Abstract
Mice of the C57BL/6ByJ (B6) and 129/J (129) strains were offered different concentrations of taste solutions in 48-hr, two-bottle choice tests. In comparison with the 129 strain, the B6 strain had higher preferences for ethanol, sucrose, and citric acid. They had lower preferences for NaCI and similar preferences for capsaicin and quinine hydrochloride. These data are consistent with the hypothesis that the higher ethanol intake by B6 mice depends, in part, on higher hedonic attractiveness of its sweet taste component.
Keywords: Mouse Strains, Ethanol Consumption, Taste Preference.
IN RODENTS, the volume of ethanol consumed depends in part on its flavor,1 but the relationship between intake and flavor is not well understood. A sweet component to alcohol taste has been demonstrated in rats,2 which is consistent with several studies showing that the proclivity to drink alcohol is associated with elevated sweet preferences.3–5 However, alcohol intake is also associated with reduced NaCI preferences,4,6 even though it is not considered salty, and is unrelated to ingestion of bitter solutions4 despite being bitter.2 In addition to its gustatory properties, alcohol activates the olfactory and trigeminal (irritation/burn) systems,7 but there is no work to determine whether these are related to differences in intake.
The goal of the present study was to characterize the relationships between genetically determined differences in ethanol consumption and perception of the four main taste qualities and burning sensation. For this purpose, preferences for various concentrations of ethanol, sucrose, citric acid, quinine, NaCI, and capsaicin were tested in mice of C57BL/6ByJ (B6) and 129/J (129) strains. The B6 and 129 mouse strains were chosen because in previous studies they revealed differences in ethanol intake,5 as well as in preferences for sweet and salty substances.5,8,9 Because few data are available concerning taste acceptance in the 129 strain, it seemed important to test animals with a range of taste solutions. Another reason for testing the 129/J strain was that the closely related 129/SvJ strain is commonly used for transgenic studies. Thus, the results found herein could potentially provide relevant background data for studies examining taste preferences in transgenic mice.
METHODS
Subjects
Male B6 and the 129 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The mice were housed in individual cages in a temperature-controlled room at 23°C on a 12-hr light/dark cycle. They had free access to water and Teklad Rodent Diet 8604.
Ethanol, sucrose, citric acid, quinine, and capsaicin were tested using eight B6 mice and twelve 129 mice. Before the experiments described in this study, the mice were used in another study that involved noninvasive measurements of 75 to 300 mM NaCI solution intake and sodium excretion. Preference measurements started when the animals were 6.5 to 7 months old, and their weight was 32.1 ± 1.0 g (B6) and 28.6 ± 0.5 g (129). After ethanol, sucrose and citric acid were tested; five 129 mice were used for another experiment, so that quinine and capsaicin were tested on only eight B6 mice and seven 129 mice.
NaCI preference was tested in a separate group of six B6 mice and six 129 mice. These were 2 months old at the beginning of the experiment, and their weight was 24.0 ± 0.5 g (B6) and 21.0 ± 0.5 g (129), respectively.
Apparatus
Tubes for measurement of fluid intakes were made of graduated 25-ml polystyrene serological pipets (Fisher, Springfield, NJ). A 6.4-cm-long stainless-steel sipper tube (UNIFAB, Kalamazoo, MI) was inserted into one end of the pipet, and the other end was plugged with a rubber stopper. The sipper tubes had 3.175-mm diameter holes. The two drinking tubes were inserted into metal tube holders and attached to the cage lids with metal springs. Tips of the sipper tubes were 15 mm apart, and each extended 25 mm into the cage.
Taste Tests
Taste solution intake was measured in 48-hr, two-bottle choice tests. Measurements were made in the middle of the light period by reading fluid volume to the nearest 0.2 ml. The positions of the tubes were switched after 24 hr of each 48-hr test. Ascending concentrations of taste solutions were tested. Mice of the first group received taste solutions in the following order: sucrose, citric acid, ethanol, capsaicin, and quinine HC1 (i.e., from most to least attractive). Testing of different taste solutions was separated by at least 2 days, when deionized water was available in both drinking tubes. This protocol was designed to minimize possible carry-over effects within and between solution series.
The following solutions were prepared in deionized water and tested in a choice test against deionized water: 1, 2, 4, 8, 16, and 32% w/v (corresponding to 29, 58, 117, 234, 467, and 935 mM) sucrose; 0.001, 0.01, 0.1, 1.0, and 10 mM citric acid (Sigma Chemical Company, St. Louis, MO); 1, 5, 10, 15, and 20% v/v (corresponding to 170, 850, 1700, 2560, and 3410 mM) ethanol (Pharmco, Bayonne, NJ); 0.003, 0.01, 0.03, 0.1, 0.3, and 1.0 mM quinine HC1; and 37.5, 75,150, 300, 450, and 600 mM NaCI (Sigma). Because capsaicin has very low solubility in water, a stock capsaicin (Sigma) solution was prepared in ethanol (0.2 g/liter). This stock solution was then diluted in deionized water to obtain 0.1, 0.5,1.0, and 5.0 mg/liter (ppm) concentrations of capsaicin. Ethanol concentration was brought up to 0.005% in all capsaicin solutions, and the solutions were tested in choice tests against 0.005% ethanol. Preliminary measurements showed that when 0.005% ethanol was given to drink in a choice test with water for 48 hr, B6 and 129 mice ingested similar amounts of ethanol solution (36.1 ± 6.8 and 44.5 ± 7.2 ml/kg, respectively) and had a similar weak preference for the ethanol solution over water (correspondingly, 65 ± 6% and 62 ± 5%). To detect any toxic effects of capsaicin ingestion, fluid intake was measured in a 48-hr, two-bottle test with 0.005% ethanol in both tubes before testing capsaicin solutions. This was compared with total fluid intake when capsaicin was available.
Body weights were measured before and after each test series.
Data Analysis
Fluid intake was expressed per kilogram of body weight. Body weights of individual mice before and after a series of concentrations of a taste solution were averaged, and used to calculate relative intake of this solution. Preference scores were calculated as a ratio of taste solution intake to total fluid intake (taste solution plus water), in percent.
Strain (between-subjects) and concentration (within-subjects) effects were evaluated by two-way ANOVA. If an interaction between the two factors was significant, post-hoc t tests were used to compare means for each concentration between strains.
RESULTS
To simplify presentation of the results, all F values and their corresponding significance levels are presented in Table 1. All analyses revealed the expected significant main effects of taste solution concentration on intake and preference, which are not discussed further.
Table 1.
ANOVA Results for Preference Tests
| Effect of: | ||||
|---|---|---|---|---|
| Solution | Index | Strain | Concentration | Strain × concentration |
| Ethanol | Intake | F(1,18) = 9.78, p < 0.01 | F(4,72) = 17.3, p < 0.001 | F(4,72) = 2.51, p < 0.05 |
| Preference | F(1,18) =10.3, p < 0.005 | F(4,72) = 11.4, p < 0.001 | F(4,72) = 2.42, p < 0.06 (NS) | |
| Sucrose | Intake | F(1,18) =43.8, p <0.001 | F(5,90) = 151.0, p < 0.001 | F(5,90) = 16.2, p < 0.001 |
| Preference | F(1,18) =15.3, p < 0.005 | F(5,90) = 16.8, p < 0.001 | F(5,90) = 5.18, p < 0.001 | |
| Citric acid | Intake | F(1,18) =3.52, p < 0.08 (NS) | F(4,72) = 14.6, p< 0.001 | F(4,72) = 0.72, NS |
| Preference | F(1,18) =6.30, p < 0.05 | F(4,72) = 13.3, p < 0.001 | F(4,72) = 0.41, NS | |
| Quinine HCI | Intake | F(1,13) =0.08, NS | F(5,65) = 26.7, p < 0.001 | F(5,65) = 2.11, p < 0.08(NS) |
| Preference | F(1,13) =1.67, NS | F(5,65) = 28.4, p < 0.001 | F(5,65) = 1.97, p <0.10(NS) | |
| Capsaicin | Intake | F(1,13) =0.29, NS | F(3,39) = 32.3, p < 0.001 | F(3,39) = 0.98, NS |
| Preference | F(1,13) =0.39, NS | F(3,39) = 30.0, p < 0.001 | F(3,39) = 0.78, NS | |
| NaCI | Intake | F(1,10) = 2.30, NS | F(5,50) = 39.4, p < 0.001 | F(5,50) = 6.03, p < 0.001 |
| Preference | F(1,10) = 2.59, NS | F(5,50) = 51.7, p <0.001 | F(5,50) = 7.83, p < 0.001 | |
NS, not significant.
Ethanol
B6 mice had significantly higher ethanol intake and preference than did 129 mice (Fig. 1, Table 1). The difference between strains was significant at ethanol concentrations of 5% and higher (5 to 15% for intake and 5 to 20% for preference). Ethanol intake and preference of B6 mice increased with increasing ethanol concentrations to peak at 10% ethanol and then decreased. On the other hand, 129 mice were indifferent to concentrations lower than 10% and avoided concentrations higher than 10%.
Fig. 1.
Ethanol intake (top) and preference (bottom) of 129 and B6 mice. Vertical bars represent SE. BW, body weight.
Sucrose
Sucrose intake and preference were higher in B6 than 129 mice (Fig. 2, Table 1). Intakes were significantly different between the two strains at 2 to 16% concentrations. Preferences were significantly different at concentrations of 1 to 4%; at concentrations 8% and above, sucrose preference of both strains was close to 100% and did not differ.
Fig. 2.
Sucrose intake (top) and preference (bottom) of 129 and B6 mice. Vertical bars represent SE. BW, body weight.
Citric Acid
Citric acid intake and preference were higher in B6 mice than in 129 mice, although the strain difference in intake was marginally nonsignificant (Fig. 3, Table 1). Mice of both strains preferred citric acid over water at concentrations between 0.001 to 1.0 mM.
Fig. 3.
Citric acid intake (top) and preference (bottom) of 129 and B6 mice. Vertical bars represent SE. BW, body weight.
Quinine HCl
No significant differences were found between the strains in quinine intake and preference (Fig. 4, Table 1). There was a nonsignificant tendency for B6 mice to have a higher preference for 0.03 mM of quinine relative to 129 mice (a priori t test, p = 0.08). The B6 mice were indifferent to 0.03 mM of quinine, whereas the 129 mice avoided it. Both strains responded indifferently to lower quinine concentrations (0.003 and 0.01 mM) and avoided higher concentrations (0.1 to 1.0 mM).
Fig. 4.
Quinine HCI intake (top) and preference (bottom) of 129 and B6 mice. Vertical bars represent SE. BW, body weight.
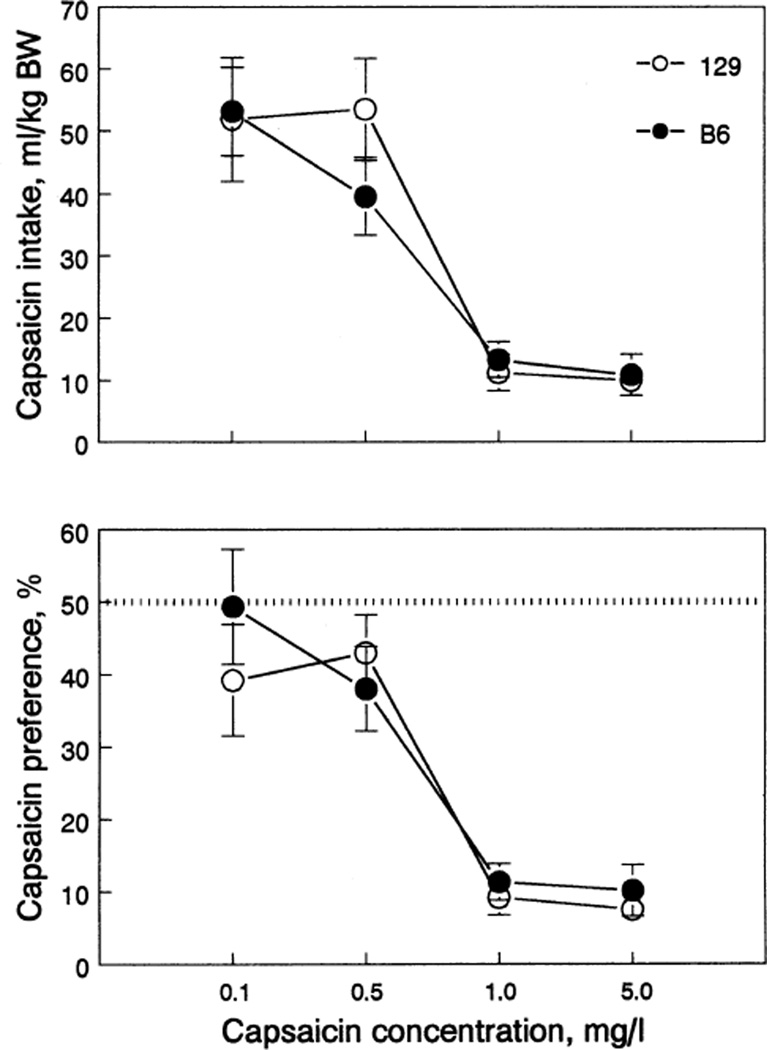
Capsaicin
Capsaicin intake and preference were similar in B6 and 129 strains (Fig. 5, Table 1). Both strains were indifferent to 0.1 and 0.5 mg/liter of capsaicin solutions, but avoided 1.0 and 5.0 mg/liter solutions.
Fig. 5.
Capsaicin intake (top) and preference (bottom) of 129 and B6 mice. Vertical bars represent SE. BW, body weight.
Because capsaicin may be toxic, total fluid intake before and during capsaicin testing was measured as an index of the general status of animals. No suppression of fluid intake and no signs of toxicity were found during capsaicin testing. When 0.005% ethanol (the vehicle for capsaicin) was given in both tubes before capsaicin testing, B6 and 129 mice drank 101.3 ± 3.4 and 116.5 ± 5.9 ml/kg, respectively. When the highest capsaicin concentration (5 mg/liter) was tested, total fluid intake was, respectively, 114.7 ± 4.9 and 133.4 ± 5.8 ml/kg. Indeed, there was a slight increase of total fluid intake when capsaicin was consumed [effect of solution tested, F(l,13) = 31.9, p < 0.001].
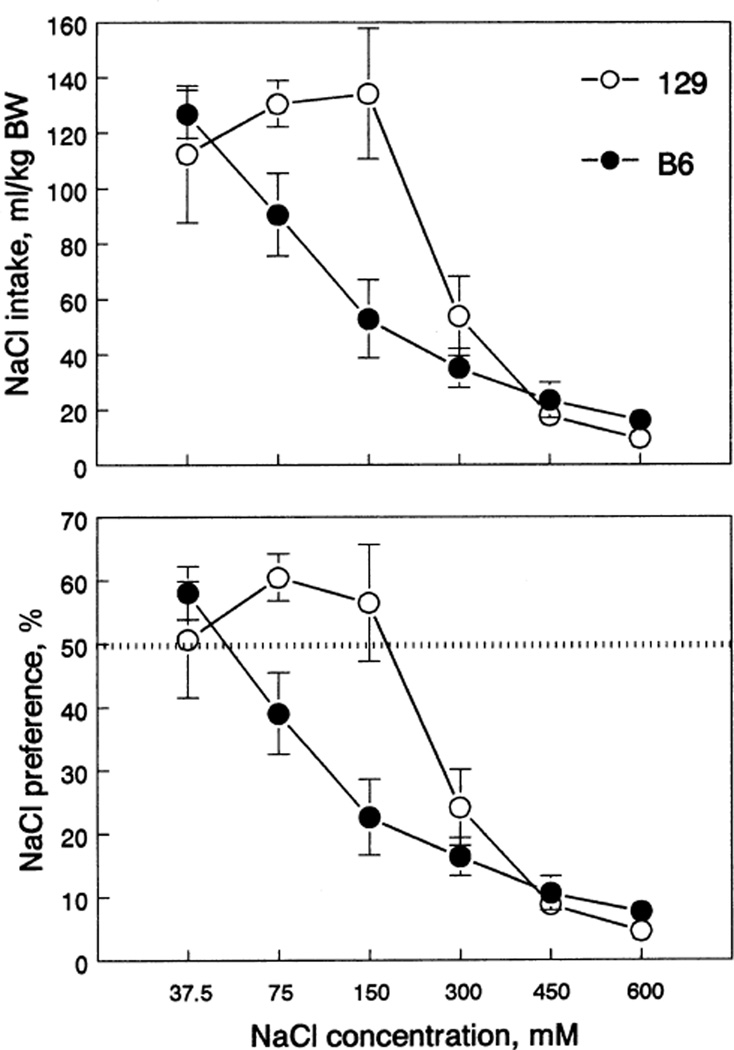
NaCl
B6 mice had lower 75 and 150 mM NaCl intake and preference in comparison with 129 mice (Fig. 6, Table 1). Both strains had similar 37.5 mM of NaCl intake and preference. Solutions of 75 and 150 mM of NaCl were preferred by 129 mice and avoided by B6 mice. Both strains avoided 300 mM and higher NaCl solutions.
Fig. 6.
NaCl intake (top) and preference (bottom) of 129 and B6 mice. Vertical bars represent SE. BW, body weight.
DISCUSSION
Compared with 129 mice, B6 mice had higher preferences for ethanol, sucrose, and citric acid, and lower preferences for NaCl. The results with quinine solutions were equivocal, but there was a tendency for B6 mice to have a higher preference for 0.03 mM of quinine. There was no difference between the strains in capsaicin solution intake or preference.
These results confirm and extend existing data. Our finding that ethanol intake was higher in B6 than 129 mice is consistent with earlier results.5 Consistent with our finding of a difference between the two strains in sucrose intake, there is evidence that B6 mice have higher intakes than do 129 mice of several sweet compounds, including sucrose,10 saccharin,5,10–12 acesulfame, dulcin,10 glycine,13 d-phenylalanine, and l-glutamine.12 A positive relationship between alcohol intake and sweet solution intake has also been found among other inbred strains of mice5 and rats,3,4 and within F2 hybrids from ethanol-preferring and ethanol-nonpreferring rat strains.3 Because these studies encompass a wide variety of nutritive and nonnutritive sweeteners, some of which are believed to have no postingestive effects, the results support the contention there is a genetically determined link between alcohol intake and sweet taste perception.
Based on findings that the taste of alcohol has a bitter component to rats,2 and that an association between a predisposition to alcoholism and lower sensitivity to bitter was found in humans,14 a lower aversion to bitter might be expected to increase ethanol consumption. However, we found little support for such a relationship. Despite the large difference in alcohol intake, male B6 mice showed only a small and nonsignificant tendency to avoid quinine less than did 129 mice. This finding is at odds with the work of Lush,15 who tested both genders together and found that 0.1 to 0.4 mM of quinine solutions were avoided less by B6 than 129 mice. We have replicated our finding of a weak, nonsignificant trend using larger groups of male mice (fourteen B6 and thirteen 129) and found that female B6 mice (n = 19) drink significantly more 0.03 mM of quinine than do female 129 mice (n = 20; Bachmanov et al., unpublished data). Thus, there may be an interaction between gender and quinine preference in these two strains, which can account for the difference in results between this and Lush's work.
There is growing evidence that several different receptors mediate bitterness perception,8 but no information about which receptors mediate ethanol taste; so, it may be that quinine is not an appropriate compound to mimic the bitter component of ethanol. However, with respect to other bitter substances, 129 and B6 strains were nontasters of strychnine,16 acetates of raffinose, galactose, and β-lactose,17 and they differed only slightly in preferences for cycloheximide13 and phenylthiourea.18 Therefore, no convincing association between alcohol consumption and bitter taste is present in these strains (see also Ref. 4). This does not rule out the possibility that such an association may be found in other models with more pronounced differences in bitter preference between strains. For instance, B6 mice with high ethanol consumption are nontasters of sucrose octaacetate, whereas SWR mice with low ethanol consumption5 are tasters of this bitter substance.19
The inverse relationship between ethanol and NaCl consumption in 129 and B6 mice found in this study was seen previously in 129 and B6 mice8,9,20 and in different sets of alcohol-preferring and alcohol-avoiding rats.4,6 It has been hypothesized that the inverse relationship between alcohol and salt intake is caused by the renin-angiotensin system, which when activated increases NaCl intake and suppresses alcohol intake.21,22 However, in unpublished experiments (Bachmanov et al., unpublished data), we found no differences between B6 and 129 strains in sodium balance or plasma concentrations of sodium, aldosterone, or renin activity. Thus, the inverse relationship between ethanol and NaCl consumption in B6 and 129 mice remains to be explained.
Although there is no evidence that alcohol is perceived as sour by rodents, we tested mice in the present study with solutions of citric acid to complete characterization of their responses to the four main taste qualities. The B6 mice showed stronger preferences for citric acid than did the 129 mice. Both strains showed marked preferences for citric acid over water, which contrasts with other work. For example, B6 mice and Sprague-Dawley rats showed indifference or avoidance to 0.1 mM and higher,23 or 1 mM and higher24 citric acid. We suspect that citric acid is attractive to mice at concentrations below 0.1 mM and avoided at concentrations above 1 mM.
A relationship between bitterness, sensitivity to the trigeminal irritant, capsaicin, and alcohol intake might be expected based on findings in humans of a linkage between alcoholism and lower sensitivity to bitter 6-n-propylthiouracil14 and between the ability to taste 6-n-propylthiouracil and the perception of bitter and irritating qualities of ethanol,25 as well as the burning quality of capsaicin.26 However, we found no difference between B6 and 129 mice in capsaicin intake, suggesting that differences in ethanol consumption are not related to differences in trigeminal sensitivity to capsaicin. To our knowledge, capsaicin preferences have not been characterized in the mouse before. We found that the avoidance threshold for capsaicin was between 0.5 and 1.0 mg/liter. In humans, capsaicin evokes a feeling of warmth at concentrations above 0.1 mg/liter; at 1 mg/liter it becomes burning, and above 10 mg/liter it is painful.26 In hamsters, 0.033 mM (≈10 mg/liter) of capsaicin was strongly aversive.27 In rats, capsaicin solutions tested in the range of 0.0016 to 0.016 mM (≈0.5 to 5.0 mg/liter) were aversive.28 A 1% (w/w) concentration of capsaicin added to food significantly reduced food consumption in rats, whereas birds were insensitive to capsaicin in concentrations up to 1% in food or water.29 Therefore, at least in mammals, capsaicin perception and avoidance thresholds seem to be fairly similar and are in the region of 0.1 to 10 mg/liter.
Because we tested several different taste stimuli in the same animals, the experimental protocol was designed to minimize interactions between solutions tested consequently. First, we used ascending concentrations of taste solutions that have smaller carry-over effects in comparison to descending order of tests.9,20 Second, because aversive stimuli are more likely to have carry-over effects, the taste solutions were tested starting from preferred sucrose and citric acid and ending with avoided capsaicin and quinine HC1. Third, several days elapsed between each test series. Although there still may have been interactions between solutions tested in this study, it seems unlikely that they would be sufficiently robust to affect the main conclusions about the direction of strain differences between B6 and 129 strains. Indeed, in our other experiments on these strains,9,20,30 as well as in studies by other authors,5,8,10–12,15 B6 and 129 mice had the same character of differences in ethanol, sucrose, saccharin, citric acid, quinine, and NaCl consumption, regardless of whether the solutions were tested in the same or in different groups of animals.
To summarize, the higher ethanol consumption of B6 mice relative to 129 mice is accompanied by higher preferences for sweet and sour, possible reduction in the aversion to bitter, no change in capsaicin sensitivity, and a lower preference for salty taste. These data are consistent with the hypothesis that the higher ethanol intake by B6 mice depends, in part, on higher hedonic attractiveness of its sweet taste component. The relevance of changes in perception of sourness and saltiness is unclear. However, we caution that any conclusions to be drawn from the data are tempered by several factors. With respect to chemosensation, the 48-hr tests used herein are difficult to interpret because chemosensory effects on intake are confounded by the postingestive effects of the solutions, and even learning. Although findings that high alcohol intake is associated with high intake of nonnutritive sweeteners point toward a taste-related mechanism, there is evidence that taste and postingestive factors interact to influence NaCl intake in the strains tested here,9,20 and there is no reason to believe the same is not true for some of the other solutions tested. There are several physiological mechanisms that could potentially explain the correspondence between alcohol and sucrose intake, including signals related to caloric value31 the brain opioid system,32,33 or intracellular calcium.34
With respect to genetics, it is possible that, rather than a gene with pleiotropic effect, two or more independent genes fortuitously fixed during inbreeding of the mouse strains might account for the differences in intake of different solutions. This can be tested by examining a genetic correlation of alcohol intake with intake of other solutions among multiple inbred strains or within a segregating cross. Such additional analyses would also give protection from possible type I errors resulting from multiple comparisons made on the two strains in this study. Thus, this work provides only a first step to understanding the genetic links between ethanol consumption and taste preferences.
ACKNOWLEDGMENTS
The advice of Drs. S. Wager-Pagé, L. Clark, and J. R. Mason in testing capsaicin is gratefully acknowledged.
This research was supported by the National Institutes of Health Grant DC 00882 and by a grant from the Howard Heinz Endowment.
Footnotes
Portions of this work were presented at the annual meeting of the Association for Chemoreception Sciences, Sarasota, FL, April 1995.
REFERENCES
- 1.Nachman M, Larue C, Le Magnen J. The role of olfactory and orosensory factors in the alcohol preference of inbred strains of mice. Physiol Behav. 1971;6:53–95. doi: 10.1016/0031-9384(71)90014-x. [DOI] [PubMed] [Google Scholar]
- 2.Kiefer SW, Lawrence GJ. The sweet-bitter taste of alcohol: Aversion generalization to various sweet-quinine mixtures in the rat. Chem Senses. 1988;13:633–641. [Google Scholar]
- 3.Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Murelle L, Halikas JA, Janowsky DS. Saccharin intake predicts ethanol intake in genetically heterogeneous rats as well as different rat strains. Alcohol Clin Exp Res. 1993;17:366–369. doi: 10.1111/j.1530-0277.1993.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 4.Stewart RB, Russell RN, Lumeng L, Li T-K, Murphy JM. Consumptions of sweet, salty, sour, and bitter solutions by selectively bred alcohol-preferring and alcohol-nonpreferring lines of rats. Alcohol Clin Exp Res. 1994;18:375–381. doi: 10.1111/j.1530-0277.1994.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 5.Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 6.Linkola J, Tikkanen I, Fyhrquist F, Rusi M. Renin, water drinking, salt preference and blood pressure in alcohol preferring and alcohol avoiding rats. Pharmacol Biochem Behav. 1980;12:293–296. doi: 10.1016/0091-3057(80)90371-8. [DOI] [PubMed] [Google Scholar]
- 7.Green BG. The sensitivity of the tongue to ethanol. Ann NY Acad Sci USA. 1987;510:315–317. [Google Scholar]
- 8.Lush IE. The genetics of bitterness, sweetness, and saltiness in strains of mice. In: Wysocki CJ, Kare MR, editors. Chemical Senses. Vol. 3. New York, Marcel Dekker: Genetics of Perception and Communication; 1991. pp. 227–241. [Google Scholar]
- 9.Beauchamp GK, Fisher AS. Strain differences in consumption of saline solutions by mice. Physiol Behav. 1993;54:179–184. doi: 10.1016/0031-9384(93)90063-l. [DOI] [PubMed] [Google Scholar]
- 10.Lush IE. The genetics of tasting in mice. VI. Saccharin, acesulfame, dulcin and sucrose. Genet Res. 1989;53:95–99. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- 11.Belknap JK, Crabbe JC, Plomin R, McClearn GE, Sampson KE, O’Toole LA, Gora-Maslak G. Single-locus control of saccharin intake in BXD/Ty recombinant inbred (RI) mice: Some methodological implications for RI strain analysis. Behav Genet. 1992;22:81–100. doi: 10.1007/BF01066794. [DOI] [PubMed] [Google Scholar]
- 12.Capeless CG, Whitney G. The genetic basis of preference for sweet substances among inbred strains of mice: Preference ratio phenotypes and the alleles of the Sac and dpa loci. Chem Senses. 1995;20:291–298. doi: 10.1093/chemse/20.3.291. [DOI] [PubMed] [Google Scholar]
- 13.Lush IE, Holland G. The genetics of tasting in mice. V. Glycine and cycloheximide. Genet Res. 1988;52:207–212. doi: 10.1017/s0016672300027671. [DOI] [PubMed] [Google Scholar]
- 14.Pelchat ML, Danowski S. A possible genetic association between PROP-tasting and alcoholism. Physiol Behav. 1992;51:1261–1266. doi: 10.1016/0031-9384(92)90318-v. [DOI] [PubMed] [Google Scholar]
- 15.Lush IE. The genetics of tasting in mice. III. Quinine. Genet Res. 1984;44:151–160. doi: 10.1017/s0016672300026355. [DOI] [PubMed] [Google Scholar]
- 16.Lush IE. The genetics of tasting in mice. II. Strychnine. Chem Senses. 1982;7:93–98. [Google Scholar]
- 17.Lush IE. The genetics of tasting in mice. IV. The acetates of raffinose, galactose and β-lactose. Genet Res. 1986;47:117–123. doi: 10.1017/s0016672300022941. [DOI] [PubMed] [Google Scholar]
- 18.Lush IE. Differences between mouse strains in their consumption of phenylthiourea (PTC) Heredity. 1986;57:319–323. doi: 10.1038/hdy.1986.129. [DOI] [PubMed] [Google Scholar]
- 19.Lush IE. The genetics of tasting in mice. I. Sucrose octaacetate. Genet Res. 1981;38:93–95. doi: 10.1017/s0016672300020425. [DOI] [PubMed] [Google Scholar]
- 20.Bachmanov AA, Tordoff MG, Beauchamp GK. Strain differences in salt appetite in mice. Abstracts of the Second Independent Meeting of the SSIB; Hamilton, Canada. 1994. (abstr A24) [Google Scholar]
- 21.Fitzsimons JT. Monographs of the Physiological Society, No. 35. Cambridge: Cambridge University Press; 1979. The Physiology of Thirst and Sodium Appetite. [PubMed] [Google Scholar]
- 22.Grupp LA, Perlanski E, Stewart RB. Regulation of alcohol consumption by the renin-angiotensin system: A review of recent findings and a possible mechanism of action. Neurosci Biobehav Rev. 1991;15:265–275. doi: 10.1016/s0149-7634(05)80006-5. [DOI] [PubMed] [Google Scholar]
- 23.Whitney G, Maggio JC, Harder DB. Manifestations of the major gene influencing sucrose octaacetate (SOA) tasting among mice: Classic taste qualities. Chem Senses. 1990;15:243–252. [Google Scholar]
- 24.Tordoff MG, Coldwell SE. Altered acceptance of taste solutions by calcium-deprived rats. Abstracts of the 17th Annual Meeting of the Association for Chemoreception Sciences; Sarasota, FL. 1995. p. 53. [Google Scholar]
- 25.Bartoshuk LM. The biological basis of food perception and acceptance. Food Qual Pref. 1993;4:21–32. [Google Scholar]
- 26.Karrer T, Bartoshuk L. Capsaicin desensitization and recovery on the human tongue. Physiol Behav. 1991;49:757–764. doi: 10.1016/0031-9384(91)90315-f. [DOI] [PubMed] [Google Scholar]
- 27.Rehnberg BG, Hettinger TP, Frank ME. The role of sucrose-sensitive neurons in ingestion of sweet stimuli by hamsters. Physiol Behav. 1990;48:459–466. doi: 10.1016/0031-9384(90)90344-4. [DOI] [PubMed] [Google Scholar]
- 28.Ganchrow JR, Seltzer Z, Bitchacho N. The effect of neonatal capsaicin treatment on gustatory behavior in the albino rat. Physiol Behav. 1992;52:1037–1042. doi: 10.1016/0031-9384(92)90456-c. [DOI] [PubMed] [Google Scholar]
- 29.Mason JR, Bean NJ, Shah PS, Clark L. Taxon-specific differences in responsiveness to capsaicin and several analogues: Correlates between chemical structure and behavioral aversiveness. J Chem Ecol. 1991;17:2539–2551. doi: 10.1007/BF00994601. [DOI] [PubMed] [Google Scholar]
- 30.Bachmanov AA, Tordoff MG, Beauchamp GK. Taste preference and ethanol consumption in mice: A genetic analysis. Abstracts of the 17th Annual Meeting of the Association for Chemoreception Sciences; Sarasota, FL. 1995. p. 267. [Google Scholar]
- 31.Gentry RT, Dole VP. Why does a sucrose choice reduce the consumption of alcohol in C57BL/6J mice? Life Sci. 1987;40:2191–2194. doi: 10.1016/0024-3205(87)90010-5. [DOI] [PubMed] [Google Scholar]
- 32.George SR, Roldan L, Lui A, Naranjo CA. Endogenous opioids are involved in the genetically determined high preference for ethanol consumption. Alcohol Clin Exp Res. 1991;15:668–672. doi: 10.1111/j.1530-0277.1991.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 33.Gosnell BA, Majchrzak MJ. Centrally administered opioid peptides stimulate saccharin intake in nondeprived rats. Pharmacol Biochem Behav. 1989;33:805–810. doi: 10.1016/0091-3057(89)90474-7. [DOI] [PubMed] [Google Scholar]
- 34.Pucilowski O, Rezvani AH, Janowsky DS. Suppression of alcohol and saccharin preference in rats by a novel Ca2+ channel inhibitor, Goe5438. Psychopharmacology. 1992;107:447–452. doi: 10.1007/BF02245174. [DOI] [PubMed] [Google Scholar]