Abstract
Interleukin-9 (IL-9) is initially described as a growth factor secreted by helper T cells. It acts on a variety of immune cells via its receptor (IL-9R). Recently, the oncogenic activities of IL-9 and IL-9R were reported in some lymphomas but not diffuse large B-cell lymphoma (DLBCL). The purpose of the present study is to investigate the expression of IL-9R in pathological tissues from patients with DLBCL and to evaluate its correlation with clinical characteristics. Tissue samples from patients with DLBCL and reactive lymphoid hyperplasia were analyzed using RT-PCR, western blot and immunohistochemical staining. There was a higher expression of IL-9R within DLBCL tissues compared with hyperplasic lymph nodes. Immunohistochemical analysis indicated membrane localization of IL-9R in 22 of 36 (61.1%) DLBCL cases. The upregulated IL-9R was correlated to the serum levels of β2 microglobulin and albumin, International Prognostic Index (IPI) score as well as Ki-67 expression within tumor tissues. Our findings suggest that overexpression of IL-9R may contribute to the pathogenesis of DLBCL and is associated with some adverse prognostic parameters.
Keywords: IL-9 receptor, diffuse large B-cell lymphoma, prognosis
Introduction
DLBCL is known as an aggressive malignancy arising from B lymphocytes. It is the most common type of non-Hodgkin lymphoma (NHL) in adults [1], with an annual incidence of 7-8 cases per 100,000 people [2,3]. Despite its greatly improved prognosis as a result of chemotherapy and immunotherapy with monoclonal antibodies [4], the exact molecular etiology of DLBCL is not well understood.
IL-9 was originally identified in mice as a helper T cell growth factor [5,6]. It binds to a heterodimeric receptor composed of a cytokine-specific α-chain (IL9Rα) and a common γ-chain shared with IL-2, IL-4, IL-7, IL-15 and IL-21 [7-9]. Signal transduction mediated by IL-9 is mainly dependent on its high affinity with IL-9R. Due to its pleiotropic functions on mast cells, IL-9 has long been recognized as an important regulator of allergic inflammation [10]. But in recent years, a resurgence of interest in IL-9 has been spurred due to an expanded identification of its receptor on various immune cells [11]. A series of observations have pointed to this cytokine as a factor promoting oncogenesis, especially lymphomagenesis [12,13]. The dysregulated expression of IL-9 and IL-9R can be detected in biopsies and serums from patients with Hodgkin’s disease (HD) , anaplastic large cell lymphomas (ALCL) [14] as well as nasal natural killer (NK)/T-cell lymphoma [15,16]. Our previous study also demonstrated that there was an elevated serum level of IL-9 in B-cell NHL patients including some DLBCL cases. Blocking the combination between IL-9 and IL-9R with neutralizing antibodies could significantly inhibit tumor growth in the murine models of lymphoma [17]. The present study is aimed to investigate the expression of IL-9R in DLBCL tissues and to illuminate its role in the pathogenesis of DLBCL.
Materials and methods
Patients and samples
Paraffin-embedded archived samples from 36 cases of DLBCL prior to therapeutic interventions were obtained at Shandong Provincial Hospital. All patients were diagnosed according to the WHO criteria between January 2010 and July 2012 [18]. Twenty biopsies of DLBCL tissues and fifteen lymph node tissues from patients with reactive lymphoid hyperplasia were frozen and stored in liquid nitrogen until further use. Clinical information, including patient gender, age, B symptoms, serum levels of lactic dehydrogenase (LDH), albumin and β2 microglobulin, and International Prognostic Index (IPI) scores, were also collected. The protocol was approved by the Shandong Provincial Hospital Ethics Committee and written informed consent was obtained from all participants involved in this study.
Reverse transcription PCR
Total RNA was extracted from DLBCL tissues and reactive hyperplasia of lymph nodes using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcription was performed using the PrimeScript RT (reverse transcription) reagent Kit (TaKaRa). The expression of IL-9R mRNA in tissues was detected by RT-PCR with the cycling parameters as follows: an initial cycle for 5 minutes at 94°C, followed by 40 cycles of 15 seconds at 95°C, 30 seconds at 59°C and 55 seconds at 72°C. PCR products were confirmed as a single product at the desired size on agarose gels and visualized by ethidium bromide staining. Specific primers for RT-PCR were obtained from Biosune (Shanghai, China), and the primer sequences were shown in Table 1.
Table 1.
Primer Sequences
| Gene Name | Sequence |
|---|---|
| IL-9R | 5’-CGTGCCCTCTCCAGCGATGTTCT-3’ |
| 5’-GACGCGCTGGGCCACAAGTG -3’ | |
| β-actin | 5’-TGACGTGGACATCCGCAAAG -3’ |
| 5’-CTGGAAGGTGGACAGCGAGG-3’ |
Western blot analysis
The expressions of IL-9R in DLBCL tissues and reactive hyperplasia of lymph node were determined by western blot. Total protein was extracted using RIPA and 1% PMSF (Shenergy Biocolor, China), according to the manufacturer’s instructions. The protein concentration of the samples was determined by the BCA assay (Shenergy Biocolor). Tissue lysate was then electrophoresed on 10% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. After the membranes were blocked with 5% skim milk in Tris-saline buffer with 0.1% Tween-20, they were subsequently probed with primary antibodies at 4°C overnight. After washing with TBST, secondary antibody conjugated with the horseradish peroxidase (Zhongshan Goldenbridge Biotechnology Company, China) was added to the membranes. Subsequently, proteins were detected using the chemiluminescence detection kit (Millipore, USA). Antibodies used in this study included rabbit anti-IL-9R polyclonal antibody (1:100; Abcam, USA) and mouse anti-β-actin monoclonal antibody (1:10,000; Abcam). Western blotting results were analyzed using the Las-4000 Image software and Multi Gauge Ver. 3.0 software (FujiFilm Life Science, Japan).
Immunohistochemical analysis
Formalin-fixed, paraffin-embedded tissue sections of 4-μm were deparaffinized and hydrated. High-pressure antigen retrieval was performed using citrate buffer (pH 6.0). Endogenous peroxidase was quenched with 3% hydrogen peroxide in methanol for 15 min, followed by incubation with normal serum to block non-specific staining. Rabbit anti-IL-9R (1:50) was then incubated with the sections overnight in a humidified chamber at 4°C; the secondary antibody was from the SP reagent kit (Zhongshan Goldenbridge Biotechnology Company). After washing, the tissue sections were treated with biotinylated anti-rabbit secondary antibody, followed by further incubation with streptavidin-horseradish peroxidase complex. After staining with diaminobenzidine kit (DAB; Zhongshan Goldenbridge Biotechnology Company), the sections were counterstained with hematoxylin and mounted.
Immunohistochemical stainings were assessed in a series of randomly selected 5 high-power fields, considered to be representative of the average in tumors at x400 magnification, by two independent observers who were blinded to all clinical data. The sections were scored according to the proportion of positively stained tumor cells. For IL-9R staining, tumors with > 20% of cells showing definitive membrane staining were regarded as positive cases.
Statistical analysis
All statistical analyses were performed using the SPSS version 16.0 for Windows. The numerical data were statistically analyzed by the 2-tailed Student’s t-test. Chi-square analysis and Fisher’s exact test was used to analyze the relationship between the positive IL-9R expression and clinicopathological features. P < 0.05 was considered to indicate a statistically significant difference.
Result
Overexpression of IL-9R in DLBCL tissues
In the present study, the expressions of IL-9R mRNA and protein were determined in DLBCL tissues and reactive hyperplasia of lymph nodes using RT-PCR and western blotting, respectively. As illustrated in Figure 1, there is a higher expression of IL-9R mRNA in DLBCL tissues in contrast to that in the reactive lymphoid hyperplasia. In addition, the analysis on the gray scale of the Western blot bands indicated that the total protein levels of IL-9R in lymphoma tissues were significantly upregulated (p < 0.05, Figure 2).
Figure 1.
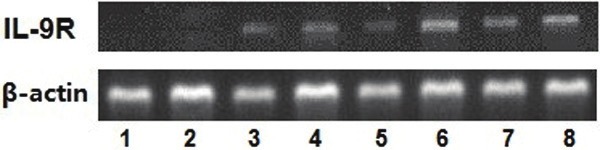
Expression of IL-9R mRNA in DLBCL tissues (lanes 5-8) and reactive hyperplasia of lymph node (lanes 1-4).
Figure 2.
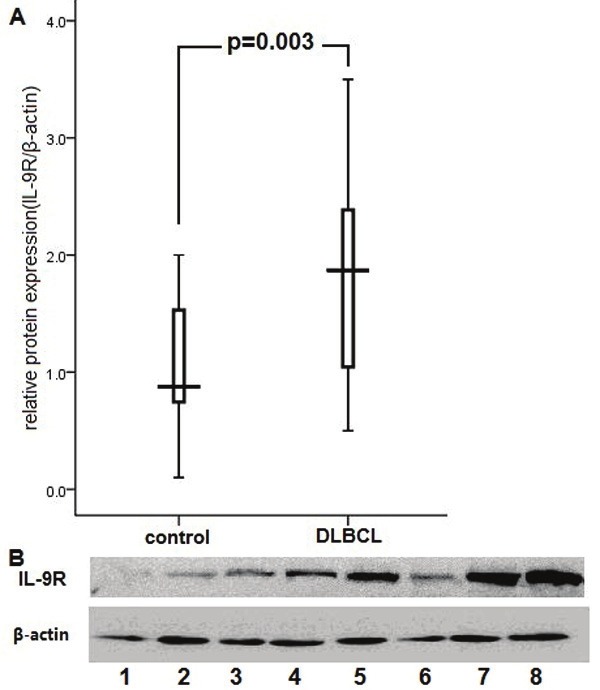
A. The analysis on the gray scale of the Western blot bands indicated that the total protein levels of IL-9R in lymphoma tissues were significantly upregulated (p=0.00 3). Expression levels were normalized with β-actin. B. Expression of total IL-9R protein in DLBCL tissues (lanes 5-8) and reactive hyperplasia of lymph node (lanes 1-4).
The correlation between positive IL-9R expression and clinical characteristics
To gain further insight into the prognostic value of IL-9R expression in DLBCL, paraffin-embedded sections from 36 DLBCL patients were examined by immunohistochemical staining. IL-9R protein was located on the tumor cell membrane (Figure 3) and positive expression was detected in 22 of 36 (61.1%) cases. Statistical analyses were performed to examine the relationship between expression of IL-9R and the clinical characteristics of DLBCL patients.
Figure 3.
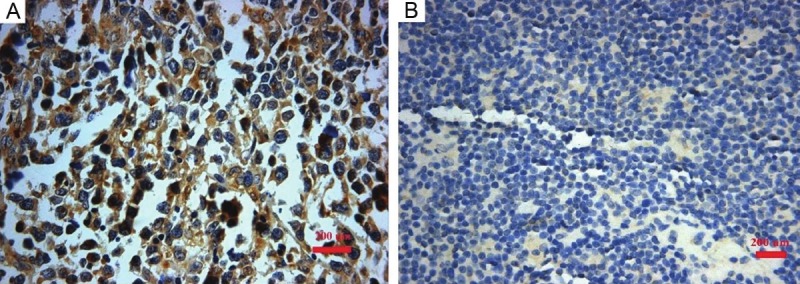
Immunohistochemical stainings located IL-9R protein on the tumor cell membrane in 23 of 36 (61.1%) DLBCL cases. A. positive expression; B. negative expression.
As shown in Table 2, no correlation was found between IL-9R expression and patient age (P=0.176), gender (P=0.733), B symptoms (p=0.14) as well as serum lactic dehydrogenase (LDH) levels (p=0.875). Nevertheless, the positive expression of IL-9R protein was correlated with serum levels of β2 microglobulin (2.15±0.43 vs 1.27±0.25, p < 0.001) and albumin (37.36±5.28 vs 41.73±5.3, p=0.024). Upregulated expression of IL-9R within tumor tissues implied higher serum β2 microglobulin and lower albumin levels.
Table 2.
Primer Sequences
| Clinicopathological Characteristics | IL-9R Expression | P value | |
|---|---|---|---|
|
| |||
| Negative (%) | Positive (%) | ||
| Age (yr) | 0.176 | ||
| <60 | 10 (71.4) | 10 (45.5) | |
| ≥60 | 4 (28.6) | 12 (54.5) | |
| Gender | 0.733 | ||
| Male | 8 (57.1) | 10 (45.5) | |
| Female | 6 (42.9) | 12 (54.5) | |
| B symptoms | |||
| yes | 6 (42.9) | 4 (18.2%) | 0.14 |
| no | 8 (57.1) | 18 (81.8%) | |
| serum LDH level | 313.17 | 326 | 0.875 |
| serum albumin level | 41.73±5.3 | 37.36±5.28 | 0.024 |
| serum β2 microglobulin level | 1.27±0.25 | 2.15±0.43 | <0.001 |
| International Prognostic Index | 0.005 | ||
| 0 | 2 (16.7) | 0 (0) | |
| 1 | 2 (16.7) | 4 (20.0) | |
| 2 | 8 (66.6) | 4 (20.0) | |
| 3 | 0 | 4 (20.0) | |
| 4 | 0 | 8 (40.0) | |
| Ki67 index | 0.023 | ||
| 0 | 4 (33.3) | 0 (0) | |
| 1 | 4 (33.3) | 4 (25.0) | |
| 2 | 4 (33.3) | 12 (75.0) | |
Besides, IPI scores, the most important prognostic indicator, were also associated with IL-9R expression (P=0.005). Patients with positive expression of IL-9R had a higher IPI score (p=0.005). Spearman correlation of IL-9R expression to IPI scores was 0.495 (p=0.004).
Expression of IL-9R protein correlates with Ki-67 expression in DLBCL
The expression of Ki-67 protein within DLBCL tissues was examined by immunohistochemical staining previously at Pathology Department of Shandong Provincial Hospital. Its immunoreactivity was evaluated as follows: 0, < 40% of tumor cells showing positive immunoreactivity; 1, 40–80% of tumor cells showing positive immunoreactivity; 2, > 80% of tumor cells showing positive immunoreactivity. Chi-square analysis indicated that there was a strong association between IL-9R overexpression and a high Ki-67 labeling index (Table 2). Bivariate correlation was also calculated by the Spearman rank correlation and the correlation coefficient is 0.483 (p=0.009).
Discussion
Interleukin-9 is able to stimulate proliferation of lymphoma cells [19] and protect them from dexamethasone (DEX)-induced apoptosis [20]. The pro-proliferative and anti-apoptotic effect of IL-9 were mainly dependent on its high-affinity binding with IL-9R. This receptor-ligand interaction activates the Janus kinase-signal transducer and activator of transcription (JAK/STAT) pathway and subsequently regulates its downstream apoptosis proteins [19,21]. Extensive studies have confirmed the oncogenic activities of IL-9 in lymphoma. Our previous study has demonstrated that there is an elevated IL-9 serum level in DLBCL patients [17]. Nevertheless, the expression IL-9R has not yet been investigated in DLBCL.
In the present study, we validated an upregulation of total IL-9R protein in DLBCL clinical specimens in contrast to reactive hyperplasia of lymph node tissues. Immunohistochemical stainings located IL-9R protein on the tumor cell membrane in 22 of 36 (61.1%) archival DLBCL paraffin sections. Further statistical analysis of the relationship between IL-9R staining and the clinical features of DLBCL patients were also performed. Our findings suggested that positive expression of IL-9R was strongly correlated with IPI scores and serum levels of β2 microglobulin and albumin. Low albumin levels might be related with aggressive progression of disease. IPI scores and serum β2 microglobulin levels are both adverse prognostic indicators of lymphoma. This correlation between IL-9R upregulation and more severe disease provided direct clinical evidence for the contribution of IL-9R to the pathogenesis of DLBCL.
In addition, we observed that IL-9R overexpression was associated with a high Ki-67 index within DLBCL tissues. The Ki-67 antigen is a cell proliferation marker; Ki-67 expression strictly correlates with cell cycle progression, and can be observed in G1, S, and G2 phase and mitotic cells [22]. Previous studies have demonstrated that IL-9 is able to promote proliferation of lymphoma cells via IL-9R [23]. We consider that the association between IL-9R and Ki-67 expression may be related to the pro-proliferation activity of IL-9.
In summary, the results clearly indicate that IL-9R is markedly overexpressed in DLBCL tissues compared to their counterparts. The expression intensity of IL-9R is correlated with adverse prognostic indicators of DLBCL and Ki-67 expression in pathological sections. Our findings suggest that the signal mediated by IL-9 via its receptor contributes to the pathogenesis of DLBCL. It helps us to get a deeper understanding about the molecular mechanism of DLBCL and could be served as a potentially therapeutic target for DLBCL patients in the future.
Acknowledgments
This study was supported by grants from the National Natural Science Fund of China, Natural Science Found of Shandong Province and the Project of Scientific and Technological Development of Shandong Province to XW.
Declaration
There are no relevant conflicts of interest to disclose.
References
- 1.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 2.Chihara T, Wada N, Ikeda J, Fujita S, Hori Y, Ogawa H, Sugiyama H, Nomura S, Matsumura I, Hino M, Kanakura Y, Morii E, Aozasa K. Frequency of intravascular large B-cell lymphoma in Japan: study of the Osaka Lymphoma Study Group. J Hematol Oncol. 2011;4:14. doi: 10.1186/1756-8722-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith A, Howell D, Patmore R, Jack A, Roman E. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer. 2011;105:1684–1692. doi: 10.1038/bjc.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Ferme C, Christian B, Lepage E, Tilly H, Morschhauser F, Gaulard P, Salles G, Bosly A, Gisselbrecht C, Reyes F, Coiffier B. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 5.Uyttenhove C, Simpson RJ, Van Snick J. Functional and structural characterization of P40, a mouse glycoprotein with T-cell growth factor activity. Proc Natl Acad Sci U S A. 1988;85:6934–6938. doi: 10.1073/pnas.85.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson RJ, Moritz RL, Rubira MR, Gorman JJ, Van Snick J. Complete amino acid sequence of a new murine T-cell growth factor P40. Eur J Biochem. 1989;183:715–722. doi: 10.1111/j.1432-1033.1989.tb21103.x. [DOI] [PubMed] [Google Scholar]
- 7.van Leeuwen BH, Martinson ME, Webb GC, Young IG. Molecular organization of the cytokine gene cluster, involving the human IL-3, IL-4, IL-5, and GM-CSF genes, on human chromosome 5. Blood. 1989;73:1142–1148. [PubMed] [Google Scholar]
- 8.Renauld JC, Druez C, Kermouni A, Houssiau F, Uyttenhove C, Van Roost E, Van Snick J. Expression cloning of the murine and human interleukin 9 receptor cDNAs. Proc Natl Acad Sci U S A. 1992;89:5690–5694. doi: 10.1073/pnas.89.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitra A, Ross JA, Rodriguez G, Nagy ZS, Wilson HL, Kirken RA. Signal transducer and activator of transcription 5b (Stat5b) serine 193 is a novel cytokine-induced phospho-regulatory site that is constitutively activated in primary hematopoietic malignancies. J Biol Chem. 2012;287:16596–16608. doi: 10.1074/jbc.M111.319756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiener Z, Falus A, Toth S. IL-9 increases the expression of several cytokines in activated mast cells, while the IL-9-induced IL-9 production is inhibited in mast cells of histamine-free transgenic mice. Cytokine. 2004;26:122–130. doi: 10.1016/j.cyto.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. 2010;10:683–687. doi: 10.1038/nri2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bittner C, Merz H, Krokowski M, Briese J, Wiedemann GJ, Feller AC. [New immunotherapeutic approaches for the treatment of anaplastic large cell lymphoma in a mouse model] . Verh Dtsch Ges Pathol. 2000;84:187–198. [PubMed] [Google Scholar]
- 13.Renauld JC, van der Lugt N, Vink A, van Roon M, Godfraind C, Warnier G, Merz H, Feller A, Berns A, Van Snick J. Thymic lymphomas in interleukin 9 transgenic mice. Oncogene. 1994;9:1327–1332. [PubMed] [Google Scholar]
- 14.Merz H, Houssiau FA, Orscheschek K, Renauld JC, Fliedner A, Herin M, Noel H, Kadin M, Mueller-Hermelink HK, Van Snick J, et al. Interleukin-9 expression in human malignant lymphomas: unique association with Hodgkin’s disease and large cell anaplastic lymphoma. Blood. 1991;78:1311–1317. [PubMed] [Google Scholar]
- 15.Nagato T, Kobayashi H, Kishibe K, Takahara M, Ogino T, Ishii H, Oikawa K, Aoki N, Sato K, Kimura S, Shimizu N, Tateno M, Harabuchi Y. Expression of interleukin-9 in nasal natural killer/T-cell lymphoma cell lines and patients. Clin Cancer Res. 2005;11:8250–8257. doi: 10.1158/1078-0432.CCR-05-1426. [DOI] [PubMed] [Google Scholar]
- 16.Fischer M, Bijman M, Molin D, Cormont F, Uyttenhove C, van Snick J, Sundstrom C, Enblad G, Nilsson G. Increased serum levels of interleukin-9 correlate to negative prognostic factors in Hodgkin’s lymphoma. Leukemia. 2003;17:2513–2516. doi: 10.1038/sj.leu.2403123. [DOI] [PubMed] [Google Scholar]
- 17.Feng LL, Gao JM, Li PP, Wang X. IL-9 contributes to immunosuppression mediated by regulatory T cells and mast cells in B-cell non-hodgkin’s lymphoma. J Clin Immunol. 2011;31:1084–1094. doi: 10.1007/s10875-011-9584-9. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe ES, Harris NL, Stein H, Isaacson PG. Classification of lymphoid neoplasms: the microscope as a tool for disease discovery. Blood. 2008;112:4384–4399. doi: 10.1182/blood-2008-07-077982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu L, Lai R, Lin Q, Lau E, Thomazy DM, Calame D, Ford RJ, Kwak LW, Kirken RA, Amin HM. Autocrine release of interleukin-9 promotes Jak3-dependent survival of ALK+ anaplastic large-cell lymphoma cells. Blood. 2006;108:2407–2415. doi: 10.1182/blood-2006-04-020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renauld JC, Vink A, Louahed J, Van Snick J. Interleukin-9 is a major anti-apoptotic factor for thymic lymphomas. Blood. 1995;85:1300–1305. [PubMed] [Google Scholar]
- 21.Shang Y, Kakinuma S, Amasaki Y, Nishimura M, Kobayashi Y, Shimada Y. Aberrant activation of interleukin-9 receptor and downstream Stat3/5 in primary T-cell lymphomas in vivo in susceptible B6 and resistant C3H mice. In Vivo. 2008;22:713–720. [PubMed] [Google Scholar]
- 22.Hu BS, Yu HF, Zhao G, Zha TZ. High RSF-1 expression correlates with poor prognosis in patients with gastric adenocarcinoma. Int J Clin Exp Pathol. 2012;5:668–673. [PMC free article] [PubMed] [Google Scholar]
- 23.Lv X, Wang X. The role of interleukin-9 in lymphoma. Leuk Lymphoma. 2012 doi: 10.3109/10428194.2012.745072. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


