Abstract
Background
The study was conducted to estimate the relative cost effectiveness of contraceptives in the United States from a payer’s perspective.
Methods
A Markov model was constructed to simulate costs for 16 contraceptive methods and no method over a 5-year period. Failure rates, adverse event rates, and resource utilization were derived from the literature. Sensitivity analyses were performed on costs and failure rates.
Results
Any contraceptive method is superior to “no method”. The three least expensive methods were the copper-T IUD ($647), vasectomy ($713) and LNG-20 IUS ($930). Results were sensitive to the cost of contraceptive methods, the cost of an unintended pregnancy, and plan disenrollment rates.
Conclusion
The copper-T IUD, vasectomy, and the LNG-20 IUS are the most cost-effective contraceptive methods available in the United States. Differences in method costs, the cost of an unintended pregnancy, and time horizon are influential factors that determine the overall value of a contraceptive method.
Keywords: Contraception, contraceptive devices, cost effectiveness, economic modeling
1. Introduction
Despite the introduction of highly effective reversible contraceptive methods, nearly half (49%) of the 6.4 million pregnancies each year in the United States are unintended; there were more than 3 million unintended pregnancies in 2001, the last year from which data are available [1]. The direct medical costs of these unintended pregnancies totaled $5 billion [2].
Contraceptive use saves nearly $19 billion in direct medical costs each year [2]. In an era of increasingly constrained resources, health care decision-makers are being held accountable for providing health care services and treatments that optimize value received for resources expended. Currently available contraceptive methods vary greatly in their efficacy and overall cost. Long-acting methods, such as intrauterine contraceptives and implants, have high effectiveness rates that do not depend on compliance or daily usage. However, their usage has been limited [3].
In contrast, user-dependent methods (e.g., condoms, oral contraceptives) may incur pregnancy-related costs that may greatly exceed the method costs themselves. Thus, there is need for good evidence on the costs and effectiveness of different contraceptive options so that individuals can make an informed choice and health plans can provide the right mix of contraceptive options.
In a previous publication on the cost effectiveness of female contraceptives, we showed that intrauterine contraceptives were more cost effective than all other female contraceptive methods [4]. In the present analysis, we update and expand our previous estimates to include no contraceptive method (chance), male contraceptive methods, and new market entrants such as implants. In addition, we adjusted the costs associated with unintended pregnancies to account for mistimed pregnancies so that the results would be generalizable to the entire population of women of reproductive age, not just those who had completed childbearing.
2. Methods
2.1. Model design
A Markov model was constructed from the health care payer perspective to evaluate the costs and effectiveness of using 16 contraceptive methods: vasectomy and tubal ligation as well as 14 reversible methods (oral contraceptives (OCs), transdermal contraceptive patch, vaginal ring, copper-T intrauterine device (IUD), levonorgestrel (LNG)-20 intrauterine system (IUS), male condom, female condom, injectable contraceptive, implant, diaphragm, spermicides, sponge, withdrawal, and fertility-awareness-based methods). We also compared these estimates with the cost and effectiveness of using no method (chance alone).
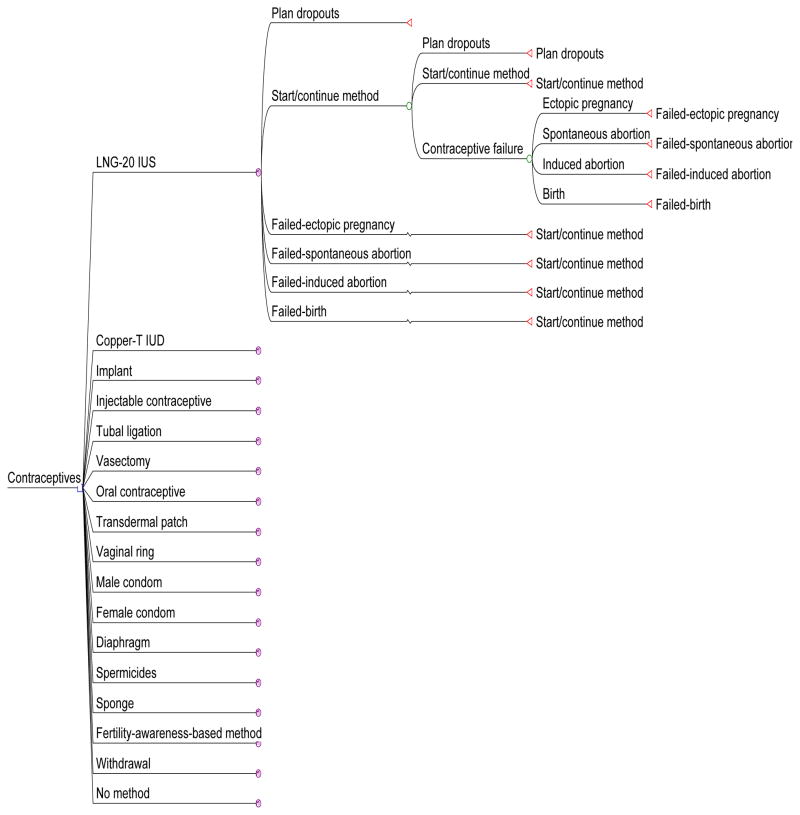
Markov processes were used to simulate contraceptive use and outcomes over a 5-year time horizon. The Markov cycle length was 1 year. Within each method, there were six states: disenrollment from the health plan (“Plan dropouts”), initial or continuing use of the method (“Start/continue method”), and outcomes related to method failure (ectopic pregnancy, birth, spontaneous abortion and induced abortion). Subjects remained on the initial method for the model duration (there was no method switching, and after a contraceptive failure, a woman resumed use of that method). Fig. 1 illustrates the model structure, with branching for one of the contraceptive methods displayed in its entirety to illustrate the format. The model structure was the same for all methods.
Fig. 1. Economic model structure.
Note: the annualized cost at year x is the total cost for x years divided by x. The annualized costs for tubal ligation for year 1 and 2 (not shown in graph) were $2,912 and $1,470 respectively. The annualized cost for implant for year 1 was $1,477.
2.2. Key model assumptions
This model applied to all couples using contraception during the time horizon of the analysis.
Individuals were not allowed to switch between contraceptive methods for the duration of the analysis. This assumption differs from our earlier analysis, which allowed switching to any of a fixed mix of choices that mirrored the prevalence of method use among older women [4]. Our current approach allows a purer comparison among the different contraceptive methods.
Contraceptive failures and health plan dropouts were assumed to occur at the midpoint of the cycle (6 months).
Method failures resulted in one of four outcomes: ectopic pregnancy, birth, spontaneous abortion or induced abortion. Pregnancies ending in a birth were assumed to be 9 months in duration. Individuals with a method failure resulting in a birth were assumed to begin using the same contraceptive again 2 months after delivery (in month 5 of the following annual cycle). Those with other outcomes continued to use the same method.
In the base case, there was a 0% dropout rate. In sensitivity analyses, the proportion of individuals dropping out of the health plan was the same regardless of method used; individuals who dropped out no longer accumulated costs payable by the plan.
In order to estimate costs for barrier methods (sponge, male condom, female condom, diaphragms, spermicides), each individual was assumed to have 83 acts of intercourse per year [5].
Costs of induced abortions and fetal losses were assumed to be avoided entirely had the pregnancy not occurred. In contrast, we assumed that most unintended births (60%) are simply mistimed [6] and would occur 2 years later.
Based on the package insert, it was assumed that 2% of individuals using an IUD or IUS require reinsertion within the first year of use.
2.3. Probability of events
Probability estimates were based on findings from a comprehensive literature review, package inserts, and expert opinion. Probabilities of failure for all methods during typical use were obtained from the literature [7] (Table 1a) and were assumed to be constant over time with the exception of those for the copper-T IUD, tubal ligation, and vasectomy. We used first-year failure rates as a proxy for annual failure rates. Decreases in failure rates are frequently observed for user-dependent products because less motivated users become pregnant and are removed from observation. However, our study assumed that all women continue to choose only the method being evaluated so that costs of different methods can be directly compared. We ignored the distinction between annual probabilities of failure and annual failure rates because the two are nearly identical.1
Table 1a.
Annual failure rates for contraceptive methods
| Method | Percent of women experiencing an unintended pregnancy within the first year of use [7]
|
|
|---|---|---|
| Typical use a | Perfect use b | |
| LNG-20 IUS | 0. 2 | 0. 2 |
| Copper-T IUD | ||
| Year 1 | 0. 8 | 0. 6 |
| Year 2 | 0. 6 | 0. 6 |
| Year 3 | 0. 4 | 0. 4 |
| Year 4 | 0. 2 | 0. 2 |
| Year 5 | 0. 1 | 0. 1 |
| Implant | 0. 05 | 0. 05 |
| Injectable contraceptive | 3 | 0. 3 |
| Tubal ligation | ||
| Year 1 | 0. 55 | 0. 55 |
| Year 2 | 0. 29 | 0. 29 |
| Year 3 | 0. 15 | 0. 15 |
| Year 4 | 0. 19 | 0. 19 |
| Year 5 | 0. 13 | 0. 13 |
| Vasectomy | ||
| Year 1 | 0. 15 | 0. 10 |
| Year 2 | 0. 01 | 0. 01 |
| Year 3 | 0. 01 | 0. 01 |
| Year 4 | 0. 01 | 0. 01 |
| Year 5 | 0. 01 | 0. 01 |
| Oral contraceptive | 8 | 0. 3 |
| Transdermal patch | 8 | 0. 3 |
| Vaginal ring | 8 | 0. 3 |
| Male condom | 15 | 2 |
| Female condom | 21 | 5 |
| Diaphragm | 16 | 6 |
| Spermicides | 29 | 18 |
| Sponge | 24c | 15c |
| Fertility-awareness-based methods | 25 | 4d |
| Withdrawal | 27 | 4d |
| No method | 85 | 85 |
IUD, intrauterine device; IUS, intrauterine system.
Among typical couples who initiate use of a method (not necessarily for the first time), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason. Estimates of the probability of pregnancy during the first year of typical use for spermicides, withdrawal, fertility-awareness-based methods, the diaphragm, the male condom, the pill, and Depo-Provera are taken from the 1995 National Survey of Family Growth corrected for underreporting of abortion.
Among couples who initiate use of a method (not necessarily for the first time) and who use it perfectly (both consistently and correctly), the percentage who experience an accidental pregnancy during the first year if they do not stop use for any other reason. See the text for the derivation of the estimate for each method.
Average of parous and nulliparous.
Natural family planning methods only.
The probability of ectopic pregnancy for each contraceptive method was obtained from the literature [8–12]. The probabilities for the remaining failures were assumed to have the same distribution as for all contraceptive failures in the United States: 37% full term pregnancies, 17% spontaneous abortions, and 46% induced abortions, regardless of method (Table 1b) (personal communication from Lawrence Finer, August 30, 2007). Probabilities of all side effects (amenorrhea, urinary tract infections, venous thromboembolism and postoperative complications) [7,13–21] were assumed to be constant for all 5 years with the exception of those for amenorrhea for the injectable contraceptive (Table 1c). Since published plan disenrollment rates vary widely, rates from 0% to 20% were examined.
Table 1b.
Probability estimates for method failure outcomes
| Method | Ectopic pregnancy a | Induced abortion c | Spontaneous abortion c | Birth c |
|---|---|---|---|---|
| LNG-20 IUS | 0.50b [8,9] | 0.2300 | 0.0850 | 0.1850 |
| Copper-T IUD | 0.03 [10] | 0.4462 | 0.1649 | 0.3589 |
| Implant | 0.01[11] | 0.4554 | 0.1683 | 0.3663 |
| Injectable contraceptive | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| Tubal ligation | 0.33[12] | 0.3082 | 0.1139 | 0.2479 |
| Vasectomy | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| Oral contraceptive | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| Transdermal patch | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| Vaginal ring | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| Male condom | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| Female condom | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| Diaphragm | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| Spermicides | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| Sponge | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| Fertility-awareness-based methods | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| Withdrawal | 0.01 | 0.4554 | 0.1683 | 0.3663 |
| No method | 0.01 | 0.4554 | 0.1683 | 0.3663 |
IUD, intrauterine device; IUS, intrauterine system.
Assumed a base case rate of 1%.
Package insert.
Probabilities of all failures except ectopic pregnancy were assumed to have the following distribution, 37% birth; 17% spontaneous abortion and 46% induced abortion, regardless of method (source: Personal communication from Lawrence Finer, August 30, 2007).
Table 1c.
Probability estimates of side effects for contraceptive methods
| Method | Amenorrhea | Venous thromboembolism | Urinary tract infections | Postoperative complications |
|---|---|---|---|---|
| LNG-20 IUS | 0.2a [13] | |||
| Copper-T IUD | ||||
| Implant | 0.222 a | |||
| Injectable contraceptive | 0.4 b[7] | |||
| Tubal ligation | 0.012c[14] | |||
| Vasectomy | 0.00043c[14] | |||
| Oral contraceptive | 0.03[15] | 0.00005[16] | 0.15[17] | |
| Transdermal patch | 0.001[18] | 0.00005 a[16] | ||
| Vaginal ring | 0.03[19] | 0.00005 a | ||
| Male condom | ||||
| Female condom | ||||
| Diaphragm | 0.31[17,20] | |||
| Spermicides | 0.31d[20,21] | |||
| Sponge | ||||
| Fertility-awareness-based methods | ||||
| Withdrawal | ||||
| No method |
IUD, intrauterine device; IUS, intrauterine system.
Source: Package Insert.
Probability of amenorrhea for 3-month injectable contraceptive increases each year to 0.7, 0.75, 0.78, and 0.8, respectively.
First year only.
Assumed to be the same as with the diaphragm.
2.4. Costs
Costs included those for the drug or device, physician services (device fitting, insertion, and removal), method failures (pregnancies), and side effects. Direct non-medical costs (e.g., cost of transportation) and indirect costs (e.g., lost productivity) were not considered. All costs were adjusted to 2007 US dollars using the Medical Care Services component of the Consumer Price Index.
2.4.1. Cost of contraceptive methods
For each year, method costs included the annual cost of drug or supplies and professional fees. Contraceptive drug and device costs were based on the 2007 Thomson Red Book Average Wholesale Price (AWP) [22], Multum [23], and Medi-Span [24] (Table 2a). Retail prices were obtained from an online pharmacy directory [25] for methods not included in the 2007 Red Book. The prices included in the model were AWP-15% to reflect drug acquisition costs that health services payers would most likely encounter. The costs of physician services, including those for device fitting, insertion/removal of intrauterine contraceptives, and an office visit, were obtained from the average fees associated with the Current Procedure Terminology (CPT) codes [26]. Costs for removing devices were incurred when there was a method failure or at the end of the device lifespan. No costs for removing devices were assigned for plan dropouts. Costs of a tubal ligation and vasectomy were calculated based on the required resource utilization [27,28] and the national average payments for relevant Diagnosis-Related Groups (DRGs) obtained from the 2007 Ingenix DRG Expert [29].
Table 2a.
Contraceptive method costs
| Contraceptive methods and codes | Cost ($) (duration of use) |
|---|---|
| LNG-20 IUS | |
| Mirena® a[24] | 585.89 (5 years) |
| IUD insertion (CPT 58300) [26] | 93.71 |
| IUD removal (CPT 58301) [26] | 105.17 |
| Office visit (CPT 99213) [26] | 64.97 |
| Copper-T IUD | |
| Paragard® a[24] | 494.00 (10 years) |
| IUD insertion (CPT 58300) [26] | 93.71 |
| IUD removal (CPT 58301) [26] | 105.17 |
| Office visit (CPT 99213) [26] | 64.97 |
| Implant | |
| Implanon® a[23] | 627.3 (3 years) |
| Cost of insertion (CPT 11975) [26] | 122.15 |
| Cost for removal (CPT 11976) [26] | 146.08 |
| Office visit (CPT 99213) [26] | 64.97 |
| Injectable contraceptive | |
| Depo Provera® a[24] | 75.53 (3 months) |
| Injection (CPT 90782) [26] | 21.63 |
| Office visit (CPT 99212) [26] | 40.55 |
| Vasectomy | |
| Procedure costb [26,29] | 707.97 |
| Postoperative infection (0.043% of $144) | 0.06 |
| Tubal ligation | |
| Procedure costc [26,29] | 2833.79 |
| Postoperative complications (1.2% of $5210) | 62.52 |
| Oral contraceptive | |
| Ortho Novum® a[24] | 52.81 (1 month) |
| Office visit (CPT 99212) [26] | 40.55 |
| Transdermal patch | |
| Ortho Evra® a[24] | 56.49 (1 month) |
| Office visit (CPT 99212) [26] | 40.55 |
| Vaginal ring | |
| NuvaRing® a[24] | 49.81 (1 month) |
| Office visit (CPT 99212) [26] | 40.55 |
| Male condom | |
| Retail-male condomd[25] | 1.00 (per use) |
| Female condom | |
| FC female condomd[25] | 3.00 (per use) |
| Diaphragm | |
| All-Flex®/Ortho® Coil Springa[22] | 41.60 (2 years) |
| Diaphragm fitting (CPT 57170) [26] | 91.12 |
| Office visit (CPT 99212) [26] | 40.55 |
| Ortho® Options-gel-prefilled applicatord[25] | 1.29 (per use) |
| Spermicides | |
| Ortho® Option-gel-prefilled applicatord[25] | 1.29 (per use) |
| Sponge | |
| Today sponged[25] | 2.83 (per use) |
| Fertility-awareness-based methods | 0 |
| Withdrawal | 0 |
| No method | 0 |
IUD, intrauterine device; IUS, intrauterine system; DRG, Diagnosis-related group; CPT, Current Procedural Terminology.
Costs are reported as average wholesale price (AWP). In the model, cost inputs are AWP-15%.
Assuming 0.2% of vasectomies are performed inpatient, 77.1% in a physician’s office and 22.7% as hospital outpatient [27].
Assuming 50% are performed postpartum and 50% as interval procedures; 96% of interval procedures are outpatient [28].
Assuming 83 acts of intercourse per year [5].
2.4.2. Cost of method failures
The costs of induced abortions and spontaneous abortions were calculated based on the assumption that 95% of the abortions are performed in the hospital [30]. The cost of hospital abortions was based on national average payments for the respective DRGs, and the cost of non-hospital abortions was based on the literature [31,32]. The average cost of a birth was obtained from the March of Dimes study [33] that reported the costs of prenatal, delivery-related and postpartum healthcare associated with having a baby in the privately insured population in the United States. The study used the 2003 – 2005 MarketScan® Commercial Claims and Encounters database, a medical and drug claims database of nearly 10 million individuals with employer-sponsored health insurance. All live-birth deliveries, including pre-term births, were included in this study. The study reported the average cost of having a baby (9-month pre and 3-month post) was $8,802 in 2004, of which $8,236 was paid by the health plan. We used the portion paid by the health plan for the purpose of this analysis (Table 2b).
Table 2b.
Cost of contraceptive method failure outcomes
| Method failure | Total average cost | Total average cost used in the model |
|---|---|---|
| Birth | $9,318 a | $4,048 b |
| Induced abortionc | $536.87 [29] | $536.87 [29] |
| Spontaneous abortiond | $536.87 [29] | $536.87 [29] |
| Ectopic pregnancy (DRG 378) | $10,613e | $10,613e |
Calculated based on the March of Dimes study [33] that reported $8,236 as health plan cost for pregnancy in 2004. Inflated using the medical component of CPI to 2007$.
Assuming 60% births are mistimed and would occur 2 years later, a 3% discount rate per year is applied. $9,318 × (1.0–0.60/(1.03)2).
Assuming 95% of abortions are performed in the hospital [30]. Cost of hospital abortions based on DRG 380 and 381. Cost of non-hospital abortions calculated from Henshaw [31] and CDC incidence rates [32].
Based on the same DRG codes and proportion of in-hospital abortions as induced abortion.
The cost of ectopic pregnancy was calculated based on the Medical Expenditure Panel Survey (MEPS) study, which reported the average expenditures for prenatal care, inpatient hospital delivery, and a combination of the two, for private and public payer [34] and the HCUP nationwide inpatient sample data [35,36]. An inflation factor (charges for an ectopic pregnancy divided by charges for a birth) was derived using information obtained from HCUP; this factor of 1.6 was then applied to the cost of inpatient delivery for a full-term birth obtained from MEPS ($5,027) [34] to estimate the cost of ectopic pregnancy. The estimates were inflated to 2007 costs using the medical component of the Consumer Price Index (CPI).
2.4.3. Cost of side effects
Costs of amenorrhea, urinary tract infections, and venous thromboembolism included the cost of office visit or inpatient stay and related care (Table 2c). Although amenorrhea is considered a positive change by many women [37], the fear of unwanted pregnancy raised by amenorrhea usually leads to a physician office visit. Costs of postoperative complications were included in the cost of surgical procedures and weighted by the incidence of the complication.
Table 2c.
Cost of contraceptive side effects
| Side effect | Cost |
|---|---|
| Amenorrheaa | $52.58 [25,26] |
| Urinary tract infectionb | $97.29 [25,26] |
| Venous thromboembolism (DRG 125) | $4,213.46 [29] |
| Postoperative complications of tubal ligation (DRG 452) | $5,209.72 [29] |
| Postoperative infections of vasectomy | $144 [14] |
Includes the cost of pregnancy test and office visit.
Includes the cost of ciprofloxacin 7-day course, office visit and lab tests.
2.5. Effectiveness
Effectiveness was defined as the estimated average annual probability of not becoming pregnant over a 5-year period, assuming typical use. Note that effectiveness is not simply the complement of the annual probability of contraceptive failure shown in Table 1a. It is always higher because women who give birth are not exposed to the risk of failure while they are pregnant.
2.6. Analyses
Costs and incremental cost-effectiveness ratios (ICERs, the cost of an additional percentage point in effectiveness) were estimated for all contraceptive methods. All costs were discounted at a rate of 3% per annum in the base case. All analyses were performed using TreeAge Pro 2006 (TreeAge Software, Williamstown, MA). One-way sensitivity analyses were conducted to identify parameters that had the greatest effect on the results. Parameters thought to have the most effect on the results of the model were tested with two-way sensitivity analyses.
3. Results
3.1. Base case analysis
Any contraceptive method is superior to no method in terms of costs and effectiveness. The average expected effectiveness ranged from 48% to nearly 100% (99.96%). The most effective methods were vasectomy (100%), the implant (100%), tubal ligation (99.8%), the LNG-20 IUS (99.8%) and the copper-T IUD (99.6%) (Table 3). Five-year costs ranged from $647 to $4,739. The three least expensive methods were the copper-T IUD ($647), vasectomy ($713) and the LNG-20 IUS ($930).
Table 3.
Cost effectiveness (C/E) of contraceptive methods at 5 years
| Method | Method-related costs ($) | Failure cost ($) | Cost of side effects ($) | Total cost (C) ($) | Marginal cost a ($) | Effectiveness b (E) | Marginal effectiveness a | C/E ($) | ICER ($) |
|---|---|---|---|---|---|---|---|---|---|
| Copper-T IUD | 605 | 42 | 0 | 647 | 99.6 | 6.50 | |||
| Vasectomy | 710 | 3 | 0 | 713 | 66 | 100 | 0.4 | 7.13 | 164 |
| LNG-20 IUS | 823 | 58 | 49 | 930 | 283 | 99.8 | 0.2 | 9.32 | 1415 |
| Male condom | 358 | 1217 | 0 | 1575 | 928 | 86.6 | −13 | 18.19 | (Dominated) c |
| Fertility-awareness-based methods | 0 | 1892 | 0 | 1892 | 1245 | 79.2 | −20.4 | 23.89 | (Dominated) c |
| Withdrawal | 0 | 2017 | 0 | 2017 | 1370 | 77.8 | −21.8 | 25.92 | (Dominated) c |
| Diaphragm | 764 | 1288 | 119 | 2171 | 1524 | 85.8 | −13.8 | 25.31 | (Dominated) c |
| Implant | 2142 | 5 | 31 | 2178 | 1531 | 100 | 0.4 | 21.78 | 3828 |
| Spermicides | 431 | 2104 | 112 | 2647 | 2000 | 76.6 | −23 | 34.55 | (Dominated) c |
| Female condom | 1043 | 1633 | 0 | 2676 | 2029 | 76.8 | −22.8 | 34.85 | (Dominated) c |
| Injectable contraceptive | 2341 | 300 | 40 | 2681 | 2034 | 97 | −2.6 | 27.64 | (Dominated) c |
| Sponge | 969 | 1829 | 0 | 2798 | 2151 | 79.8 | −19.8 | 35.06 | (Dominated) c |
| Tubal ligation | 2866 | 59 | 53 | 2978 | 2330 | 99.8 | 0.2 | 29.84 | (Dominated) c |
| Vaginal ring | 2467 | 683 | 8 | 3158 | 2511 | 92.4 | −7.2 | 34.18 | (Dominated) c |
| Oral contraceptive | 2630 | 682 | 69 | 3381 | 2734 | 92.4 | −7.2 | 36.59 | (Dominated) c |
| Transdermal patch | 2774 | 683 | 1 | 3458 | 2811 | 92.4 | −7.2 | 37.42 | (Dominated) c |
| No method | 0 | 4739 | 0 | 4739 | 4091 | 48 | −51.6 | 98.72 | (Dominated) c |
IUD, intrauterine device; IUS, intrauterine system; ICER, incremental cost-effectiveness ratio.
Compared to the least costly method over 5 years (i.e., copper-T IUD).
Average annual rate of not becoming pregnant over 5 years
Dominated means this contraceptive option cost more and was less effective than the reference contraceptive, in this case, copper-T IUD.
Results at 5 years show that costs of unintended pregnancies reflect the majority (>90%) of the total costs for contraceptive methods that have low effectiveness rates (no method, withdrawal, fertility-awareness-based methods, and the male condom). In contrast, for highly effective methods, such as tubal ligation, vasectomy, the implant, the copper-T IUD, and the LNG-20 IUS, the method or device cost represents the majority of the costs. The copper-T IUD dominated all other methods except the LNG-20 IUS, vasectomy, tubal ligation and the implant (Table 3). However, the implant and tubal ligation cost substantially more than the copper-T IUD, with an ICER of $3,828 and $11,652 per additional percentage point of effectiveness, respectively. Vasectomy and the LNG-20 IUS had better effectiveness than the copper-T IUD but cost only marginally more, with an ICER of $164 and $1,415 per additional percentage point of effectiveness, respectively.
3.2. Sensitivity analysis
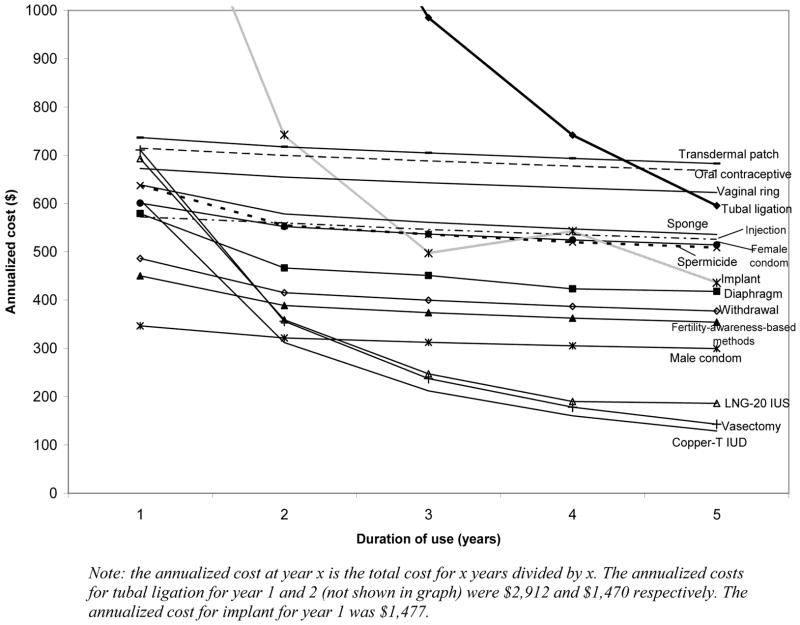
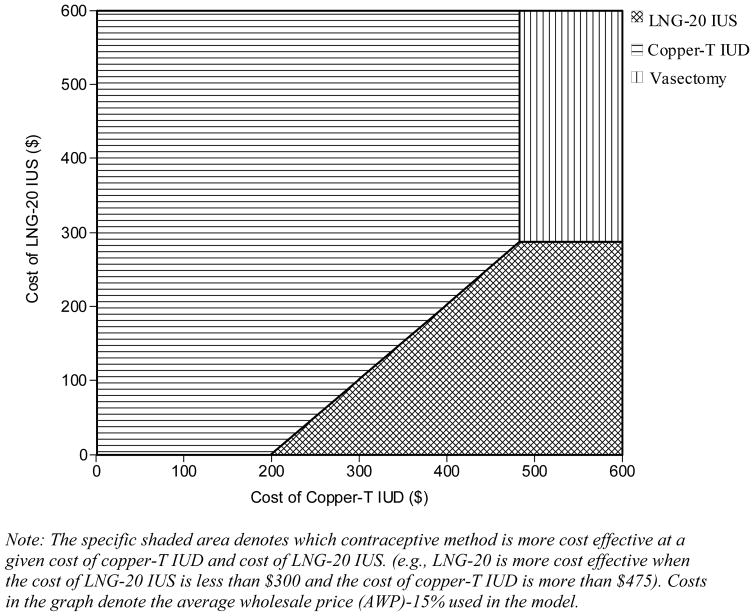
Results were sensitive to variations in costs of contraceptive methods and the cost of an unintended pregnancy. Moreover, with a longer time horizon, methods with high initial costs and high effectiveness (vasectomy, implant, tubal ligation, LNG-20 IUS and copper-T IUD) become more cost effective (Fig. 2). One- and two-way sensitivity analysis on method costs showed that the LNG-20 IUS dominated the copper-T IUD when the cost of LNG-20 IUS was approximately less than $300 (Fig. 3) and vasectomy was the dominant method when its cost was less than $640. However, the implant and tubal ligation, the other two highly effective methods, remained significantly more expensive than the copper-T IUD, even when the costs were decreased by 50%. Even when the failure rates for the highly effective methods (vasectomy, implant, tubal ligation, LNG-20 IUS and copper-T IUD) were varied by 20%, there was no change in the cost-effectiveness rankings.
Fig. 2. Annualized costs associated with contraceptive methods.
Note: The specific shaded area denotes which contraceptive method is more cost effective at a given cost of copper-T IUD and cost of LNG-20 IUS. (e.g., LNG-20 is more cost effective when the cost of LNG-20 IUS is less than $300 and the cost of copper-T IUD is more than $475). Costs in the graph denote the average wholesale price (AWP)-15% used in the model.
Fig. 3.
Two-way sensitivity analysis on cost of LNG-20 IUS and copper-T IUD
Among the costs of method failures, those for a birth and an ectopic pregnancy were the most sensitive in the model. When the cost of a birth was increased to $9,318, corresponding to a scenario in which all women want no more children, the ICER for vasectomy and the LNG-20 IUS compared to the copper-T IUD dropped to $78 and $1,270 per percent increase in effectiveness, respectively. The cost of no method rose to $9,493, twice as much as in the base model, and the cost savings from use of vasectomy, the LNG-20 IUS, and the copper-T IUD compared with use of no method rose by approximately 100%. When the cost of ectopic pregnancy is decreased, the cost of the LNG-20 IUS drops relative to the cost of the copper-T IUD. In general, when the cost of an unintended pregnancy is increased, contraceptive methods with high effectiveness rates become more cost effective.
Increasing the plan disenrollment rates from 0% to 20% improved the ICERs for methods with low method costs. The largest changes were for the male condom, the female condom, the vaginal ring, the transdermal patch, spermicides and the sponge. However, even when the plan disenrollment rates were increased to 20%, the copper-T IUD remained the most cost effective, followed by vasectomy and the LNG-20 IUS.
The cost and effectiveness for the copper-T IUD, LNG-20 IUS, and vasectomy did not change when estimates of method failure during perfect use (Table 1) were applied to the model. In contrast, the cost effectiveness of barrier methods (the diaphragm, the sponge, spermicides, and the male and female condoms) improved with perfect use. Fertility-awareness-based methods became the least costly, followed by withdrawal and the male condom.
Chance (no method) remained the least cost-effective method in all scenarios except when the cost of a birth was lower than $2,400, in which case the transdermal patch became the least cost-effective contraceptive option. Since the costs for a public payer are different (often lower) than for a private payer, we ran the model using the costs of an unintended pregnancy for public payers reported by the MEPS study and applied a deeper discount (AWP-25%) for the methods. The results were similar to that of the base case, private payer model, demonstrating that our results are robust. The remaining sensitivity analyses (changes in the costs of spontaneous and induced abortion and the costs of other contraceptive methods) did not result in any change in the cost-effectiveness rankings.
4. Discussion
Using a 5-year Markov model, we examined the cost effectiveness of contraceptives (including chance, or no method) in the United States from a health care payer’s perspective. The findings from this study highlighted the fact that all contraceptive methods were more cost effective when compared to no method. Results indicated that long-term contraceptives prevent unintended pregnancies without relying on user compliance while providing excellent value for money. The three least expensive methods were the copper-T IUD, vasectomy, and the LNG-20 IUS, and the most effective methods (≥99.6% effectiveness) were vasectomy, the implant, tubal ligation, the LNG-20 IUS and the copper-T IUD.
The current results were sensitive to variations in costs of contraceptive methods, the cost of an unintended pregnancy, and time horizon. Because of the similarity in effectiveness among the copper-T IUD, vasectomy and the LNG-20 IUS, the results were very cost-sensitive, particularly between IUD and IUS devices. Only a small change in costs alters the cost-effectiveness rankings, and for all practical purposes, the IUD and IUS could be considered to have equivalent cost effectiveness. These results are likely to vary across health plans depending on the negotiated discount rates.
Our analysis updated and expanded a previously published cost-effectiveness analysis [4] to include no contraceptive method, male contraceptive methods, and new market entrants such as implants, along with cost updates. Results of our previous analysis [4] indicated that the LNG-20 IUS and the copper-T IUD cost less and are more effective than all other methods except tubal ligation. This analysis found similar results where, along with vasectomy, intrauterine contraceptive methods had better cost-effectiveness profiles than most other methods. Similarly, earlier analyses by Trussell et al. [14,38], which also included male contraceptives, found that vasectomy, the copper-T IUD, and the contraceptive implant were the most cost effective. Recently, the U.K. National Institute for Clinical Excellence conducted a cost-effectiveness analysis [39,40] that found that despite high initiation costs, the implant, IUD, and IUS were more cost effective than the injectable or combined oral contraceptive pill. Varney et al. [41] and Phillips [42] also evaluated the cost effectiveness of long-term reversible contraceptive methods and reported that the LNG-20 IUS and implant were cost-effective strategies, respectively.
This analysis has several limitations. In our model we assumed that individuals use the same contraceptive method throughout the 5-year period, and switching between methods was not allowed even when failure occurs. In reality, given their changing preferences and situations, individuals do switch between different methods. There are, however, no nationally representative data from which to estimate probabilities of switching among all methods. Moreover, allowing switches precludes a pure comparison of different contraceptive methods. If all switches are assigned an average cost of a mix of contraceptive methods, as in our prior model [4], then the costs of the different methods will converge over time.
Another limitation relates to the model time horizon, which was restricted to 5 years. With a longer time horizon, the copper-T IUD, vasectomy, and tubal ligation become more cost effective, because they provide longer-term contraceptive protection at no additional cost. We chose a 5-year time horizon, as short time horizons are most relevant from a payer’s perspective and few women would use most methods beyond that horizon [43,44]. Estimates of cost savings for shorter time horizons are shown in Fig. 2. Although our analysis compared reversible methods with non-reversible contraception (female and male sterilization), sterilization is not a realistic alternative until the desired family size has been reached.
At the same time, it should be noted that the model did not account for certain costs incurred by women with tubal ligation, including reversal costs for those desiring pregnancy. Non-contraceptive beneficial effects and associated cost savings (e.g., the reduction in need for surgical treatment of menorrhagia following IUS use [45] and the protective role of condoms against sexually transmitted infections) were not considered. In the case of LNG-20 IUS, including such a beneficial effect might substantially improve the method’s relative cost effectiveness compared with other methods. In contrast, inclusion of sexually transmitted infections, including HIV, has been shown to have only a minimal effect on cost savings associated with condom use [14,38]. Additionally, the study excluded any indirect costs associated with changes in emotional and psychological well being due to unintended pregnancy.
The strength of this analysis is that the cost of a birth was reduced to account for the fact that most births are mistimed. Preventing a mistimed birth today does not avoid its cost altogether but simply postpones that cost until a later time. Failure to recognize this distinction is common [46]. Estimates of the cost effectiveness of contraceptives that assume that all births avoided today are avoided forever seriously overstate cost savings from contraceptive use. In our analysis, if we had counted the full cost of a birth, we would have overestimated the cost of no method and the cost savings from use of the most effective methods by about 100%.
In conclusion, our study showed that using any contraceptive method is a cost-effective strategy. The copper-T IUD, vasectomy and the LNG-20 IUS are the most cost-effective methods currently available in the United States. Differences in method costs, the cost of an unintended pregnancy, and time horizon are important factors that affect the cost effectiveness of contraceptive methods.
Acknowledgments
This study was supported by a research grant from Bayer HealthCare Pharmaceuticals, Wayne, NJ, USA.
Footnotes
Consider the following simple birth-interval model for women aged 15–44 using withdrawal. An annual probability of failure of 0.27 during typical use implies an average monthly probability of 0.0259 (=1.0-[1.0–0.27]1/12). Assuming this probability is constant over time, the average waiting time to conception while using spermicides is 38.61 months (=1/0.0259). Then, ignoring ectopic pregnancies, spontaneous abortions, and stillbirths and assuming that every other pregnancy ends in induced abortion, the waiting time to a conception leading to a live birth would be 81.2 months: 38.6 months to get pregnant the first time, 3 months’ gestation until the abortion, 1 month of postpartum non-susceptibility following the abortion, and 38.6 months to get pregnant again. The entire interval from one birth to the next would be 92.2 months: 2 months of postpartum non-susceptibility following the birth (assuming minimal breastfeeding), 81.2 months of waiting time to the next live-birth conception, and 9 months for gestation. Hence, a birth occurs every 92.2 months or 7.68 years, so the birth rate per year is 0.130 (=1/7.68). Because there are two pregnancies for each birth, the pregnancy rate per year is 0.26 (=2×0.130), which is very close to the annual probability of failure of 0.27. Differences are even smaller for more effective methods. For use of no method, the pregnancy rate is 0.84 versus an annual probability of failure of 0.85.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38:90–6. doi: 10.1363/psrh.38.090.06. [DOI] [PubMed] [Google Scholar]
- 2.Trussell J. The cost of unintended pregnancy in the United States. Contraception. 2007;75:168–70. doi: 10.1016/j.contraception.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004:1–36. [PubMed] [Google Scholar]
- 4.Chiou CF, Trussell J, Reyes E, et al. Economic analysis of contraceptives for women. Contraception. 2003;68:3–10. doi: 10.1016/s0010-7824(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 5.Trussell J, Koenig J, Ellertson C, Stewart F. Preventing unintended pregnancy: the cost-effectiveness of three methods of emergency contraception. Am J Public Health. 1997;87:932–7. doi: 10.2105/ajph.87.6.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra A, Martinez GM, Mosher WD, Abma JC, Jones J. Fertility, family planning, and reproductive health of U.S. women: data from the 2002 National Survey of Family Growth. Vital Health Stat. 2005;23:1–160. [PubMed] [Google Scholar]
- 7.Hatcher RA, Trussell J, Nelson AL, Cates W, Stewart FH, Kowal D. Contraceptive Technology, nineteenth revised edition. New York NY: Ardent Media; 2007. [Google Scholar]
- 8.Backman T, Rauramo I, Huhtala S, Koskenvuo M. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50–4. doi: 10.1016/j.ajog.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Backman T, Huhtala S, Blom T, Luoto R, Rauramo I, Koskenvuo M. Length of use and symptoms associated with premature removal of the levonorgestrel intrauterine system: a nation-wide study of 17,360 users. BJOG. 2000;107:335–9. doi: 10.1111/j.1471-0528.2000.tb13228.x. [DOI] [PubMed] [Google Scholar]
- 10.Westrom L, Bengtsson LP, Mardh PA. Incidence, trends, and risks of ectopic pregnancy in a population of women. Br Med J (Clin Res Ed) 1981;282:15–8. doi: 10.1136/bmj.282.6257.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croxatto HB, Makarainen L. The pharmacodynamics and efficacy of Implanon. An overview of the data. Contraception. 1998;58:91S–97S. doi: 10.1016/s0010-7824(98)00118-8. [DOI] [PubMed] [Google Scholar]
- 12.Peterson HB, Xia Z, Hughes JM, Wilcox LS, Tylor LR, Trussell J. The risk of ectopic pregnancy after tubal sterilization. U.S. Collaborative Review of Sterilization Working Group. N Engl J Med. 1997;336:762–7. doi: 10.1056/NEJM199703133361104. [DOI] [PubMed] [Google Scholar]
- 13.Lahteenmaki P, Rauramo I, Backman T. The levonorgestrel intrauterine system in contraception. Steroids. 2000;65:693–7. doi: 10.1016/s0039-128x(00)00176-8. [DOI] [PubMed] [Google Scholar]
- 14.Trussell J, Leveque JA, Koenig JD, et al. The economic value of contraception: a comparison of 15 methods. Am J Public Health. 1995;85:494–503. doi: 10.2105/ajph.85.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Endrikat J, Dusterberg B, Ruebig A, Gerlinger C, Strowitzki T. Comparison of efficacy, cycle control, and tolerability of two low-dose oral contraceptives in a multicenter clinical study. Contraception. 1999;60:269–74. doi: 10.1016/s0010-7824(99)00097-9. [DOI] [PubMed] [Google Scholar]
- 16.Jick SS, Kaye JA, Russmann S, Jick H. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 2006;73:223–8. doi: 10.1016/j.contraception.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Fihn SD, Latham RH, Roberts P, Running K, Stamm WE. Association between diaphragm use and urinary tract infection. JAMA. 1985;254:240–5. [PubMed] [Google Scholar]
- 18.Audet MC, Moreau M, Koltun WD, et al. Evaluation of contraceptive efficacy and cycle control of a transdermal contraceptive patch vs an oral contraceptive: a randomized controlled trial. JAMA. 2001;285:2347–54. doi: 10.1001/jama.285.18.2347. [DOI] [PubMed] [Google Scholar]
- 19.Barreiros FA, Guazzelli CA, de Araujo FF, Barbosa R. Bleeding patterns of women using extended regimens of the contraceptive vaginal ring. Contraception. 2007;75:204–8. doi: 10.1016/j.contraception.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Hooton TM, Scholes D, Hughes JP, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. N Engl J Med. 1996;335:468–74. doi: 10.1056/NEJM199608153350703. [DOI] [PubMed] [Google Scholar]
- 21.Fihn SD, Boyko EJ, Normand EH, et al. Association between use of spermicide-coated condoms and Escherichia coli urinary tract infection in young women. Am J Epidemiol. 1996;144:512–20. doi: 10.1093/oxfordjournals.aje.a008958. [DOI] [PubMed] [Google Scholar]
- 22.Drug Topics Red Book. Montvale, NJ: Thomson Healthcare; 2007. [Google Scholar]
- 23.Cerner Multum™. Lexicon: 2007. [Google Scholar]
- 24.Wolters Kluwer Health. [Accessed October 2007];Price Rx Drug Price and Reference Tool (Medi-Span Master Drug Database) Available at http://www.MediSpan.com.
- 25. [Accessed March 2008];Pharmacy directory: drug prices. Available at http://www.drugstore.com.
- 26.Current procedural terminology (CPT) Chicago, IL: American Medical Association; 2007. [Google Scholar]
- 27.Marquette CM, Koonin LM, Antarsh L, Gargiullo PM, Smith JC. Vasectomy in the United States, 1991. Am J Public Health. 1995;85:644–9. doi: 10.2105/ajph.85.5.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacKay AP, Kieke BA, Jr, Koonin LM, Beattie K. Tubal sterilization in the United States, 1994–1996. Fam Plann Perspect. 2001;33:161–5. [PubMed] [Google Scholar]
- 29.Ingenix DRG Expert. Eden Prairie, MN: Ingenix; 2007. [Google Scholar]
- 30.Finer LB, Henshaw SK. Abortion incidence and services in the United States in 2000. Perspect Sex Reprod Health. 2003;35:6–15. doi: 10.1363/3500603. [DOI] [PubMed] [Google Scholar]
- 31.Henshaw SK, Finer LB. The accessibility of abortion services in the United States, 2001. Perspect Sex Reprod Health. 2003;35:16–24. doi: 10.1363/3501603. [DOI] [PubMed] [Google Scholar]
- 32.Abortion Surveillance - United States. Morbidity and Mortality Weekly Report. SS-11. Vol. 55. Centers for Disease Control and Prevention; 2003. [Accessed March 2008]. Surveillance Summaries. Available at http://www.cdc.gov/mmwr/PDF/ss/ss5511.pdf. [PubMed] [Google Scholar]
- 33.March of Dimes. [Accessed June 2008];The healthcare costs of having a baby. Available at http://www.marchofdimes.com/aboutus/14817_25927.asp.
- 34.Machlin SR, Rohde F. Health Care Expenses for Uncomplicated Pregnancies. [Accessed March 2008];Research Findings No 27. Available at http://www.meps.ahrq.gov/mepsweb/data_files/publications/rf27/rf27.pdf.
- 35.Merrill C, Steiner C. HCUP Statistical Brief #11. Agency for Healthcare Research and Quality; Rockville, MD: [Accessed November 2007]. Hospitalizations Related to Childbirth, 2003. Available at www.hcup-us.ahrq.gov/reports/statbriefs/sb11.pdf. [PubMed] [Google Scholar]
- 36.Agency for Healthcare Research and Quality. [Accessed June 2008];Healthcare Cost and Utilization Project (HCUP) Available at http://hcupnet.ahrq.gov/ [PubMed]
- 37.Baldaszti E, Wimmer-Puchinger B, Loschke K. Acceptability of the long-term contraceptive levonorgestrel-releasing intrauterine system (Mirena): a 3-year follow-up study. Contraception. 2003;67:87–91. doi: 10.1016/s0010-7824(02)00482-1. [DOI] [PubMed] [Google Scholar]
- 38.Trussell J, Koenig J, Stewart F, Darroch JE. Medical care cost savings from adolescent contraceptive use. Fam Plann Perspect. 1997;29:248–55. 295. [PubMed] [Google Scholar]
- 39.National Collaborating Centre for Women & Children’s Health. Long-acting reversible contraception: the effective and appropriate use of long-acting reversible contraception. London (UK): Royal College of Obstetricians and Gynecologists (RCOG); 2005. [PubMed] [Google Scholar]
- 40.Mavranezouli I. The cost-effectiveness of long-acting reversible contraceptive methods in the UK: Analysis based on a decision-analytic model developed for a National Institute for Health and Clinical Excellence (NICE) clinical practice guideline. Hum Reproduc. 2008;23:1338–45. doi: 10.1093/humrep/den091. [DOI] [PubMed] [Google Scholar]
- 41.Varney SJ, Guest JF. Relative cost effectiveness of Depo-Provera, Implanon, and Mirena in reversible long-term hormonal contraception in the UK. Pharmacoeconomics. 2004;22:1141–51. doi: 10.2165/00019053-200422170-00004. [DOI] [PubMed] [Google Scholar]
- 42.Phillips CJ. Economic analysis of long-term reversible contraceptives. Focus on Implanon Pharmacoeconomics. 2000;17:209–21. doi: 10.2165/00019053-200017020-00009. [DOI] [PubMed] [Google Scholar]
- 43.Trussell J, Vaughan B. Contraceptive failure, method-related discontinuation and resumption of use: results from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:64–72. 93. [PubMed] [Google Scholar]
- 44.Vaughan B, Trussell J, Kosta K, Singh S, Jones R. Discontinuation and resumption of contraceptive use: results from the 2002 National Survey of Family Growth. Contraception. 2008 doi: 10.1016/j.contraception.2008.05.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao B, Wu SC, Chong J, Zeng T, Han LH, Luukkainen T. Therapeutic effects of the levonorgestrel-releasing intrauterine system in the treatment of idiopathic menorrhagia. Fertil Steril. 2003;79:963–9. doi: 10.1016/s0015-0282(02)04913-0. [DOI] [PubMed] [Google Scholar]
- 46.Trussell J. Overstating cost savings from contraceptive use. Eur J Contraception Reprod Health Care. 2008 doi: 10.1080/13625180802359263. in press. [DOI] [PubMed] [Google Scholar]