Abstract
Carcinoid tumor is one of the commonly encountered primary pulmonary neoplasms. Although it has been known to be accompanied by calcification and/or ossification, presentation with a large ossified mass is rare. We describe here the case of a 29-year-old female with the radiological finding of a single bony nodular lesion. Pathological examination of the surgically resected specimen led to the diagnosis of carcinoid tumor of the lung with massive ossification. Although histological features showed the tumor of low grade malignancy, subcarinal and right hilar lymph nodes were found to be positive for metastasis. Further immunohistochemical analysis revealed that the tumor cells expressed the osteogenic inducer protein, bone morphogenic protrein-2 [BMP-2] and osteoblastic marker protein, osteocalcin. We interpreted this to mean that the carcinoid tumor cells had acquired an osteoblastic phenotype and had subsequently developed marked intratumoral ossification. The relevant literature is reviewed and possible mechanisms of tumor-related osteogenesis are discussed.
Keywords: Carcinoid tumor, osteomimicry, bone morphogenic protein, osteocalcin
Introduction
Pulmonary or bronchial carcinoid tumors account for 1 to 2% of all pulmonary neoplasms and over 25% of all carcinoid tumors [1]. Approximately, 10 to 20% of pulmonary carcinoids are atypical carcinoids, and therefore, the remaining 80 to 90% are typical carcinoids [1]. Although it is known that carcinoid tumors may be accompanied by calcification and/or ossification, presentation as a single tumor nodule with massive ossification is rare. Herein, we describe a case of a young female with typical carcinoid with contra-lateral lymph node metastasis and massive intratumor ossification.
Material and methods
Tissue processing
Tissue samples were fixed in formalin, dehydrated by ethanol and embedded in paraffin. Serial sections of 3.5 µm thickness were prepared and used for hematoxylin-eosin and immunohistochemical (IHC) stains. For IHC staining, sections were stained with the primary antibodies at the following dilutions; antibody against chromogranin (monoclonal, clone DAK-A3, DAKO, Carpinteria, CA, U.S.A.) 1:100, synaptophysin (polyclonal, DAKO) 1:150, cytokeratin (monoclonal, clone AE1/AE3, DAKO) 1:100, bone morphogenic protein (BMP-2, polyclonal, Santa Cruz Biotech., Santa Cruz, CA) 1:200, osteocalcin (monoclonal, clone OCG3, TaKaRa Biomedicals, Ohtsu, Shiga, Japan) at 1:500 dilution. Antibodies were visualized by ChemMate Envision/peroxidase complex kit (DAKO, Glostrup, Denmark).
Case report
A 29-year-old female was referred to our hospital with an abnormal chest X-ray shadow. She had an unremarkable medical and family history and was a non-smoker. Computed tomography (CT) imaging of the chest revealed a 4.5-cm diameter tumor with coarse calcification in the lower lobe of the left lung (Figure 1A). [18F]-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET) showed an increased uptake by the tumor with a maximum standardized uptake value of 4.0. No significant FDG accumulation was noted both in the hilar and mediastinal lymph nodes. Laboratory testing showed slightly elevated serum levels of neuron-specific enolase (12 U/mL), while other tumor markers were all within normal limits. Bronchoscopy showed a hemorrhagic tumor occluding the basal segmental bronchus (Figure 1B). Transbronchial biopsy was not performed to avoid tumor bleeding. No metastases were detected by brain MRI or FDG-PET. Based on the radiological and bronchoscopic findings, the tumor was suspected to be a carcinoid, and surgery was planned. Dissection of the left lower lobectomy and hilar and mediastinal lymph nodes were done via a left thoracotomy through the sixth intercostal space. The patient had an uneventful postoperative course and continues to do well 4 years postoperatively, with no evidence of recurrence.
Figure 1.
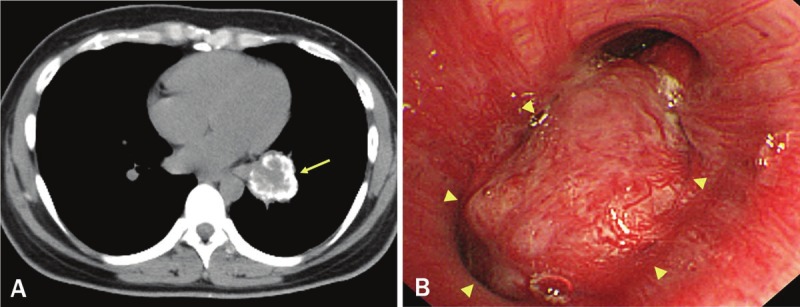
A. Computed tomography (CT) image, showing a 4.5 cm tumor with coarse calcification in the left lower lobe (arrow). B. Endoscopically, a hypervascularized tumor occluding the basal segmental bronchus was observed (arrowheads).
Macroscopically, the specimen obtained from the left lower lobectomy measured 16.5 x 10.7 x 8.5 cm. The tumor was a well-circumscribed and encapsulated yellow-white nodular mass measuring 4.7 x 3.6 x 3.5 cm, and calcification or ossification was noted. (Figure 2A). No bronchi or pleura were involved within the tumor, although secondary bronchiole (b8-10) showed stenosis due to compression by the adjacent tumor nodule (Figure 2B). Histologically, the tumor cells consisted of the nests or interconnecting trabeculae of uniformly arranged polygonal cells with a wide cytoplasm and homogeneous round nuclei, occasionally with prominent nucleoli (Figure 3A). There were no definitive features suggestive of high grade malignancy, i.e. no evidence of necrosis and no more than 1 mitotic figure per 10 high-power fields (HPFs). In between the tumor cells, stroma containing numerous thin-walled vascular structures and mature trabecular bone were present (Figure 3B). Among the dissected lymph nodes, subcarinal and right hilar lymph nodes were positive for metastasis. IHC study revealed that the tumor cells showed positive staining for cytokeratin, chromogranin and synaptophysin (Figure 3C and 3D). Since no tumor was found in other site, the final pathological diagnosis of “carcinoid tumor of the lung, the central type” was made. Furthermore, the osteogenic inducer protein, BMP-2 and osteoblastic marker proteins, osteocalcin were found to be positive in the cytoplasm of these tumor cells (Figure 3E and 3F).
Figure 2.
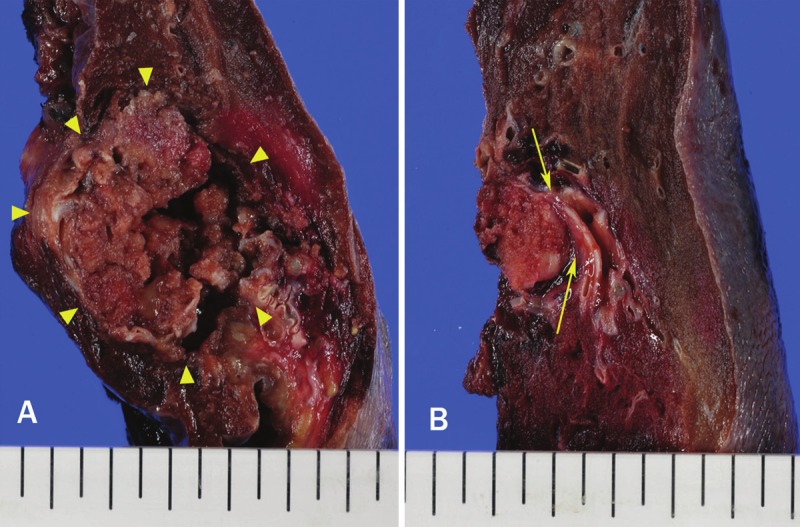
Gross appearance of the cut surface of the left/lower lobectomy specimen, containing a hard, well-circumscribed nodular lesion (A, arrowheads). Bronchiole (b8-10) showed stenosis due to compression by the tumor nodule (B, arrows).
Figure 3.
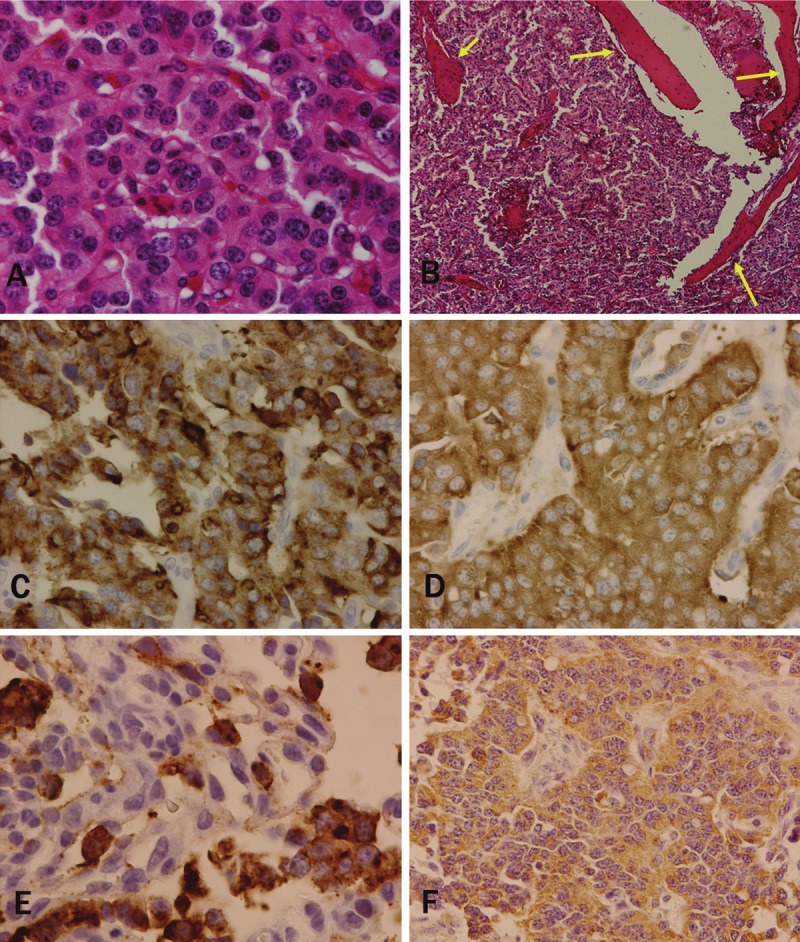
A. Histological features of the tumor, showing the nests of uniformly proliferated polygonal cells with an eosinophilic cytoplasm and round nuclei. Necrosis or mitosis was not observable. B. Conspicuous thin-walled vascular structures and mature trabecular bone present in between the tumor nests (arrows). Immunohistochemical staining of the tumor cells, showing positive reactivity for (C) chromogranin, (D) synaptophysin, (E) bone morphogenic protein-2, and (F) osteocalcin. Original magnification: A, F, x200; B, x100. C to E, x400.
Discussion
Carcinoid tumors are classified into two histological types; typical carcinoid and atypical carcinoid. Both share the common histological features, but they are distinguished by strict histological criteria [1]. The histological features in this case did not exhibit those of atypical carcinoid or high grade malignancy (small cell carcinoma), as we did not observe high mitotic activity or necrosis, etc. Nevertheless, extensive lymph node metastasis was found. The reason for such discrepancy was unclear and no obvious explanation could be found even in the literature. One speculation from the literature is that the intratumoral ossification may reflect the aggressiveness of the tumor [2], while little empirical data exists to support this idea. Therefore, the current recommendation is to treat and follow the patients with this kind of carcinoid tumor more carefully versus the more common forms [1].
One of the remarkable pathological findings in the current case was the presence of extensive ossification. Calcification has been described in up to 30% of pulmonary carcinoid tumors, usually in the vascular stroma and intersecting fibrous strands, and is more frequent in central carcinoids than in peripheral carcinoids [3]. Ossification within carcinoid tumors is less common, found exclusively in pulmonary carcinoid patients in up to 10% of the cases, and is particularly found in those of long duration [4]. One proposed mechanism for this calcification is the production of an osteogenic factor by the tumor cells, in coordination with surrounding normal tissue that induces local ossification [4]. Since an osteoblastic reaction around the tumor cells can also be observed in the metastatic foci of carcinoids, especially in bone, it is possible that ossification is initiated by the osteogenic factor(s) secreted by the tumor cells that then acts on the surrounding stroma [4].
It has been recognized that ossification requires three conditions: i) osteogenic (precursor) cells, ii) ossification-inducing agents and iii) an environment permitting osteogenesis [2,5]. First, in normal bone development and in several pathological conditions such as tumor or inflammation, osteogenic (precursor) cells derive from mesenchymal stem cells (MSCs). Human MSCs (hMSCs) have been reported to be present in bone marrow, adipose tissues, dermis, muscles, and other tissues, and have the potential to differentiate into various lineages, including those forming bone, cartilage, muscle, and neurons [6,7]. Second, ossification is initiated by the metaplasia or differentiation of pluripotent hMSCs into osteoblastic cells induced by osteogenic inducer protein. The major inducer proteins are the bone morphogenetic proteins (BMPs), which are members of the TGFβ superfamily. Stimulation by BMPs has been reported to differentiate skeletal muscle-derived cells into osteogenic lineages [7,8]. Among the BMPs, BMP 2, 4, 6 and 7 have demonstrated potent osteogenic effects [9-11]. In particular, the involvement of BMP-2 produced by tumor cells in promoting osteogenesis have been extensively investigated [2,7,8]. BMP-2 is an active inducer of osteoblastic differentiation of both immature osteoblasts and less-committed cells [2] via the induction of a network of transcription factors and molecular switches that regulate osteoblast differentiation and bone development [8-10]. Lastly, the regulators downstream of BMP-2 include Runx2 (Cbfa1/AML3) [12,13], the osteogenic master gene specific for bone formation, Tbx3 as well as homeodomain proteins and osterix [11]. This complex network of signaling cascades mediates the upregulation of osteoblastic marker proteins by BMP2 [2,7].
IHC staining in this case revealed clear cytoplasmic expression of BMP-2, which therefore may be the principal inducers of ossification. On the other hand, ossification and cartilage formation are commonly observed in lung tumors, as in carcinoid and hamartoma, respectively, suggesting that the bronchial epithelium itself may play a role as an inducer of ossification and cartilage formation. Indeed, transitional epithelium is also a well-known bone-inducing tissue [2]. Even in other lung tumors, intratumoral ossification has been reported predominantly in adenocarcinomas, which frequently exhibit BMP-2 production, in up to 90% of the cases [14].
A number of proteins have been identified as markers of osteogenic/osteoblastic differentiation to date, such as osteocalcin [11]. Demonstration of osteocalcin expression in carcinoid tumor cells is consistent with the idea that secreted BMP-2 may have induced osteoblastic differentiation of the carcinoid tumor cells in an autocrine manner, followed by ossification in the surrounding stroma. We consistently observed calcification and ossification exclusively within the tumor nodule, i.e., dystrophic type [15], as well as bony trabeculae that were tightly surrounded by tumor cells without osteoblastic rimming cells. This indicates that ossification was executed directly by the tumor cells. Collectively, overall data suggests that the current case is an example of osteomimicry, wherein the tumor cell acquires the properties of osteoblasts [12,13].
In conclusion, we have described a case of pulmonary carcinoid with peculiar clinicopathological features as follows; i) presentation of a single bony mass in a young female, ii) aggressive lymph node metastasis, despite ordinary histological appearance, other than extreme intratumoral trabecular bone formation, and iii) expression by tumor cells of both BMP-2, an ossification-inducing protein, and osteocalcin, an osteoblastic marker protein.
Acknowledgments
This work was partly supported by Japan Society for the Promotion of Science C23590409 (Y.D.) and Smoking Research Foundation (Y.D). We have no financial interest or conflict of interest in association with this work.
References
- 1.Chong S, Lee KS, Chung MJ, Han J, Kwon OJ, Kim TS. Neuroendocrine tumors of the lung: clinical, pathologic, and imaging findings. Radiographics. 2006;26:41–57. doi: 10.1148/rg.261055057. discussion 57-48. [DOI] [PubMed] [Google Scholar]
- 2.Komai Y, Morimoto S, Saito K, Urushibara M, Sakai K, Ikeda S. Possible involvement of bone morphogenetic protein 2 in heterotopic ossification in metastatic lesion from urothelial carcinoma of bladder. Int J Urol. 2006;13:1126–1128. doi: 10.1111/j.1442-2042.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- 3.Zwiebel BR, Austin JH, Grimes MM. Bronchial carcinoid tumors: assessment with CT of location and intratumoral calcification in 31 patients. Radiology. 1991;179:483–486. doi: 10.1148/radiology.179.2.2014296. [DOI] [PubMed] [Google Scholar]
- 4.Vanmaele L, Noppen M, Frecourt N, Impens N, Welch B, Schandevijl W. Atypical ossification in bronchial carcinoid. Eur Respir J. 1990;3:927–929. [PubMed] [Google Scholar]
- 5.Chalmers J, Gray DH, Rush J. Observations on the induction of bone in soft tissues. J Bone Joint Surg Br. 1975;57:36–45. [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Claros S, Alonso M, Becerra J, Andrades JA. Selection and induction of rat skeletal muscle-derived cells to the chondro-osteogenic lineage. Cell Mol Biol (Noisy-le-grand) 2008;54:1–10. [PubMed] [Google Scholar]
- 8.Luo X, Chen J, Song WX, Tang N, Luo J, Deng ZL, Sharff KA, He G, Bi Y, He BC, Bennett E, Huang J, Kang Q, Jiang W, Su Y, Zhu GH, Yin H, He Y, Wang Y, Souris JS, Chen L, Zuo GW, Montag AG, Reid RR, Haydon RC, Luu HH, He TC. Osteogenic BMPs promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab Invest. 2008;88:1264–1277. doi: 10.1038/labinvest.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He TC. Distinct osteogenic activity of BMPs and their orthopaedic applications. J Musculoskelet Neuronal Interact. 2005;5:363–366. [PubMed] [Google Scholar]
- 10.Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning D, Xue A, Montag AG, Luu HH, Haydon RC, He TC. Regulation of osteogenic differentiation during skeletal development. Front Biosci. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 11.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 12.Hall CL, Kang S, MacDougald OA, Keller ET. Role of Wnts in prostate cancer bone metastases. J Cell Biochem. 2006;97:661–672. doi: 10.1002/jcb.20735. [DOI] [PubMed] [Google Scholar]
- 13.Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39:246–261. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Kuribayashi H, Tsuta K, Mizutani E, Maeshima AM, Yoshida Y, Gemma A, Kudoh S, Asamura H, Matsuno Y. Clinicopathological analysis of primary lung carcinoma with heterotopic ossification. Lung Cancer. 2009;64:160–165. doi: 10.1016/j.lungcan.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Pantongrag-Brown L, Buetow PC, Carr NJ, Lichtenstein JE, Buck JL. Calcification and fibrosis in mesenteric carcinoid tumor: CT findings and pathologic correlation. Am J Roentgenol. 1995;164:387–391. doi: 10.2214/ajr.164.2.7839976. [DOI] [PubMed] [Google Scholar]


