Abstract
Although an influence of adult neurogenesis in mediating some of the effects of antidepressants has received considerable attention in recent years, much less is known about how alterations in this form of plasticity may contribute to psychiatric disorders such as anxiety and depression. One way to begin to address this question is to link the functions of adult-born hippocampal neurons with specific endophenotypes of these disorders. Recent studies have implicated adult-born hippocampal neurons in pattern separation, a process by which similar experiences or events are transformed into discrete, non-overlapping representations. Here we propose that impaired pattern separation underlies the overgeneralization often seen in anxiety disorders, specifically post-traumatic stress disorder and panic disorder, and therefore represents an endophenotype for these disorders. The development of new, pro-neurogenic compounds may therefore have therapeutic potential for patients who display pattern separation deficits.
Anxiety disorders have a lifetime prevalence of over 25%1, making them the most common of psychiatric disorders. They account for more than 30% of the United States’s total expenditure on mental illness, costing the country an estimated $45 billion annually. Moreover, anxiety disorders are often chronic conditions that severely affect quality of life and work productivity and, in the common situation in which they begin early in life (for example, by adolescence), they substantially disrupt personal and social development2. Developing novel and effective therapeutics for improving outcome in these disorders will therefore be a major benefit to society.
Panic disorder has a lifetime prevalence of 2–4% and is characterized by recurrent unexpected panic attacks with intense physical symptoms and persistent fear of future attacks. Persons with panic disorder often avoid situations (for example, public transportation) that they have come to associate with panic attacks. Post-traumatic stress disorder (PTSD), which has a lifetime prevalence of 5–8%, is an anxiety disorder that can occur after a severely traumatic event. The disorder is characterized by re-experiencing the trauma through intrusive thoughts; memories and nightmares; autonomic hyperarousal symptoms and avoidance of reminders of the trauma, with certain reminders often gaining heightened salience for the patient3. Recently, high rates of PTSD among veterans returning from war have given extra impetus to developing better treatments for this disorder. To achieve this, a greater understanding of the neural substrates of PTSD and related anxiety disorders is of utmost importance. Such an understanding requires a delineation of the processes that go awry in these disorders and the identification of the neural circuit malfunctions and how they might be rehabilitated.
Anxiety disorders can be viewed as maladaptive fear responses that may result from dysregulation of brain circuits involved in generating fearfulness, a trait that has notable survival value. Animals demonstrate learned fear, which, on the basis of previous experiences, allows them to generate adaptive responses to situations that are likely to threaten their safety. As neutral stimuli present during an aversive experience acquire the ability to engender a conditioned fear response upon subsequent exposures, and they bear similarity to many other stimuli in changing environments, it is imperative to compare present experiences to stored associations, so that fear or anxiety should only be evoked by cues that truly predict danger. Healthy individuals are able to successfully assess whether day-to-day situations are different or similar to those previously encountered and to elicit measured responses in appropriate contexts. In contrast, individuals with anxiety disorders such as PTSD exhibit heightened reactivity to neutral stimuli resembling the aversive event, and this occurs even in presence of cues that convey safety.
Consistent with these observations, learning theories have implicated a range of associative learning processes, such as extinction learning, extinction learning recall, fear inhibition and overgeneralization of conditioned fear, as well as non-associative learning based mechanisms such as habituation and sensitization, in the development of anxiety disorders4,5. Although the neural circuits underlying some of these processes, such as extinction learning4 and fear inhibition5, have begun to be delineated in preclinical studies and in humans, the neural substrates of processes such as overgeneralization of fear are much less understood.
In this review, we provide a new framework for the role of the dentate gyrus, and specifically adult hippocampal neurogenesis, in anxiety disorders. We propose that impairments in pattern separation in the dentate gyrus may underlie overgeneralization of fear in anxiety disorders and suggest that a pattern separation deficit is an endophenotype of anxiety disorders such as PTSD and panic disorder. On the basis of recent studies implicating adult hippocampal neurogenesis in pattern separation, we hypothesize that reductions in adult hippocampal neurogenesis as a consequence of, or preceding, traumatic stress manifests as a deficit in pattern separation and an overgeneralization of memory. We conclude by proposing that interventions that target neurogenesis may be of therapeutic value in the treatment of these disorders.
Generalization and anxiety disorders
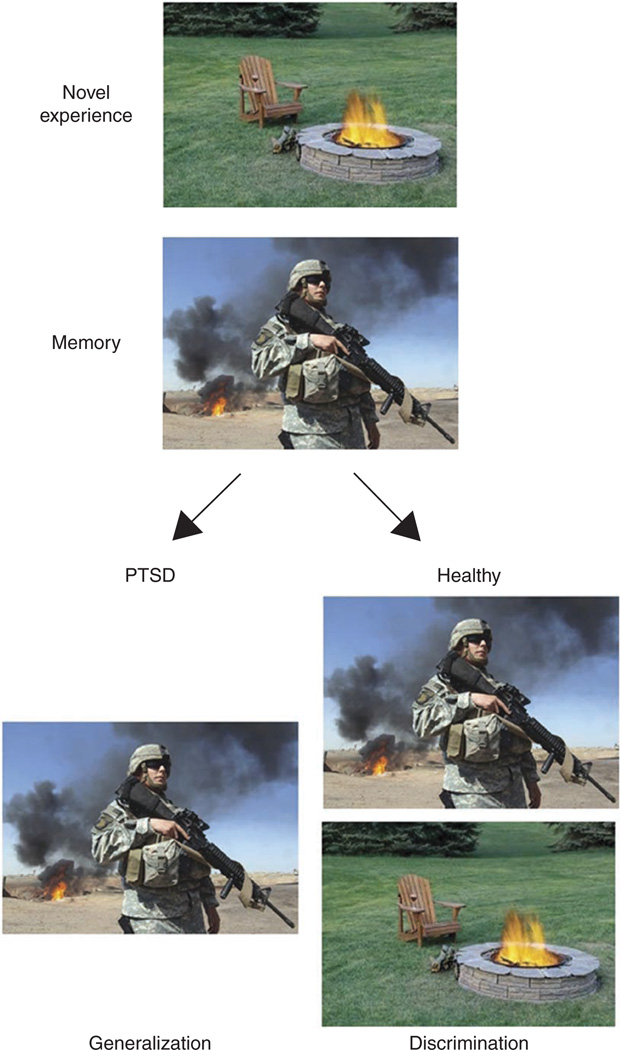
Many patients with anxiety disorders display an overgeneralization of fear responses to emotional stimuli. For example, a soldier with PTSD may experience heightened arousal and anxiety to a cue that resembles the traumatic experience (such as a campfire), even when other contextual cues indicate safety (such as absence of combat and a park setting) (Fig. 1). This phenomenon may be characteristic of several anxiety disorders; for example, a person with social anxiety disorder generalizes from one embarrassing experience to fear of embarrassment in many friendly social situations6,7, a person with panic disorder may have one panic attack on a bridge and subsequently fear traveling on any bridges, tunnels or highways8,9. The excessive and unregulated fear response in these examples may be due to alterations in several underlying processes, such as lack of ability to suppress fear in presence of safety cues, increased fear responses to novel cues (sensitization) or a deficit in extinction of the aversive cue. In addition, in each of these examples, the fear response is evoked by experiences or cues that bear resemblance to the original aversive experience. Therefore, it is possible that there are impairments in other mechanisms that act upstream of fear inhibition to differentiate between perceptually similar cues or events. We suggest that one such mechanism is pattern separation.
Figure 1.
Excessive generalization in PTSD. Pattern separation serves to disambiguate a new experience from stored memories. When a novel experience (campfire) contains some shared features with a previously stored experience (war zone), it evokes retrieval of that memory. Upon experiencing a situation that shares features of a traumatic event, a healthy individual is able to discriminate the novel experience from the fearful one and successfully encode this experience as novel and safe. Alternatively, in an individual suffering from PTSD, excessive generalization manifests as a failure to discriminate the novel, safe experience from the previously stored emotional memory, leading to excessive arousal and fear responses.
Pavlovian conditioning has been implicated in the pathogenesis of panic disorder, as neutral stimuli present during the onset of a panic attack can acquire salience owing to pairing with the attack and can elicit anxiety during future encounters with these stimuli7,8,10. In addition, as with PTSD, there is an overgeneralization of memory for the stimuli associated with the aversive event: for example a panic attack in a parking garage may elicit anxiety in all parking garages. Overgeneralization of fear has now been systematically tested in the laboratory using a stimulus-generalization task in which rings of different sizes are morphed parametrically from one to the other and responses to intermediate size rings are treated as a measure of generalization9. Panic disorder patients do not show any difference in fear responses to the conditioned danger cue but do show enhanced responses to the cues that are similar to the conditioned danger cue, suggesting that panic disorder involves an overgeneralization of the conditioned fear. Moreover, panic disorder patients also show elevated responses to the non-danger cue, which could indicate reduced fear inhibition or increased response to all novel cues; that is, increased sensitization. Thus, it is likely that overgeneralization of fear is accompanied by other alterations that together constitute the symptoms seen in panic disorder. Future studies like these in which perceptual similarities are parametrically varied while the subject is imaged may address the mechanisms underlying overgeneralization.
Hippocampus and anxiety disorders
One function of the hippocampus is to form new memories and guide behavior by comparing new sensory input to stored representations. Most models of hippocampal function propose that memory traces are stored in the autoassociative network of CA3 pyramidal neurons, where, owing to collateral connectivity, memories can be recalled from partial cues by a process termed ‘pattern completion’11. In contrast, ‘pattern separation’ is thought to be essential for the successful encoding of distinct memory traces of similar experiences. Pattern separation is an active process that disambiguates overlapping, perceptually similar sensory inputs. On the basis of its functional anatomy and its physiological properties, this process of pattern separation is believed to occur upstream of CA3, in the dentate gyrus11–14. In vivo electrophysiological recordings and lesions in rodents and functional magnetic resonance imaging (fMRI) studies in humans support this function of the dentate gyrus in pattern separation13,15,16. A postulated behavioral deficit resulting from a failure in pattern separation is impaired ability to distinguish between two similar sensory inputs and grouping of multiple contexts or items together even if they are dissimilar11. Such a maladaptive response may contribute to generalizing new innocuous experiences with previously encountered aver-sive events, as seen in individuals with panic disorder and PTSD9,17. The Benton Visual Retention Task, an element of neuropsychological testing in which memory for specific patterns and designs is tested, has been specifically associated with pattern separation. Patients with social anxiety disorder and PTSD have poor performance in this task18,19. Hippocampus-dependent impairments in processing of spatial cues have also been observed in PTSD patients as compared to twin brothers that never developed PTSD20
Changes in hippocampal volume have been reported across several anxiety disorders. Structural MRI studies documented a decrease in hippocampal volume in PTSD patients as compared to healthy or trauma-exposed controls21,22, as well as in adults with social anxiety disorder23. Recent evidence indicates that this reduction in volume may represent a risk factor for vulnerability to PTSD24–26. In fMRI studies, a positive correlation between PTSD severity and activation in the hippocampus and amygdala in response to negative imagery has been documented, and recalling negative imagery related to the initial trauma can reduce blood oxygen level–dependent (BOLD) fMRI responses in PTSD patients as compared to trauma-exposed controls27,28. High-resolution MRI has indicated a specific reduction in volume of the dentate gyrus and CA3 subfields in PTSD, with sparing of other hippocampal subregions29. This selective vulnerability of the dentate gyrus and CA3 is consistent with results in animal models of chronic stress30. Thus, alterations in the dentate gyrus and CA3 circuit may confer vulnerability to development of PTSD or be symptomatic of impairments in underlying mnemonic processes.
Dentate gyrus and pattern separation
The theoretical foundations of pattern separation in the hippocampus were first set out in a computational theory11 that remains a dominant hypothesis31. It was proposed that the hippocampus performs two distinct serial computations. The first, pattern separation, takes similar patterns of neural activity and converts them into distinct representations. The second computation, pattern completion, operates on these distinct representations and either ignores the differences if they are negligible or, alternatively, generates an orthogonal representation if the differences are sufficiently large14
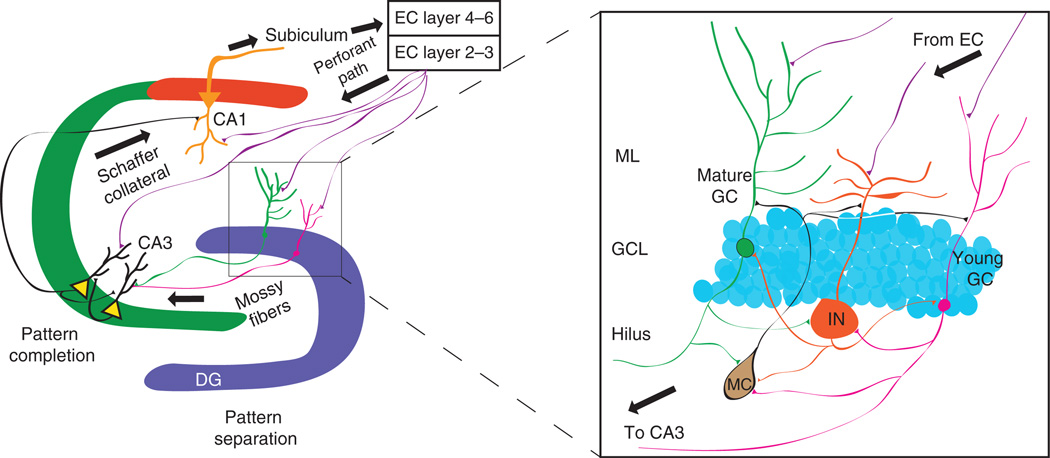
The anatomical and network properties of the dentate gyrus make it well suited to facilitate pattern separation. This includes the density of granule cells, their sparse pattern of activation, and the powerful synapses they make onto CA3 pyramidal cells. The sparse activation of granule cells allows inputs carrying information about similar contextual representations to be distributed into non-overlapping populations of granule cells12,32. One way sparseness may be achieved is through robust inhibition of granule cells by inhibitory interneurons residing in the hilus and the granule cell layer that target the somata and proximal dendrites of granule cells33,34. These inhibitory interneurons receive input from the perforant path, as well as from granule cell mossy fibers, and thus through their elaborate axonal plexus can mediate both feedforward and feedback inhibition to constrain dentate gyrus firing (Fig. 2). This pattern of activation can in turn instruct CA3 with high fidelity owing to the robust mossy fiber synapses onto CA3 pyramidal neurons35. Thus, a contextual representation encoded in a small cohort of dentate gyrus granule cells can create a new autoassociative network in CA3 for future memory retrieval12,36
Figure 2.
Local circuit properties of the dentate gyrus that facilitate pattern separation. Perforant path inputs from the entorhinal cortex carrying processed sensory information innervate granule cells of the dentate gyrus and pyramidal cells of both CA3 and CA1. In the dentate gyrus, sparseness is achieved by inhibitory interneurons that constrain granule cell firing through feedforward and feedback inhibition. The sparse cohort of granule cells that fire in a specific context can drive CA3 with high fidelity, owing to the robust mossy fiber synapses on CA3 pyramidal neurons. In this way, sparse coding and the mossy fiber–CA3 synapse can facilitate pattern separation. Activation of CA3 can instruct the generation of autoassociative networks in CA3 to facilitate future pattern completion for retrieval of stored representations. DG, dentate gyrus; EC, entorhinal cortex; GC, granule cell; IN, inhibitory interneuron; MC, mossy cell; ML, molecular layer.
In vivo recordings of hippocampal ensemble activity have begun to identify the neuronal correlates of pattern separation in the dentate gyrus. Parametric morphing of a rat’s environment (which in some ways is homologous to a stimulus-generalization task used in humans9) is sufficient to elicit remapping of firing rates of place cells in the dentate gyrus, suggesting that small changes in spatial input can produce highly divergent output13. Unlike CA3 pyramidal cells, which tend to have one place field, granule cells have multiple place fields that remap quickly with small changes in environmental context, supporting their role in fine discrimination of overlapping contextual representations. These highly distinct outputs are sent to CA3, where recurrent collaterals between CA3 pyramidal neurons are thought to perform pattern completion.
A function of the dentate gyrus in behavioral pattern separation has been documented using ablation and genetic manipulation techniques. Lesions of the dentate gyrus have resulted in deficits in tasks where there is either a spatial overlap in distal cues or when the contextual cues are highly similar16,37. In this pattern-separation task, rats are trained to find a food reward at a specific location and then probed with a foil placed at differing distances from the correct choice. Dentate gyrus–lesioned rats cannot discriminate between the two choices when the two objects are spatially close together, but performance approaches normal as the distance between the objects increases. Recent results also indicate that dentate gyrus–lesioned rats cannot recognize small displacement of object locations, further supporting a function for the dentate gyrus in spatial pattern separation38.
The first evidence for neural substrates of pattern separation in humans used high-resolution BOLD fMRI of activity in hippocampal subfields during a pattern-separation task and took advantage of the habituation of BOLD activity with familiarity15. In these studies, subjects are presented with an object and then later presented with the same object or a novel object that is either similar or distinct. When an object similar to one previously encountered is presented, the BOLD signal in CA1 is of a magnitude similar to that when an object is presented for a second time, suggestive of pattern completion; that is, the object does not elicit a novel pattern of activity in CA1. In contrast, activity in the dentate gyrus and CA3 when a subject sees a similar object are comparable to activity when seeing a novel object, as if this region is engaged in pattern separation; that is, encoding that similar object as a distinct entity.
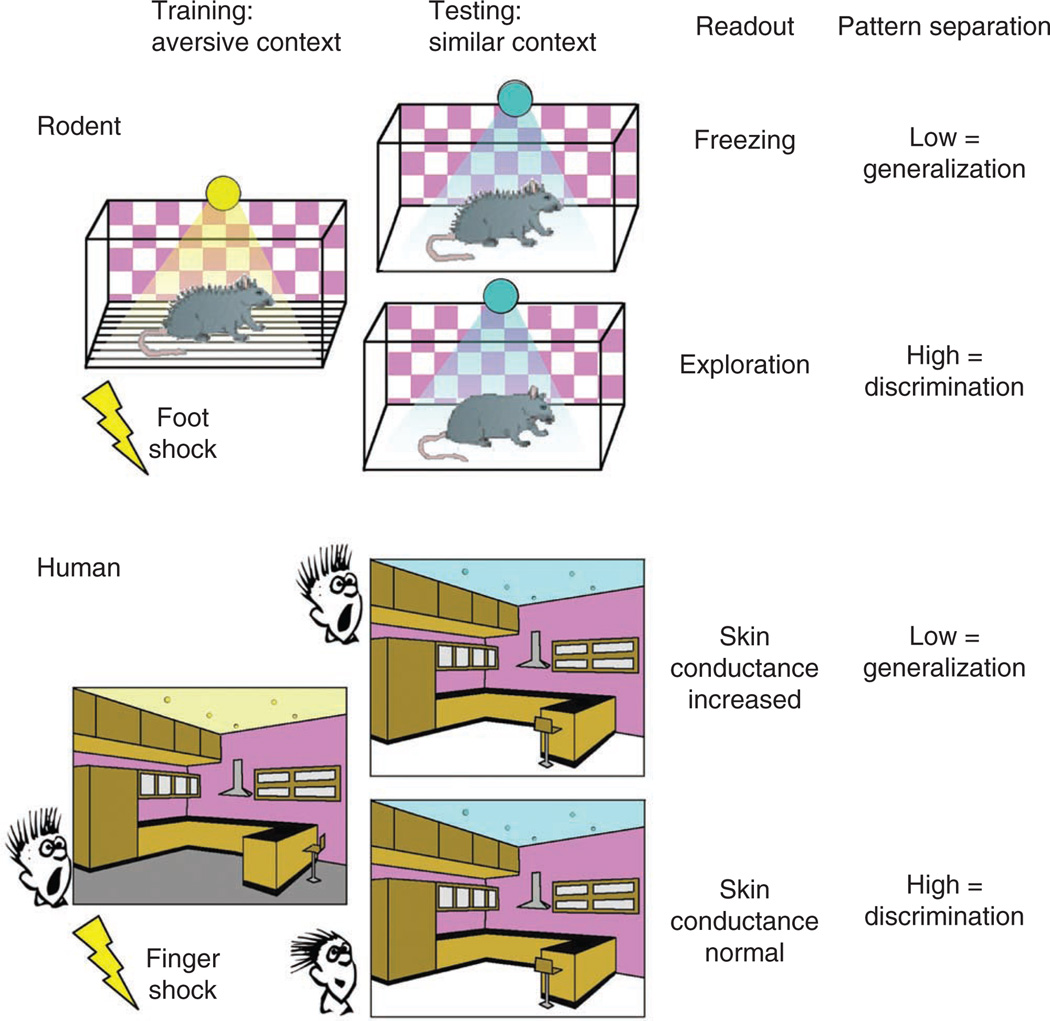
Although these studies have elegantly documented a function for the dentate gyrus in rapid pattern separation of neutral or appetitive stimuli, they do not recapitulate the emotionally charged stimuli that can lead to anxiety disorders such as PTSD and panic disorder. Pavlovian fear conditioning and extinction, in which a neutral stimulus (contextual or discrete) is paired with an aversive stimulus (mild shock; unconditioned stimulus), have been used to model PTSD. Re-exposure to the conditioned stimulus elicits a stereotypical conditioned response, freezing in rodents or arousal responses in humans, used as a measure of fear. These conditioned fear responses can be extinguished with repeated exposure to the conditioned stimulus in the absence of the reinforcer, thus forming a new memory trace indicating that the conditioned stimulus is now safe39. Yet in some cases, it would be beneficial to extinguish response to stimuli that resemble the original traumatic stimulus, so the memory of the trauma is not generalized to all stimuli that resemble the cues associated with the traumatic event. To model this situation, a contextual fear discrimination task has been used40–42. In this task, mice are trained to fear an aversive context (as in classical contextual conditioning) and then to discriminate between the aversive context and a highly similar ‘safe, no shock’ context (Fig. 3, top). In addition, the mice are exposed to a third novel context that is completely different from the aversive and the similar contexts. In this way, contextual features maybe parametrically varied to the extent allowed by measurements of freezing behavior. Because the two contexts share many similar features, mice freeze the same amount in the training context as in the similar context, thus generalizing their fear to the two contexts. Upon repeated training sessions, where mice are always shocked in the training context but never the similar context, mice will eventually reduce their freezing to the similar context, effectively separating the two similar contextual patterns (Fig. 3, top). A role for plasticity in the dentate gyrus has recently been documented in this form of pattern separation. Selective deletion of the NR1 subunit of the NMDA receptor from dentate gyrus granule cells impairs this ability to discriminate between the two similar contexts, suggesting that plasticity in the perforant path synapses onto granule cells is essential for discrimination learning40.
Figure 3.
Testing pattern separation with contextual fear discrimination in rodents and humans. Top: in the contextual fear discrimination task, rodents are trained to associate a neutral context with an aversive foot shock. Placing animals in a similar context that shares some, but not all, of the contextual cues present in the training context tests the ability to discriminate. Freezing in this similar context indicates a generalization of the new context to the training context, whereas exploration indicates discrimination and recognition that the context is novel. Bottom: testing pattern separation in humans using a virtual reality fear discrimination task. Humans freely navigate through a virtual reality environment while receiving unsignaled finger shocks. Upon exposure to a similar environment, measures of increased arousal indicate a generalization of the new context to the training context, whereas low arousal indicates discrimination of the two contexts.
In humans, modified versions of this discrimination task can be applied to directly test this type of pattern separation (Fig. 3, bottom). Virtual reality environments have been shown to be sufficiently complex to model real-life experiences and used successfully for the treatment of anxiety disorders43,44. Furthermore, virtual reality environments have also been used for classic cued and contextual fear conditioning with distinct training and testing contexts, using skin conductance as a measure of fear or arousal45,46. We propose that these highly controlled virtual reality environments could be used to test generalization to emotionally charged contexts. In this design, a virtual reality environment can acquire negative emotional valence through presentation of finger shocks during free navigation. In a similar fashion as in rodent experiments, the features of the virtual reality environment can be parametrically changed to test the ability to discriminate between the shocked environment and either highly similar contexts or distinct contexts (Fig. 3, bottom). It is possible for subjects to navigate and interact with virtual reality environments during fMRI scanning studies, and as spatial resolution and computational techniques improve, it is becoming possible to isolate activity within subregions of the hippocampus during functional imaging studies47,48. These techniques will allow mapping of the human hippocampal regions activated by this task, correlate neural activation with emotional reactivity, and investigate how hippocampal activity underlying pattern separation may differ in individuals with anxiety disorders such as PTSD.
Adult neurogenesis
In the mammalian adult brain, there are two regions where stem cells continuously give rise to new neurons, a process termed neu-rogenesis: the subventricular zone and the subgranular zone of the dentate gyrus. Adult-born neurons functionally integrate into the dentate gyrus49–51, exhibit heightened synaptic plasticity during a specific window of their maturation52,53 and can account for up to 10% of the entire granule cell population54. Hippocampal neurogenesis is increased by several categories of antidepressants, and some of the anxiolytic and antidepressant effects of antidepressants require intact hippocampal neurogenesis55,56. Moreover, the fate of neural progenitors is substantially affected by emotional state. Specifically, in impoverished environments such as isolation, hippocampal stem cells give rise to more stem cells and fewer neurons than in enriched environments57. Neurogenesis has also been shown to modulate the stress response both in baseline conditions and in response to antidepressants and enrichment58–60. However, the role of neurogenesis in regulating emotionality or response to stress remains controversial, with some groups seeing robust effects and others not61,62. Indeed, interventions that affect mood, such as stress or antidepressants, can also induce remodeling of mature networks via dendritogenesis or spinogenesis, thereby modulating hippocampal plasticity independently of neurogenesis63.
The extent of adult neurogenesis in humans, and its ability to affect behavior, remains to be fully explored. A recent post-mortem study identified an increase in proliferation of neural precursors in response to antidepressants64, whereas another study found no change65. In addition, recent studies of the olfactory bulb in humans revealed that neurogenesis is very limited in adulthood66,67.
Adult neurogenesis in the dentate gyrus and pattern separation
A function for neurogenesis in pattern separation has recently been proposed68,69. Mice in which adult neurogenesis has been ablated with X-irradiation exhibit specific deficits in a delayed nonmatching-to-place radial arm maze task where the spatial separation between the choice and sample arm is low70. Marked deficits in this task have also been reported in mice in which the fragile-X mental retardation protein (FMRP) has been deleted specifically in young granule cells, effectively reducing neurogenesis in vivo71. In addition, ablating neurogenesis with X-rays or increasing neurogenesis with voluntary running have bidirectional effects in a spatial discrimination touch-screen task that requires mice to choose the correct visual cue based on separation distance between the cues70,72. Evidence for a function of adult-born granule cells in pattern separation tasks with an emotional component has also been demonstrated. Ablation of adult-born granule cells with X-rays results in impaired pattern separation in contextual fear discrimination learning tasks41,69,73,74. This led to the hypothesis that increasing the pool of adult-generated neurons would increase the ability to discriminate between similar contexts. Indeed, when the proapoptotic gene Bax is deleted from the young neurons, promoting their survival, mice are better at distinguishing similar contexts, suggesting that expansion of a functional pool of adult-generated neurons is sufficient to facilitate pattern separation41. More recently, it was shown that a specific form of plasticity exhibited by young adult-born granule cells is required for contextual fear discrimination learning42, further highlighting the contribution of adult generated granule cells to pattern separation.
Although the mechanisms underlying the contribution of adult-born granule cells to pattern separation are unknown, recent evidence suggests that these cells can modulate sparseness and local inhibitory tone in the dentate gyrus. Computational modeling and electrophysiological studies have proposed a function for sparse coding in pattern separation31,75,76. Sparse coding is thought to facilitate the transformation of overlapping sensory inputs into non-overlapping representations. Whether this occurs owing to input expansion, whereby similar inputs arising in the entorhinal cortex are distributed across the granule cell layer, or by other means is not known. It is plausible that regulation of inhibition in the dentate gyrus is important in the maintenance of sparse coding and that young adult-born granule cells modulate local network inhibition within the dentate gyrus. One distinct physiological characteristic of young neurons is their lack of GABAergic inhibition early in their development77. Granule cells receive feedforward inhibitory input from perforant path activation of GABAergic interneurons, as well as feedback inhibition, as granule cells target tens of hilar inhibitory interneurons for each innervated downstream CA3 neuron78. Yet, during the first 2–3 weeks of their development, GABA depolarizes young neurons77, suggesting that increases in GABAergic tone mediated by feedback inhibition would have very different effects in a mature versus immature granule cells. In support of this, in vivo recordings have indicated that ablation of adult born granule cells increases gamma-frequency bursts and synchronization of dentate gyrus neuron firing to these bursts, suggesting that young neurons may inhibit network activity in the dentate gyrus79. In addition, ablation of young granule cells decreases inhibition on mature granule cells, suggesting a reduction in inhibitory tone after ablation of adult-born granule cells80. Another indication that young neurons may exert an inhibitory influence on the dentate gyrus comes from the recent observation that after a behavioral experience there is an increase in induction of immediate-early genes in the dentate gyrus of mice lacking neurogenesis81. Thus, immature granule cells may influence pattern separation by directly modulating the excitability of the dentate gyrus. Future studies examining the consequence of modulating neurogenesis on sparseness in the dentate gyrus will elucidate this specific contribution of young neurons.
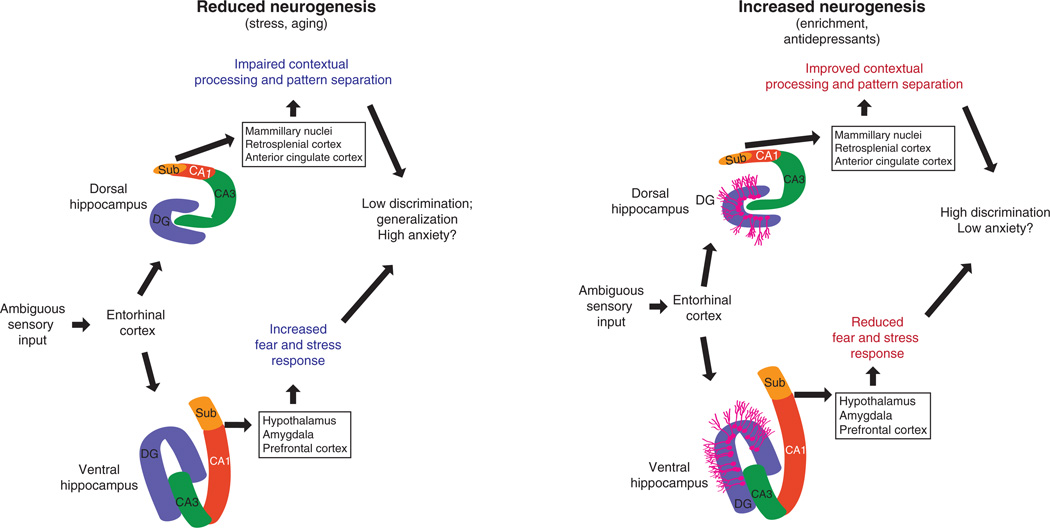
The dentate gyrus network performs pattern separation on its inputs from the entorhinal cortex, regardless of the particular information content of those inputs (Fig. 4). We propose that pattern separation of spatial information is computed by the dentate gyrus and then, depending on the position along the dorso-ventral axis, transmitted to downstream structures to control aspects of emotional behavior such as exploration, anxiety and stress responses. Whether contextual representations are differentially computed along the dorso-ventral axis remains an open question, as neurons in ventral CA1 exhibit place fields, albeit at a lower proportion and with larger place fields than those in dorsal CA1, suggesting that space is computed differently in ventral CA1 (ref. 82). Manipulations of the ventral hippocampus affect contextual, and in some cases cued, fear conditioning, though these studies have produced some inconsistent results83–86. These deficits may arise from either impaired contextual processing or modulation of emotional state, as the amygdala only receives hippocampal input from the ventral hippocampus87.
Figure 4.
Modulation of pattern separation by adult neurogenesis and its impact on mood. Novel sensory representations can be disambiguated from stored representations through pattern separation on inputs from entorhinal cortex. Under stressful conditions, neurogenesis is low, leading to low discrimination and to generalization of contexts with substantial emotional valence. This may lead to increased anxiety owing to impaired contextual processing through modulation of exploration through dorsal outputs and increased stress responses by influencing outputs from the ventral pole. In conditions with high neurogenesis, such as enrichment or antidepressant treatment, enhanced pattern separation leads to high discrimination and greater ability to disambiguate similar contexts with differing emotional valence. This may influence output of the dorsal hippocampus to increase exploration and contextual encoding, and the ventral hippocampus to modulate stress responses. DG, dentate gyrus; Sub, subiculum.
The hippocampus is connected with several components of the limbic system, and synchrony between hippocampus and limbic regions have been reported in emotionally charged situations88–90. Outputs from the ventral hippocampus project directly to the prefrontal cortex (PFC), the amygdala, the shell of the nucleus accumbens, the bed nuclei of the stria terminalis and the hypothalamus, to control the autonomic, neuroendocrine and motivational responses to emotionally charged stimuli. The dorsal hippocampus is connected to the retrosplenial and anterior cingulate cortex, as well as to mammillary nuclei to influence exploratory behavior and contextual encoding (Fig. 4)90. In addition, indirect dorsal hippocampal outputs to the midbrain dopaminergic neurons of the ventral tegmental area have been recently linked to attribution of positive salience to contextual representations91. Although segregation of outputs has been noted along the dorso-ventral axis, connectivity in the dentate gyrus can extend along the longitudinal axis, as mossy fiber collaterals, the axon plexus of inhibitory interneurons, entorhinal cortex input and mossy cell projections extend considerably along the dorso-ventral axis of the hippocampus78,92,93.
Functional differences along the septo-temporal axis hippocampus have been noted in humans, with greater activity seen in more anterior regions of the temporal lobe when presented with emotionally charged stimuli or faces94 and more neutral stimuli recruiting more posterior areas95. In humans, selective serotonin reuptake inhibitors and tricyclic antidepressants increase neuronal precursor cells more prominently in the anterior portion (ventral) of the dentate gyrus of patients with major depressive disorder as compared to controls and untreated subjects64. In rodents, chronic treatment with agomelatine, a melatonin receptor agonist and 5-hydroxytryptamine receptor 2C (5-HT2C) antagonist with efficacy in animal models and in human major depressive disorder, increases neurogenesis selectively in the ventral dentate gyrus96.
Stimulating adult neurogenesis to restrain overgeneralization
Here we have hypothesized that anxiety disorders such as PTSD are associated with pattern separation deficits that are attributable to dentate gyrus dysfunction. As adult neurogenesis influences pattern separation, we hypothesize that targeting adult neurogenesis to improve pattern separation, particularly for situations and contexts that are emotionally charged, may be beneficial for the treatment of anxiety disorders.
The identification of specific endophenotypes such as pattern separation deficits creates opportunities to develop approaches targeting specific underlying neural circuits and mnemonic processes. Moreover, it facilitates diagnosis based on circuit-based changes that are assessed with imaging rather than on qualitative observation of disease symptoms. Anxiety disorders are likely to be associated with a variety of endophenotypes, including fear inhibition and pattern separation deficits. The recent progress in understanding the mechanisms underlying pattern separation40,41,69, improved behavioral assessments of human pattern separation97 and improved techniques for imaging function of hippocampal subregions15,29 offer an opportunity to develop treatments targeting specific endophenotypes. The emerging influence of adult hippocampal neurogenesis in modulation of pattern separation motivates development of pro-neurogenic strategies to restrain the overgeneralization seen in anxiety disorders. The genetic and epigenetic mechanisms that regulate the proliferation, survival and integration of young granule cells into the hippocampal circuit have begun to be elucidated (Fig. 5). Targeting these mechanisms to selectively modulate each of these processes may have beneficial effects on pattern separation by either increasing the available pool of adult generated neurons or modifying their properties to increase their capacity for information processing.
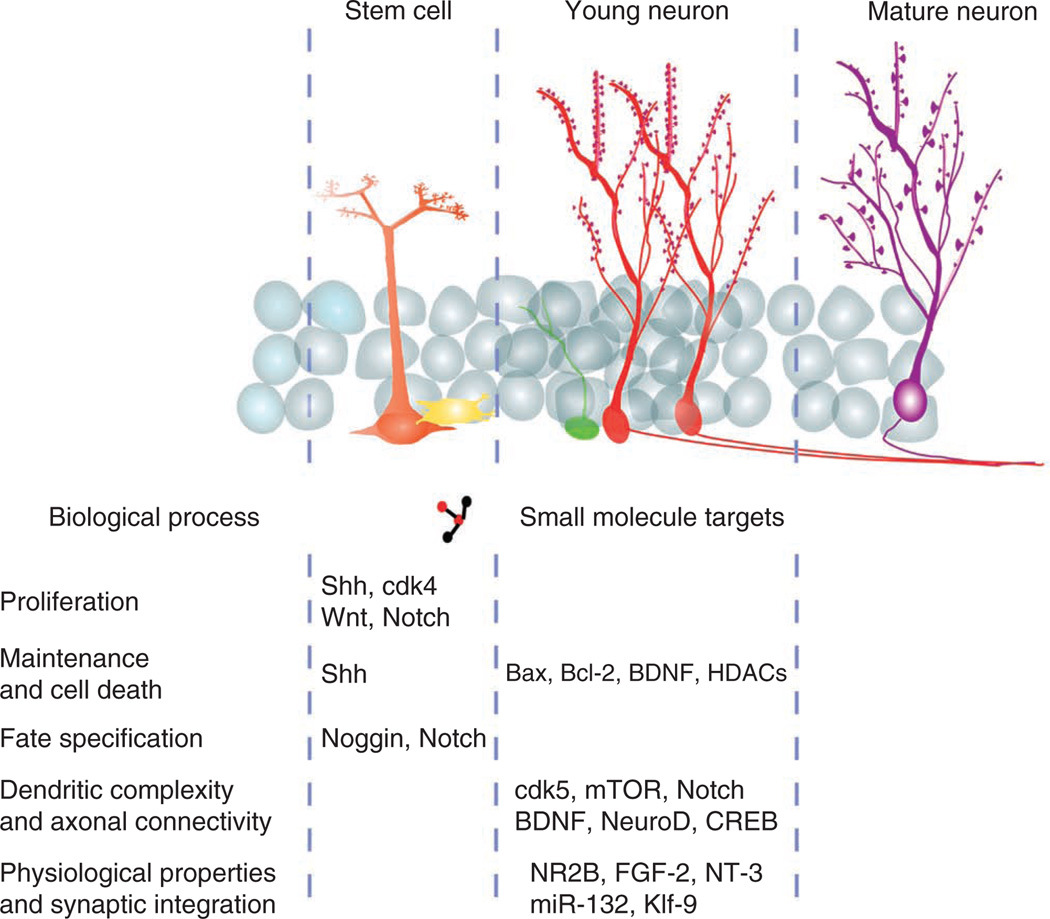
Figure 5.
Targets for stimulating neurogenesis to enhance pattern separation and restrain overgeneralization. The mechanisms that regulate the proliferation, integration and maturation of adult born granule cells provide targets to generate new therapies aimed at increasing neurogenesis to reduce overgeneralization in anxiety disorders. Targets aimed at increasing proliferation (Sonic hedgehog (Shh), cyclin-dependent kinase 4 (cdk4), Wnt, Notch) or those that increase the survival of young neurons (Bax, brain-derived neurotrophic factor (BDNF), histone deacetylases (HDACs)) can provide an increased available pool of young neurons. Expanding the pool of young neurons has recently been shown to increase pattern separation in rodent models41. Alternatively, targeting the distinct physiological properties or the structural properties of young neurons will provide further insight into the specific function of young neurons in pattern separation, and in turn in anxiety disorders such as PTSD, and may provide more specific targets for the treatment of overgeneralization in anxiety disorders.
Future studies will examine the effect of modulating neurogenesis in mouse models of anxiety disorders. It is crucial that such efforts are mirrored by development of imaging approaches to capture ongoing neurogenesis in the adult human dentate gyrus98. We expect that patients who have pattern separation deficits belong to a subgroup whose disorder is caused by a dysfunction of the dentate gyrus and are as a result more likely to benefit from treatments aimed at stimulating neurogenesis. Once candidate compounds are identified, clinical trials may target those patients with anxiety disorders who display both an impairment in pattern separation tasks and a dysfunction in the dentate gyrus as assessed by MRI-based imaging studies. Such clinical trials will assess the extent to which clinical improvement can be achieved by targeting pattern separation.
ACKNOWLEDGMENTS
We thank L. Drew for comments on the manuscript. The authors are supported by US National Institute of Mental Health grants 1F32MH092101-01A1 and 1K01MH099371-01; a Sackler Institute Award and a NARSAD Young Investigator Award (M.A.K.); US Nationa l Institute of Mental Health grant 4R00MH086 615-03, the Ellison Medical Foundation New Scholar in Aging and the Whitehall Foundation (A.S.); and NARSAD, the New York Stem Cell Initiative, US National Institutes of Health grant R01 MH068542, and Hope for Depression Research Foundation grants (R.H.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Kessler RC, et al. Lifetime prevalence age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch.Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.DuPont RL, et al. Economic costs of anxiety disorders. Anxiety. 1996;2:167–172. doi: 10.1002/(SICI)1522-7154(1996)2:4<167::AID-ANXI2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 4.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jovanovic T, Ressler KJ. How the neurocircuitry genetics of fear inhibition may inform our understanding of PTSD. Am. J. Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lissek S, et al. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am. J. Psychiatry. 2008;165:124–132. doi: 10.1176/appi.ajp.2007.06091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it’s not what you thought it was. Am. Psychol. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- 8.Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol. Rev. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- 9.Lissek S, et al. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am. J. Psychiatry. 2010;167:47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolpe J, Rowan VC. Panic disorder: a product of classical conditioning. Behav. Res. Ther. 1988;26:441–450. doi: 10.1016/0005-7967(88)90138-6. [DOI] [PubMed] [Google Scholar]
- 11.Marr D. Simple memory: a theory for archicortex. Phil. Trans. R. Soc. Lond. B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 12.Treves A, Tashiro A, Witter ME, Moser EI. what is the mammalian dentate gyrus good for? Neuroscience. 2008;154:1155–1172. doi: 10.1016/j.neuroscience.2008.04.073. [DOI] [PubMed] [Google Scholar]
- 13.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 14.Fenton AA. Neuroscience. Where am I? Science. 2007;315:947–949. doi: 10.1126/science.1139146. [DOI] [PubMed] [Google Scholar]
- 15.Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 17.Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol. Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- 18.Cohen LJ, et al. Specificity of neuropsychological impairment in obsessive-compulsive disorder: a comparison with social phobic and normal control subjects. J. Neuropsychiatry Clin. Neurosci. 1996;8:82–85. doi: 10.1176/jnp.8.1.82. [DOI] [PubMed] [Google Scholar]
- 19.Šodic´ L, Anticevic V, Britvic D, Ivkosic N. Short-term memory in Croatian war veterans with posttraumatic stress disorder. Croat. Med. J. 2007;48:140–145. [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbertson MW, et al. Configural cue performance in identical twins discordant for posttraumatic stress disorder: theoretical implications for the role of hippocampal function. Biol. Psychiatry. 2007;62:513–520. doi: 10.1016/j.biopsych.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J. Affect. Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Karl A, Malta LS, Maercker A. Meta-analytic review of event-related potential studies in post-traumatic stress disorder. Biol. Psychol. 2006;71:123–147. doi: 10.1016/j.biopsycho.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Irle E, et al. Reduced amygdalar and hippocampal size in adults with generalized social phobia. J. Psychiatry Neurosci. 2010;35:126–131. doi: 10.1503/jpn.090041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbertson MW, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbertson MW, et al. Is trauma a causal agent of psychopathologic symptoms in posttraumatic stress disorder? Findings from identical twins discordant for combat exposure. J. Clin. Psychiatry. 2010;71:1324–1330. doi: 10.4088/JCP.10m06121blu. [DOI] [PubMed] [Google Scholar]
- 26.Dannlowski U, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Hayes JP, et al. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. J. Psychiatr. Res. 2011;45:660–669. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brohawn KH, Offringa R, Pfaff DL, Hughes KC, Shin LM. The neural correlates of emotional memory in posttraumatic stress disorder. Biol. Psychiatry. 2010;68:1023–1030. doi: 10.1016/j.biopsych.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, et al. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch. Gen. Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEwen BS. Stress and hippocampal plasticity. Annu. Rev. Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 31.O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 32.Lisman J. Formation of the non-functional and functional pools of granule cells in the dentate gyrus: role of neurogenesis, LTP and LTD. J. Physiol. (Lond.) 2011;589:1905–1909. doi: 10.1113/jphysiol.2010.201137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- 34.Patton PE, McNaughton B. Connection matrix of the hippocampal formation: I The dentate gyrus. Hippocampus. 1995;5:245–286. doi: 10.1002/hipo.450050402. [DOI] [PubMed] [Google Scholar]
- 35.Henze DA, Wittner L, Buzsáki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat. Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- 36.McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information-storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- 37.Kim J, Lee I. Hippocampus is necessary for spatial discrimination using distal cue-configuration. Hippocampus. 2011;21:609–621. doi: 10.1002/hipo.20784. [DOI] [PubMed] [Google Scholar]
- 38.Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- 39.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol. Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 40.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 41.Sahay A, et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kheirbek MA, Tannenholz L, Hen R. NR2B–dependent plasticity of adult-born granule cells is necessary for context discrimination. J. Neurosci. 2012;32:8696–8702. doi: 10.1523/JNEUROSCI.1692-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ressler KJ, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch. Gen. Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 44.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol. Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J. Neurosci. 2008;28:6211–6219. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huff NC, et al. Revealing context-specific conditioned fear memories with full immersion virtual reality. Front. Behav. Neurosci. 2011;5:75. doi: 10.3389/fnbeh.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CE. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learn. Mem. 2010;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suthana N, Ekstrom A, Moshirvaziri S, Knowlton B, Bookheimer S. Dissociations within human hippocampal subregions during encoding and retrieval of spatial information. Hippocampus. 2011;21:694–701. doi: 10.1002/hipo.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toni N, et al. Synapse formation on neurons born in the adult hippocampus. Nat. Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 51.Toni N, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 53.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 55.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 56.David DJ, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dranovsky A, et al. Experience dictates stem cell fate in the adult hippocampus. Neuron. 2011;70:908–923. doi: 10.1016/j.neuron.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Surget A, et al. Antidepressants recruit new neurons to improve stress response regulation. Mol. Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol. Psychiatry. 2010;15:1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 62.DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58:884–893. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 64.Boldrini M, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reif A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol. Psychiatry. 2006;11:514–522. doi: 10.1038/sj.mp.4001791. [DOI] [PubMed] [Google Scholar]
- 66.Bergmann O, et al. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 67.Sanai N, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo W, et al. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat. Med. 2011;17:559–565. doi: 10.1038/nm.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc. Natl. Acad. Sci. USA. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tronel S, et al. Adult-born neurons are necessary for extended contextual discrimination. Hippocampus. 2012;22:292–298. doi: 10.1002/hipo.20895. [DOI] [PubMed] [Google Scholar]
- 74.Nakashiba T, et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149:188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Prog. Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Rolls ET. An attractor network in the hippocampus: theory and neurophysiology. Learn. Mem. 2007;14:714–731. doi: 10.1101/lm.631207. [DOI] [PubMed] [Google Scholar]
- 77.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 79.Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2012;22:106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singer BH, et al. Compensatory network changes in the dentate gyrus restore long-term potentiation following ablation of neurogenesis in young-adult mice. Proc. Natl. Acad. Sci. USA. 2011;108:5437–5442. doi: 10.1073/pnas.1015425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22:1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci. 1994;14:7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maren S, Holt WG. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behav. Neurosci. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- 84.Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol. Learn. Mem. 2008;89:61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Czerniawski J, Ree F, Chia C, Otto T. Dorsal versus ventral hippocampal contributions to trace and contextual conditioning: differential effects of regionally selective NMDA receptor antagonism on acquisition and expression. Hippocampus. 2012;22:1528–1539. doi: 10.1002/hipo.20992. [DOI] [PubMed] [Google Scholar]
- 86.Wang SH, Finnie PS, Hardt O, Nader K. Dorsal hippocampus is necessary for novel learning but sufficient for subsequent similar learning. Hippocampus. 2012 doi: 10.1002/hipo.22036. [DOI] [PubMed] [Google Scholar]
- 87.Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo . J. Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bienvenu TC, Busti D, Magill PJ, Ferraguti F, Capogna M. Cell-type-specific recruitment of amygdala interneurons to hippocampal theta rhythm and noxious stimuli in vivo . Neuron. 2012;74:1059–1074. doi: 10.1016/j.neuron.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 93.Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J. Comp. Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- 94.Lau JY, et al. BDNF gene polymorphism (Val66Met) predicts amygdala and anterior hippocampus responses to emotional faces in anxious and depressed adolescents. Neuroimage. 2010;53:952–961. doi: 10.1016/j.neuroimage.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- 96.Banasr M, Soumier A, Hery M, Mocaer E, Daszuta A. Agomelatine, a new antidepressant, induces regional changes in hippocampal neurogenesis. Biol. Psychiatry. 2006;59:1087–1096. doi: 10.1016/j.biopsych.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 97.Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn. Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sierra A, Encinas JM, Maletic-Savatic M. Adult human neurogenesis: from microscopy to magnetic resonance imaging. Front. Neurosci. 2011;5:47. doi: 10.3389/fnins.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]