Abstract
Individual variability in sucrose consumption is prominent in humans and other species. To investigate the genetic contribution to this complex behavior, we conducted behavioral, electrophysiological, and genetic studies, using male progeny of two inbred mouse strains (C57BL/6ByJ [B6] and 129/J [129]) and their F2 hybrids. Two loci on Chromosome (Chr) 4 were responsible for over 50% of the genetic variability in sucrose intake. These loci apparently modulated intake by altering peripheral neural responses to sucrose. One locus affected the response threshold, whereas the other affected the response magnitude. These findings suggest that the majority of difference in sucrose intake between male B6 and 129 mice is due to polymorphisms of two genes that influence receptor or peripheral nervous system activity.
Introduction
The consumption of sucrose and other carbohydrate sweeteners requires a complex integration of peripheral sensory, central nervous system, and post-ingestive events. For example, not only does sucrose taste pleasantly sweet to humans and is highly preferred by many other species (Dobbing 1987; Bolles 1991), but it also stimulates release of endorphins (Melchoir et al. 1991; Blass and Shah 1995) and provides a rich source of calories. It is clear that sensory factors predominate in short-term tests (Pfaffmann 1959; Davis and Smith 1988), but the relative contribution of these factors to long-term sucrose intake is unknown. To test the hypothesis that peripheral sensory factors also play a major role in controlling long-term (that is, daily) consumption, we conducted behavioral, electrophysiological, and genetic studies with two strains of inbred mice (C57BL/6ByJ [B6] and 129/J [129]) that differ greatly in sucrose intake and preference (Lush 1989, Bachmanov et al. 1996a, 1996b).
We determined the chromosomal positions of genes underlying the strain differences by examining sucrose intake and gustatory neural responses to sucrose in the segregating F2 (B6 × 129) hybrid generation in relation to a dense microsatellite map (Lander and Botstein 1989; Dietrich et al. 1994). We focused on Chromosome (Chr) 4 because two loci, one assigned to the proximal (dpa) and one assigned to the distal (Sac) end of this chromosome, have been implicated in sweet-taste function. The dpa locus, associated with the perception of the sweetness of the amino acid d-phenylalanine, was postulated based on conditioned taste aversion generalization between d-phenylalanine and sucrose in C57BL/6 and BALB/c mice and their hybrids (Ninomiya et al. 1991). The Sac locus, which influences preferences for saccharin and some other sweet substances, was suggested based on two-bottle preference tests in C57BL/6 and DBA/2 mice, their hybrids and recombinant-inbred strains (Fuller 1974; Phillips et al. 1994; Lush et al. 1995; Blizard and McClearn 1996).
Materials and methods
Animals
Adult male and female C57BL6/ByJ (B6) and 129/J (129) mice obtained from The Jackson Laboratory (Bar Harbor, Me.) were used for breeding. The F1 was generated by reciprocal crosses with both strains and genders (female 129 × male B6; male 129 × female B6). Two types of the F2 hybrids were produced by intercrossing the two types of reciprocal F1 hybrids [(129 × B6 F1 females) × (129 × B6 F1 males) and (B6 × 129 F1 females) × (B6 × 129 F1 males)]. Mice from the parental strains were bred, raised, and tested simultaneously with the F2. Pups were weaned at 21–30 days of age and reared in same-sexed groups of four to six. Because no differences in sucrose intake were found between the hybrids from reciprocal crosses (Bachmanov et al. 1996a), the data for 171 male F2 mice from both crosses were analyzed together as a single group. We studied only males, not females, in order to reduce gender-related variability and therefore increase power for the detection of quantitative trait loci.
The 21 male F2 mice were selected for the electrophysiological experiments after genotyping all F2 hybrids for the D4Mit4 and D4Mit42 markers. Mice carrying different combinations of alleles for these markers were included in the group. Because of this selection, the proportion of individuals homozygous for these markers was higher than in the overall F2 population, which approximated the Mendelian ratios of 0.25 (B6/B6), 0.5 (B6/129), 0.25 (129/129). The selected group included F2 mice with a broad range of sucrose intakes.
All mice were housed at 23°C on a 12:12 h light:dark cycle and had free access to water and Teklad Rodent Diet 8604. Details of the breeding protocol, the behavioral testing, and the results of biometric analyses are given in Bachmanov and colleagues (1996a).
Behavioral testing
Taste solution intake was measured in two-bottle tests in individually caged adult mice (~60 days old). Construction of the drinking tubes and other experimental details have been previously described (Bachmanov et al. 1996a, 1996b). Mice were presented with two drinking tubes; one tube contained deionized water and the other tube contained 4% wt/vol (0.12 m) sucrose (Sigma Chemical Company, St. Louis, Mo.) for 4 days. The positions of the tubes were switched every 24 h of the 96-h test to control for side preferences. The body weight of each mouse was measured before and after the 96-h test, averaged, and used to calculated the relative intake of sucrose solution. Fluid intake for the 4-day test was averaged and expressed per 30 g of body weight (the approximate weight of an adult mouse) per day.
The 0.12 m sucrose solution tastes sweet to humans. Both B6 and 129 mice preferred this solution over water in the two-bottle test, with the B6 strain having a higher preference score than the 129 strain. Genetic analyses of sucrose preference and intake gave similar results, and so only analyses of intake are presented here.
Electrophysiology
In mice anesthetized with an intraperitoneal injection of sodium pentobarbital (40–50 mg/kg), the right chorda tympani nerve was dissected at the point of its entry to the bulla and placed on an electrode. Test solutions were prepared in deionized water and flowed over the anterior part of the tongue at a flow rate of 0.5 ml/s. Integrated whole nerve responses to chemical stimulation of the tongue were recorded. The magnitude of the integrated response at 15 s after stimulus onset was measured and expressed relative to the response to 0.1 m NH4Cl (Ninomiya et al. 1996).
Genotypic and linkage analysis
Genomic DNA was purified from the mouse tails by phenol/chloroform extraction and precipitation by alcohol (Hogan et al. 1986). Microsatellite markers were amplified by the polymerase chain reaction (PCR) with primers purchased from Research Genetics Inc. (Huntsville, AL), with a protocol modified slightly from that described by Dietrich and coworkers (1992). PCR reaction mixtures included 50-ng template genomic DNA, 100 µm dNTPs, 0.5 µCi 33P-α-dCTP(Amersham, Arlington Heights, Ill.), 50 mm KCl, 10 mm Tris-HCl, pH 8.3, 1.5 mm MgCl2, 1.65 pmol of the forward, 1.65 pmol of the reserve primer, and 1 U of Taq DNA polymerase. PCR conditions were as follows: 35 cycles of 1 min at 94°C, 2 min at 55°C, and 2 min at 72°C, followed by one cycle of 7 min at 72°C. The denaturated PCR products were electrophoresed on a 6% polyacrylamide, 8.3 m urea sequencing gel, and the polymorphic sequences were visualized by autoradiography. We have genotyped D1Mit21, D1Mit46, D1Mit48, D1Mit14, D1Mit17, D2Mit9, D3Mit10, D3Mit103, D3Mit16, D3Mit199, D4Mit264, D4Mit97, D4Mit4, D4Mit7, D4Mit17, D4Mit58, D4Mit204, D4Mit71, D4Mit33, D4Mit42, D4Mit256, D5Mit1, D6Mit9, D6Mit25, D6Mit14, D7Mit44, D7Mit38, D7Mit7, D11Mit21, D11Mit4, D12Mit34, D16Mit3, D18Mit23, and D19Mit11 markers. Because significant linkages were found only for the markers on Chr 4, data for the other markers are not shown in the paper.
Interval mapping based on maximum likelihood estimation was conducted with MAPMAKER/QTL 1.1 software (Lander et al. 1987), which calculated chromosome positions, confidence intervals, and percentage of variance explained by quantitative trait loci. Thresholds for significant linkage were estimated for 2 degrees of freedom, assuming that both additive and dominant components were estimated in the intercross (Lander and Kruglyak 1995). The most likely loci positions were determined at LOD score maximums. Confidence intervals for the loci were estimated as LOD drops of 1.0 proximal and distal to the LOD maximums.
The Pearson correlation coefficients and the analysis of variance (ANOVA) were used to estimate relationships between the F2 animals’ phenotypes and their genotypes expressed as zero, one, or two B6 alleles carried at each marker. When chorda tympani responses were analyzed, one-way ANOVA for each sucrose concentration was conducted. A Bonferroni correction for multiple comparisons requires the level of statistical significance to be set for the matrix of 55 correlation coefficients (5 sucrose concentrations by 11 markers in F2) at 0.05/55, or p < 0.0009, and for 15 one-way ANOVA tests (5 sucrose concentrations by 2 markers in F2 plus a pair of parental strains) at 0.05/15, or p < 0.0033, in order to provide p < 0.05 protection against any false relationship in the matrices.
Differences between genotype group means were assessed by post hoc Tukey tests for unequal sample sizes (in two-way ANOVAs, analyzing effects of two marker loci simultaneously) or by planned a priori comparisons (in one-way ANOVAs, analyzing effect of each marker locus separately). The presence of dominance was assessed by planned comparisons, when a heterozygote mean was compared with (a) each of the homozygote means and (b) an average of the two homozygote means.
Results
Sucrose consumption
In a standard two-bottle test with water presented in one drinking tube and 0.12 m (4%) sucrose solution in another, nondeprived adult male B6 mice (n = 14) consumed more sucrose than did 129 mice (n = 13, Fig. 1A). Genotypes for microsatellite markers were determined by PCR in 171 F2 hybrid male mice. Interval mapping with maximum likelihood estimation identified two independently segregating loci influencing sucrose intake in the F2 mice. One locus with an incompletely recessive B6 allele was mapped to D4Mit4 on proximal Chr 4 (LOD score = 3.47). The other locus with a dominant B6 allele was mapped to D4Mit42 on distal Chr 4 (LOD score = 5.82, Fig. 2A). From known marker positions for these loci (respectively 13.55 cM and 81.0 cM from centromere: Mouse Genome Database) and their confidence intervals (−5/+8 cM and −3/+7 cM respectively), the two loci identified in this study are located between 8.55 and 21.55 cM and between 78.0 and 88.0 cM from the centromere on Chr 4. When these two loci were mapped simultaneously, their combined effect accounted for 22% of the phenotypic variation in sucrose consumption. Because total genetic variance in the F2 (based on difference between phenotypic variance in the F2 and nongenetic variance calculated from phenotypic variances in the parental strains and F1; Bachmanov et al. 1996a) for sucrose intake was estimated as 41% of phenotypic variance, the proportion of total genetic variance explained by these two loci was 53%.
Fig. 1.
Row 1. Average daily intake of 0.12 m (4%) sucrose solution in 96-h two-bottle tests expressed per 30 g of body weight (BW) in: (A) B6 (n = 14) and 129 (n = 13) mice; (B) their F2 hybrids with different genotypes for the D4Mit4 marker (B6/B6, n = 39; B6/129, n = 84; 129/129, n = 37); (C) their F2 hybrids with different genotypes for the D4Mit42 marker (B6/B6, n = 45; B6/129, n = 84; 129/129, n = 39). Row 2: Lowest sucrose concentration (threshold) that evoked chorda tympani response for: (D) B6 (n = 5) and 129 (n = 5) mice: (E) their F2 hybrids with different genotypes for the D4Mit4 marker (B6/B6, n = 8; B6/129, n = 4; 129/129, n = 8); (F) their F2 hybrids with different genotypes for the D4Mit42 marker (B6/B6, n = 7; B6/129, n = 7; 129/129, n = 7). Row 3: Magnitude of chorda tympani responses to different sucrose concentrations relative to 0.1 m NH4Cl in the same three groups as in the Row 2. Vertical bars represent standard errors. *p < 0.05 for effect of genotype by ANOVA.
Fig. 2.
(A) Chromosome 4 LOD scores for 0.12 m sucrose intake under free (unconstrained), B6 dominant, B6 recessive, and additive models. Dominance of the B6 allele is rejected for the locus on the proximal part of Chr 4 (the LOD score under the dominant model is more than 1.0 lower than that for the free model). The additive and recessive modes of inheritance are rejected for the B6 allele at the locus on the distal part of Chr 4. The dotted horizontal lines show thresholds for significant linkage (LOD = 4.3) and suggestive linkage (LOD = 2.8; Lander and Kruglyak 1995). (B) Correlations between the number of the B6 alleles (0, 1, 2) for the corresponding markers and threshold and relative magnitude of electrophysiological chorda tympani responses to different sucrose concentrations in selected F2 mice (n = 13 – 21). ns = p ≥ 0.05; * = p < 0.05; **p < 0.01; ***p < 0.001. The microsatellite markers and map distances between them in centimorgans (cM) are shown on the X axis. At the bottom of the figure, arrows show the loci positions at the peak of the LOD scores; the solid horizontal lines show confidence intervals for the loci (LOD Drops of 1.0).
The modes of inheritance for these two loci were confirmed when the effects of genotypes at D4Mit4 and D4Mit42 were analyzed separately. Mice homozygous for the B6 alleles at D4Mit4 consumed more sucrose than did mice homozygous for the 129 alleles [Fig. 1B; F(1,156) = 16.1, p = 0.0001, planned comparison test]. Heterozygotes at this locus were closer to the 129/129 homozygotes [F(1,156) = 1.65, ns (not significant)] than to the B6/B6 homozygotes [F(1,156) = 11.8, p = 0.0008], although their deviation from the average of the two homozygote means did not reach significance [F(1,156) = 1.68, ns]. B6/B6 homozygotes at D4Mit42 consumed more sucrose than did 129/129 homozygotes [Fig. 1C; F(1,164) = 20.8, p = 0.00001]. Sucrose intake by heterozygotes at D4Mit42 was similar to that of B6/B6 homozygotes [Fig. 1C; F(1,164) = 0.09, ns] and exceeded both the mean of the 129/129 homozygotes [F(1,164) = 23.2, p = 0.000003] and an average of the two homozygote means [F(1,164) = 8.05, p = 0.005].
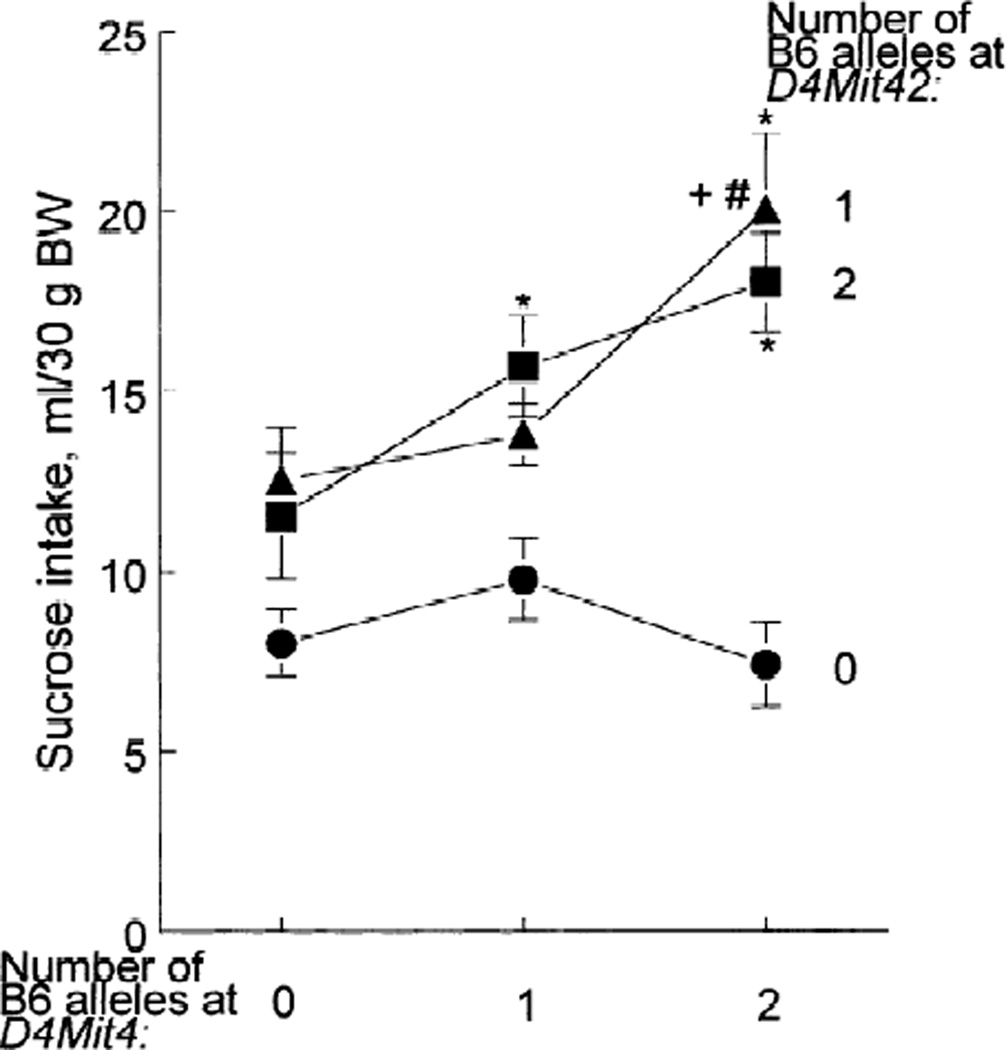
In order to estimate a possible interaction between the two loci, we analyzed the sucrose consumption of animals of nine genotypes (every combination of 129/129, 129/B6 and B6/B6 genotypes for the D4Mit4 and D4Mit42 markers; Fig. 3). The D4Mit4 genotype did not affect sucrose intake if animals had no B6 alleles at D4Mit42, and the D4Mit4 genotype had a bigger effect on sucrose intake if animals had one or two B6 alleles at D4Mit42. Similarly, the genotype for D4Mit42 did not significantly affect sucrose intake in animals with no B6 alleles at D4Mit4, and it did affect it in mice with one or two B6 alleles at D4Mit4. Therefore, the two loci closely linked to the D4Mit4 and D4Mit42 markers exert nonadditive effects on sucrose intake.
Fig. 3.
Average daily 0.12 m sucrose intake in 96-h two-bottle tests expressed per 30 g of body weight (BW) in F2 hybrids with different genotypes for the D4Mit4 and D4Mit42 markers (7 ≤ n ≤ 37 in each of the nine groups). Lines correspond to D4Mit42 genotypes, with numbers of the B6 alleles shown to the right. Each marker genotype affected sucrose consumption [D4Mit4: F(2,151) = 4.14, p = 0.018; D4Mit42: F(2,151) = 14.4, p = 0.000002; two-way ANOVA]. Although interaction between the D4Mit4 and D4Mit42 genotypes was marginally nonsignificant when all nine genotypes were analyzed [F(4,151) = 2.13, p = 0.08], it was significant when analysis of only four homozygous genotypes (combinations of 129/129 and B6/B6 genotypes for the two markers) was conducted [F(1,32) = 5.89, p = 0.02]. The group differences significant at p < 0.05 in the post hoc Tukey tests are shown as * (compared with D4Mit42 = 0 within the same D4Mit4 group), + (compared with D4Mit4 = 0 within the same D4Mit42 group), and # (compared with D4Mit4 = 1 within the same D4Mit42 group). Vertical bars represent standard errors.
Gustatory neural response to sucrose
Responses of the whole chorda tympani gustatory nerve to stimulation of the tongue with sucrose solutions were higher and detectable at lower sucrose concentrations in B6 than in 129 mice (Fig. 1D,G). Similar recordings of chorda tympani responses in 21 selected F2 mice revealed that responses to sucrose solution concentrations below 0.1 m (close to the electrophysiological threshold) correlated significantly with the number of B6 alleles at the markers on the proximal part of Chr 4, whereas responses to 0.1–1.0 m sucrose solutions correlated with the number of B6 alleles at the markers on the distal part of Chr 4 (Fig. 2B). Mice with two B6 alleles at the D4Mit4 marker (Fig. 1E, H) responded at lower concentrations of sucrose solution than did mice with two 129 alleles, but they did not differ at higher concentrations. Conversely, the responses of the mice with one or two B6 alleles at the D4Mit42 marker (Fig. 1F, I) did not differ from those of the mice with no B6 alleles at lower sucrose concentrations, but they were higher for 0.1–1.0 m sucrose solutions. In both correlational and ANOVA analyses, levels of significance required by Bonferroni corrections for multiple comparisons were attained: for the correlation between the D4Mit42 genotype and the response to 1 m sucrose, p < 0.0009; for the effect of the D4Mit4 genotype on response to 0.01 m sucrose, and for the effect of the D4Mit42 genotype on response to 1 m sucrose, p < 0.0033.
Discussion
We have identified two loci on mouse Chr 4 that accounted for over 50% of the genetic variability in sucrose intake and modulated peripheral neurophysiological responses to sucrose in male F2 hybrids of the B6 and 129 mouse strains. On the basis of our mapping data and other studies (Fuller 1974; Ninomiya et al. 1991; Phillips et al. 1994; Lush et al. 1995; Blizard and McClearn 1996), it is most likely that the two loci on Chr 4 correspond to the previously postulated dpa (proximal) and Sac (distal) loci. The LOD score attained by the locus on proximal Chr 4 corresponds to the level accepted for “suggestive linkage” (Lander and Kruglyak 1995). However, identification of linkage for sweet taste responses to the dpa locus in an independent experiment with a cross between different mouse strains (Ninomiya et al. 1991) provides additional evidence for the existence of this locus. No other obvious candidate genes that could be involved in sweet-taste responses are located near the locus on the proximal portion of Chr 4 (this region shows conserved synteny with the region p22-p32 of human Chr 9). The Gnb1 gene encoding one of the β-subunits of retinal transducin has been mapped (Danciger et al. 1990) on the distal portion of Chr 4 within 2 cM from the D4Mit42 marker. Alteration of a G protein α-subunit affected sweet-taste responses (Wong et al. 1996). Thus, a polymorphism at the β-subunit encoded by the Gnb1 gene also could possibly affect sweet-taste responsiveness. However, involvement of the Gnb1 in sweet-taste responses is difficult to evaluate, because no data are available about the Gnb1 polymorphism among mouse strains and about expression of the corresponding protein in the taste cells. The distal portion of mouse Chr 4, including Gnb1, is syntenic to the region p35-p36 of human Chr 1.
Our data indicate that the two loci on mouse Chr 4 influence different components of the neural response to sucrose. The proximal locus affects sensitivity (that is, response threshold), whereas the distal locus affects response magnitude at suprathreshold concentrations. It is possible that response sensitivity depends on properties of the sweet-taste receptor, whereas response magnitude depends on the intensity of the intracellular signal, with both mechanisms contributing to the behavioral response to sucrose. Interaction between the mechanisms encoded by the two loci on Chr 4 is evident from the nonadditive effects of these loci on sucrose consumption. This could be explained by an interaction between the taste receptor(s) and intracellular transduction, as well as by other mechanisms, but this remains to be tested. The non-additive effects of the two loci on sucrose consumption demonstrate that the effect of each locus depends on background genotype and therefore may vary across mouse strains.
Besides peripheral gustatory responsiveness, intake of sweeteners depends also on brain hedonic mechanisms (Gosnell and Majchrzak 1989; Melchior et al. 1991; Blass and Shah 1995; Koch et al. 1995), which may be common to that influencing consumption of alcohol and other euphoria-producing drugs (George et al. 1991). B6 mice consume large amounts of sweeteners (Lush 1989; Bachmanov et al. 1996a, 1996b), alcohol (Belknap et al. 1993; Bachmanov et al. 1996a, 1996b) and morphine (Forgie et al. 1988) compared with the other mouse strains, and genetic correlations have been found between sweetener and alcohol intake in mice (Belknap et al. 1993; Blizard and McClearn 1996; Bachmanov et al. 1996a), suggesting that these behaviors may be affected by a common mechanism. However, quantitative trait loci modulating alcohol and morphine preference of B6 mice have been localized to Chrs 1, 2, 6, 10, and 11, but not to Chr 4 (Berettini et al. 1994; Melo et al. 1996). Although these results do not exclude the existence of loci with pleiotropic effects on these behaviors, they indicate that there are independent mechanisms influencing high consumption of sucrose, alcohol, and morphine by B6 mice.
To summarize, our data demonstrate that a potentially complex behavior—48-h sucrose intake—is directly related to the electro-physiologically measured peripheral responsiveness to sucrose, which in turn is strongly influenced by two genetic loci on Chr 4.
Acknowledgments
This research was supported by National Institutes of Health grant DC 00882 from the National Institute on Deafness and Other Communication Disorders (G.K. Beauchamp), Howard Heinz Endowment (A.A. Bachmanov), Weight Watchers Foundation (D.R. Reed), NIH-R01-DK 44073 and R01-DK 48095 (R.A. Price). We thank R. Hughes and D. Pilchak for technical assistance, and Dr. D. Nagle for advice on DNA extraction.
References
- Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Intake of ethanol, sodium chloride, sucrose, critic acid and quinine hydrochloride solutions by mice: A genetic analysis. Behav Genet. 1996a;26:563–573. doi: 10.1007/BF02361229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 1996b;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacol. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Berettini WH, Ferraro TN, Alexander RC, Buchberg AM, Vogel WH. Quantitative trait loci mapping of three loci controlling morphine preference using inbred mouse strains. Nature Genet. 1994;7:54–58. doi: 10.1038/ng0594-54. [DOI] [PubMed] [Google Scholar]
- Blass EM, Shah A. Pain-reducing properties of sucrose in human newborns. Chem Senses. 1995;20:29–35. doi: 10.1093/chemse/20.1.29. [DOI] [PubMed] [Google Scholar]
- Blizard DA, McClearn GE. Sweet genes and ethanol intake in mice. Alcohol Clin Exp Res. 1996;20:25A. [PubMed] [Google Scholar]
- Bolles RC, editor. The Hedonics of Taste. Hillsdale, NJ: Lawrence Erlbaum; 1991. [Google Scholar]
- Danciger M, Farber DB, Peyser M, Kozak CA. The gene for the beta-subunit of retinal transducin (Gnb-1) maps to distal mouse chromosome 4, and related sequences map to mouse chromosomes 5 and 8. Genomics. 1990;6:428–435. doi: 10.1016/0888-7543(90)90472-7. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of lick rate measures: the positive and negative feedback effects of carbohydrates on eating. Appetite. 1988;11:229–238. doi: 10.1016/s0195-6663(88)80005-9. [DOI] [PubMed] [Google Scholar]
- Dietrich W, Katz H, Lincoln SE, Shin H-S, Friedman J, Dracopoli N, Lander ES. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992;131:423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich WF, Miller JC, Steen RG, Merchant M, Damron D, Nahf R, Gross A, Joyce DC, Wessel M, Dredge RG, Marquis A, Stein LD, Goodman N, Page DC, Lander ES. A genetic map of the mouse with 4,006 simple sequence length polymorphisms. Nature Genet. 1994;7:220–245. doi: 10.1038/ng0694supp-220. [DOI] [PubMed] [Google Scholar]
- Dobbing J, editor. Sweetness. London: Springer-Verlag; 1987. [Google Scholar]
- Forgie ML, Beyerstein BL, Alexander BK. Contributions of taste factors and gender to opioid preference in C57BL and DBA mice Psychopharmacol. 1988;95:237–244. doi: 10.1007/BF00174516. [DOI] [PubMed] [Google Scholar]
- Fuller JL. Single-locus control of saccharin preference in mice. J Hered. 1974;65:33–36. doi: 10.1093/oxfordjournals.jhered.a108452. [DOI] [PubMed] [Google Scholar]
- George SR, Roldan L, Lui A, Naranjo CA. Endogenous opioids are involved in the genetically determined high preference for ethanol consumption. Alcohol Clin Exp Res. 1991;15:668–672. doi: 10.1111/j.1530-0277.1991.tb00576.x. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Majchrzak MJ. Centrally administered opioid peptides stimulate saccharin intake in nondeprived rats. Pharmacol Biochem Behav. 1989;33:805–810. doi: 10.1016/0091-3057(89)90474-7. [DOI] [PubMed] [Google Scholar]
- Hogan B, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1986. [Google Scholar]
- Koch JE, Glass MJ, Cooper ML, Bodnar RJ. Alterations in deprivation, glucoprivic and sucrose intake following general, mu and kappa opioid antagonists in the hypothalamic paraventricular nucleus of rats. Neuroscience. 1995;66:951–957. doi: 10.1016/0306-4522(95)00001-y. [DOI] [PubMed] [Google Scholar]
- Lander ES, Botstein D. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E, Green P, Abrahamson J, Barlow A, Daley M, Lincoln S, Newburg L. MAPMAKER: An interactive complex package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Lander ES, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage findings. Nature Genetics. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Lush IE. The genetics of tasting in mice. VI. Saccharin, acesulfame, dulcin and sucrose. Genet Res. 1989;53:95–99. doi: 10.1017/s0016672300027968. [DOI] [PubMed] [Google Scholar]
- Lush IE, Hornigold N, King P, Stoye JP. The genetics of tasting in mice. VII. Glycine revisited, and the chromosomal location of Sac and Soa. Genet Res. 1995;66:167–174. doi: 10.1017/s0016672300034510. [DOI] [PubMed] [Google Scholar]
- Melchoir JC, Rigaurd D, Colas-Linhart N, Petiet A, Girard A, Apfelbaum M. Immunoreactive beta-endorphin increases after an aspartame chocolate drink in healthy human subjects. Physiol Behav. 1991;50:941–944. doi: 10.1016/0031-9384(91)90418-n. [DOI] [PubMed] [Google Scholar]
- Melo JA, Shendure J, Pociask K, Silver LM. Identification of sexspecific quantitative trait loci controlling alcohol preference in C57BL/6 mice. Nature Genet. 1996;13:147–153. doi: 10.1038/ng0696-147. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Fukami Y, Yamazaki K, Beauchamp GK. Amiloride inhibition of chorda tympani responses to NaCl and its temperature dependency in mice. Brain Res. 1996;708:153–158. doi: 10.1016/0006-8993(95)01218-4. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Sako N, Katsukawa H, Funakoshi M. Taste receptor mechanisms influenced by a gene on chromosome 4 in mice. In: Wysocki CJ, Kare MR, editors. Chemical Senses, vol 3: Genetics of Perception and Communication. New York NY: Marcel Dekker; 1991. pp. 267–278. [Google Scholar]
- Pfaffmann C. The sense of taste. In: Field J, Magoun HW, Hall VE, editors. Handbook of Physiology, Section 1: Neurophysiology. vol I. Washington DC: American Physiological Society; 1959. pp. 507–533. [Google Scholar]
- Phillips TJ, Crabbe JO, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Wong GT, Gannon KS, Margolskee RF. Transduction of bitter and sweet taste by gustducin. Nature. 1996;381:796–800. doi: 10.1038/381796a0. [DOI] [PubMed] [Google Scholar]