Abstract
MALT lymphoma of the ileum is extremely rare: only several cases have been reported. A 34-year-old woman presented abdominal pain and melena. Colorectal and small intestinal endoscopes revealed multiple tumors and ulcers of the entire ileum. Biopsy was taken. Histologically, the biopsy consisted of 6 tissue specimens taken from the various sites of the ileum. All the tissue specimens showed infiltration of small atypical cells resembling centrocyte-like cells (CLC). Immunoblastic cells were scattered, though the number was scant. Monocytoid, plasma cell differentiation, and germinal centers were seen. Lymphoepithelial lesions (LEL) were scattered. Some small atypical lymphocyte were destructive the vessels and stromal tissues. Giemsa and Gram stains demonstrated no Helicobacter pylori and any bacteria. Immunohistochemically, the atypical small lymphocytes were positive for vimentin, but negative for various kinds of cytokeratins (CKs), EMA, CEA and CA19-9. The CK highlighted the LEL. They were positive for CD45, and B-cell markers (CD20, CD79a, CD10, CD23, bcl-2). CD138-positive plasma cells were seen in large number. CD68-positive macrophages were scattered. CD30- and CD15-positive immunoblastic cells were scattered. Most of the lymphoid cells were negative for T-cell markers (CD3, CD4, CD5, CD45RO, and CD43) and negative for NK cell markers (CD56 and CD57). The lymphoid cells were positive for κ-chain but negative for λ-chain; thus the light chain restriction was seen. TdT and cyclin D1 were negative. P53 was positive and Ki-67 labeling index was 67%. The lymphoid cells were negative for neuroendocrine markers (NCAM, NSE, chromogranin, and synaptophysin). The pathological diagnosis was MALT lymphoma of the ileum. Post-biopsy imaging techniques including CT, MRI, PET endoscope and gallium scintigraphy identified no tumors and no lymphadenopathy in the body except the ileum. The stomach was free from MALT lymphoma. She was treated by low dose chemotherapy and strictly followed up.
Keywords: Ileum, MALT lymphoma, histopathology, immunohistochemistry
Introduction
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) is defined as an extranodal lymphoma composed of morphologically heterogenous small B-cells including marginal (centrocyte-like) cells, cell resembling monocytoid cells, small lymphocytes, and scattered immunoblasts and centroblast-like cells [1,2]. This entity was first described by Isaacson and White in 1983 [2]. There is a plasma cell differentiation in a proportion of cases. The infiltrate is in the marginal zone of reactive B-cell follicles and extends into the interfollicular region. In epithelial tissues, the neoplastic cells typically infiltrate the epithelium forming lymphoepithelial lesions.
MALT lymphoma most commonly involves gastrointestinal (GI) tract (50%), followed in order by salivary glands, lung (14%), head and neck (15%), ocular adnexa (12%), skin (11%), thyroid (4%) and breast (4%). In the GI tract, the majority of MALT lymphoma occurs in the stomach [3-14], where Helicobacter Pylori (HP) are regarded as the causative agent [1]. Elimination of HP commonly cures the gastric MALT lymphoma.
Primary gastrointestinal lymphoma comprises 10-15% of all non-Hodgkin lymphomas and encompasses 30-40% of the total extranodal lymphomas. Approximately 60-75% of cases occur in the stomach, followed by colon, cecum, jejunum, ileum, and rectum [3-14]. Lymphoid neoplasms may consist of mature B, T and less commonly extranodal NK/T cells. Of these, the two most frequently encountered histologic subtypes are MALT lymphoma, diffuse large B cell lymphoma (DLBCL). Enteropathy-associated T cell lymphoma, type I in particular, usually arises in a background of celiac disease. T cell gene rearrangement confirms clonality. NK/T cell neoplasms are invariably associated with Epstein-Barr virus infection.
Primary ileal MALT lymphoma is extremely rare; only several cases have been reported in the literature [15-19]. Herein reported is a case of ileal MALT lymphoma occurred in a young woman.
Case report
A 34-year-old woman complained of abdominal pain and melena, and admitted to our hospital. Colorectal and small intestinal endoscopes revealed multiple tumors and ulcers of the entire ileum (Figure 1). Endoscopic diagnosis was ileitis, mesenchymal tumor, or lymphoma. A biopsy was taken.
Figure 1.
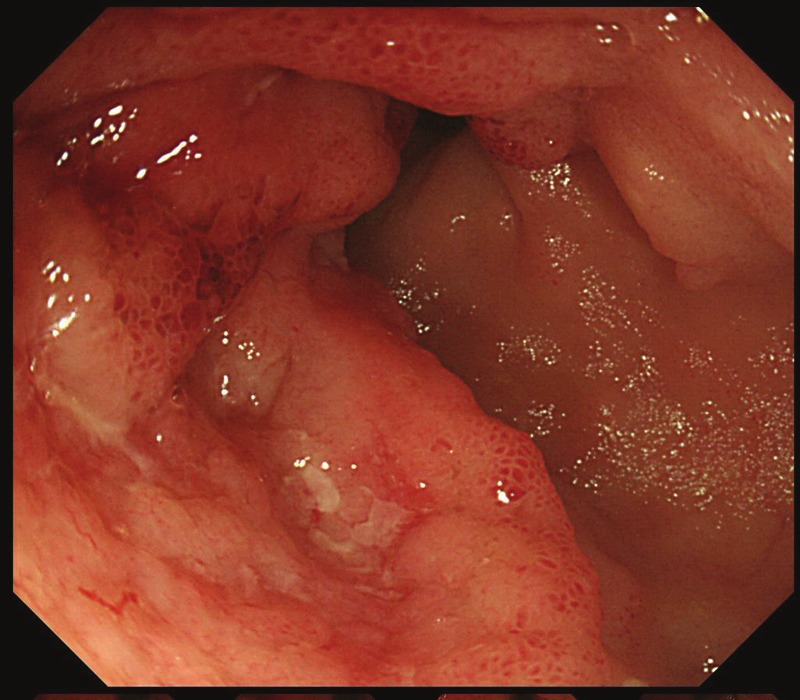
Ileal endoscopy. Multiple tumors and ulcers are seen.
Histologically, the biopsy consisted of 6 tissue specimens taken from the various sites of the ileum (Figure 2A). All the tissue specimens showed infiltration of small atypical cells resembling centrocyte-like lymphocytes (CLL) (Figure 2B and 2D). Immunoblasts-like cells were scattered, though the number was scant. Monocytoid, plasma cell differentiation, germinal centers were seen (Figure 2B-D). Lymphoepithelial lesions (LEL) were scatted (Figure 2E). The CK immunostaining highlighted the LEL. Some atypical lymphocyte were destructive the vessels (Figure 2F) and stromal tissues. Giemsa and Gram stains identified no HP and no bacteria.
Figure 2.

Histological findings. A. Very low power view of the one specimen of the ileal biopsy. Severe proliferation of atypical small lymphocytes with destruction of normal architectures is seen. HE, x20. B. Medium size view. Proliferation of small atypical lymphocytes is seen. HE, x200. C. High power view. Proliferation of small atypical lymphocytes is seen. Monocytoid cells, centrocytes, immunoblastic cells and plasm cells are also seen. HE, x400. D. Lymphoepithelial lesions. The glands (center) are infiltrated by atypical lymphocytes. HE, x200. E. The angiodestructive features are seen. HE, x200.
An immunohistochemical study was performed with the use of Dako-Envision method, as previously described [20-25]. Immunohistochemically, the atypical small lymphocytes were positive for vimentin, but negative for various kinds of cytokeratins (CKs), EMA, CEA and CA19-9. They were positive for CD45, and B-cell markers (CD20, CD79a, CD10, CD23, bcl-2) (Figure 3A). CD138-positive plasma cells were seen in large number (Figure 3B). CD68-positive macrophages were scatted. CD30- and CD15-positive immunoblastic cells were scattered. Most of the atypical lymphoid cells were negative for T-cell markers (CD3, CD4, CD5, CD45RO, and CD43) and negative for NK cell markers (CD56 and CD57). The lymphoid cells were positive for κ-chain (Figure 3C) but negative for λ-chain (Figure 3D); thus the light chain restriction was seen. TdT and cyclin D1 was negative. P53 was positive (Figure 3E) and Ki-67 labeling index was 67% (Figure 3F). The lymphoid cells were negative for neuroendocrine markers (NSE, chromogranin, and synaptophysin).
Figure 3.
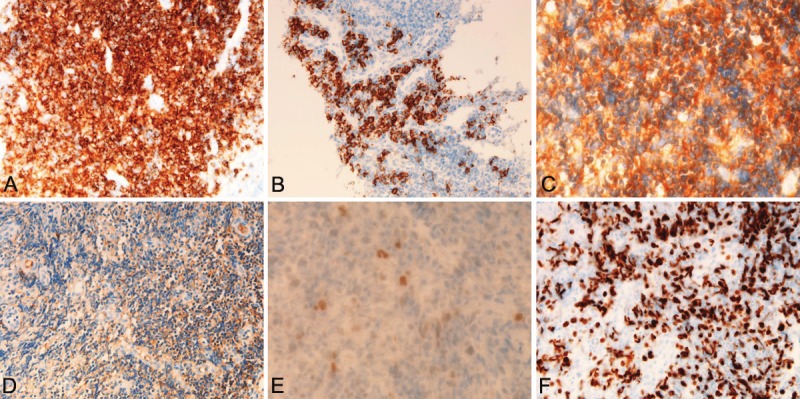
Immunohistochemical findings of the ileal MALT lymphoma. A. The atypical lymphocytes are strongly and diffusely positive for CD20. X200. B. The plasma cell differentiation cells are strongly positive for CD138. X200. C. The atypical lymphocytes are strongly positive for κ-light chain. x200. D. In contrast, the atypical lymphocytes are almost negative for λ-light chain. x200. E. p53 is expressed in the atypical lymphocytes. X200. F. The Ki-67 labeling is very high. X200.
The epithelial components were negative for lymphocytes markers, and showed focal LELs which were highlighted by CK and CD45 immunostaining. The epithelial cells were positive for CKAE1/3, CA CAM5.2, CK8, CK18, CK19, CK20, CEA, cyclin D1, CD138, and CA19-9, but negative for CK5, CK6, CK7, CK14, EMA, NSE, NCAM (CD56), chromogranin A, synaptophysin, KIT, and PSGFRA.
The pathological diagnosis was MALT lymphoma of the ileum. Post-biopsy imaging techniques including CT, MRI, PET endoscope and gallium scintigraphy identified no tumors and no lymphadenopathy in the body except the ileum. The stomach was free from MALT lymphoma. She was treated by low dose chemotherapy and strictly followed up.
Discussion
The author reported a very rare case of MALT lymphoma of the ileum. In general, MALT lymphoma is almost always seen in the stomach, where HP infection is very prevalent. HP is thought to the etiology of MALT lymphoma in addition to gastric carcinoma. The lesion (MALT lymphoma) was once called reactive lymphoid hyperplasia and pseudolymphoma, but recently it is called as MALT lymphoma since the paper of Isaacson and Wright in 1983 [2]. Gastric MALT lymphoma usually cures by eradication of HP. In the present case, Giemsa and Gram stains revealed no HP. The present case did not examined HP culture. In other case reports of ileal MALT lymphoma did not show HP [15-19]. Thus, the etiology of ileal MALT lymphoma is unknown. It may be true low grade malignancy developed by genetic alterations.
The diagnosis of the present study was MALT lymphoma of the ileum. Most of the GI lymphoma except for the stomach is diffuse large B-cell lymphoma, followed by follicular lymphoma and mantle cell lymphoma and T-cell neoplasm is very rare [3-14]. The present study showed tumorous proliferation of small atypical lymphocytes. They resembled centrocyte-like lymphocytes and monocytoid cells. Germinal centers were scattered and plasma cell differentiation, as evidenced by CD138 immunostaining, was broad. In addition, apparent LELs were seen. The tumor was seen to be destructive vessels and stromal cells, but no angiocentric lymphoma (T-cells) features were seen. These histological features are highly suggestive or confirmative of MALT lymphoma. However, the involvement site is not stomach; the author investigated an immunohistochemical study.
Immunohistochemistry revealed the tumor was composed largely of B-cells, though a small number of T-cells were seen. Plasma cells were confirmed by CE138 immunostaining. CD30-, CD15-positive immunoblastic cells were scattered in a very small number. The LEL was highlighted by immunostaining of CK and B cell markers. There were germinal centers not stained completely by bcl-2. Importantly, the present tumor was positive κ-light chain and negative for λ-light chain; thus the so called light chain restriction was seen. This phenomenon implies the monoclonality of the lesion and strongly suggest the lesion is true neoplasm. The p53 was positive, suggesting p53 gene mutations and malignant potentials of the present lesion. Ki-67 labeling index (67%) was very high, suggesting that the cell proliferation is severe and the lesion is malignant. TdT was negative, suggesting that the present tumor is not precursor lymphoma. These immunohistochemical data, together with histological findings, confirms that the present ileal lesion is MALT lymphoma.
The differential diagnosis includes low-grade small B-cell neoplasms including small lymphocytic lymphoma/CLL, lymphoplasmacytic lymphoma, follicular lymphoma, and mantle cell lymphoma, and plasmacytoma [26-30]. The present case is not small lymphocytic lymphoma, in which the lymphoid proliferation is monotonous and no LEL, plasma cell differentiation, monocytoid B-cells, CLC, or germinal centers are present. The present case is different from follicular lymphoma, in which the lymphoid proliferation is monotonous and no other above mentioned MALT features were seen. In the diagnosis of follicular lymphoma, the bcl-2 and bcl-6 immunostaining are mandatory; in follicular lymphoma, the follicles were strongly stained with bcl-2 and bcl-6. In the present case, the nodular aggregate of lymphocytes are not follicles but germinal centers, and they were only faintly stained by bcl-2 immunostaining. Histologically and immunohistochemically, the present tumor is entirely different from follicular lymphoma. The present case is not mantle cell lymphoma, in which the lymphoma cells have characteristic nuclear groove seen in cells of mantle zone and are positive for cyclin D1. In the present tumor, no such nuclear features were seen, and cyclin D1 was negative. The present case is apparently not plasmacytoma or myeloma.
In conclusion, the author reported an extremely rare case of MALT lymphoma of the ileum in a young Japanese woman.
Declaration
The author has no conflict of interest.
References
- 1.Isaacson PG, Chott A, Nakamura S, Muller-Hermelink HK, Harris NL, Swerdlow SH. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 214–217. [Google Scholar]
- 2.Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue: a distinctive type of B-cell lymphoma. Cancer. 1983;52:1410–1416. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Terada T. Gastrointestinal malignant lymphoma: a pathologic study of 37 cases in a single Japanese institution. Am J Blood Res. 2012;2:194–200. [PMC free article] [PubMed] [Google Scholar]
- 4.Bautista-Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J Gastrointest Oncol. 2012;3:209–225. doi: 10.3978/j.issn.2078-6891.2012.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terada T. One patient with double lymphomas: simultaneous gastric MALT lymphoma and ileal diffuse large B-cell lymphoma. Int J Clin Exp Pathol. 2012;5:260–263. [PMC free article] [PubMed] [Google Scholar]
- 6.Terada T. Histopathologic study of the rectum in 1,464 consecutive rectal specimens in a single Japanese hospital: II. malignant lesions. Int J Clin Exp Pathol. 2013;6:385–394. [PMC free article] [PubMed] [Google Scholar]
- 7.Terada T. A clinicopathologic study of esophageal 860 benign and malignant lesions in 910 cases of consecutive esophageal biopsies. Int J Clin Exp Pathol. 2013;6:191–198. [PMC free article] [PubMed] [Google Scholar]
- 8.Terada T. Malignant tumors of the small intestine: a histopathologic study of 41 cases among 1,312 consecutive specimens of small intestine. Int J Clin Exp Pathol. 2012;5:203–209. [PMC free article] [PubMed] [Google Scholar]
- 9.Terada T. Esophageal cancers: a clinicopathologic and immunohistochemical study of 223 cases. Gastroenterol Res. 2009;2:148–151. doi: 10.4021/gr2009.05.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: II. Expresssion of MUC1, MUC2, MUC5AC, and MUC6 in normal mucosa and in 42 cases. Int J Clin Exp Pathol. 2013;6:613–21. [PMC free article] [PubMed] [Google Scholar]
- 11.Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: I. Cytokeratin profile in 42 cases. Int J Clin Exp Pathol. 2013;6:703–10. [PMC free article] [PubMed] [Google Scholar]
- 12.Terada T. An immunohistochemical study of primary signet-ring cell carcinoma of the stomach and colorectum: III. Expressions of EMA, CEA, CA19-9, CDX-2, p53, Ki-67 antigen, TTF-1, vimentin, and p63 in normal mucosa and in 42 cases. Int J Clin Exp Pathol. 2013;6:630–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Terada T. Histopathologic study of the stomach using computer database in 10,000 consecutive gastric specimens: II. Malignant lesions. Int J Clin Exp Pathol. 2013 in press. [Google Scholar]
- 14.Terada T. Histopathologic study using computer database of the stomach in 10,000 consecutive gastric specimens: I. Benign conditions. Int J Clin Exp Pathol. 2013 in press. [Google Scholar]
- 15.Makino Y, Suzuki H, Nishizawa T, Kameyama K, Hisamatsu T, Imaeda H, Mukai M, Hibi T. Ileal mucosa-zssociated lymphoid tissue (MALT) lymphoma with a large-cell component that regressed spontaneously. Gut Liver. 2010;4:117–121. doi: 10.5009/gnl.2010.4.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohashi S, Yazumi S, Watanabe N, Matsumoto S, Fukui T, Nishio A, Chiba T. Education and imaging. Gastrointestinal: MALT lymphoma of the terminal ileum. J Gastroenterol Hepatol. 2006;21:1495. doi: 10.1111/j.1440-1746.2006.04634.x. [DOI] [PubMed] [Google Scholar]
- 17.Saito T, Toyoda H, Yamaguchi M, Nakamura T, Nakamura S, Mukai K, Fuke H, Wakita Y, Iwata M, Adachi Y, Shiku H. Ileocolonic lymphomas: a series of 16 cases. Endoscopy. 2005;37:466–469. doi: 10.1055/s-2005-861093. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto Y, Matsumoto T, Nakamura S, Kawasaki A, Aso M, Aoyagi K, Sadoshima S, Onoyama K, Fujishima M. Primary ileal plasmacytoma arising in mixed low- and high-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Abdom Imaging. 2000;25:139–141. doi: 10.1007/s002619910033. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa N, Tsuboi Y, Kato K, Yamada K, Morita K, Kuroiwa M, Ito H, Matsushima T, Ono K, Oshiro M. Endoscopic diagnosis of ileocecal mucosa-associated lymphoid tissue lymphoma. Gastrointest Endosc. 1999;50:115–117. doi: 10.1016/s0016-5107(99)70360-3. [DOI] [PubMed] [Google Scholar]
- 20.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 21.Terada T, Takeuchi T, Taniguchi M. Hepatobiliary cystadenocarcinoma with cystadenoma elements of the gall bladder in an old man. Pathol Int. 2003;53:790–795. doi: 10.1046/j.1440-1827.2003.01559.x. [DOI] [PubMed] [Google Scholar]
- 22.Terada T, Tanigichi M. Intraductal oncocytic papillary neoplasm of the liver. Pathol Int. 2004;54:116–123. doi: 10.1111/j.1440-1827.2004.01594.x. [DOI] [PubMed] [Google Scholar]
- 23.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;206:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 24.Terada T. Extramuscular subcutaneous fibrolipoma containing foci of striated muscle cells: a hitherto unreported condition. Int J Clin Exp Pathol. 2013;6:113–115. [PMC free article] [PubMed] [Google Scholar]
- 25.Terada T. Vascular leiomyoma of the lung arising from pulmonary artery. Int J Clin Exp Pathol. 2013;6:97–99. [PMC free article] [PubMed] [Google Scholar]
- 26.Muller-Hermlink HK, Montserrat E, Catovsky D, Campo E, Harris NL, Stein H. Chronic lymphocytic leukemia/small lymphocytic lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 180–182. [Google Scholar]
- 27.Harris NL, Nathwani BN, Swerdlow SH, de Jong D, Jaffe ES, Yoshino T, Ott G, Spagnoto D. Follicular lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 220–226. [Google Scholar]
- 28.Swerdlow SH, Campo E, Seto M, Muller-Hermlink HK. Mantle cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 229–232. [Google Scholar]
- 29.McKenna RW, Kyle RA, Kuehl WM, Grogan TM, Harris NL, Coupland RW. Extraosseous plasmacytoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of tumours of haematopietic and lymphoid tissues. Lyon: IARC; 2008. pp. 208–209. [Google Scholar]
- 30.McKenna RW, Kyle RA, Kuehl WM, Grogan TM, Harris NL, Coupland RW. Plasma cell myeloma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of tumours of haematopietic and lymphoid tissues. Lyon: IARC; 2008. pp. 202–208. [Google Scholar]


