Abstract
Background
Recently, several studies reported that the cancer incidence in type 2 diabetes patients is higher than in the general population. Although a number of risks are shared between cancer and diabetes patients, there have been few studies of its correlation. We evaluated the influences of several factors including low density lipoprotein cholesterol (LDL-C), albuminuria and use of metformin on the risk of cancer in patients with type 2 diabetes.
Methods
We enrolled 1,320 patients with at least 5 years of follow-up and 73 patients were diagnosed with cancer during this period. The associations of the risk factors with cancer incidence were evaluated by multiple regression analysis. The subjects were placed into two subgroups based on metformin dosage (<1,000 mg/day, ≥1,000 mg/day) and we compared cancer incidence using analysis of covariance.
Results
LDL-C and albuminuria were not significantly correlated with cancer risk. In contrast, metformin showed a reverse correlation with cancer risk (P=0.006; relative risk, 0.574). In the metformin nonadministration group, smoking, male gender, and high triglyceride levels tended to be contributing factors without statistical significance. Cancer occurence was lower in the low dose metformin group (less than 1,000 mg/day) (P=0.00).
Conclusion
These results suggest that the administration of low dose metformin in patients with type 2 diabetes may be associated with a reduced risk of cancer.
Keywords: Diabetes mellitus, type 2; Metformin; Neoplasms
INTRODUCTION
The prevalence of diabetes mellitus and cancer are on the rise throughout the world. According to a World Cancer Report [1], the estimated number of cancer diagnoses was 124 million in 2008 with highest rates in lung, breast, colon and rectal cancer and the highest mortality in lung, stomach and liver cancer. The prevalence of cancer is estimated to be approximately 6.6% and, the morbidity of diabetes was 10.7% in 2007, of which type 2 diabetes accounted for about 95% in the United States [2]. Cancer is the second largest cause of death in the world and diabetes is the twelfth [3]. In Korea, cancer is the leading cause of death and diabetes is the fifth largest cause of death [4].
According to a number of epidemiologic studies, the risk of cancer is significantly higher in patients with diabetes [5,6]. Joslin et al. [7] stated, "Studies of the association of diabetes and cancer have been conducted over a period of years, but evidence of a positive relation remains inconclusive." However, a study in Switzerland on type 1 diabetes reported that cancer incidence in patients with diabetes was more than 20% higher than in patients without diabetes [8]. Also, Park et al. [9] reported that the mortality of cancer rose from 4.7% to 21.9% over the past 10 years in patients with diabetes in Korea. Cancer and diabetes share a number of risk factors such as hyperglycemia, hyperinsulinemia, and dyslipidemia, but they cannot wholly elucidate the correlation between these two diseases [10]. There are also numerous reports of studies on the association between cancer risk and hypoglycemic agents [6, 11,12], which is still under debate. Metformin, in particular, was found to have a reduced cancer risk in a controlled study of Scottish patients [13]. Jiralerspong et al. [14] reported that diabetic patients with breast cancer receiving metformin and neoadjuvant chemotherapy have a higher pathologic complete response rate than diabetic patients not receiving metformin. Another study reported that the association between low density lipoprotein cholesterol (LDL-C) and cancer was V-shaped among patients with type 2 diabetes [15]. In the present study, we investigated the influences of LDL-C, albuminuria and the use of metformin on the cancer risk in patients with type 2 diabetes.
METHODS
Subjects
Diabetes patients at the Division of Endocrinology and Metabolism of Yeungnam University Medical Center from January 2003 to December 2009 were included in this study. Among 1,320 patients with a diabetes history of 5 years or more, 1,217 patients were finally included in this retrospective observational study, excluding 103 patients who had type 1 diabetes, or had already been diagnosed with cancer before the commencement of this study, or whose data were insufficient. The study protocol was approved by the Institutional Review Board of Yeungnam University Medical Center.
Methods
Blood pressure (BP) was measured with a standardized sphygmomanometer after at least 10 minutes of rest. Measurements of LDL-C were taken and body mass index (BMI) was calculated by dividing the weight (kg) with the height squared (m2). The microalbuminuria test and liver function test were performed at baseline. History of smoking, drinking, and medication (metformin, insulin, angiotensin-converting-enzyme inhibitors, and statin) were taken from the medical records at the hospital. Measurements of fasting blood glucose, bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transferase (GGT), blood urea nitrogen, and creatinine were taken using the hexokinase method (AU 5400 Autoanalyser; Olympus, Tokyo, Japan). Total cholesterol was measured using enzyme colorimetry (Kyowa Medex Co., Ltd., Tokyo, Japan) and triglyceride was measured using the glycerol elimination method. High density lipoprotein cholesterol (HDL-C) and LDL-C were measured using the direct enzymatic assay (Kyowa Medex Co., Ltd.). Glycated hemoglobin (HbA1c) and microalbuminuria were performed using HLC-723G7 (Tosoh, Tokyo, Japan) with high performance liquid chromatography.
Statistical analysis
Statistical analyses were performed using the statistical program IBM PASW version 18.0 for Windows (IBM, Armonk, NY, USA) and values were expressed as mean±standard deviation. Causal analysis was performed to examine the confounding, inhibiting, and interactive factors among the risk factors for cancer occurrence. Multiple regression analysis was performed to identify significant predicting factors for cancer occurrence among the factors with a Kaiser-Meyer-Olkin measure of 0.6 or higher. Also, the Student's t-test and chi-square test were used to compare the significance in the metformin administration group and nonadministration group and analysis of covariance was performed to compare the difference of cancer occurrences between the two groups for different doses of metformin. In all statistical analysis, P<0.05 with a 95% confidence interval was applied in determining the significance.
RESULTS
Clinical characteristics and prevalence of cancer in subjects
There were 1,217 subjects in this study consisting of 586 males (48.6%) and their mean age was 62.9±11.4 years. The mean BMI was 23.5±3.2 kg/m2 and the mean period of diabetes history 12.1±5.3 years. The 44.9% of all subjects had a history of smoking and the mean HbA1c was 8.4%±1.9%. A lipid assay showed that a mean LDL-C of 104.2±36.7 mg/dL, HDL-C of 52.2±15.9 mg/dL, and triglyceride of 157.3±136.5 mg/dL. Microalbuminuria was detected in 47.7% of all subjects and the glomerular filtration rate (GFR) was 77.1±26.7 mL/min/1.73 m2. Smoking history and LDL-C were higher in males, while triglyceride levels were higher in females. The 656 patients (53.9%) were on metformin, 521 (42.5%) on insulin, and 672 (55.2%) on statin (Table 1). Of all 1,217 patients, cancer was diagnosed in 71 patients (5.6%) consisting of gastric cancer (n=15), thyroid cancer (n=11), prostate cancer (n=8), hepatocellular carcinoma (n=8), pancreatic cancer (n=3), and colon cancer (n=3). Among 15 patients with stomach cancer, 11 patients had Helicobacter pylori infection. There were three patients with hepatitis B virus infection of the hepatocellular carcinoma (n=5), but no patients with a positive antihepatitis C virus antibody.
Table 1.
Baseline characteristics
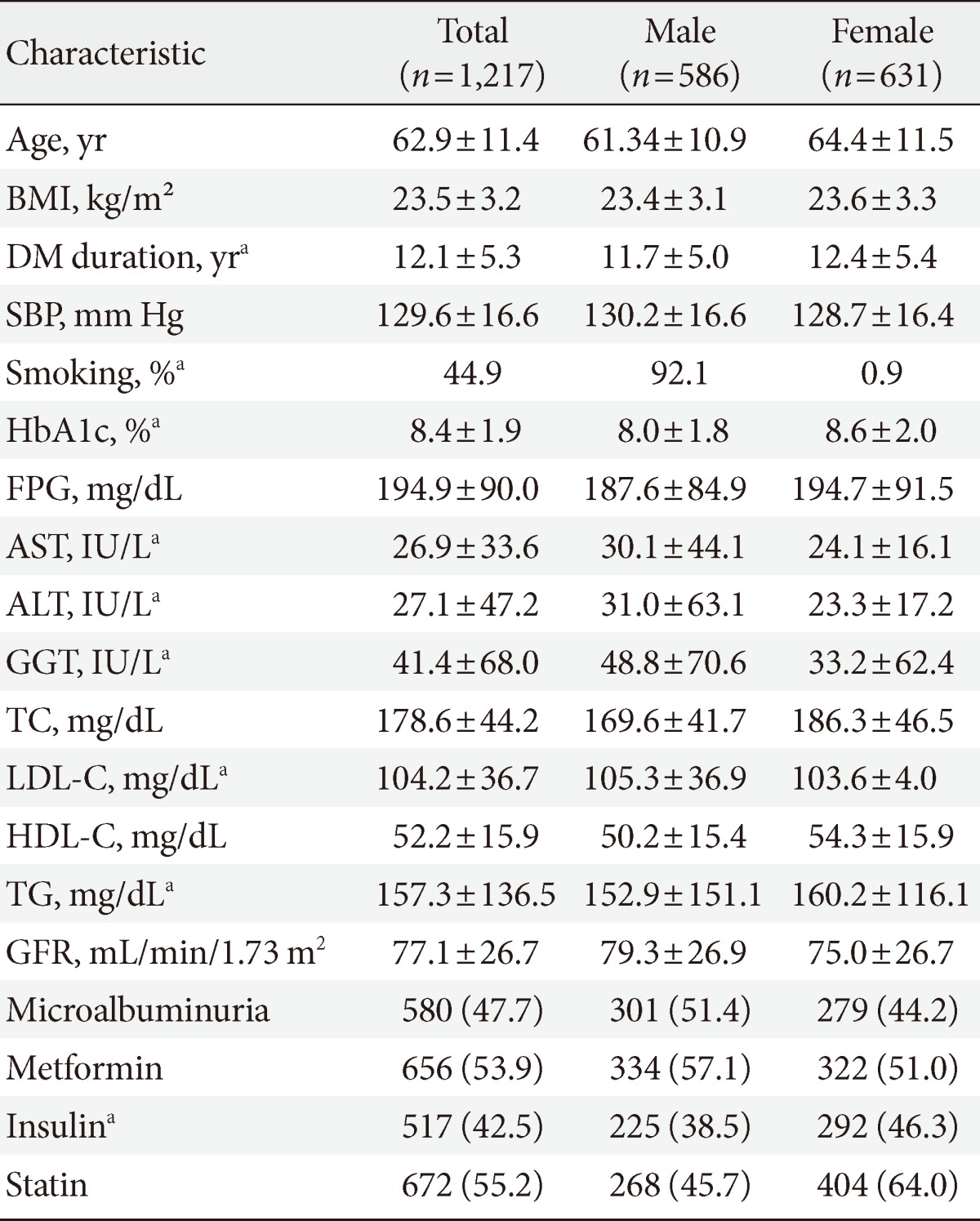
Values are presented as mean±standard deviation or number (%).
BMI, body mass index; DM, diabetes mellitus; SBP, systolic blood pressure; HbA1c, glycated hemoglobin; FPG, fasting plasma glucose; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl transferase; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglyceride; GFR, glomerular filtration rate.
aP<0.05.
Correlations between cancer risk and several other factors
Causal analysis was performed to investigate the factors in type 2 diabetes patients that can affect the occurrence of cancer Regression analysis on factors of LDL-C, microalbuminuria, and metformin, which were selected based on a Kaiser-Meyer-Olkin measure of 0.6, showed that only metformin had a significant correlation with cancer occurrence (P=0.006; relative risk [RR], 0.574) (Table 2).
Table 2.
The risk of cancer among patients with type 2 diabetes
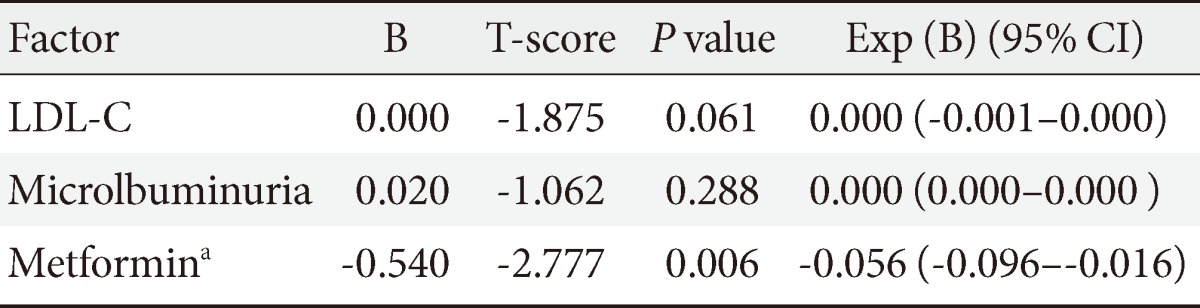
Multivariate linear regression analysis.
CI, confidence interval; LDL-C, low density lipoprotein cholesterol.
aP<0.05.
Clinical characteristics of subjects with or without metformin administration
In order to examine other factors besides metformin that may affect the occurrence of cancer, causal analysis was performed by dividing the subjects into a metformin administration group (n=551) and nonadministration group (n=656) (Table 3). In cross-sectional analysis, there was no difference in BMI, duration of diabetes, systolic and diastolic BP, HbA1c, and fasting blood glucose, but history of smoking and drinking showed higher levels in the metformin administration group. The levels of ALP, LDL-C, high sensitivity C-reactive protein, and microalbuminuria were significantly higher in the nonadministration group, while triglyceride and creatinine levels were higher in the administration group. Regression analysis was then performed on smoking history, total cholesterol, triglyceride, LDL-C, length of diabetes history, systolic pressure, which were selected based on a Kaiser-Meyer-Olkin measure of 0.6, and results revealed no factor of significance (Table 4).
Table 3.
Baseline characteristics grouped by metformin use
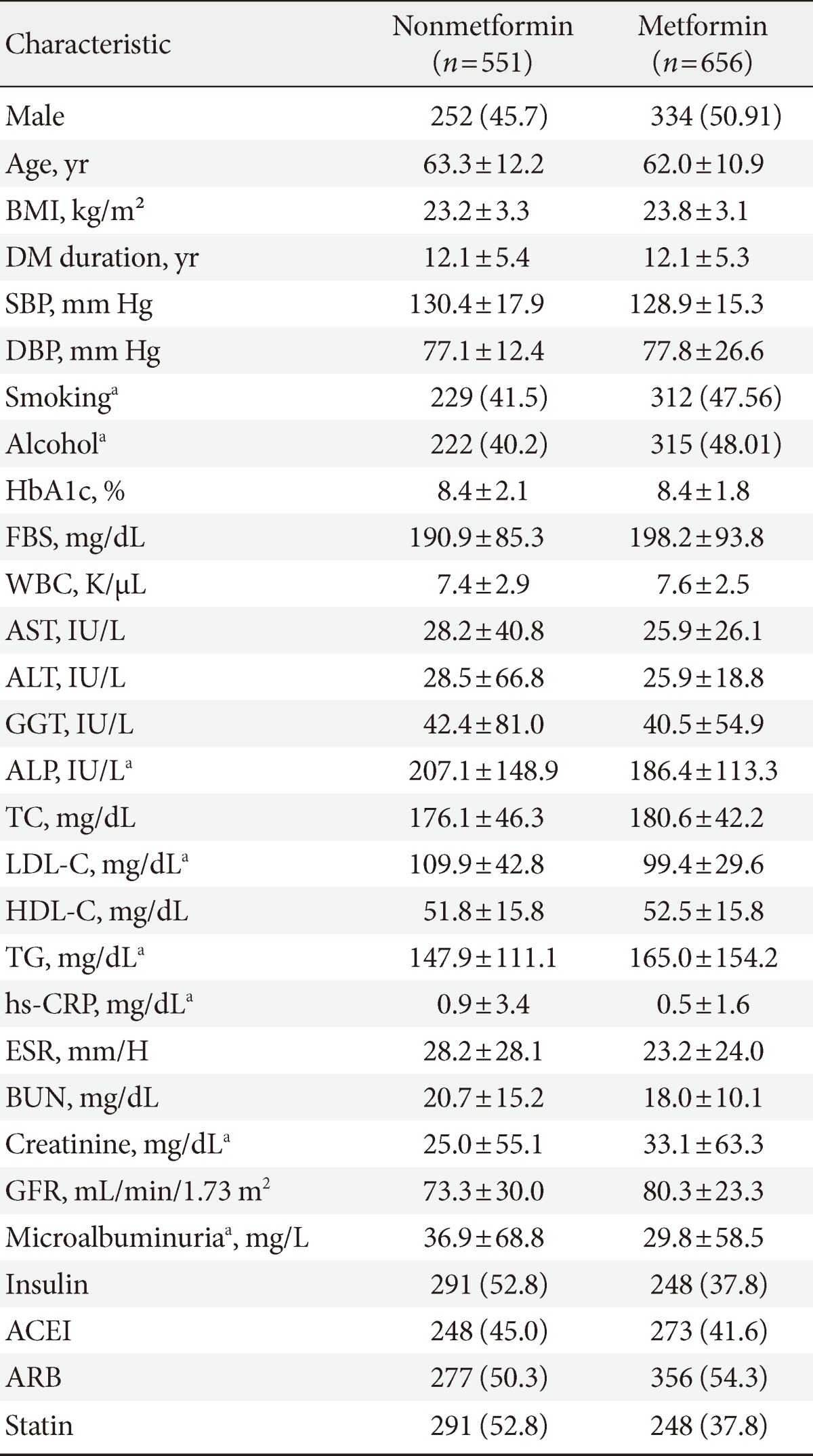
Values are presented as mean±standard deviation or number (%).
BMI, body mass index; DM, diabetes mellitus; SBP, systolic blood pressure; HbA1c, glycated hemoglobin FBS, fasting blood sugar; WBC, white blood cell; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyl transferase; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglyceride; hs-CRP, high sensitivity C-reactive protein; BUN, blood urea nitrogen; GFR, glomerular filtration rate; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers.
aP<0.05.
Table 4.
The risk of cancer among patients with type 2 diabetes mellitus who did not use metformin
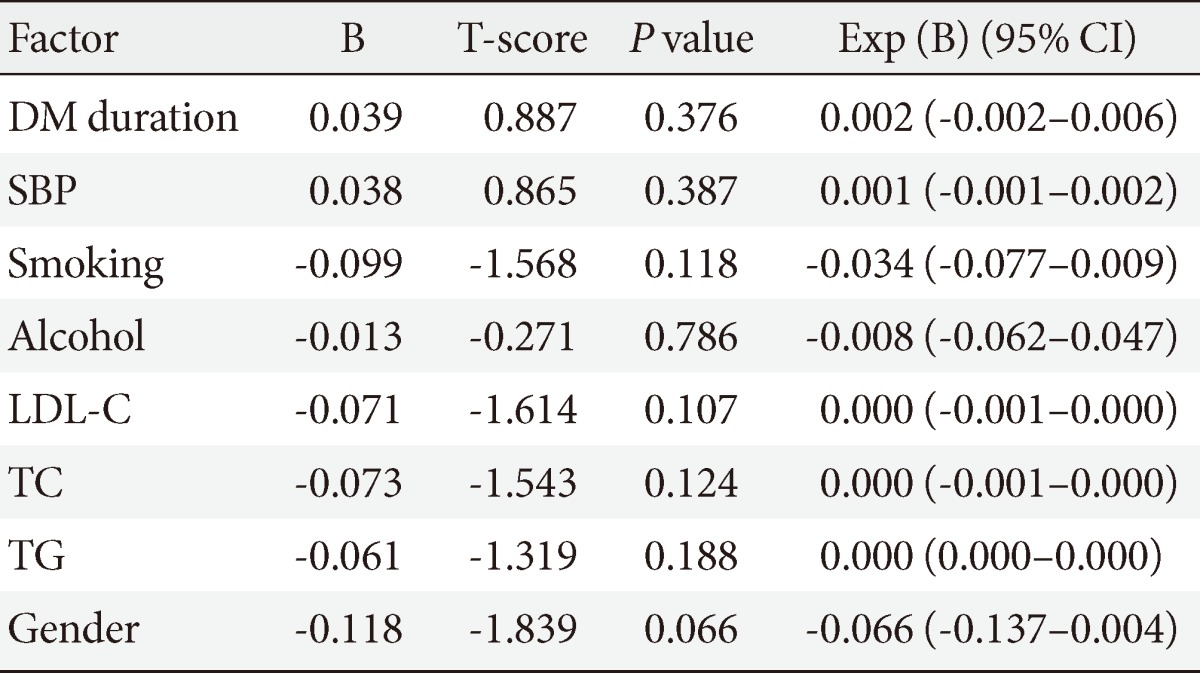
Multivariate linear regression analysis.
CI, confidence interval; DM, diabetes mellitus; SBP, systolic blood pressure; LDL-C, low density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Cancer occurence by metformin dose
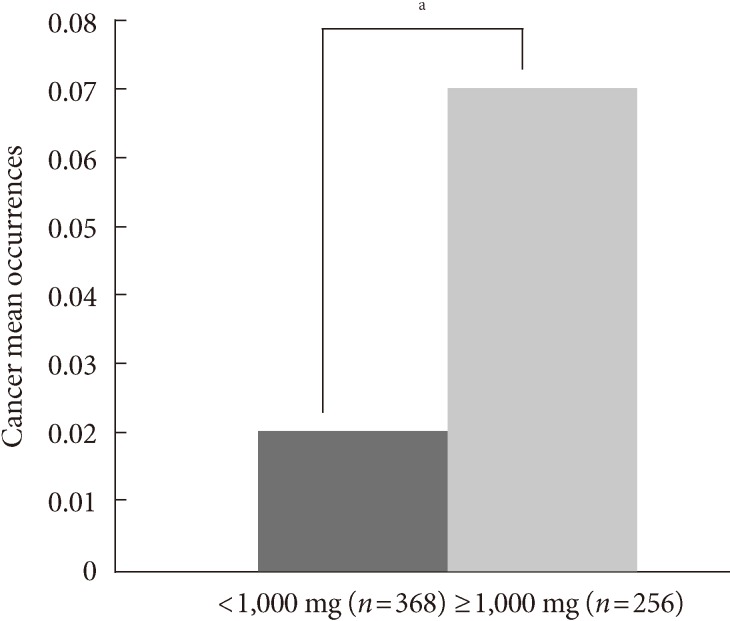
A Student's t-test showed no significant difference between the groups with different doses of metformin administration (<1,000 mg/day, ≥1,000 mg/day) in diabetes mellitus duration, systolic BP, fasting blood glucose, AST, ALT, GGT, GFR, total cholesterol, triglyceride, LDL-C, HDL-C, and medication history including insulin. However, there was a significant difference in HbA1c (8.3±1.7% vs. 8.2±0.2%, P=0.05). The low dose metformin group (<1,000 mg/day) showed a significantly lower cancer occurrence than the high dose group (≥1,000 mg/day, P<0.0001) (Fig. 1).
Fig. 1.
The relationship between metformin dose and cancer. The cancer occurrence rate mean was significantly lower in the low dose metformin group (<1,000 mg/day) compared to the high dose group (≥1,000 mg/day). aP<0.001.
DISCUSSION
According to recent studies on diabetes and cancer risk, patients with diabetes are known to have a higher risk of liver, pancreatic, colorectal, breast and endometrial cancer, while prostate cancer has been reported to have reduced incidence in patients with diabetes [10]. Hyperinsulinemia is suggested as a possible mechanism that increases the occurrences of liver and pancreatic cancer because the liver and pancreas are exposed to a higher endogenous insulin concentration via portal circulation [6]. Diabetes-related factors such as adiposity, nonalcoholic fatty liver, and liver cirrhosis were also reported to have the potential to increase the occurrence of liver cancer. On the contrary, the RR of prostate cancer in diabetes is known to be lower and this is most likely attributed to the decreased testosterone levels in diabetic patients [16]. In our study, the cancer incidence was 5.6% in order of gastric, thyroid, prostate, hepatocellular, pancreatic, and colon cancer. Compared with the Statistics Korea [4], our results showed higher incidences in pancreatic cancer (3.7% vs. 2.5%) and hepatocellular carcinoma (11.2% vs. 7.7%), while no reduced risk of prostate cancer (11.2% vs. 2.9%) was observed, which may be attributable to the higher mean age of the subjects in our study.
The common potential risk factors for cancer and diabetes include increased age, gender, obesity, physical activity, diet, drinking, and smoking. Individuals that are overweight (25≤BMI<30 kg/m2) or obese (BMI ≥30 kg/m2) are reported to have a higher cancer risk than those with a normal BMI [5,17]. In particular, obesity was consistently associated with breast (among women in menopause), colorectal, endometrial, pancreatic, esophageal, renal, and gallbladder cancer and increased the mortality of some cancers including prostate cancer [10,17]. Among these risk factors, we performed causal analysis using BMI, smoking and drinking factors but the results did not show any significant relationship. In order to eliminate the influence of metformin, we divided the subjects into a metformin administration group and nonadministration group and performed a regression analysis on the nonadministration group, but the results were still not significant.
The risk association between LDL-C and cancer remains controversial [15,18]. Although its mechanism is not known, the pathway of L-mevalonic acid synthesis, which produces isoprenoid intermediates of the cholesterol biosynthetic pathway such as farnesylpyrophosphate and geranylgeranylpyrophosphate, has been suggested. These intermediates are important for posttranslational modification of proteins, such as Ras and Rho GTPases, which play important roles in cellular pathways that are critical for cancer formation and progression [19]. In addition, it has been suggested that HMG-CoA reductase inhibitors (statins) have antitumor effects [19]. In our study, no significant correlation was observed between LDL-C and cancer incidence, which is assumed to be related to the low LDL-C levels among the subjects and antitumor effects of statins in part because 55.2% of subjects were on statin medication.
It has been suggested that the use of insulin and sulfonylurea increases cancer risk [10,20], whereas metformin reduces it [13]. However in our study, the use of insulin or sulfonylurea did not show significant correlation with cancer occurrence, which coincided with a recent cohort study conducted in France in which the use of insulin glargine did not increase the risk of cancer in patients with type 2 diabetes [21]. On the other hand, metformin was also observed to have significantly reduced the cancer risk by 43% in our study. Metformin is the most frequently used oral hypoglycemic agent and the glucose lowering effect is mediated by decreasing hepatic gluconeogenesis [22]. Metformin is known to inhibit cellular proliferation, reduce colony formation, and cause partial cell cycle arrest of cancer cells [11]. Dowling et al. [23] suggested that metformin-mediated AMP-activated protein kinase (AMPK) activation leads to inhibition of mammalian target of rapamycin (mTOR) and a reduction in translation initiation, thus providing a possible mechanism of action of metformin in the inhibition of cancer cell growth. Metformin attenuated the increased insulin receptor activation associated with a high-energy diet and also led to increased phosphorylation of AMP kinase, leading to decrease neoplastic proliferation [24]. These results, therefore, contribute to the rationale for evaluation of antineoplastic activity of metformin in hyperinsulinemic cancer patients. The association between metformin doses and cancer risk is the subject of much debate. Libby et al. [25] reported that high maximum doses of metformin were related with a large reduction in the risk of cancer. However, metformin dose usually increases with increasing duration of use and this could produce a survival bias, with higher doses spuriously associated with reduced cancer because patients have survived to receive a higher dose. In contrast, it was observed in our study that cancer incidence was significantly lower in the low dose group (≤1,000 mg/day). We consider this finding a new result for several reasons. First, there was a significant difference in glycosylated hemoglobin between the two groups (P=0.05) and no significant intergroup differences were observed for other factors such as the duration of diabetes and BMI. These results imply that the state of blood glucose control can affect the incidence of malignancy. Second, the incidence of malignancy may be more closely related with the 'total exposure amount' of metformin rather than daily dose, so the days with using metformin will be needed to clarify the dose-response relationship. However, practically we cannot evaluate the accurate duration and amount of metformin in this study and thus, further studies are needed. Finally as stated above, a survival bias can be included in this result.
This study had several limitations. First, being a retrospective observation study, we could not clearly determine the correlation between diabetes and cancer risk. Second, investigation of morbidity and mortality was limited because of the short follow-up duration of 6 years. The lack of correlation of the interaction between drugs and the small number of subjects was also a limitation of our study.
Based on our results, it is suggested that metformin administration in patients with type 2 diabetes reduces the risk of cancer and low dose metformin may be associated with a reduced risk of cancer as well as blood glucose control. Large-scale prospective long-term studies will be needed for further clarification of the correlation between diabetes and the occurrence of cancer.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.International Agency for Research on Cancer: World cancer report 2008. [cited 2010 Apr 1]. Available from: http://www.iarc.fr/en/publications/pdfs-online/wcr/2008/index.php.
- 2.Centers for Disease Control and Prevention: General information and national estimates on diabetes in the United States, 2007. [cited 2010 Apr 1]. Available from: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf.
- 3.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 4.Statistics Korea: Statistics of cause of death. [updated 2010 Sep 9]. Available from: http://www.kostat.go.kr.
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Chari ST, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, Petersen GM. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology. 2008;134:95–101. doi: 10.1053/j.gastro.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joslin EP, Lombard HL, Burrows RE, Manning MD. Diabetes and cancer. N Engl J Med. 1959;260:486–488. doi: 10.1056/NEJM195903052601007. [DOI] [PubMed] [Google Scholar]
- 8.Zendehdel K, Nyren O, Ostenson CG, Adami HO, Ekbom A, Ye W. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst. 2003;95:1797–1800. doi: 10.1093/jnci/djg105. [DOI] [PubMed] [Google Scholar]
- 9.Park SK, Park MK, Suk JH, Kim MK, Kim YK, Kim IJ, Kang YH, Lee KJ, Lee HS, Lee CW, Kim BH, Lee KI, Kim MK, Kim DK. Cause-of-death trends for diabetes mellitus over 10 years. Korean Diabetes J. 2009;33:65–72. [Google Scholar]
- 10.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 11.Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, Thor AD. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 12.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 13.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi GN, Gonzalez-Angulo AM. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X, So W, Ko GT, Ma RC, Kong AP, Chow CC, Tong PC, Chan JC. Independent associations between low-density lipoprotein cholesterol and cancer among patients with type 2 diabetes mellitus. CMAJ. 2008;179:427–437. doi: 10.1503/cmaj.071474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 17.World Cancer Research Fund, American Institute for Cancer Research: Food, nutrition, physical activity, and the prevention of cancer: a global perspective. [updated 2007 Nov 1]. Available from: http://www.dietandcancerreport.org.
- 18.Schuit AJ, Van Dijk CE, Dekker JM, Schouten EG, Kok FJ. Inverse association between serum total cholesterol and cancer mortality in Dutch civil servants. Am J Epidemiol. 1993;137:966–976. doi: 10.1093/oxfordjournals.aje.a116769. [DOI] [PubMed] [Google Scholar]
- 19.Liao JK. Clinical implications for statin pleiotropy. Curr Opin Lipidol. 2005;16:624–629. doi: 10.1097/01.mol.0000191913.16321.60. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, So WY, Ma RC, Yu LW, Ko GT, Kong AP, Ng VW, Luk AO, Ozaki R, Tong PC, Chow CC, Chan JC. Use of sulphonylurea and cancer in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Res Clin Pract. 2010;90:343–351. doi: 10.1016/j.diabres.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Blin P, Lassalle R, Dureau-Pournin C, Ambrosino B, Bernard MA, Abouelfath A, Gin H, Le Jeunne C, Pariente A, Droz C, Moore N. Insulin glargine and risk of cancer: a cohort study in the French National Healthcare Insurance Database. Diabetologia. 2012;55:644–653. doi: 10.1007/s00125-011-2429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 24.Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocr Relat Cancer. 2008;15:833–839. doi: 10.1677/ERC-08-0038. [DOI] [PubMed] [Google Scholar]
- 25.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–1625. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]