Abstract
The Sac (saccharin preference) locus affecting mouse behavioral and neural responsiveness to sweeteners has been mapped to distal Chr 4. A putative sweet taste receptor, T1R1, has been recently cloned, and the gene encoding it, Gpr70, has also been mapped to mouse distal Chr 4. To assess Gpr70 as a candidate gene for Sac, we compared the Gpr70 sequences of C57BL/6ByJ and 129P3/J mouse strains with different alleles of Sac. Using Gpr70 sequence variation between the C57BL/6ByJ and 129P3/J strains, we conducted a high-resolution analysis of the chromosomal localization of the Gpr70 and Sac loci in the F2 hybrids and 129.B6-Sac partially congenic mice originating from these two strains. The Gpr70 gene maps proximal to Sac, which demonstrates that they are different loci.
Introduction
Sugars, and a number of other chemicals, evoke the sensation of sweetness in humans and are palatable to many other animals. Sweetness perception is initiated in taste receptor cells in taste buds of the oral cavity, and presumably involves the interaction of sweet-tasting compounds with a sweet taste receptor or receptors (DuBois 1995).
The Sac (saccharin preference) locus was initially identified on the basis of behavioral acceptance tests with saccharin and some other sweeteners in C57BL/6 and DBA/2 mice, their hybrids, and recombinant-inbred strains (Fuller 1974; Lush 1989; Phillips et al. 1994; Lush et al. 1995; Blizard et al. 1999). Our studies demonstrate that Sac influences both behavioral acceptance and the afferent responses of gustatory nerves to sweeteners (Bachmanov et al. 1997). The latter finding strongly suggests that Sac determines peripheral sweet taste perception and thus may code for a sweet taste receptor. Recently,Hoon et al. (1999) have identified a putative sweet taste receptor, TR1 [later named T1R1 (Adler et al. 2000); the gene symbol for the T1R1 protein is Gpr70]. This protein has a deduced amino acid sequence representative of a seven-transmembrane domain G protein-coupled receptor. Its role in sweet taste reception was suggested based in part on its expression in the apical ends of a subset of taste receptor cells. The other potential evidence implicating T1R1 in sweetness detection involves co-localization of the Gpr70 and Sac loci on mouse distal Chr 4 (Hoon et al. 1999).
To assess Gpr70 as a candidate gene for Sac, we compared T1R1 cDNA sequences expressed in the tongue tissues of C57BL/6ByJ (B6) and 129P3/J (129) mouse strains. These two strains have different alleles of Sac, with the B6 allele determining higher behavioral and neural taste responses to sweeteners (Bachmanov et al. 1997). Next, using Gpr70 sequence variation between the B6 and 129 strains and our genetic mapping panels originating from these two strains, we have conducted a high-resolution linkage analysis of the chromosomal locations of Gpr70 and Sac.
Materials and methods
Animals
C57BL6/ByJ (B6) and 129P3/J (129) mice were purchased from The Jackson Laboratory (Bar Harbor, Me.). The B6 and 129 mice were outcrossed to produce the first filial generation of hybrids (F1), and these were intercrossed to produce the second hybrid generation (F2, n = 629). The F2 mice were genotyped with several markers on Chr 4 (D4Mit264, D4Mit4, D4Mit7, D4Mit58, D4Mit33, D4Mit190, D4Mit42, D4Mit254, D4Mit209, D4Mit256, and D18346). Three F2 mice with distal ends of Chr 4 from the B6 strain and with recombinations between D4Mit254 and D4Mit209, D4Mit209 and D4Mit256, or between D4Mit256 and D18346 were used as founders of strains congenic with the 129 strain. These F2 founders were backcrossed to the 129 strain to produce the N2 generation. Mice from this and subsequent backcross generations were phenotyped by using 96-h two-bottle tests with saccharin solutions, and genotyped by using markers on distal Chr 4 (D4Mt209, D4Mit256, and D18346) and on other autosomes. The markers unlinked to distal Chr 4 were chosen for genotyping the backcross mice based on results of a genome screen of the F2 mice, including the congenic founders. We tested markers that had at least one B6 allele in at least one founder mouse: D1Mit15, D1Mit21, D2Mit9, D3Mit86, D4Mit4, D5Mit1, D6Mit9, D8Mit29, D11Mit4, D11Mit21, D12Mit34, D14Mit11, D17Mit10, and D18Mit23. Mice with high saccharin intake, carrying a fragment of distal Chr 4 from the B6 strain, and with the highest homozygosity for 129 alleles of markers on other chromosomes, were selected for subsequent backcrossing. This marker-assisted selection produced three segregating 129.B6-Sac partially congenic substrains. Mice from the N2 backcross generation (n = 11) were used in electrophysiological experiments, and mice from the N4 backcross generation (n = 178) were used to genotype Gpr70.
Behavioral testing
Consumption of 120 mm sucrose and 17 mm saccharin (Sigma Chemical Company, St. Louis, Mo.) was measured in individually caged mice by using 96-h two-bottle tests, with water as the second choice (see details in Bachmanov et al. 1996). The positions of the tubes were switched every 24 h. Fluid intakes were expressed per 30 g of body weight (BW, the approximate weight of an adult mouse) per day, or as a preference score (ratio of average daily solution intake to total fluid intake, in percent).
Electrophysiological recording
In mice anesthetized with an intraperitoneal injection of sodium pentobarbital (40–50 mg/kg BW), the right chorda tympani nerve was dissected at the point of its entry to the bulla and placed on an electrode (see details in Bachmanov et al. 1997, 1999). Test solutions were prepared in deionized water and flowed over the anterior part of the tongue at a flow rate of 0.5 ml/s. Integrated whole-nerve responses to chemical stimulation of the tongue were recorded. The magnitude of the integrated response at 15 s after stimulus onset was measured and expressed relative to the response to 0.1 m NH4Cl.
Genotyping
Genomic DNA was purified from mouse tails by using the NaOH/Tris method (Truett et al. 2000). PCR conditions for amplification of genomic DNA were: 95°C for 5 min; 2 cycles of 94°C for 1 min, 55°C for 2 min, and 72°C for 2 min; 34 cycles of 94°C for 30 s, 55°C for 45 s, and 72°C for 30 s; 72°C for 7 min, and a 4°C hold. SSLP (microsatellite) polymorphisms were tested by using PAGE as described earlier (Bachmanov et al. 1997). The D18346 sequence tag site (STS) marker was identified from YAC contig (singly-linked WC4.59, or doubly-linked WC-829), amplified by using primers 5′-TGTCCGCAGTGTGGAAACTA-3′ (forward), 5′-ATGTCCAGGGTAGAGAGCCC-3′ (reverse), and the polymorphic bands were separated by using SSCP analysis (Orita et al. 1989).
To compare T1R1 cDNA sequences of the B6 and 129 mice, we extracted total RNA from the anterior parts of their tongues, using TRIZOL Reagent (Life Technologies, Rockville, Md.). Total RNA (200 ng) was reverse transcribed by using the Life Technologies SuperScript Kit. Following the reverse transcription, the samples were amplified by using Advantage® cDNA PCR Kit (Clontech, Palo Alto, Calif.) and primers 5 ′-CACGGGAAGAACAATCAGGT-3′ (forward), 5 ′-GTGGGCACCTTGGTAGAAAA-3′ (reverse) based on rat T1R1 cDNA (Hoon et al. 1999), GenBank Accession ID AF127389. The PCR parameters were: 94°C for 3 min; 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 2 min; 72°C for 10 min, and a 4°C hold. PCR products were separated on 2% agarose gels (FMC Bioproducts, Rockland, Me.) containing 0.5 mg/ml ethidium bromide, visualized by UV transillumination, and subcloned by using pGEM-T Easy Vector Systems (Promega, Madison, Wis.). The RT-PCR products and the clones with expected sizes (548 bp) were sequenced at the Genetics Core Facility of the University of Pennsylvania. The nucleotide sequences were translated into amino acid sequences by using Gene Runner software. The nucleotide and amino acid sequences were aligned by using the ClustalW 1.7 method (http://dot.imgen.bcm.tmc.edu:9331/multi-align/multi-align.html). BLAST search of the GenBank and aligning the B6 and 129 cDNA and protein sequences against the rat cDNA or protein (GenBank ID AF127389) or mouse protein (Hoon et al. 1999) sequences have confirmed that the cloned product is a fragment of mouse T1R1 cDNA. Fragments of Gpr70 genomic DNA encoding T1R1 were amplified by using primers flanking regions with sequence variation between B6 and 129 strains, and then they were separated with SSCP. Four pairs of primers were tested in the B6 and 129 mice, and in a subset of the F2 mice (n = 81): (i) 5′-GTCTTCACACGCCTGTTGTG-3′ (forward), 5′-CTGTAGAGGCTGCAACTCCC-3′ (reverse), product size 91 bp; (ii) 5′-CTGGGAGTTGCAGCCTCTAC-3′ (forward), 5′-ACCCGAGAGAAAAGAGGG-3′ (reverse), 86 bp; (iii) 5′-GGAGTTGCAGCCTCTACAGC-3′ (forward), 5′-CAGGAGAGGAAAATGGCAAA-3′ (reverse), 100 bp; (iv) 5′-GGAGTTGCAGCCTCTACAGC-3′ (forward), 5′-AAACCCGAGAGAAAAGAGGG-3′ (reverse), 83 bp. As a control, we used cDNA template reverse-transcribed from lingual RNA of the B6 and 129 mice. Product sizes for genomic and cDNA were identical, indicating that the amplified fragments reside within exons. Individual genotypes of the F2 mice were identical for all four primer pairs, and thus the rest of the F2 mice and the 129.B6-Sac partially congenic mice were genotyped only with the first pair of primers.
Interval mapping was conducted with MAPMAKER software (Lander et al. 1987). Thresholds for significant linkage were estimated for 2 degrees of freedom, assuming that both additive and dominant components were estimated in the intercross (Lander and Kruglyak 1995). Chromosomal positions of microsatellite markers are given in cM from the beginning of a linkage group on the MIT map (http://www-genome.wi.mit.edu).
Results
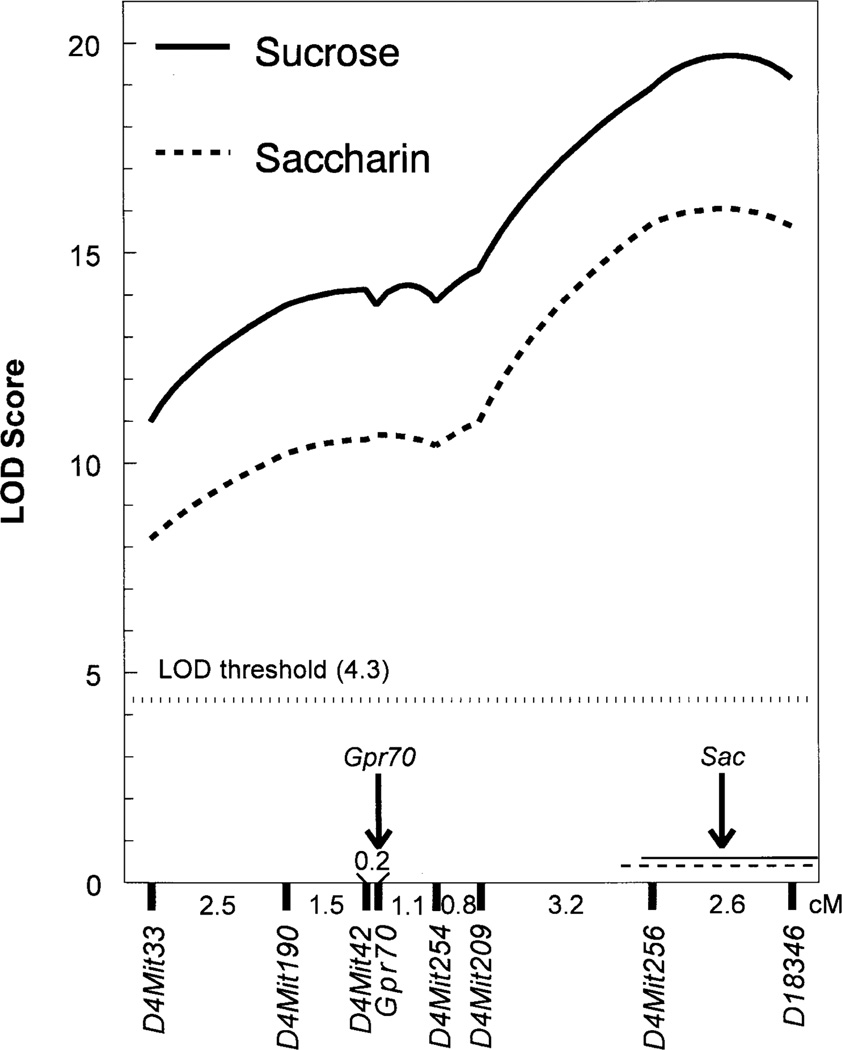
Using a pair of oligonucleotide primers designed to anneal to rat T1R1 cDNA, we amplified a 548-bp fragment of T1R1 from B6 and 129 mouse lingual tissues cDNAs (Fig. 1). This fragment had six single nucleotide substitutions between these two strains, and it was 92.7% (B6) or 92.5% (129) identical to a corresponding part of the rat T1R1 cDNA. A 91-bp fragment of mouse Gpr70 gene (corresponding to the sequenced part of T1R1 cDNA), including two single nucleotide polymorphisms between the B6 and 129 strains, was amplified from genomic DNA and used for genotyping. In the F2 intercross, Gpr70 was mapped 0.2 cM distal to D4Mit42, within a D4Mit42–D4Mit254 interval (76.5–80.9 cM, MIT map; Fig. 2).
Fig. 1.
ClustalW alignment of partial-length T1R1 cDNAs from the 129 and B6 mice (GenBank accession numbers AF301162 and AF301161). The nucleotide substitutions between the 129 and B6 strains are marked by bold letters and arrows. Horizontal lines indicate primers used for amplification of genomic DNA for genotyping.
Fig. 2.
Genetic map of mouse distal Chr 4. Genetic distances between markers were estimated by using MAPMAKER/EXP based on data from the B6 × 129 F2 intercross (n = 629). Curves show LOD scores for 120 mm sucrose and 17 mm saccharin intakes/30 g BW estimated under the free model. Peak LOD scores indicating the most likely loci positions are shown by the arrow and are located 1.3 cM distal to D4Mit256 for both sucrose (LOD 19.7) and saccharin (LOD 16.0) intakes. These loci explained 14.0% and 15.4% of phenotypical variance of sucrose and saccharin intakes respectively. The confidence intervals (LOD drops of 1.0 shown by the horizontal lines) extend 0.2 cM (sucrose) or 0.6 cM (saccharin) proximally to D4Mit256, and distally beyond D18346 (the distal boundary could not be estimated in cM because of the absence of markers distal to D18346). The B6 allele was dominant over the 129 allele because the peak LOD scores estimated under the free model were within 1 LOD unit from those estimated under B6 dominant model, whereas they were more than 1 LOD unit higher than those estimated under additive or B6 recessive models (not shown). Analysis of preference scores showed similar results (not shown). Gpr70 maps 0.2 cM distal to D4Mit42 (shown by the arrow).
Previously, we mapped the Sac locus to D4Mit42 (76.5 cM from the beginning of the linkage group, MIT map; Bachmanov et al. 1997). In this study, using a larger number of the B6 × 129 F2 hybrids and several additional polymorphic markers, we mapped Sac to a shorter and more distal fragment of Chr 4. From the analysis of the B6 × 129 F2 intercross, the most likely position of Sac is between D4Mit256 (82.0 cM, MIT map) and D18346, with a 1 LOD confidence interval ending proximally between D4Mit209 and D4Mit256 (Fig. 2). Thus, Gpr70 is located outside of the Sac confidence interval.
Haplotype analysis of mice from the 129.B6-Sac partially congenic strains (Fig. 3) provided further evidence that the Sac and Gpr70 loci have distinct chromosomal locations. The Sac locus must be included in the part of the B6 donor fragments of distal Chr 4 that overlaps among the three congenic substrains. This overlapping part ends proximally between D4Mit256 and D18346 (Fig. 3), which proves that Sac is positioned distal to D4Mit256. Mice from all congenic substrains were homozygous for the 129 allele of Gpr70. Thus, Gpr70 is located outside of the Sac-containing donor fragment of distal Chr 4 from the B6 strain.
Fig. 3.
Average daily saccharin solution consumption by segregating 129.B6-Sac partially congenic (N4) mice in 96-h two-bottle tests (Means ± Standard Errors). Haplotypes of three congenic substrains are shown at the bottom of the figure. Each substrain carries a B6 donor chromosome fragment of different length, with recombinations between D4Mit254 and D4Mit209, D4Mit209 and D4Mit256, or between D4Mit256 and D18346. The partially congenic mice with one copy of the Sac-containing chromosome fragment from the B6 strain (n = 81, filled bar) had higher saccharin intakes than did the mice homozygous for the 129 Sac allele [n = 97, open bar; F(1,166) = 75.5, p < 0.00001, 3-way ANOVA]. The saccharin intakes by the Sac-heterozygous (B6/129) and homozygous (129/129) mice from each of the three substrains were respectively 12.6 ± 0.7 (n = 39) and 7.3 ± 0.2 (n = 62) ml/30 g BW (the substrain with the shortest donor fragment); 11.7 ± 0.9 (n = 26) and 7.9 ± 0.5 (n = 24) ml/30 g BW (the substrain with the intermediate donor fragment); and 10.7 ± 0.7 (n = 16) and 6.6 ± 0.4 (n = 11) ml/30 g BW (the substrain with the longest donor fragment). The differences among the substrains were non-significant [F(2,166) = 2.0, p = 0.14]. Females had higher intakes than did males [F(1,166) = 9.6, p = 0.002], but the effect of genotype was similar within each gender [gender × genotype interaction, F(1,166) = 0.4, p = 0.54]. Analysis of preference scores showed similar results (not shown). Because the overlapping part of the donor chromosome fragments in the three substrains ends proximally between D4Mit256 and D18346, Sac must be located distal to D4Mit256. Because neither 129.B6-Sac substrain carries the B6 allele of Gpr70, it is located outside of the Sac-containing donor fragment and proximally to D4Mit209 (the marker included in the largest donor fragment).
Previously, we found that Sac affects gustatory afferent input by using recording of the chorda tympani nerve responses of the B6 × 129 F2 hybrids to lingual application of sucrose (Bachmanov et al. 1997). In this study, integrated chorda tympani electrical activity was recorded in the B6, 129, and 129.B6-Sac N2 mice in response to lingual application of an artificial sweetener, saccharin (Fig. 4). Compared with the 129 mice, the B6 mice had larger neural responses to saccharin (Fig. 4a). The 129.B6-Sac N2 mice with one copy of the distal Chr 4 from the B6 strain had larger neural responses than did the 129.B6-Sac N2 mice with both copies from the 129 strain (Fig. 4b). The Sac-containing donor fragments of these mice did not extend proximally beyond D4Mit254, and thus did not include Gpr70. This experiment has confirmed that neural as well as behavioral taste responses to sweeteners are influenced by the Sac genotype, but they do not depend on Gpr70.
Fig. 4.
The chorda tympani responses to lingual application of saccharin (relative to 0.1 m NH4Cl; Means ± Standard Errors). Asterisks indicate significant differences between genotypes (p < 0.05, t-tests). (a) 129 (n = 5–6) and B6 (n = 5) male mice. (b) 129.B6-Sac partially congenic (N2) mice. 129/129—mice homozygous for the 129 Sac allele (n = 4; 2 males and 2 females); B6/129—mice with one copy of the Sac-containing chromosome fragment from the B6 strain (n = 7; 3 males and 4 females). The presence of the Sac-containing donor fragments from the B6 strain was determined based on genotypes at D4Mit209 and D4Mit256. In the mice used in this experiment, the donor chromosome fragments ended proximally between D4Mit254 and D4Mit209, or between D4Mit209 and D4Mit256, and thus did not include Gpr70.
Discussion
The goal of this study was to evaluate the Gpr70 gene encoding a putative sweet taste receptor T1R1 as a candidate for the Sac locus. Consistent with this hypothesis, the B6 and 129 mouse strains with different alleles of Sac had variable Gpr70 sequences. Using these polymorphisms, we mapped Gpr70 in a segregating cross between these two strains. The linkage analysis confirmed that Gpr70 is located on distal Chr 4, as was initially reported (Hoon et al. 1999), but it maps proximal to Sac. Marker-assisted selection of the 129.B6-Sac congenic mice further demonstrated that Sac affects behavioral and neural taste responses to saccharin, but the Sac-containing donor chromosomal fragment from the B6 strain does not include Gpr70. We therefore conclude that Sac and Gpr70 are different loci.
Based on predominant expression of T1R1 in fungiform papillae, and chromosomal co-localization of the Gpr70 gene encoding T1R1 and the Sac locus, it was suggested that T1R1 might be a sweet taste receptor (Hoon et al. 1999). However, the involvement of T1R1 in sweet taste still remains open. The significance of the spatial distribution of T1R1 expression on the tongue has been questioned (Lindemann 1999; Smith and Margolis 1999). Distinct chromosomal positions of the Gpr70 and Sac loci were demonstrated in this study.
It is interesting to note that the V2r2 (vomeronasal organ family 2, receptor 2) gene has a sequence related to Gpr70 (Hoon et al. 1999) and maps to the same region on mouse distal Chr 4 (Matsunami and Buck 1997). Thus, there may be a cluster of related chemosensitive receptor genes on mouse distal Chr 4. It is conceivable that one of these genes may correspond to Sac. However, it is also possible that phenotypical variation determined by Sac (i.e., effects on behavioral and neural taste responses to sweeteners) may be explained by non-receptor mechanisms, such as intracellular events in taste receptor cells, or synaptic transmission from the taste cells to nerve fibers.
In conclusion, this study shows that the putative taste receptor, T1R1, and the saccharin preference locus, Sac, are coded by different genes. T1R1 expression in the apical ends of a subset of taste receptor cells (Hoon et al. 1999) strongly suggests its involvement in taste reception. However, the T1R1 gene, Gpr70, is not involved in the behavioral and neural effects determined by Sac. Thus, further studies are needed to elucidate the role of T1R1 and to identify a gene product of the Sac locus.
Acknowledgments
We thank Dr. J.G. Brand for help with collecting lingual tissues, Dr. D.L. Kalinoski for advice on sequence analysis, Dr. S. Brazier for advice on RNA extraction and RT-PCR, and Dr. W. Grosvenor and Mr. D. Bayley for their valuable help and advice. Supported by National Institutes of Health grants R01DC00882, R01AA11028, R01DK44073, and R01DK48095.
Footnotes
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, et al. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 1996;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Reed DR, Ninomiya Y, Inoue M, Tordoff MG, et al. Sucrose consumption in mice: major influence of two genetic loci affecting peripheral sensory responses. Mamm Genome. 1997;8:545–548. doi: 10.1007/s003359900500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Inoue M, Ninomiya Y, Beauchamp GK. Modification of behavioral and neural taste responses to NaCl in C57BL/6 mice: effects of NaCl exposure and DOCA treatment. Physiol Behav. 1999;65:817–822. doi: 10.1016/s0031-9384(98)00239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA, Kotlus B, Frank ME. Quantitative trait loci associated with short-term intake of sucrose, saccharin and quinine solutions in laboratory mice. Chem Senses. 1999;24:373–385. doi: 10.1093/chemse/24.4.373. [DOI] [PubMed] [Google Scholar]
- DuBois GE. New insights on the coding of the sweet taste message in chemical structure. In: Salvadori G, editor. Firmenich Jubilee Symposium 1895–1995: Olfaction and Taste: A Century for the Senses; Geneva, Switzerland. Carol Stream, IL, USA: Allured Publishing Corp.; 1995. pp. 32–95. [Google Scholar]
- Fuller JL. Single-locus control of saccharin preference in mice. J Hered. 1974;65:33–36. doi: 10.1093/oxfordjournals.jhered.a108452. [DOI] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, et al. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 1999;96:541–551. doi: 10.1016/s0092-8674(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Lander E, Green P, Abrahamson J, Barlow A, Daley M, et al. MAPMAKER: An interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Lander ES, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage findings. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Lindemann B. Receptor seeks ligand: on the way to cloning the molecular receptors for sweet and bitter taste. Nat Med. 1999;5:381–382. doi: 10.1038/7377. [DOI] [PubMed] [Google Scholar]
- Lush IE. The genetics of tasting in mice. VI. Saccharin, acesulfame, dulcin and sucrose. Genet Res. 1989;53:95–99. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- Lush IE, Hornigold N, King P, Stoye JP. The genetics of tasting in mice. VII. Glycine revisited, and the chromosomal location of Sac and Soa. Genet Res. 1995;66:167–174. doi: 10.1017/s0016672300034510. [DOI] [PubMed] [Google Scholar]
- Matsunami H, Buck LB. A multigene family encoding a diverse array of putative pheromone receptors in mammals. Cell. 1997;90:775–784. doi: 10.1016/s0092-8674(00)80537-1. [DOI] [PubMed] [Google Scholar]
- Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as singlestrand conformation polymorphisms. Proc Nat Acad Sci USA. 1989;86:2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Smith DV, Margolis FL. Taste processing: whetting our appetites. Curr Biol. 1999;9:R453–R455. doi: 10.1016/s0960-9822(99)80280-2. [DOI] [PubMed] [Google Scholar]
- Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, et al. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) Biotechniques. 2000;29:52–54. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]