Abstract
Background: C4d has been used as an evaluation marker for antibody-mediated rejection for solid organ transplantation. Although some studies have proposed that complement activation is involved in renal diseases, very little information is available on pathogenesis. This study was conducted to investigate C4d deposition in IgA nephropathy and to find its relations with histopathology and albuminuria. Methods: This retrospective study included 23 patients who underwent renal biopsy at our medical center. The WHO grade of IgA nephropathy, interstitial inflammation and fibrosis, C4d staining and medical records including sex, age, and urine albumin were reviewed. Results: Thirteen patients (56.5%) were positive for C4d staining in the glomerulus and eleven patients (47.8%) were positive in the tubular epithelium. Glomerular C4d deposition was associated with albuminuria (p=0.044), and tubular C4d deposition was associated with a higher grade of IgA nephropathy (p=0.014). Conclusions: Activation of the complement system was involved in renal damage and was identified through deposition of C4d in the glomerulus and tubules. Positive C4d staining in the glomerulus and the tubules may be associated with functional damage related to glomerular filtration and poor renal outcome.
Keywords: Albuminuria, C4d, grade, IgA, nephropathy
Introduction
C4d is a well-known biomarker of the complement cascade. Although C4d itself has no biological function, it is recognized for its function of complement activation through the classical, alternative or lectin pathway. C4d is derived from cleavage of the labile thioester bond of C4b. This cleavage provides C4d a covalent bond which helps C4d to anchor to nearby cells where immune complexes are deposited. Antibodies dissociate naturally because of relatively weak hydrostatic and Van der Waals forces between antigens and antibodies, whereas covalent bond of C4d has a much longer half-life [1]. For this reason, C4d serves as a footprint for complement activation.
The usefulness of C4d in the identification of antibody-mediated rejection (AMR) has generally been accepted. C4d was incorporated in the Banff classification in 2003 [2]. Clinically, C4d immunohistochemistry has been used as an evaluation marker for AMR for solid organ transplantation. In particular, deposition of C4d in the peritubular capillary endothelium serves crucial role in the rejection mechanism of kidney transplantation. Therefore, many studies have been focusing on C4d as a diagnostic tool for AMR.
Recently, many researchers have turned their attention to C4d deposition in native renal disease. Xing et al [3] recently investigated that complement activation is involved in renal damage of pauci-immune crescentic glomerulonephritis. Espinosa et al [4] suggested that C4d is a useful tool for the differential diagnosis of membranous nephropathy and minimal change disease. However, very little information is available on the pathogenesis of various diseases that are activated by the complement system.
Therefore, it is reasonable to expand the study of C4d to other immunologic diseases to prove C4d deposition on immune-complexes and to find a new role of C4d in other diseases. We undertook a study on C4d deposition with 23 cases of IgA nephropathy by immunohistochemistry. In addition, we connected C4d deposition to histopathology and albuminuria. The aim of our study was to investigate deposition of C4d in IgA nephropathy and to find its relations with histopathology and albuminuria.
Materials and methods
This retrospective study incorporated all consecutive patients who underwent renal biopsy at our medical center between January 2010 and December 2011. The diagnosis of IgA nep-hropathy and minimal change disease (MCD) was based on the histological assessment of renal biopsy specimens by light microscopy, with H&E, Masson’s trichrome, periodic acid-Schiff (PAS), and periodic acid methenamine (PAM) silver stain. Immunofluorescence (IF) study was performed with polyclonal IgG, IgM, IgA, C3, and C1q. Electronic microscopic study was performed with standard laboratory protocols. The study group was divided into IgA group and control group (Figure 1). As complement activation has been considered not to be invo-lved in MCD [5], renal biopsy samples from 11 patients with MCD were included as controls.
Figure 1.
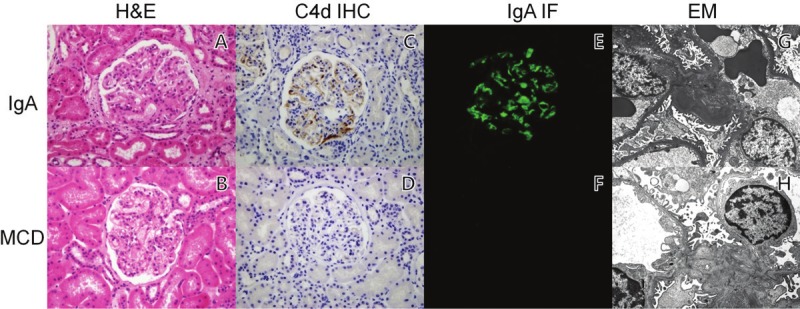
Hematoxylin and eosin (A and B), immunohistochemistry, immunofluorescence with IgA antibodies, and electron microscopic studies in IgA nephropathy and minimal change disease patient. The same granular pattern of IgA and C4d deposits are observed by immunohistochemical stain (C) and immunofluorescence (E). Electron-density deposits in the subendothelium were detected by electron microscopy (G). C4d depositions were not observed in minimal change disease (D, F and H).
Medical records and laboratory findings were reviewed at the time of renal biopsy. The following data, including patient sex, age, and urine albumin, were recorded: urine albumin was scored as 0 when <15 mg/dL, 1+ when 15 mg/dL-30 mg/dL, 2+ when 30 mg/dL-100 mg/dL, and 3+ when >100 mg/dL.
Histologic evaluation
Routine H&E, PAS, PAM silver stain, and Masson’s trichrome were evaluated on renal biopsy specimens. Assessment of histological compartments in the kidney (glomeruli, tubules, interstitiums, and blood vessels) was performed separately. For the IgA nephropathy group, the histologic grade was assessed using the WHO classification.
C4d immunohistochemical staining
Staining of C4d was performed by immunohistochemical staining. Paraffin embedded renal tissues were cut into 4 μm sections and deparaffinized. The sections underwent immunohistochemical staining using anti-human C4d polyclonal antibodies as primary antibodies (Biomedica, Vienna, Austria). Immunoreactivity in each compartment of the kidney was analyzed as below:
Glomerular C4d staining
C4d staining was observed in both endothelial and mesangial cells. Glomerular C4d was defined positive when more than 50% of glomeruli staining were observed.
Tubular C4d staining
Tubular basement membrane (TBM) C4d staining was considered to be present if more than half of the circumference of TBM was stained [6]. Tubular epithelial staining was considered to be positive if the granular pattern of C4d staining was present.
Peritubular capillary and vascular staining
Peritubular capillary staining and vascular were classified as absent or present in <10% or >10% of stained tissues available for evaluation as recommended by Banff 2007 [7].
Statistical analysis
The data are expressed as mean ± standard deviation for continuous variables and percentage for categorical variables. Fisher’s exact test or the Pearson Chi-square test was used to compare the differences in qualitative data. A P-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS statistics software version 19.0 (IBM corp., Armonk, NY, USA).
Results
Twenty three patients diagnosed with IgA nephropathy were included in our study group and 11 patients with MCD were included as the control group.
Histologic evaluation
Renal biopsy of the IgA nephropathy group was reviewed in each compartment of histological structure and graded according to the WHO classification. Eleven subjects (47.8%) had no inflammation, 10 subjects (43.5%) had mild inflammation, and 1 subject (4.3%) had moderate inflammation in the tubule and interstitium in the IgA group.
The numbers of IgA patients according to the WHO grade were as follows: grade I (17.4%), grade II (52.2%), grade III (26.1%), and grade IV (4.3%). There was no patient with grade V IgA nephropathy in our study.
Fibrosis in the tubules and interstitium was scored as: none (60.9%), mild (34.8%), moderate (0%), and severe (4.3%), respectively. Thickening of blood vessels was identified as unremarkable (60.9%) and mild thickening (39.1%).
Immunohistochemical C4d staining
Staining of C4d in the tubular basement membrane, peritubular capillary, and vascular structure was not detected in both the IgA and MCD groups. Therefore, they were not included in the statistical analysis.
Glomerular C4d staining
In the IgA nephropathy group, glomerular C4d staining was observed in 13 out of 23 patients (56.5%). The staining pattern of glomerular C4d showed granular distributions outlining the capillary loops and in the mesangium (Figure 2A). On the other hand, in the MCD group, no glomerular C4d staining was detected in the glomeruli.
Figure 2.
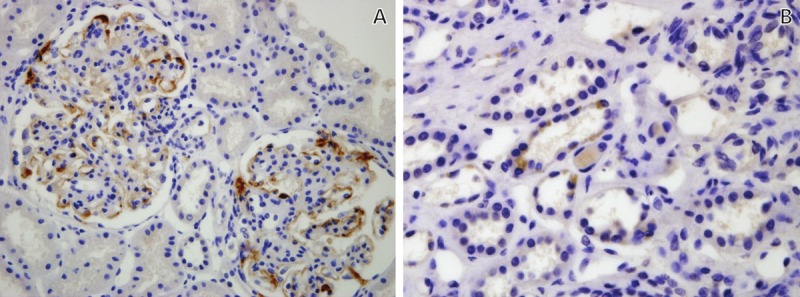
Immunohistochemical C4d staining in IgA nephropathy patient. A. Glomerular C4d staining was distributed along the capillary loops in the glomerulus. The mesangial staining was suggested in small focus. B. Granular distribution of C4d staining was observed in tubular epithelium. A, B. Original magnification X400.
In IgA nephropathy, the amount of albuminuria is significantly correlated with glomerular C4d staining (p=0.044) (Figure 3A). However, there was no significant correlation between C4d staining and the WHO grade (p=0.562), inflammation (p=0.463), fibrosis (p=0.318), or thickening of blood vessels (p=0.102) (Table 1).
Figure 3.
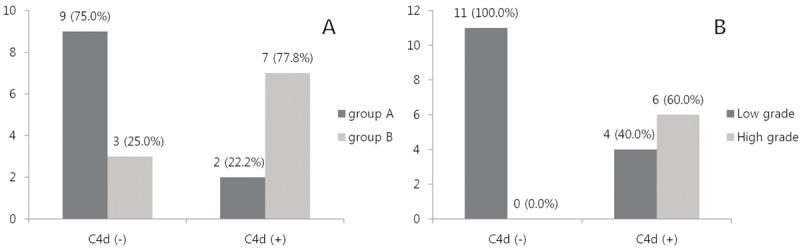
The number and percentage of IgA nephropathy patients with albuminuria and the grade of WHO classification. A. The degree of albuminuria was divided into group A (0, 1+: albuminuria < 30 mg/dL) and group B (2+, 3+: albuminuria > 30 mg/dL). There was statistical significance between group A and B, according to positivity of the glomerular C4d staining (p=0.044). B. The grade of WHO classification was sub-grouped into low grade (class I and II) and high grade (class III and IV). There was significant difference between two groups, according to C4d staining in tubular epithelium (p=0.014).
Table 1.
C4d staining in glomerulus and association with histopatholgy and albuminuria in IgA nephropathy
| G-C4d‡ | Grade, N (%) | p-value† | ||||
|---|---|---|---|---|---|---|
| Inflammation | 0 | 1+ | 2+ | 3+ | Total | 0.463 |
| C4d (+) | 7 (30.4%) | 1 (4.3%) | 4 (17.4%) | 0 (0%) | 12 (52.1%) | |
| C4d (-) | 4 (17.4%) | 0 (0%) | 5 (21.7%) | 1 (4.3%) | 10 (43.4%) | |
| Fibrosis | 0 | 1+ | 2+ | 3+ | Total | 0.318 |
| C4d (+) | 9 (39.1%) | 3 (13.0%) | 0 (0%) | 1 (4.3%) | 13 (56.5%) | |
| C4d (-) | 5 (21.7%) | 5 (21.7%) | 0 (0%) | 0 (0%) | 10 (43.5%) | |
| Blood vessel | Unremarkable | Thickening | Total | 0.102 | ||
| C4d (+) | 10 (43.5%) | 3 (13.0%) | 13 (56.5%) | |||
| C4d (-) | 4 (28.6%) | 6 (66.7%) | 10 (43.5%) | |||
| WHO classification | I | II | III | IV | Total | 0.562 |
| C4d (+) | 3 (13.0%) | 7 (30.4%) | 2 (8.7%) | 1 (4.3%) | 13 (56.5%) | |
| C4d (-) | 1 (4.3%) | 5 (21.7%) | 4 (17.4%) | 0 (0%) | 10 (43.5%) | |
| Albuminuria | 0 | 1+ | 2+ | 3+ | Total | 0.044 |
| C4d (+) | 6 (28.6%) | 3 (14.3%) | 1 (4.8%) | 2 (9.5%) | 12 (57.1%) | |
| C4d (-) | 1 (4.8%) | 1 (4.8%) | 6 (28.6%) | 1 (4.8%) | 9 (42.9%) | |
Inflammation and fibrosis score in inerstitium: (0) negative, (1+) mild, (2+) moderate, (3+) severe. Albuminuria score: (0) < 15 mg/dL, (1+) 15 mg/dL-30 mg/dL, (2+) 30 mg/dL-100 mg/dL, (3+) > 100 mg/dL.
Calculated by Fisher’s exact tests or Pearson chi-square tests.
G-C4d: Glomerular C4d staining.
Missing data and obscure cases of C4d staining were excluded from the analysis.
Tubular C4d staining
Eleven out of 21 patients (47.8%) had C4d staining in the tubular epithelium in the IgA nephropathy group (Figure 2B). Two cases were classified as indeterminate for C4d staining in the tubular epithelium. However, C4d staining in the tubular epithelium was not detected in the MCD group.
The higher grade of the WHO classification was significantly correlated with C4d staining in the tubular epithelium (p=0.014) (Figure 3B). How-ever, positive C4d in the tubular epithelium did not correlate with albuminuria (p=0.173), inf-lammation (p=0.066), fibrosis (p=0.124), or thickening of blood vessels (p=0.183) (Table 2).
Table 2.
C4d staining in tubular epithelium and association with histopatholgy and albuminuria in IgA nephropathy
| T-C4d‡ | Grade, N (%) | p-value† | ||||
|---|---|---|---|---|---|---|
| Inflammation | 0 | 1+ | 2+ | 3+ | Total | 0.066 |
| C4d (+) | 8 (38.1%) | 1 (4.8%) | 1 (4.8%) | 1 (4.8%) | 11 (52.4%) | |
| C4d (-) | 3 (14.3%) | 0 (0%) | 6 (28.6%) | 0 (0%) | 10 (47.6%) | |
| Fibrosis | 0 | 1+ | 2+ | 3+ | Total | 0.124 |
| C4d (+) | 9 (42.9%) | 2 (9.5%) | 0 (0%) | 0 (0%) | 11 (52.4%) | |
| C4d (-) | 4 (19.0%) | 5 (23.8%) | 0 (0%) | 1 (4.8%) | 10 (47.6%) | |
| Blood vessel | Unremarkable | Thickening | Total | 0.183 | ||
| C4d (+) | 9 (42.9%) | 2 (9.5%) | 11 (52.4%) | |||
| C4d (-) | 5 (23.8%) | 5 (23.8%) | 10 (47.6%) | |||
| WHO classification | I | II | III | IV | Total | 0.014 |
| C4d (+) | 3 (14.3%) | 8 (38.1%) | 0 (0%) | 0 (0%) | 11 (52.4%) | |
| C4d (-) | 1 (4.8%) | 3 (14.3%) | 5 (23.8%) | 1 (4.8%) | 10 (47.6%) | |
| Albuminuria | 0 | 1+ | 2+ | 3+ | Total | 0.173 |
| C4d (+) | 6 (28.6%) | 2 (9.5%) | 2 (9.5%) | 1 (4.8%) | 11 (52.4%) | |
| C4d (-) | 1 (4.8%) | 2 (9.5%) | 5 (23.8%) | 2 (9.5%) | 10 (47.6%) | |
Inflammation and fibrosis score in inerstitium: (0) negative, (1+) mild, (2+) moderate, (3+) severe. Albuminuria score: (0) < 15 mg/dL, (1+) 15 mg/dL-30 mg/dL, (2+) 30 mg/dL-100 mg/dL, (3+) > 100 mg/dL.
Calculated by Fisher’s exact tests or Pearson chi-square tests.
T-C4d: tubular epithelial C4d staining.
Missing data and obscure cases of C4d staining were excluded from the analysis.
Tubular basement C4d staining was not found either in the IgA nephropathy group or in the MCD group.
Discussion
C4d is a degradation product of the complement cascade. Although C4d has no biological effect, it is a well-known biomarker as a footprint of complement activation. During complement activation, C4d is derived from cleavage of C4b and binds covalently to the endothelium or adjacent tissue. Until recently, the majority of C4d studies have been focusing on AMR in renal allografts as a diagnostic tool. Recent discoveries regarding C4d deposition in native renal diseases have been reported, yet there are only few studies.
IgA nephropathy is believed to be associated with immunoglobulin deposition in the kidney but the exact pathogenesis remains unknown. Our study demonstrated C4d deposition in the glomerulus and tubular epithelium in the renal tissue previously diagnosed with IgA nephropathy. This deposition of C4d was shown to matched with the histology and the degree of albuminuria. Glomerular C4d staining was observed in 13/23 (56.5%) in the IgA group. The staining area of C4d immunohistochemistry and IgA IF coincided with the same granular pattern in the endothelium and mesangium in the glomeruli. Our study suggests that glomerular C4d probably reflects in situ activation of the complement pathway by deposition of immune complexes.
In renal allotransplantation, glomerular C4d staining is regarded as a non-specific indication of glomerular damage [6,8]. However, in our study, positive glomerular C4d staining was associated with a higher degree of albuminuria (p=0.044) in IgA nephropathy. Tamouza et al. [9] showed that IgA1 immune complex-mediated activation of the MAPK/ERK pathway in mesangial cells in IgA nephropathy is associated with glomerular damage and filtration barrier manifested by proteinuria. These findings imply that immune complex mediated complement activation might be involved in glomerular damage, either in a direct or indirect fashion. Therefore, we propose that glomerular C4d staining could be considered as an indication of glomerular damage in IgA nephropathy. We are also consistent with the idea that positive mesangial C4d staining in the glomerulus is associated with poor prognosis in IgA nephropathy [10].
A granular pattern of C4d staining in the tubular epithelium was observed in 11/21 (52.4%) in the IgA group. Positive epithelial C4d staining in the tubules was associated with a higher grade of the WHO classification (p=0.014). Although it was not statistically significant, the degree of interstitial inflammation also appeared to have an association tendency with staining in the tubular epithelium (p=0.066). Van Es et al. [11] revealed intraepithelial lymphocytes in the tubules and interstitial B cells as a predictive marker of progression in the early stage of IgA nephropathy. Our previous observation which showed an association of a higher WHO grade and a higher degree of interstitial inflammation with positive C4d staining in the tubular epithelium explains that the complement system may be involved in the progression in IgA nephropathy.
In renal allograft biopsy, peritubular capillary C4d was correlated with AMR and was associated with poor renal outcome [2,12-14]. Peritubular capillary C4d staining was investigated in various native kidney diseases [3,15-19], where peritubular capillary C4d was never observed, except for lupus nephritis [A]. In our study, we did not detect peritubular capillary C4d staining in both the IgA and control groups. In native renal diseases, such as IgA nephropathy and MCD in our case, C4d does not seem to deposit nor activate the complement system in peritubular capillaries. Overall, C4d staining indicates a probable local activation of the complement system in each compartment in IgA nephropathy [10,20], while complement activation plays no role in the pathogenesis of MCD [4].
Our study has some limitations. This was an observational study; the relations with C4d staining should be interpreted carefully as associations rather than casualties. Including more IgA nephropathy patients and MCD patients would have provided more significant data.
In summary, this is the first study to show the relation between C4d staining in different histologic compartments, histopathology, and degree of albuminuria in IgA nephropathy. This study revealed that C4d depositions were observed in the glomerulus and tubular epithelium. Glomerular C4d staining reflects in situ activation of the complement pathway by immune complex deposition and shows an association with a higher degree of albuminuria. Also, positive staining in the tubular epithelium correlates significantly with a higher grade of WHO classification. Our study suggests that C4d deposition in each compartment indicates poor prognosis and identifies glomerular and tubular damage through C4d immunohistochemical staining.
References
- 1.Cohen D, Colvin RB, Daha MR, Drachenberg CB, Haas M, Nickeleit V, Salmon JE, Sis B, Zhao MH, Bruijn JA, Bajema IM. Pros and cons for C4d as a biomarker. Kidney Int. 2012;81:628–639. doi: 10.1038/ki.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K. Antibody-mediated rejection criteria - an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 3.Xing GQ, Chen M, Liu G, Zheng X, E J, Zhao MH. Differential deposition of C4d and MBL in glomeruli of patients with ANCA-negative pauci-immune crescentic glomerulonephritis. J Clin Immunol. 2010;30:144–156. doi: 10.1007/s10875-009-9344-2. [DOI] [PubMed] [Google Scholar]
- 4.Espinosa-Hernandez M, Ortega-Salas R, Lopez-Andreu M, Gomez-Carrasco JM, Perez-Saez MJ, Perez-Seoane C, Aljama-Garcia P. C4d as a diagnostic tool in membranous nephropathy. Nefrologia. 2012;32:295–299. doi: 10.3265/Nefrologia.pre2012.Feb.11224. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Horita S, Kadoya K, Mitsuiki K, Aita K, Harada A, Nitta K, Nagata M. C4d Immunohistochemistry in glomerulonephritis with different antibodies. Clin Exp Nephrol. 2007;11:287–291. doi: 10.1007/s10157-007-0496-1. [DOI] [PubMed] [Google Scholar]
- 6.Batal I, Girnita A, Zeevi A, Saab BA, Stockhausen S, Shapiro R, Basu A, Tan H, Morgan C, Randhawa P. Clinical significance of the distribution of C4d deposits in different anatomic compartments of the allograft kidney. Mod Pathol. 2008;21:1490–1498. doi: 10.1038/modpathol.2008.152. [DOI] [PubMed] [Google Scholar]
- 7.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 8.Seemayer CA, Gaspert A, Nickeleit V, Mihatsch MJ. C4d staining of renal allograft biopsies: a comparative analysis of different staining techniques. Nephrol Dial Transplant. 2007;22:568–576. doi: 10.1093/ndt/gfl594. [DOI] [PubMed] [Google Scholar]
- 9.Tamouza H, Chemouny JM, Raskova Kafkova L, Berthelot L, Flamant M, Demion M, Mesnard L, Paubelle E, Walker F, Julian BA, Tissandie E, Tiwari MK, Camara NO, Vrtovsnik F, Benhamou M, Novak J, Monteiro RC, Moura IC. The IgA1 immune complex-mediated activation of the MAPK/ERK kinase pathway in mesangial cells is associated with glomerular damage in IgA nephropathy. Kidney Int. 2012;82:1284–96. doi: 10.1038/ki.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinosa M, Ortega R, Gomez-Carrasco JM, Lopez-Rubio F, Lopez-Andreu M, Lopez-Oliva MO, Aljama P. Mesangial C4d deposition: a new prognostic factor in IgA nephropathy. Nephrol Dial Transplant. 2009;24:886–891. doi: 10.1093/ndt/gfn563. [DOI] [PubMed] [Google Scholar]
- 11.van Es LA, de Heer E, Vleming LJ, van der Wal A, Mallat M, Bajema I, Bruijn JA, de Fijter JW. GMP-17-positive T-lymphocytes in renal tubules predict progression in early stages of IgA nephropathy. Kidney Int. 2008;73:1426–1433. doi: 10.1038/ki.2008.66. [DOI] [PubMed] [Google Scholar]
- 12.Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046–1056. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 13.Herzenberg AM, Gill JS, Djurdjev O, Magil AB. C4d deposition in acute rejection: an independent long-term prognostic factor. J Am Soc Nephrol. 2002;13:234–241. doi: 10.1681/ASN.V131234. [DOI] [PubMed] [Google Scholar]
- 14.Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmuller G, Land W, Albert E. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993;43:1333–1338. doi: 10.1038/ki.1993.187. [DOI] [PubMed] [Google Scholar]
- 15.Zwirner J, Felber E, Herzog V, Riethmuller G, Feucht HE. Classical pathway of complement activation in normal and diseased human glomeruli. Kidney Int. 1989;36:1069–1077. doi: 10.1038/ki.1989.302. [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Xing GQ, Yu F, Liu G, Zhao MH. Complement deposition in renal histopathology of patients with ANCA-associated pauci-immune glomerulonephritis. Nephrol Dial Transplant. 2009;24:1247–1252. doi: 10.1093/ndt/gfn586. [DOI] [PubMed] [Google Scholar]
- 17.Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276–286. doi: 10.1016/j.jaut.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Kusunoki Y, Itami N, Tochimaru H, Takekoshi Y, Nagasawa S, Yoshiki T. Glomerular deposition of C4 cleavage fragment (C4d) and C4-binding protein in idiopathic membranous glomerulonephritis. Nephron. 1989;51:17–19. doi: 10.1159/000185234. [DOI] [PubMed] [Google Scholar]
- 19.Cohen D, Koopmans M, Kremer Hovinga IC, Berger SP, Roos van Groningen M, Steup-Beekman GM, de Heer E, Bruijn JA, Bajema IM. Potential for glomerular C4d as an indicator of thrombotic microangiopathy in lupus nephritis. Arthritis Rheum. 2008;58:2460–2469. doi: 10.1002/art.23662. [DOI] [PubMed] [Google Scholar]
- 20.Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, Stahl GL, Matsushita M, Fujita T, van Kooten C, Daha MR. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734. doi: 10.1681/ASN.2005090923. [DOI] [PubMed] [Google Scholar]


