scholar]
- 18.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 19.Wang SE, Wu FY, Shin I, Qu S, Arteaga CL. Transforming growth factor β (TGFβ)-smad target gene protein tyrosine phosphatase receptor type kappa is required for TGFβ function. Mol Cell Biol. 2005;25:4703–4715. doi: 10.1128/MCB.25.11.4703-4715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saika S, Ikeda K, Yamanaka O, Flanders KC, Ohnishi Y, Nakajima Y, Muragaki Y, Ooshima A. Adenoviral gene transfer of BMP-7, Id2, or Id3 suppresses injury-induced epithelial-to-mesenchymal transition of lens epithelium in mice. Am J Physiol Cell Physiol. 2006;290:C282–289. doi: 10.1152/ajpcell.00306.2005. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Wan J, Jiang D, Wu X. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition in human renal proximal tubular epithelial cells. J Nephrol. 2009;22:403–410. [PubMed] [Google Scholar]
- 22.Gould SE, Day M, Jones SS, Dorai H. BMP-7 regulates chemokine, cytokine, and hemodynamic gene expression in proximal tubule cells. Kidney Int. 2002;61:51–60. doi: 10.1046/j.1523-1755.2002.00103.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee MJ, Yang CW, Jin DC, Chang YS, Bang BK, Kim YS. Bone morphogenetic protein-7 inhibits constitutive and interleukin-1 beta-induced monocyte chemoattractant protein-1 expression in human mesangial cells: Role for jnk/ap-1 pathway. J Immunol. 2003;170:2557–2563. doi: 10.4049/jimmunol.170.5.2557. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Hirschberg R. Bone morphogenetic protein-7 signals opposing transforming growth factor beta in mesangial cells. J Biol Chem. 2004;279:23200–23206. doi: 10.1074/jbc.M311998200. [DOI] [PubMed] [Google Scholar]
- 25.Benchabane H, Wrana JL. Gata- and smad1-dependent enhancers in the smad7 gene differentially interpret bone morphoge-netic protein concentrations. Mol Cell Biol. 2003;23:6646–6661. doi: 10.1128/MCB.23.18.6646-6661.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harada N, Hatano E, Koizumi N, Nitta T, Yoshida M, Yamamoto N, Brenner DA, Yamaoka Y. Akt activation protects rat liver from ischemia/reperfusion injury. J Surg Res. 2004;121:159–170. doi: 10.1016/j.jss.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Carini R, Grazia De Cesaris M, Splendore R, Baldanzi G, Nitti MP, Alchera E, Filigheddu N, Domenicotti C, Pronzato MA, Graziani A, Albano E. Role of phosphatidylinositol 3-kinase in the development of hepatocyte preconditioning. Gastroenterology. 2004;127:914–923. doi: 10.1053/j.gastro.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-beta and the smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell. 2005;16:1987–2002. doi: 10.1091/mbc.E04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tacke F, Gäbele E, Bataille F, Schwabe RF, Hellerbrand C, Klebl F, Straub RH, Luedde T, Manns MP, Trautwein C, Brenner DA, Schölmerich J, Schnabl B. Bone morphogenetic protein 7 is elevated in patients with chronic liver disease and exerts fibrogenic effects on human hepatic stellate cells. Dig Dis Sci. 2007;52:3404–3415. doi: 10.1007/s10620-007-9758-8. [DOI] [PubMed] [Google Scholar]
- 30.Schnabl B, Kweon YO, Frederick JP, Wang XF, Rippe RA, Brenner DA. The role of smad3 in mediating mouse hepatic stellate cell activation. Hepatology. 2001;34:89–100. doi: 10.1053/jhep.2001.25349. [DOI] [PubMed] [Google Scholar]


