Abstract
Uncontrolled asthma is a major cause of hospitalizations and emergency room visits. Factors including obesity, African ancestry and childhood are associated with increased asthma severity. Considering the high morbidity caused by asthma, relatively few classes of drugs exist to control this common disease. Therefore, new therapeutic strategies may be needed to reduce asthma’s impact on public health. Data suggest that a high fat diet that is deficient in omega-3 fatty acids could promote both obesity and excessive inflammation, resulting in greater asthma severity. Small trials with supplemental omega-3 fatty acids have been conducted with encouraging but inconsistent results. The variability in response seen in past trials may be due to the past subjects’ genetics (specifically ALOX5 rs59439148) or their particular asthma phenotypes. Therefore, the “Nutrigenetic response to Omega-3 Fatty acids in Obese Asthmatics (NOOA)” trial is currently underway and was designed as a randomized, double-blind, placebo controlled intervention study to determine if supplemental omega-3 fatty acids improves symptoms among obese adolescents and young adults with uncontrolled asthma. Here we report the design and rationale for the NOOA trial. Participants were given either 3.18g daily of eicosapentaenoic acid and 822mg daily docosahexaenoic acid, or matched control soy oil, for 24 weeks. Change in the asthma control questionnaire score was the primary outcome. Secondary outcomes included spirometry, impulse oscillometry, exacerbation rate, airway biomarkers, systemic inflammation, leukotriene biosynthesis and T-lymphocyte function. NOOA may lead to a new therapeutic treatment strategy and greater understanding of the mechanistic role of diet in the pathogenesis of asthma.
Keywords: Asthma Control, Obesity, Children, Nutrigenetics, Omega-3 Fatty Acids
1. INTRODUCTION
Asthma is a common, complex disease of the bronchial airways that involves diverse underlying mechanisms and clinical phenotypes [1, 2]. Uncontrolled asthma symptoms continue to cause impaired quality of life and urgent healthcare utilization. Factors such as obesity and younger age are risk factors for poor symptom control. Relatively few classes of pharmacologic medications exist to control this common disease. Therefore, new therapeutic interventions to facilitate improved asthma control are greatly needed, particularly for subgroups with severe disease.
External factors such as diet and obesity-status may alter the risk for incident asthma and may worsen existing disease. Obesity is associated with incident asthma [3, 4], greater asthma-related symptoms, and altered treatment response [5, 6]. In addition, a poor diet (low in vegetables and fish, and high in saturated fats and omega-6 fatty acids) has been associated with obesity[7] and onset of asthma symptoms[8–10]. Observational studies note a lower rate of asthma among populations consuming high amounts of cold-water fish [11–15]. Cold-water fish are rich in the omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Plasma and intracellular concentrations of EPA and DHA increase following ingestion of omega-3 fatty acid [16–19] and increase within inflammatory cell phosholipid membranes. Supplementation also increases the ratio of omega-3 versus omega-6 polyunsaturated fatty acids (PUFA) within cell phosholipid membranes[20–24]. There exist several plausible mechanisms by which EPA and DHA may reduce inflammation important in asthma. First, omega-3 PUFAs serve as a competitive inhibitor of the arachidonic acid cascade enzymes phospholipase A2, 5-lipoxygenase and cyclooxygenase. Greater omega-3 PUFA availability diverts from inflammatory pathways that lead to eicosanoids (leukotrienes, thromboxanes and prostaglandins) which have asthma-promoting features[25]. Next, EPA and DHA inhibit production of TNF-alpha [17, 20, 26] and IL-1[20, 26–28] through altered NF-kappaB activity and reduced gene transcription. EPA/DHA may affect T-regulatory cell (Treg) production and activity [29] , FoxP3 expression[30–32], and effector T-lymphocyte proliferation[33–35]. Finally, omega-3 PUFAs are precursor molecules to the anti-inflammatory mediators, resolvins and protectins[36–40], which function to curtail neutrophil chemotaxis, and reduce chemokine and cytokine production[38, 39]. All of these molecular mechanisms are relevant to asthma.
Supplementation of the diet of asthmatics with omega-3 PUFA has been evaluated in several small trials (see table 1), the results of which are encouraging but inconsistent[16, 41–43]. Several studies report significant asthma-related improvements [16, 19, 22, 25, 44–48]. The inconsistent past results could be explained by inadequate dose or duration of EPA/DHA, or by reduced response among participants with polymorphisms in arachidonic acid pathway genes. The ALOX5 promoter polymorphism rs59439148 has been shown to be an important modifier of treatments acting on the leukotriene pathway[49] including fish oil[50]. Further studies involving a longer duration of treatment and involving consideration of possible nutrigenetic effects are needed.
Table 1.
Asthma Trials involving omega-3 fatty acids
| Study | age | Size (n) | Daily EPA/DHA dose (g)1 | Control agent | duration | Effect seen |
|---|---|---|---|---|---|---|
| Hodge, 1998 | 8–12 | 39 | .72/.036 | Safflower/Sunflower | 6 months | No |
| Broughton, 1997 | 19–25 | 19 | -- | -- | 1 month | Reduced AHR |
| Arm, 1988 | 15–42 | 25 | 3.2/2.2 | Olive oil | 10 weeks | Reduced inflammation |
| Arm, 1989 | 15–42 | 17 | 3.2/2.2 | Olive oil | 10 weeks | Lung function |
| Thien, 1993 | 15–65 | 25 | 3.2/2.2 | Olive oil | 6 months | No |
| Surette, 2003 | 18–56 | 43 | .5–.75/-- | -- | 4 weeks | Reduced LT production |
| Emelyanov, 2002 | 18–56 | 23 | Combined | Olive oil | 8 weeks | Reduced symptoms |
| Mickleborough, 2006 | Mean = 23 | 16 | 3.2/2.0 | Olive oil | 3 weeks | Lung function |
AHR – airway hyperresponsiveness, LT – leukotriene.
Institute of Medicine’s adequate intake for omega-3 polyunsaturated fatty acids is 1.1 and 1.6 grams/day for females and males, respectively (IOM report, 2002). A typical serving of Salmon contains just less than 1 gram of EPA + DHA.
Therefore, the “Nutrigenetic response to Omega-3 Fatty acids in Obese Asthmatics (NOOA)” trial was designed as a randomized, double-blind, placebo controlled 24 week intervention study to determine if supplemental omega-3 fatty acids improves symptoms among obese adolescents and young adults with uncontrolled asthma. NOOA will measure change in asthma control questionnaire score as its primary outcome, while evaluating nutrigenetics, safety and tolerability.
2. STUDY DESIGN AND RATIONALE
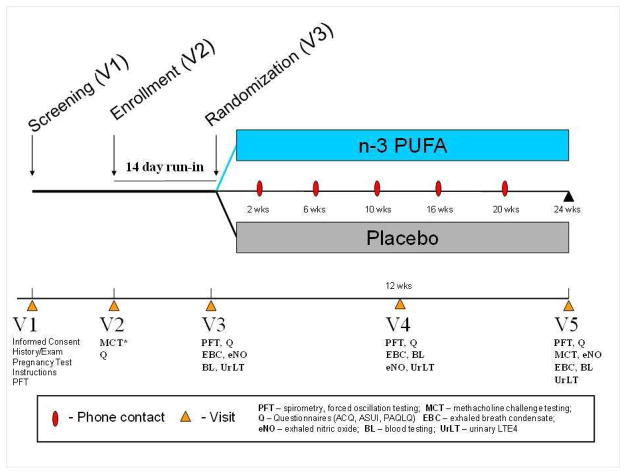
The NOOA study is a controlled, 24-week parallel group intervention trial involving 100 obese children and young adults with asthma randomized to either omega-3 PUFA treatment (3.18g EPA, 822mg DHA per day) or similar soy oil control (fig 1). The study was reviewed by the National Institutes of Health (NIH), the Food and Drug Administration (FDA) and by the Institutional Review Boards (IRB) at all participating sites.
Figure 1.
Schema for the Nutrigenetic response to Omega-3 fatty acids in Obese Asthmatics (NOOA) trial. NOOA is a 24-week randomized, controlled, double-blinded parallel intervention trial. Acronyms not depicted above: ACQ – asthma control questionnaire, ASUI – asthma symptom utility index, PAQLQ – paediatric asthma quality of life questionnaire.
2.1 Study Cohort and Rationale
NOOA selection criteria (Table 2) were established to study obese asthmatic adolescents and young adults with inadequately controlled asthma. Trained nurse coordinators measured height, weight and waist circumference at visit 1 and subsequent visits using standardized procedures[51, 52]. We utilized body mass index (BMI)-based CDC guidelines for the definition of obesity[53, 54]. We included only participants with a waist-circumference above the 90th percentile to target participants with central obesity[53]. We enrolled only asthma patients with recent inadequate control (Table 3) similar to recent large asthma trials[55].
Table 2.
Participant Selection
Criteria for Enrollment - Inclusion
|
Criteria for Enrollment – Exclusion (participant cannot have any of the following):
|
Table 3.
Definition of Inadequately Controlled Asthma
Participants were considered to have evidence of inadequately controlled asthma if one or more of the following criteria were met.
|
2.2 Study Medication and Dosing
Participants were randomized to either oral omega-3 PUFA supplementation (3.18g EPA, 822mg DHA, 101mg other omega-3 fatty acids), or similar weight control soy oil. The daily dose for both treatments comes in the form of six softgel capsules and was justified based on past studies (Table 1) and recent recommendations[56]. Nordic Naturals, Inc (NNI) (Watsonville, CA USA) was the supplier of active treatment (EPA/DHA) and control treatment (soy oil) and reports third-party testing results that show precision of dosing and purity from contaminants[57]. Testing of content purity was performed by Nutrasource Diagnostics, Inc (Guelph, Candada) which is a certified and accredited reference laboratory. Both EPA/DHA and soy oil treatments were similar in look, taste and feel..
2.3 Randomization Procedure
Eligible participants were monitored for 14–28 days prior to randomization to assess asthma control and asthma diary card adherence. Participants were randomized in a 3:1 ratio, with 3 participants receiving EPA/DHA for each participant receiving control soy oil. This unbalanced randomization scheme was devised to increase the power to detect a nutrigenetic effect of EPA/DHA. Participants were also stratified into one of two groups according to their BMI in order to ensure that there was a balanced distribution of participants with an extreme BMI between treatments assignments. The randomization scheme was generated using the SAS procedure PROC PLAN. A block randomization was used (instead of a completed randomized design) by dividing subjects into 2 BMI blocks or strata (details of BMI strata exist in the online supplement). Neither participants nor the research staff were informed of the treatment group assignment to maintain the double-mask. The randomization scheme was devised by our statistical consultant (YG), and was controlled and implemented by an unblinded on-site collaborator (EM).
2.4 Safety Monitoring
EP A and DHA in the form of nutritional oils have been found to be safe and well-tolerated in several post-marking studies [16, 18, 19, 21–23, 25, 41, 44, 58]. There have been fewer safety studies in children. Therefore, all patients had blood work for alanine aminotransferase (ALT), hemagram and prothrombin time (PT)/partial thromboplastin time (PTT) drawn at baseline and 12 weeks. We assessed for side-effects at visits 3, 4 and 5, and via phone contacts at weeks 2, 4, 6, 10, 16, and 20.
All subjects continued on their previously prescribed asthma control regimen that included an inhaled corticosteroid (either from previous asthma plan or conversion from leukotriene modifier (LTM) monotherapy (see table 2 and online supplement for details). The participant’s asthma during the intervention period was managed by the participant’s designated asthma physician.
2.5 Data collection and study visits
Certified research staff determined eligibility using a baseline medical history, anthropometrics, pulmonary function/lung responsiveness testing, asthma symptom questionnaires, and pregnancy testing (females) at visit 1. Participants or guardians (if needed) signed informed consent documents. Patients who met all requirements for inclusion with the sole exception of the lung responsiveness criteria were scheduled for methacholine challenge at visit 2. Once lung responsiveness criterion was met, participants started on a monitored 2–4 week run-in period. If participants were adherent to peak flow and current asthma controller on 5 out of 7 days (on average), they met eligibility for randomization at visit 3.
Willing participants previously on LTM were taken off LTM according to the step-down protocol. Because a possible mechanism of EPA/DHA may be reduced leukotriene production, participants were taken off all leukotriene modifying agents at visits 2. The timing and details of the baseline data collection are in table 4. The screening visit (V1) included informed consent, instructions regarding study format and asthma action plan, and testing per table 4. Participants with documented lung responsiveness criteria at visit 1 or within the past 24 months had visits 1 and 2 combined in the same day. Visit 2 signified the start of the run-in period. At visit 2, staff reviewed the participant’s asthma action plan and the participant and accompanying parent were told to continue with their normal diet and level of activity. Participants were given a peak flow device (and trained on its use) and daily diary cards to document peak flow values and controller medicine use.
Table 4.
Schedule for Collection of Screening and Baseline Data
| Visit 1 Screening | Visit 2 Enrollment | Visit 3 Randomization | |
|---|---|---|---|
| Timeline | Minus 2–8 weeks | Minus 2–4 weeks | 0 |
| Consent/Assents and eligibility evaluation | • | • | • |
| Medical History, participant instructions | • | ||
| Anthropometrics | • | ||
| Review inhaler technique | • | • | |
| Distribute Asthma Action Plan | • | ||
| Issue Asthma Diary Cards | • | • | |
| Review Asthma Diary | • | • | |
| Asthma Questionnaire Scoring | • | • | |
| Pregnancy test (females) | • | • | • |
| Methacholine Challenge (if needed for inclusion) | • | ||
| Block Food Frequency Questionnaire | • | ||
| 72 hour Dietary Recall | • | ||
| Spirometry, Forced oscillation | • | • | |
| Exhaled nitric oxide and breath condensate | • | ||
| Urinary LTE4 | • | ||
| Blood for CRP, ALT, F2-isoprostane | • | ||
| Blood for T-regulatory Cell analysis | • | ||
| Granulocyte membrane PUFA analysis | • |
LTE4 – leukotriene E4, CRP – C-reactive protein, ALT – alanine aminotransferase, CBC – complete blood count, PUFA – polyunsaturated fatty acid
At the randomization visit (V3), participants returned diary cards and were assessed for randomization. Testing occurred per table 5 and fig 1. If subjects did not meet inclusion criteria for randomization due to adherence and the study personnel agreed, they were permitted to repeat visit 3 after continuing on their routine daily asthma medications and run-in specifications for an additional 2 weeks. Participants who had one or more of the following: 1) took daily asthma treatment < 10 of the preceding 14 days as documented by diary cards. 2) FEV1 ≤ 60% predicted pre-bronchodilator; or 3) febrile illness (>38.0ºC or 100.4ºF) within last 24 hours, had the opportunity to be re-evaluated and randomized after an additional 2 week monitoring period.
Table 5.
Schedule for Collection of Response Data during Intervention
| Visit 3 Randomization | Visit 4 Midpoint | Visit 5 Termination | |
|---|---|---|---|
| Timeline | 0 | +12 (10–14) | +24 (22–26) |
| Anthropometrics | • | • | • |
| Review inhaler technique | • | • | • |
| Issue Asthma Diary Cards | • | • | |
| Asthma Questionnaire Scoring (ACQ, ACT) | • | • | • |
| Asthma-related Quality of Life questionnaires1 | • | • | • |
| Pregnancy test (females) | • | • | • |
| Adverse Event screening | • | • | • |
| Interval Health/Asthma assessment | • | • | • |
| Block Food Frequency Questionnaire | • | • | |
| 24 hour Dietary Recall | • | ||
| 72 hour Dietary Recall | • | • | |
| Spirometry and Forced oscillation testing | • | • | • |
| Exhaled nitric oxide and breath condensate | • | • | • |
| Urinary LTE4 | • | • | • |
| Blood for CRP, ALT, CBC, F2-isoprostane | • | • | • |
| Blood for T-regulatory Cell analysis | • | • | • |
| Granulocyte membrane PUFA analysis | • | • | • |
ACQ – asthma control questionnaire, ACT – asthma control test; LTE4 – leukotriene E4, CRP – C-reactive protein, ALT – alanine aminotransferase, CBC – complete blood count, PUFA – polyunsaturated fatty acid,
consists of the asthma quality of life questionnaire, the pediatric asthma quality of life questionnaire, and the pediatric caregiver asthma quality of life questionnaire;
After 12 weeks of intervention, participants returned their daily diary cards at visit 4 (V4) and had testing performed per table 5. At the termination visit (V5), participants returned their diary cards. They received an exit letter unmasking their treatment assignment and counseling information regarding a healthy lifestyle and diet. Testing proceeded per table 5.
2.6 Outcome Measures
2.6.1 Asthma Symptom Scoring
Change in the ‘Juniper’ Asthma Control Questionnaire (ACQ) was the primary outcome. The ACQ instrument has been validated in children[59] and adults, and used in several multi-center asthma trials[55, 60]. The ACQ ranges from 0 to 6 (higher values indicate worse asthma control) and considered a broad set of control indicators including use of bronchodilators, cough, nocturnal symptoms, level of activity, and pulmonary function. At all visits we also performed the Asthma Control Test (ACT), Asthma Symptom Utility Index (ASUI) and several tools measuring quality of life. We computed rate and prevalence of asthma symptom exacerbations for both treatment groups (see online supplement for details).
2.6.2 Lung Function
The Koko spirometric system was used per American Thoracic Society standards[61]. Outcomes included forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and forced expiratory flows at 25–75% of vital capacity (FEF25–75). Raw data and percent predicted values were recorded before and after bronchodilator. Forced oscillation testing was also performed (Jaeger MasterScreen Impulse Oscillometry system, Jaeger Co, Germany)[62–68] at all clinic visits (at two sites, Jacksonville and Orlando). Impedance, resistance and reactance values were calculated at discrete frequencies from 5 to 35 Hz. Lastly, expiratory peak flow measurements were measured at home daily during the intervention period in order to calculate peak expiratory flow variability (see on-line repository).
2.6.3 Inflammatory mechanisms
Exhaled nitric oxide, exhaled breath condensate (pH, 8-isoprostane), plasma C-reactive protein and urinary LTE4 were collected at visits 3, 4 and 5. (See online web repository for details on methodology.) Omega-3 to omega-6 PUFA ratio within leukocyte plasma membranes was assessed at visits 3–5. Phospholipids from cell lysates were purified and transmethylated. The fatty acid methyl esters were analyzed by gas-liquid chromatography at Nemours Bioanalytic Lab and the amounts of individual fatty acids will be expressed as relative percentages, together totaling 100 percent.
2.6.4 Regulatory T-lymphocyte (CD4+-CD25+-CD127--FoxP3+) number and function
We collected blood and sputum on a subset of participants to measure regulatory T-lymphocytes. CD4+-CD25hi-CD127lo/- regulatory T cells were magnetically isolated from whole blood, and counted by flow cytometry[69]. The level of FoxP3 expression will be measured by RT-qPCR and by flow cytometry, and Treg activity will be assessed by chemotaxis and T cell proliferation-inhibition assays[70] (See online repository for more detail on methodology).
2.7 Assessment of Dietary Intake
We assessed each participant’s diet using a Block validated food frequency questionnaire (at randomization and again at week 24) (Block Kids FFQ 2004, Block Adult FFQ 2005) [71–74]. Two 72 hour dietary recalls were performed at the beginning and the end of the study period, along with three additional 24-hour dietary recalls periodically throughout the intervention period. We had access to the Food Processor 9.3 (Standard Version, Esha Research, Salem, OR USA) software package that analyzed intake in order to describe dietary patterns among obese asthmatics, and to report the comparability in diet (vitamin D, antioxidant vitamins, soy, saturated fats) between intervention groups. Our research nutritionist (KK) assisted in quantifying particular dietary components including omega-3 and omega-6 PUFA, antioxidant vitamins (such as vitamin A, vitamin E, Vitamin C and Selenium), and total fat intake.
2.8 Genetic analysis
The ALOX5 promoter SP1 tandem repeat polymorphism genotype has been shown to affect responses to drugs acting on the 5-lipoxygenase/leukotriene pathway[49, 75]. Therefore, the ALOX5 promoter SP1 tandem repeat polymorphism (marker rs59439148) will be genotyped at the completion of the study as previously described [50] (see online repository for details). Participant genomic DNA was prepared from mononuclear cells in whole blood samples. Hardy-Weinberg equilibrium (HWE) between expected and observed genotype distributions was calculated using χ2 goodness-of-fit tests. We planned to test both additive (5/5 vs. 5/X vs. X/X) and dominant (5/5 vs. 5/X + X/X) general linear models. The final model will depend on how accurately each model describes the distribution of the data, and will be consistent with past analyses[49, 76, 77]. Accounting for attrition and a racially heterogenous sample, we estimate that we should have at minimum > 70% power to detect a nutrigenetic effect if one exists.
2.9 Organization of the Study
The NOOA study utilized the Nemours Network for Asthma Research. The study was initiated at the Nemours Clinic in Jacksonville, Florida and was expanded to the Nemours Children’s Hospital, Orlando and three other sites in Florida and Delaware. A data and safety monitoring board was created to monitor for safety and data integrity and met yearly. An investigational new drug application was submitted under the section 505(i) of the Federal Food, Drug, and Cosmetic Act for the omega-3 polyunsaturated fatty acid intervention (ProEPA Xtra) and granted in March 2010 (IND107443).
2.10 Data management
Study data were collected and managed using the Research Electronic Data Capture (REDCap) electronic data capture system and tools, hosted by collaborators within Nemours Bioinformatics/Nemours Foundation[78]. REDCap was chosen because it is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
2.11 Analysis
2.11.1 Sample size calculation
We determined that 100 participants will need to be randomized according to a 3:1 allocation ratio, with a goal of 75 receiving EPA/DHA and 25 receiving control. We assumed a 10% dropout rate with a final goal of 90 participants completing intervention. Power estimation for EPA/DHA effect on asthma control was performed depending on a two-sided t-test of a hypothesis about two population means (EPA/DHA-treated and control-treated). We let n be the minimum sample size per group to detect a deviation of magnitude Δ from the null value in the direction of the research hypothesis. The appropriate sample size per group to reach a defined power would be calculated as:
| [[ 79]] |
(Where σ was the population standard deviation; v denoted the degree of freedom, and v = 2(n −1) for a two-sample t test; α is type I error set as 0.05; β is type II error and the power can be estimated as 1 − β). With large sample size (e.g. n ≥ 30), the t-distribution can be approximated by a standard normal distribution. In this case, the above inequality could be simplified as the following equation:
| [[ 79]] |
Sample size was determined based on the ACQ (primary outcome measure). Power to detect a treatment effect using alternate clinical outcomes (assuming 90 participants with type I error α = .05)are shown in table 6.
Table 6.
Power Analysis for EPA/DHA Treatment
| Outcome | Standardized effect size (change/standard deviation) | Estimated Power1 |
|---|---|---|
| ACQ | 0.5/0.45 = 1.1 | 98 |
| ASUI | 0.1/0.1 = 1.0 | 98 |
| 8-isoprostane | 1.0/0.7 = 1.43 | 99 |
| FEV1/FVC | 0.05/0.07 = 0.71 | 83 |
| FEV1 | 0.07/0.11 = 0.64 | 75 |
Based on 90 completing intervention, 67 treated, 23 controls;
ACQ – asthma control questionnaire; ASUI – asthma symptom utility index, FEV – forced expiratory volume, FVC – forced vital capacity
The trial had at least 98% power to detect a significant effect size in clinical outcomes of ACQ, ASUI and 8-isoprostane. We sought a power >70% to improve the likelihood of detecting a nutrigenetic effect (see on-line supplement).
2.11.2 Data analysis
We assumed an intention-to-treat approach. When the trial ends, we will utilize a two sample t-test to determine whether the change in ACQ from the randomization to termination visit differs between treatment groups, (α=.05). Other secondary outcomes that are continuous numeric variables will be analyzed similarly. We will also compare the proportion of diary card asthma-free days between the two treatment arms using the Mann-Whitney-Wilcoxon test. Additionally, we will use an aligned rank test (non-parametric) test to account for the multiple strata (two BMI strata X 4 clinic sites). For asthma exacerbations, we will likely employ Poisson regression analysis because of its relatively low event frequency. The statistical packages SAS 9.2 (SAS Institute Inc, Cary NC, USA) and STATA 11 (College Station, TX: StataCorp, 2005) were used. All tests were two-tailed at a level of significance of 0.05 (see online supplement for additional details).
3. Discussion
The NOOA study aimed to determine if the addition of oral EPA/DHA to corticosteroid therapy in obese children and young adults with inadequately controlled asthma leads to improved asthma control. Though there have been several small interventional trials involving EPA/DHA for asthma, past results have been inconsistent (Table 1). Considering the large problem that uncontrolled asthma symptoms pose to public health, the relative paucity of effective therapies for asthma control, and the general safety of EPA/DHA, further exploration of the efficacy of EPA/DHA for the treatment of asthma is warranted. In addition, increased omega-3 fatty acid intake leading to altered leukotriene production may prove to be particularly effective in asthmatics with obesity. Obesity has been associated with greater leukotriene production, while evidence also suggests that the montelukast, a leukotriene receptor antagonist, may have a modestly increased effect with higher body mass index[80].
We chose to use softgel capsules to deliver 4g of daily omega-3 fatty acids primarily in the form of EPA and DHA, or matching control soy oil, over a 24 week duration. A 24 week intervention was chosen to ensure adequate time to: 1) alter omega-3/omega-6 fatty acid ratios in leukocyte plasma membranes, and 2) establish effectiveness (that fish oil could be tolerated and adhered to among patients with persistent asthma). The primary outcome measure was change in Asthma Control Questionnaire on therapy, which included assessment of lung function and several clinically relevant symptom measures. The NOOA study also evaluated several likely molecular mechanisms before, during and after intervention to try to determine causal effects of omega-3 fatty acid therapy on inflammation. Data collected as a part of NOOA will be analyzed to determine is omega-3 fatty acid treatment is modified secondary factors such as level of obesity, dietary omega-3 fatty acid intake, urinary leukotriene levels or previous response to LTRAs. Our current hypothesis is that omega-3 fatty acids will improve asthma particularly in those with obesity and leukotriene-driven disease. However, because omega-3 fatty acids affect other mechanisms, it is possible that larger studies will be needed testing the effect of omega-3 fatty acids in both obese and non-obese asthmatics.
As depicted in table 1, smaller studies have been completed that included a range of ages, dosing regimens and clinical phenotypes. Ages have ranged from 8–65 years, with the majority of studies involving young and middle-aged adults. Very few randomized controlled studies have used an intervention more than 12 weeks, and the largest 6 month trial in asthmatics to our knowledge involved 39 participants[17]. A systematic review by Reisman in 2006 attempted to perform a meta-analysis of omega-3 fatty acid supplementation for asthma, however the authors were not able due to widely varying reporting of allocation concealment, baseline characteristics, asthma severity, disease phenotype, primary outcome and missing data[41]. Additionally, there may be a dose-response effect, with Reisman et al speculating that a daily dose greater 3 grams may be required to see a significant and clinically meaningful effect[41]. Several studies with encouraging results have shown that doses in the range of greater than 5 grams/day succeed in reducing bronchial reactivity among persistent asthmatics or asthmatics with exercise-induced bronchospasm[22, 23, 48]. One of the largest and most controlled studies used dietary manipulation to increase omega-3 fatty acid intake but failed to show clinical improvement[17]. Inconsistent past results could reflect a nutrigenetic or phenotype-specific effect with EPA/DHA. The current NOOA study focused on the important problem of poorly controlled asthma symptoms despite inhaled corticosteroids, and used a 24 week intervention period at a high dose of greater than 4g per day. In addition, we focus on obese asthmatics, a subset of asthmatics who have been shown to be more resistant to inhaled steroids. Importantly, obese asthmatics may be more responsive to omega-3 fatty acid supplementation if their past diets are excessively high in n-6 fatty acids. Dietary assessment and intervention (‘medical nutrition therapy’) has even been advocated for asthmatic children[81]. With our detailed dietary assessments, we will report the nature of the dietary intake among obese asthmatics and evaluate how diet affects asthma control and response to omega-3 fatty acids.
Nausea and abdominal discomfort were consistently reported, and could limit the future effectiveness of EPA/DHA supplementation as a treatment strategy. Concerns about taste and tolerability led us to use a pharmaceutical-grade product that was lemon-flavored and masked the commonly reported fishy taste and dyspepsia. So far, gastrointestinal complaints have been rarely reported and have not led to problems of withdrawal.
The choice of specifically which omega-3 PUFAs to use, the daily dose and treatment duration, and the choice of optimal ‘placebo’ control agent to use were important design questions. A fixed daily dose of 4g using a discrete number of softgels has optimize adherence and maintain blinding. We used a similar number of softgels as our control treatment which were matched for taste, appearance and consistency. Various products have been used in past trials as a ‘placebo’. The choice of soy oil seemed to be the best option after considering the factors of safety, need for blinding, and need for a true inert placebo. The soy oil control is isovolumetric and similar in caloric content to EPA/DHA treatment. The control intervention constitutes a negligible fraction of the daily omega-6 PUFA consumption in a typical Western diet and therefore should not significantly alter omega-3/omega-6 ratios within inflammatory cells[24]. Soy oil contains a small fraction of (non-EPA, non-DHA) α-linolenic acid, however conversion of α-linolenic acid into EPA is limited. Soybeans contain isoflavone glycoproteins that theoretically could be anti-inflammatory, however our soy oil product has been filtered and highly purified to remove proteins and glycoproteins, and any likely anti-inflammatory effect.
Several drugs show inter-individual variability of response due to nutrigenetic effects which can limit their efficacy within populations. If responses to EPA/DHA were dependent on genetics (as we are postulating), results of EPA/DHA intervention trials would vary depending on the genetic make-up of the treated group. Past results from asthma trials cited above have been consistent with a nutrigenetic effect. Because EPA/DHA appears to reduce inflammation through action on the arachidonic acid pathway, we chose to evaluate associations between common gene variants in the arachidonic acid and leukotriene pathways and treatment response. The ALOX5 gene is located on Chromosome 10q11.21 and has been associated with altered response to asthma therapy[49, 76] and risk for obesity in both asthmatics and non-asthmatics. Dwyer and Alayee showed that omega-3 PUFA displays a protective effect on carotid intimal thickening[50] that is also dependent on ALOX5 rs59439148 promoter polymorphism. This promoter polymorphism in the gene encoding 5-lipoxygenase leads to increased production of inflammatory leukotrienes, and to a gene-environment interaction[50]. Compared to heterozygotes and wild type homozygotes, individuals that are mutant homozygotes (carrying 2 mutant alleles) have increased carotid intima-media thickness, an atherogeneic effect that was exacerbated by increased intake of dietary arachidonic acid (n-6 PUFA). Increased dietary intake of n-3 PUFA blunted the atherogenic effect in carriers of the mutant variant. These data suggest that the mutant allele of the addition/deletion promoter polymorphism for the ALOX5 gene up-regulates production of proinflammatory leukotrienes, and that n-3 PUFA supplementation is effective in one genotype but not the other at ameliorating the inflammatory and atherogenic effect.
In addition, Tantisira recently concluded that pharmacogenetic variability exists in the response to zileuton therapy (a 5-lipoxygenase inhibitor). The results of this study are important because it is reasonable to hypothesize that these same genetic variants may also influence response to EPA/DHA[82].In a clinical trial of montelukast (selective cysLTR1 antagonist), Lima et al reported that asthmatics carrying one or 2 mutant alleles responded better to montelukast treatment compared to individuals carrying 2 wild type alleles[83], suggesting that mutant variants in asthmatics up-regulated 5-LO activity. Since obesity is accompanied by up-regulation of pro-inflammatory mediators, it could be hypothesized that the mutant allele for the addition/deletion promoter polymorphism for the ALOX5 gene might be more prevalent in obese compared to non-obese individuals. Consistent with this, other authors have shown improved clinical response to montelukast with increasing BMI[80]. Taken together these data make a compelling argument that n-3 PUFA supplementation will reduce leukotriene-mediated inflammation and improve asthma control particularly among obese asthmatics.
In summary, we have presented a review of the largest clinical trial to assess the efficacy of omega-3 fatty acids for the treatment of asthma. Furthermore, this is the first trial to our knowledge that targets inadequately controlled obese asthmatics with a novel nutrient with potential pharmacologic effects. We are assessing for a nutrigenetic effect by interrogating a select arachidonic acid pathway polymorphism. If this trial discovers that omega-3 fatty acids improve asthma control, a new class of therapy will potentially become available and could significantly improve the way asthma is managed in the future.
Supplementary Material
Acknowledgments
This research was performed by the Nemours Network for Asthma Research (NNAR). The members of the NNAR research group for the trial were as follows:
Nemours Network for Asthma Research
Nemours Children’s Clinic, Jacksonville, Florida: Jason E. Lang, M.D. (principal investigator, 2010–2012), Burnese Rutledge, R.N. (Primary Research Coordinator), David Schaeffer, M.D (Study Physician), Sharon Leonard, M.D. (Study Physician), Roni Socher (Study Physician), Mary Warde, R.N., BSN (Research Coordinator) .
Nemours Children’s Hospital/Nemours Children’s Clinic, Orlando, Florida: Jason E. Lang, M.D. (principal investigator, 2012-present), Floyd Livingston, M.D. (Co-Investigator/Study Physician), Joi Lucas (Study Physician), Bert Kesser, RT (Primary Site Coordinator), Angela Price, RN, BSN (Research Coordinator).
Partnering Sites:
University of South Florida, Tampa: Richard Lockey (principal investigator, Tampa), Michelle Grandstaff, Sarah Croker (coordinators).
Data and Safety Monitoring Board: Lewis Smith (chair), Theresa Guilbert, Elizabeth Garret-Mayer, Laurie Duckworth.
FUNDING
Support: Supported by a grant from the National Heart Lung and Blood Institute [K23HL096838-01] and from the Office of Dietary Supplements (ODS)
Footnotes
COMPETING INTERESTS
Disclosures:
Dr. Lang has no conflicts of interest in the subject matter of this manuscript
Dr. Mougey has no conflicts of interest in the subject matter of this manuscript
Dr. Blake has no conflicts of interest in the subject matter of this manuscript
Dr. Yong as no conflicts of interest in the subject matter of this manuscript
Dr. Lockey has no conflicts of interest in the subject matter of this manuscript
Dr. Hossain has no conflicts of interest in the subject matter of this manuscript
Dr. Lima has no conflicts of interest in the subject matter of this manuscript
(A complete listing of the Nemours Network for Asthma Research (NNAR) and collaborating partners can be found at the end of this article.)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jason E. Lang, Email: jelang@nemours.org.
Edward Mougey, Email: emougey@nemours.org.
Hooman Allayee, Email: hallayee@usc.edu.
Kathryn V. Blake, Email: kblake@nemours.org.
Richard Lockey, Email: rlockey@health.usf.edu.
Yan Gong, Email: gong@cop.ufl.edu.
Jobayer Hossain, Email: jhossain@nemours.org.
Kelleigh Killen, Email: kdkillen@hotmail.com.
John J. Lima, Email: jlima@nemours.org.
References
- 1.Stream AR, Sutherland ER. Obesity and asthma disease phenotypes. Curr Opin Allergy Clin Immunol. 2012 Feb;12(1):76–81. doi: 10.1097/ACI.0b013e32834eca41. [DOI] [PubMed] [Google Scholar]
- 2.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012 May;18(5):716–25. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 3.Camargo CA, Jr, Weiss ST, Zhang S, Willett WC, Speizer FE. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Arch Intern Med. 1999 Nov 22;159(21):2582–8. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 4.Gold DR, Damokosh AI, Dockery DW, Berkey CS. Body-mass index as a predictor of incident asthma in a prospective cohort of children. Pediatr Pulmonol. 2003 Dec;36(6):514–21. doi: 10.1002/ppul.10376. [DOI] [PubMed] [Google Scholar]
- 5.Forno E, Lescher R, Strunk R, Weiss S, Fuhlbrigge A, Celedon JC. Decreased response to inhaled steroids in overweight and obese asthmatic children. J Allergy Clin Immunol. 2011 Mar;127(3):741–9. doi: 10.1016/j.jaci.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutherland ER, Goleva E, Strand M, Beuther DA, Leung DY. Body mass and glucocorticoid response in asthma. Am J Respir Crit Care Med. 2008 Oct 1;178(7):682–7. doi: 10.1164/rccm.200801-076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira MA, Kartashov AI, Ebbeling CB, Van Horn L, Slattery ML, Jacobs DR, Jr, et al. Fast-food habits, weight gain, and insulin resistance (the CARDIA study): 15-year prospective analysis. Lancet. 2005 Jan 1–7;365(9453):36–42. doi: 10.1016/S0140-6736(04)17663-0. [DOI] [PubMed] [Google Scholar]
- 8.Nagel G, Weinmayr G, Kleiner A, Garcia-Marcos L, Strachan DP. Effect of diet on asthma and allergic sensitisation in the International Study on Allergies and Asthma in Childhood (ISAAC) Phase Two. Thorax. 2010 Jun;65(6):516–22. doi: 10.1136/thx.2009.128256. [DOI] [PubMed] [Google Scholar]
- 9.Antova T, Pattenden S, Nikiforov B, Leonardi GS, Boeva B, Fletcher T, et al. Nutrition and respiratory health in children in six Central and Eastern European countries. Thorax. 2003 Mar;58(3):231–6. doi: 10.1136/thorax.58.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatzi L, Torrent M, Romieu I, Garcia-Esteban R, Ferrer C, Vioque J, et al. Diet, wheeze, and atopy in school children in Menorca, Spain. Pediatr Allergy Immunol. 2007 Sep;18(6):480–5. doi: 10.1111/j.1399-3038.2007.00596.x. [DOI] [PubMed] [Google Scholar]
- 11.Hodge L, Salome CM, Peat JK, Haby MM, Xuan W, Woolcock AJ. Consumption of oily fish and childhood asthma risk. Med J Aust. 1996 Feb 5;164(3):137–40. doi: 10.5694/j.1326-5377.1996.tb122010.x. [DOI] [PubMed] [Google Scholar]
- 12.Herxheimer H, Schaefer O. Letter: Asthma in Canadian Eskimos. N Engl J Med. 1974 Dec 26;291(26):1419. doi: 10.1056/NEJM197412262912621. [DOI] [PubMed] [Google Scholar]
- 13.Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950–1974. Acta Med Scand. 1980;208(5):401–6. [PubMed] [Google Scholar]
- 14.Bang HO, Dyerberg J, Hjoorne N. The composition of food consumed by Greenland Eskimos. Acta Med Scand. 1976;200(1–2):69–73. doi: 10.1111/j.0954-6820.1976.tb08198.x. [DOI] [PubMed] [Google Scholar]
- 15.Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985 May 9;312(19):1205–9. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 16.Nagakura T, Matsuda S, Shichijyo K, Sugimoto H, Hata K. Dietary supplementation with fish oil rich in omega-3 polyunsaturated fatty acids in children with bronchial asthma. Eur Respir J. 2000 Nov;16(5):861–5. doi: 10.1183/09031936.00.16586100. [DOI] [PubMed] [Google Scholar]
- 17.Hodge L, Salome CM, Hughes JM, Liu-Brennan D, Rimmer J, Allman M, et al. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur Respir J. 1998 Feb;11(2):361–5. doi: 10.1183/09031936.98.11020361. [DOI] [PubMed] [Google Scholar]
- 18.Surette ME, Koumenis IL, Edens MB, Tramposch KM, Clayton B, Bowton D, et al. Inhibition of leukotriene biosynthesis by a novel dietary fatty acid formulation in patients with atopic asthma: a randomized, placebo-controlled, parallel-group, prospective trial. Clin Ther. 2003 Mar;25(3):972–9. doi: 10.1016/s0149-2918(03)80117-0. [DOI] [PubMed] [Google Scholar]
- 19.Mihrshahi S, Peat JK, Webb K, Oddy W, Marks GB, Mellis CM. Effect of omega-3 fatty acid concentrations in plasma on symptoms of asthma at 18 months of age. Pediatr Allergy Immunol. 2004 Dec;15(6):517–22. doi: 10.1111/j.1399-3038.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 20.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996 Jan;63(1):116–22. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 21.Arm JP, Horton CE, Mencia-Huerta JM, House F, Eiser NM, Clark TJ, et al. Effect of dietary supplementation with fish oil lipids on mild asthma. Thorax. 1988 Feb;43(2):84–92. doi: 10.1136/thx.43.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mickleborough TD, Lindley MR, Ionescu AA, Fly AD. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006 Jan;129(1):39–49. doi: 10.1378/chest.129.1.39. [DOI] [PubMed] [Google Scholar]
- 23.Mickleborough TD, Murray RL, Ionescu AA, Lindley MR. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. 2003 Nov 15;168(10):1181–9. doi: 10.1164/rccm.200303-373OC. [DOI] [PubMed] [Google Scholar]
- 24.Romieu I, Tellez-Rojo MM, Lazo M, Manzano-Patino A, Cortez-Lugo M, Julien P, et al. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med. 2005 Dec 15;172(12):1534–40. doi: 10.1164/rccm.200503-372OC. [DOI] [PubMed] [Google Scholar]
- 25.Broughton KS, Johnson CS, Pace BK, Liebman M, Kleppinger KM. Reduced asthma symptoms with n-3 fatty acid ingestion are related to 5-series leukotriene production. Am J Clin Nutr. 1997 Apr;65(4):1011–7. doi: 10.1093/ajcn/65.4.1011. [DOI] [PubMed] [Google Scholar]
- 26.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989 Feb 2;320(5):265–71. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 27.Bryan DL, Forsyth KD, Hart PH, Gibson RA. Polyunsaturated fatty acids regulate cytokine and prostaglandin E2 production by respiratory cells in response to mast cell mediators. Lipids. 2006 Dec;41(12):1101–7. doi: 10.1007/s11745-006-5059-9. [DOI] [PubMed] [Google Scholar]
- 28.Mantzioris E, Cleland LG, Gibson RA, Neumann MA, Demasi M, James MJ. Biochemical effects of a diet containing foods enriched with n-3 fatty acids. Am J Clin Nutr. 2000 Jul;72(1):42–8. doi: 10.1093/ajcn/72.1.42. [DOI] [PubMed] [Google Scholar]
- 29.Iwami D, Nonomura K, Shirasugi N, Niimi M. Immunomodulatory effects of eicosapentaenoic acid through induction of regulatory T cells. International immunopharmacology. 2011 Mar;11(3):384–9. doi: 10.1016/j.intimp.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 30.Iwami D, Zhang Q, Aramaki O, Nonomura K, Shirasugi N, Niimi M. Purified eicosapentaenoic acid induces prolonged survival of cardiac allografts and generates regulatory T cells. Am J Transplant. 2009 Jun;9(6):1294–307. doi: 10.1111/j.1600-6143.2009.02641.x. [DOI] [PubMed] [Google Scholar]
- 31.Yessoufou A, Ple A, Moutairou K, Hichami A, Khan NA. Docosahexaenoic acid reduces suppressive and migratory functions of CD4+CD25+ regulatory T-cells. J Lipid Res. 2009 Dec;50(12):2377–88. doi: 10.1194/jlr.M900101-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodworth HL, McCaskey SJ, Duriancik DM, Clinthorne JF, Langohr IM, Gardner EM, et al. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res. Oct 15;70(20):7960–9. doi: 10.1158/0008-5472.CAN-10-1396. [DOI] [PubMed] [Google Scholar]
- 33.Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with gamma-linolenic acid or fish oil decreases T lymphocyte proliferation in healthy older humans. J Nutr. 2001 Jul;131(7):1918–27. doi: 10.1093/jn/131.7.1918. [DOI] [PubMed] [Google Scholar]
- 34.Soyland E, Nenseter MS, Braathen L, Drevon CA. Very long chain n-3 and n-6 polyunsaturated fatty acids inhibit proliferation of human T-lymphocytes in vitro. Eur J Clin Invest. 1993 Feb;23(2):112–21. doi: 10.1111/j.1365-2362.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- 35.Purasiri P, McKechnie A, Heys SD, Eremin O. Modulation in vitro of human natural cytotoxicity, lymphocyte proliferative response to mitogens and cytokine production by essential fatty acids. Immunology. 1997 Oct;92(2):166–72. doi: 10.1046/j.1365-2567.1997.d01-2308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004 Nov;39(11):1125–32. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 37.Yacoubian S, Serhan CN. New endogenous anti-inflammatory and proresolving lipid mediators: implications for rheumatic diseases. Nat Clin Pract Rheumatol. 2007 Oct;3(10):570–9. doi: 10.1038/ncprheum0616. quiz 1 p following 89. [DOI] [PubMed] [Google Scholar]
- 38.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000 Oct 16;192(8):1197–204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002 Oct 21;196(8):1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003 Apr 25;278(17):14677–87. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 41.Reisman J, Schachter HM, Dales RE, Tran K, Kourad K, Barnes D, et al. Treating asthma with omega-3 fatty acids: where is the evidence? A systematic review. BMC Complement Altern Med. 2006;6:26. doi: 10.1186/1472-6882-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodge AM, Dowse GK, Gareeboo H, Tuomilehto J, Alberti KG, Zimmet PZ. Incidence, increasing prevalence, and predictors of change in obesity and fat distribution over 5 years in the rapidly developing population of Mauritius. Int J Obes Relat Metab Disord. 1996 Feb;20(2):137–46. [PubMed] [Google Scholar]
- 43.Marks GB, Mihrshahi S, Kemp AS, Tovey ER, Webb K, Almqvist C, et al. Prevention of asthma during the first 5 years of life: a randomized controlled trial. J Allergy Clin Immunol. 2006 Jul;118(1):53–61. doi: 10.1016/j.jaci.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Emelyanov A, Fedoseev G, Krasnoschekova O, Abulimity A, Trendeleva T, Barnes PJ. Treatment of asthma with lipid extract of New Zealand green-lipped mussel: a randomised clinical trial. Eur Respir J. 2002 Sep;20(3):596–600. doi: 10.1183/09031936.02.02632001. [DOI] [PubMed] [Google Scholar]
- 45.Okamoto M, Mitsunobu F, Ashida K, Mifune T, Hosaki Y, Tsugeno H, et al. Effects of perilla seed oil supplementation on leukotriene generation by leucocytes in patients with asthma associated with lipometabolism. Int Arch Allergy Immunol. 2000 Jun;122(2):137–42. doi: 10.1159/000024369. [DOI] [PubMed] [Google Scholar]
- 46.Villani F, Comazzi R, De Maria P, Galimberti M. Effect of dietary supplementation with polyunsaturated fatty acids on bronchial hyperreactivity in subjects with seasonal asthma. Respiration. 1998;65(4):265–9. doi: 10.1159/000029274. [DOI] [PubMed] [Google Scholar]
- 47.Dry J, Vincent D. Effect of a fish oil diet on asthma: results of a 1-year double-blind study. Int Arch Allergy Appl Immunol. 1991;95(2–3):156–7. doi: 10.1159/000235421. [DOI] [PubMed] [Google Scholar]
- 48.Arm JP, Horton CE, Spur BW, Mencia-Huerta JM, Lee TH. The effects of dietary supplementation with fish oil lipids on the airways response to inhaled allergen in bronchial asthma. Am Rev Respir Dis. 1989 Jun;139(6):1395–400. doi: 10.1164/ajrccm/139.6.1395. [DOI] [PubMed] [Google Scholar]
- 49.Drazen JM, Yandava CN, Dube L, Szczerback N, Hippensteel R, Pillari A, et al. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nat Genet. 1999 Jun;22(2):168–70. doi: 10.1038/9680. [DOI] [PubMed] [Google Scholar]
- 50.Dwyer JH, Allayee H, Dwyer KM, Fan J, Wu H, Mar R, et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004 Jan 1;350(1):29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 51.Callaway CW, Chumlea WCCB. Circumference. Human Kinetics Books; 1988. [Google Scholar]
- 52.CDC. National Health and Nutrition Examination Survey: Anthropometry Procedures Manual. 2009 [cited 2008; Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf.
- 53.McDowell MA, National Center for Health Statistics (U.S.) Anthropometric reference data for children and adults, United States, 2003–2006. Hyattsville, MD: U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2008. [Google Scholar]
- 54.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007 Dec;120( Suppl 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 55.Holbrook JT, Wise RA, Gold BD, Blake K, Brown ED, Castro M, Dozor AJ, Lima J, Mastronarde JG, Sockrider M, Teague WG. Lansoprazole for Children With Poorly Controlled Asthma: A Randomized Clinical Trial. JAMA. 2012 doi: 10.1001/jama.2011.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002 Nov;102(11):1621–30. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 57.Diagnostics IFOS-N. International Fish Oil Standards Approvals Program Consumer Report. 2009 [cited; Available from: http://www.nordicnaturals.com/images/ifoscharts/20.pdf.
- 58.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006 Jul;84(1):5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 59.Juniper EF, Gruffydd-Jones K, Ward S, Svensson K. Asthma Control Questionnaire in children: validation, measurement properties, interpretation. Eur Respir J. Dec;36(6):1410–6. doi: 10.1183/09031936.00117509. [DOI] [PubMed] [Google Scholar]
- 60.Mastronarde JG, Anthonisen NR, Castro M, Holbrook JT, Leone FT, Teague WG, et al. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009 Apr 9;360(15):1487–99. doi: 10.1056/NEJMoa0806290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995 Sep;152(3):1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 62.Bates JH, Allen GB. The estimation of lung mechanics parameters in the presence of pathology: a theoretical analysis. Ann Biomed Eng. 2006 Mar;34(3):384–92. doi: 10.1007/s10439-005-9056-6. [DOI] [PubMed] [Google Scholar]
- 63.Bates JH, Lutchen KR. The interface between measurement and modeling of peripheral lung mechanics. Respir Physiol Neurobiol. 2005 Aug 25;148(1–2):153–64. doi: 10.1016/j.resp.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 64.Bates JH, Mishima M, Balassy Z. Measuring the mechanical properties of the lung in vivo with spatial resolution at the acinar level. Physiol Meas. 1995 Aug;16(3):151–9. doi: 10.1088/0967-3334/16/3/002. [DOI] [PubMed] [Google Scholar]
- 65.Davey BL, Bates JH. Regional lung impedance from forced oscillations through alveolar capsules. Respir Physiol. 1993 Mar;91(2–3):165–82. doi: 10.1016/0034-5687(93)90097-t. [DOI] [PubMed] [Google Scholar]
- 66.Kaminsky DA, Irvin CG, Lundblad L, Moriya HT, Lang S, Allen J, et al. Oscillation mechanics of the human lung periphery in asthma. J Appl Physiol. 2004 Nov;97(5):1849–58. doi: 10.1152/japplphysiol.00300.2004. [DOI] [PubMed] [Google Scholar]
- 67.Kaminsky DA, Irvin CG, Moriya HT, Lynn M, Lang S, Bates JH. Peripheral lung responsiveness assessed by forced oscillations through the wedged bronchoscope. Chest. 2003 Mar;123(3 Suppl):363S. doi: 10.1378/chest.123.3_suppl.363s. [DOI] [PubMed] [Google Scholar]
- 68.Sly PD, Brown KA, Bates JH, Spier S, Milic-Emili J. Noninvasive determination of respiratory mechanics during mechanical ventilation of neonates: a review of current and future techniques. Pediatr Pulmonol. 1988 Jan-Feb;4(1):39–47. doi: 10.1002/ppul.1950040109. [DOI] [PubMed] [Google Scholar]
- 69.Fazekas de St Groth B, Zhu E, Asad S, Lee L. Flow cytometric detection of human regulatory T cells. Methods Mol Biol. 2011;707:263–79. doi: 10.1007/978-1-61737-979-6_17. [DOI] [PubMed] [Google Scholar]
- 70.Nadeau K, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes J, et al. Ambient air pollution impairs regulatory T-cell function in asthma. J Allergy Clin Immunol. 2010 Oct;126(4):845–52. e10. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 71.Johnson BA, Herring AH, Ibrahim JG, Siega-Riz AM. Structured measurement error in nutritional epidemiology: applications in the Pregnancy, Infection, and Nutrition (PIN) Study. Journal of the American Statistical Association. 2007;102(479):856–66. doi: 10.1198/016214506000000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr. 2006 Feb;9(1):84–93. doi: 10.1079/phn2005763. [DOI] [PubMed] [Google Scholar]
- 73.Cullen KW, Watson K, Zakeri I. Relative reliability and validity of the Block Kids Questionnaire among youth aged 10 to 17 years. J Am Diet Assoc. 2008 May;108(5):862–6. doi: 10.1016/j.jada.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 74.Tucker KL. Assessment of usual dietary intake in population studies of gene-diet interaction. Nutr Metab Cardiovasc Dis. 2007 Feb;17(2):74–81. doi: 10.1016/j.numecd.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 75.Lima JJ. Treatment heterogeneity in asthma: genetics of response to leukotriene modifiers. Mol Diagn Ther. 2007;11(2):97–104. doi: 10.1007/BF03256228. [DOI] [PubMed] [Google Scholar]
- 76.Lima JJ, Zhang S, Grant A, Shao L, Tantisira KG, Allayee H, et al. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. Am J Respir Crit Care Med. 2006 Feb 15;173(4):379–85. doi: 10.1164/rccm.200509-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vikman S, Brena RM, Armstrong P, Hartiala J, Stephensen CB, Allayee H. Functional analysis of 5-lipoxygenase promoter repeat variants. Hum Mol Genet. 2009 Dec 1;18(23):4521–9. doi: 10.1093/hmg/ddp414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rao PV. Statistical research methods in the life sciences. Pacific Grove, CA: Duxbury Press; 1998. [Google Scholar]
- 80.Peters-Golden M, Swern A, Bird SS, Hustad CM, Grant E, Edelman JM. Influence of body mass index on the response to asthma controller agents. Eur Respir J. 2006 Mar;27(3):495–503. doi: 10.1183/09031936.06.00077205. [DOI] [PubMed] [Google Scholar]
- 81.McCloud E, Papoutsakis C. A medical nutrition therapy primer for childhood asthma: current and emerging perspectives. J Am Diet Assoc. 2011 Jul;111(7):1052–64. doi: 10.1016/j.jada.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 82.Tantisira KG, Lima J, Sylvia J, Klanderman B, Weiss ST. 5-lipoxygenase pharmacogenetics in asthma: overlap with Cys-leukotriene receptor antagonist loci. Pharmacogenet Genomics. 2009 Mar;19(3):244–7. doi: 10.1097/FPC.0b013e328326e0b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dixon AE, Shade DM, Cohen RI, Skloot GS, Holbrook JT, Smith LJ, et al. Effect of obesity on clinical presentation and response to treatment in asthma. J Asthma. 2006 Sep;43(7):553–8. doi: 10.1080/02770900600859123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.