Abstract
Background
Potentially inappropriate medication use in hospitalized older patients is common. Our objective was to determine whether a computerized provider order entry (CPOE) drug warning system can decrease orders for potentially inappropriate medications in hospitalized older patients.
Methods
We used a prospective pre-post design among patients 65 years and older admitted to a large, urban, academic medical center in Boston, Massachusetts, from March 2005 through August 2008. We instituted a medication-specific warning system within CPOE that alerted ordering providers at point of care when ordering a potentially inappropriate medication that advised alternative medication or dose reduction. The main outcome measure was the rate of orders for potentially inappropriate medications before and after the warning system was deployed.
Results
The rate of ordering medications that were not recommended dropped from 11.56 ±0.36 to 9.94 ±0.12 orders/day after the implementation of a CPOE warning system (difference 1.62 ±0.33; p<0.001), with no evidence that the effect waned over time. There were no changes in the rate of ordering medications for which only dose reduction was recommended or that were not targeted following implementation. These effects persisted in autoregressive models that accounted for secular trends and season (p<0.001).
Conclusions
Specific alerts embedded into a CPOE system, used in patients 65 years and older, can decrease the number of orders of potentially inappropriate medications quickly and specifically.
Older people admitted to the hospital are especially vulnerable to adverse drug events (ADEs),1 which occur in up to 40% of hospital admissions.2 ADEs increase the length of stay, the cost of caring for patients admitted to the hospital, and increase the risk of death.3
Some medications may predispose vulnerable elders to ADEs. Beers et al. proposed a list of drugs identified by a panel of geriatric medicine experts that should be avoided in older persons.4 Despite the publication of the “Beers medications,” the prescription of potentially inappropriate medications to elderly patients remains common.5, 6
Up to 60% of ADEs during hospitalization occur at the time of ordering;3, 6, 7 the remainder occur downstream, during delivery or omission (not giving a medication as prescribed). Computerized provider order entry (CPOE) systems provide an opportunity for intervention to change prescribing practices before potentially inappropriate medications are ordered. However, to our knowledge, no CPOE system has previously been described that utilizes a warning system built around PIMs in older, hospitalized adults.
The purpose of this study was to determine if the number of orders for potentially inappropriate medications in hospitalized patients 65 years and older could be decreased using a computerized warning system linked to CPOE. We studied the ordering patterns before and after the implementation of such a system for three groups of drugs – a larger group of drugs included in the original Beers list (‘Beers drugs’) that were flagged not to be used, a second group of Beers drugs that were flagged to be used at reduced doses, and a third group of Beers drugs not flagged.
Methods
Patient Population
We studied all inpatients age 65 years and older hospitalized at a single academic medical center in the North Eastern US. The hospital provides primary and tertiary care with 621 inpatient beds with approximately 40,000 inpatient admissions annually. The medical center’s institutional review board approved this study.
CPOE Warning System
The CPOE system at the medical center was developed by programmers at the institution and is not commercially available. All medications prescribed to inpatients are ordered through the CPOE system, although dispensed medications are tracked through a separate pharmacy program. With hospital Pharmacy and Therapeutics Committee feedback, we developed medication-specific alerts that were built into the hospital’s CPOE system. A series of step-by-step screen shots illustrating the ordering process for a typical flagged medication is available on-line (Appendix).
From the larger list of potentially inappropriate medications listed in the Beers article4, we identified three primary classes of medications for study a priori. These were: those that were flagged as not recommended for use in older patients (not recommended medications), those for which only a reduced dose was advised (dose reduction medications), and those that were not flagged because no safer alternative was considered equally efficacious (unflagged medications - amiodarone, digoxin, disopyramide, and indomethacin); the last group represented controls in our analyses. Table 1 shows the targeted drugs and the exact wording of the alerts used. A geriatrician and a pharmacist proposed the specific groups of medications utilizing literature where possible to support their decisions, and the Pharmacy and Therapeutics Committee at the medical center, comprised of senior hospital pharmacists and clinicians, revised and approved the list.
Table 1.
Specific Beers drugs targeted and the warning received by the ordering physician. All warnings state, “Precaution is necessary for use of [drug name] in geriatric patients.” All warnings provide a reference to the Beers article.4
| Medication with recommendation NOT to order | |
|---|---|
| Drug | Warning |
| Amitriptyline | Because of its strong anticholinergic and sedation properties, amitriptyline should be used rarely in the elderly. ** Degree of Severity: HIGH **` Risk is increased in patients with Hepatic, Cardiovascular or Neurologic/Psychiatric impairment. |
| Chlordiazepoxide | This drug has a long half-life in elderly patients (often several days), producing prolonged sedation and increasing the risk of falls and fractures. Short- and intermediate-acting benzodiazepines are preferred if a benzodiazepine is required. ** Degree of severity: HIGH ** Risk is increased in patients with Renal, Hepatic or Neurologic/Psychiatric impairment. |
| Clonidine | Potential for orthostatic hypotension and CNS adverse effects. ** Degree of severity: LOW ** Risk is increased in patients with Renal or Neurologic/Psychiatric impairment. |
| Clorazepate | This drug has a long half-life in elderly patients (often several days), producing prolonged sedation and increasing the risk of falls and fractures. Short- and intermediate-acting benzodiazepines are preferred if a benzodiazepine is required. ** Degree of severity: HIGH ** Risk is increased in patients with Renal, Hepatic or Neurologic/Psychiatric impairment. |
| Cyclobenzaprine | Most muscle relaxants and antispasmodic drugs are poorly tolerated by elderly patients, since these cause anticholinergic side effects, sedation and weakness. Additionally, their effectiveness at doses tolerated by elderly patients is questionable. ** Degree of severity: HIGH ** Risk is increased in patients with Renal, Hepatic, Cardiovascular or Neurologic/Psychiatric impairment |
| Diazepam | This drug has a long half-life in elderly patients (often several days), producing prolonged sedation and increasing the risk of falls and fractures. Short- and intermediate-acting benzodiazepines are preferred if a benzodiazepine is required. ** Degree of severity: HIGH ** Risk is increased in patients with Hepatic or Neurologic/Psychiatric impairment. |
| Diphenhydramine | May cause confusion and sedation. Should not be used as a hypnotic, and when used to treat emergency allergic reactions, it should be used in the smallest possible dose. ** Degree of severity: HIGH ** Risk is increased in patients with Neurologic/Psychiatric impairment. |
| Doxazosin | Potential for hypotension, dry mouth, and urinary problems. ** Degree of severity: LOW ** Risk is increased in patients with Renal, Cardiovascular or Neurologic/Psychiatric impairment. |
| Fluoxetine | Long half-life of drug and risk of producing excessive CNS stimulation, sleep disturbances, and increasing agitation. Safer alternatives exist. ** Degree of severity: HIGH ** Risk is increased in patients with Hepatic or Neurologic/Psychiatric impairment. |
| Hydroxyzine | All non prescription and many prescription antihistamines may have potent anticholinergic properties. Nonanticholinergic antihistamines are preferred in elderly patients when treating allergic reactions. ** Degree of severity: HIGH ** Risk is increased in patients with Neurologic/Psychiatric impairment |
| Ketorolac | Immediate and long-term use should be avoided older persons, since a significant number have asymptomatic GI pathologic conditions. ** Degree of severity: HIGH ** Risk is increased in patients with Renal, Hepatic or Cardiovascular impairment. |
| Naproxen | Has the potential to produce GI bleeding, renal failure, high blood pressure, and heart failure. ** Degree of severity: HIGH ** Risk is increased in patients with Renal, Hepatic or Cardiovascular impairment. |
| Oxybutynin | Most muscle relaxants and antispasmodic drugs are poorly tolerated by elderly patients, since these cause anticholinergic side effects, sedation and weakness. Additionally, their effectiveness at doses tolerated by elderly patients is questionable. ** Degree of severity: HIGH ** Risk is increased in patients with Renal, Hepatic, Cardiovascular or Neurologic/Psychiatric impairment. |
| Piroxicam | Has the potential to produce GI bleeding, renal failure, high blood pressure, and heart failure. ** Degree of severity: HIGH ** Risk is increased in patients with Renal, Hepatic or Cardiovascular impairment. |
| Propoxyphene | Offers few analgesic advantages over acetaminophen, yet has the adverse effects of other narcotic drugs. ** Degree of severity: LOW ** Risk is increased in patients with Renal, Hepatic or Neurologic/Psychiatric impairment. |
| Thioridizine | Greater potential for CNS and extrapyramidal adverse effects. Other antipsychotic agent might be more appropriate. ** Degree of severity: HIGH ** Risk is increased in patients with Renal, Hepatic, Cardiovascular or Neurologic/Psychiatric impairment. |
| Medications with recommendation to DECREASE dosage | |
| Drug | Warning |
| Lorazepam | Because of increased sensitivity to benzodiazepines in elderly patients, smaller doses may be as effective as well as safer. Total daily dose should rarely exceed the suggested maximum of 3 mg. ** Degree of severity: HIGH ** Risk is increased in patients with Renal, Hepatic or Neurologic/Psychiatric impairment. |
| Ferrous sulfate | Doses greater than 325 mg/d do not dramatically increase the amount of absorbed but greatly increase the incidence of constipation. ** Degree of severity: LOW ** |
| Medications not flagged | |
| Amiodarone | |
| Digoxin | |
| Disopyramide | |
| Indomethacin | |
We did not target two other general categories of medications from the original Beers list: 1) those drug classes for which individual drugs were not consistently on formulary throughout the study period or that were extremely infrequently used among elderly inpatients, and 2) classes with very broad and heterogeneous use (e.g., NSAIDs and calcium-channel blockers) left unflagged to minimize fatigue. We included these latter two classes as controls in secondary analyses.
For all flagged medications, the ordering provider had the option to bypass the warning and order the medication; no prior approval was required. Each time, however, the ordering provider had to choose a reason. From March 2004 to October 2006, there were three possible reasons the clinician could choose: 1) “Patient stabilized on regimen; will monitor appropriate drug levels or laboratory values” or 2) “Interaction noted, regimen clinically indicated, will closely monitor,” or 3) Other. In October 2006, a fourth choice was added: 4) “Warning noted, will use smaller dose and monitor for side effects.”
The warning system applied to all patients admitted to the hospital who were 65 years or older at the time of the order regardless of location within the hospital or admitting service, although meperedine and promethazine were part of a fixed post-anesthesia care unit (PACU) order set that was not flagged and thus are not included. There were no other concurrent efforts made to educate providers about medication safety, and the warnings suggested no specific alternatives.
Outcome Measures
From July 1 through November 30, 2004, the five months prior to the deployment of the CPOE warning system, the number of orders in hospitalized patients 65 years and older for the selected medications were recorded. All orders, whether as needed or standing, are included; the hospital does not have an electronic medication administration record, and hence we were not able to record the number or dosage of medications actually given to the patient. We excluded the time period between December 2004 and March 2004, the period of beta testing of the warning system. We then recorded all orders after the warning system was deployed in March 2004 through August 31, 2008.
Statistical Analyses
We computed two measures of the rate of prescribing of Beers medications – the daily number of medications in each class divided by either the total number of hospitalized patients 65 years and older or the number of newly admitted hospitalized patients 65 years and older each day. Because medications are differentially more likely to be prescribed on the first hospital day, these denominators represent complementary estimates of the number of patients ‘at risk’ for inappropriate prescriptions.
We first plotted the daily rate of each outcome measure against calendar time and fit separate smoothed splines for the time periods before and after intervention. In initial analyses, we calculated the mean daily rates of each of the three classes of drugs before and after the warning system was instituted and compared these with t-tests. Because the smoothed splines indicated that the underlying trend of the outcome rate over time was linear, we assumed linearity in time series models and fit autocorrelative regression models that accounted for the serial correlation in the measurement errors of the daily outcome rates. These models included calendar time, period (before versus after intervention), the product (or interaction) of period and time (i.e., change in the secular trend following intervention), and season. Regression analyses were performed in SAS 9.2 (Cary, NC) using the statistical procedure PROC AUTOREG.
Results
During the period of study, there was a secular trend in both the number of patients in the hospital 65 years and older and the mean number of all orders, resulting in larger numbers of orders over time.
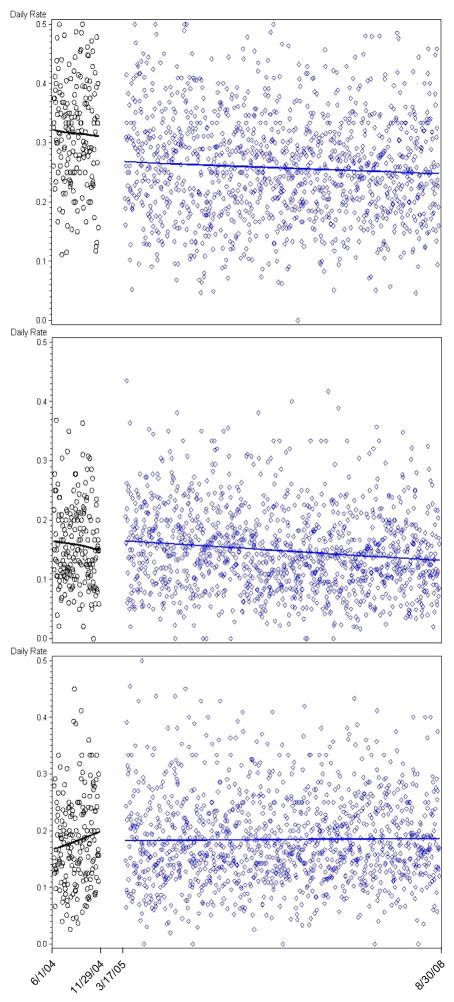
Figure 1 shows the temporal trends in the rate of orders of the three classes of medications we studied. After the warning system was deployed, there was an immediate and sustained decrease in the rate of orders for the not recommended group of medications. There was a modest secular trend resulting in decreased use of unflagged medications that did not change appreciably following warning system implementation, and no change in the dose reduction group.
Figure 1.
Daily rate of medication orders among hospitalized elders for each of three medication classes before and after implementation of a computerized provider order entry alert system. The vertical axis indicates the number of medications ordered on a daily basis for patients aged 65 and older divided by the number of older adults admitted on the corresponding day. The horizontal axis indicates time, with ticks at the beginning and end dates of follow-up and the dates that the alert system was first and then completely implemented. Black circles indicate the pre-intervention period and blue diamonds the post-intervention period. The upper panel shows medications that were flagged by the alert system. The middle panel shows medications that were flagged with a recommendation for dose reduction. The lower panel shows medications that were not flagged. The lines in each panel indicate smoothed splines fit separately for the pre- and post-intervention periods. There was a significant change in the rate of ordering following intervention only for the middle panel (p<0.001).
In pre-post comparisons (Table 2), the rate of prescribing of the group of not recommended medications dropped by 20–30% (p<0.001). There was a modest decrease in use of unflagged medications of borderline statistical significance, consistent with the observed secular trend, and no change in medications in which only a dose reduction was advised.
Table 2.
Number of orders per day in three groups of drugs before and after the start of the CPOE warning system.
| Medications Recommended Not to Order | Medications Recommended for Dose Reduction | Medications Not Flagged | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | P Value | Before | After | P Value | Before | After | P Value | |
| Orders/day | 11.56 ±0.36 | 9.94 ±0.12 | 6.09 ±0.20 | 6.87 ±0.08 | 5.40 ±0.20 | 5.55 ±0.07 | |||
| Orders/total patients/day | 0.070 ±0.002 | 0.054 ±0.001 | <0.001 | 0.037 ± 0.001 | 0.037 ± 0.001 | 0.71 | 0.033 ± 0.001 | 0.030 ± 0.001 | 0.03 |
| Orders/new patients/day | 0.333 ±0.001 | 0.263 ±0.003 | <0.001 | 0.182 ± 0.006 | 0.186 ± 0.002 | 0.51 | 0.158 ± 0.005 | 0.148 ± 0.002 | 0.08 |
Data are presented as mean ±SE.
Autoregressive models yielded similar results. There was a highly significant and immediately observed drop in the rate of use of not recommended medications (p<0.001) with no change in the secular trend following intervention (p=0.11). There were no significant changes in the absolute rate of prescribing or in the secular trend of prescribing for the other two classes of medications following intervention in these models.
In secondary analyses, we also examined rates of prescribing of all unflagged medications on the original Beers list, rather than the four medications selected a priori. In autoregressive models, there was no significant effect of the intervention on the daily rate of prescribing in absolute terms (p=0.44) or on the secular trend of prescribing (p=0.17).
Among the medications not recommended, the most commonly prescribed was diphenhydramine, accounting for approximately one-third of all prescriptions in that group prior to implementation. Both its use and use of other targeted medications dropped markedly following implementation of the warning system, although we had insufficient power to examine other medications individually. For example, the daily rate of orders for not recommended medications per new admission (±SE) dropped by 0.070 ±0.008 (p<0.001) following implementation; the corresponding drops were 0.043 ±0.004 (p<0.001) for diphenhydramine alone and 0.027 ±0.006 (p<0.001) for other targeted medications. The drops following implementation were also significant in autoregressive models for both diphenhydramine (p<0.001) and other targeted medications (p=0.001).
All orders recorded in this study on flagged medications reflect orders where the ordering provider bypassed the warning; the CPOE does not track prescriptions that are started but not completed. In our study, users provided “Interaction noted, regimen clinically indicated” as the reason for overriding the warning about half of the time. “Patient Stabilized on Regimen, will monitor” was given as the second most common reason for overriding the warning. (Table 3). A third option that indicated the prescriber intended to use a low dose was instituted in October 2006; as intended, this option was used more frequently for those drugs for which dose reduction was recommended (19%) than for those that were recommended not to be used (13%; p heterogeneity <0.001 across categories).
Table 3.
Reasons for overrides of medications recommended not to be ordered and recommended for dose reduction.
| Medications Recommended Not to Order | Medications Recommended for Dose Reduction | |||
|---|---|---|---|---|
| 3/05–10/06 | 10/06–8/08 | 3/05–10/06 | 10/06–8/08 | |
| Patient stabilized on regimen; will monitor appropriate drug levels or laboratory values | 1863 (38%) | 2673 (40%) | 1147 (33%) | 1564 (31%) |
| Interaction noted, regimen clinically indicated, will closely monitor | 2828 (58%) | 3015 (44%) | 2204 (62%) | 2326 (46%) |
| Warning noted, will use smaller dose and monitor for side effects. | N/A | 859 (13%) | N/A | 948 (19%) |
| Other | 215 (4%) | 214 (3%) | 177 (5%) | 173 (4%) |
Three override options were available up to October 2006; four were available afterward.
Discussion
In this quasi-experimental study of a large urban medical center, the rate of orders for potentially inappropriate medications in older patients was markedly decreased by the use of a CPOE warning system targeting a subset of Beers medications. The intervention showed no signs of fatigue, and other medications that were either not flagged or flagged only for dose adjustment continued to be prescribed at unchanged rates.
Specific Features and Findings
After our alert system was implemented, the rate of ordering of the targeted medications declined immediately in the study population. Others have found similar results in the outpatient setting.8, 9 This may reflect our restriction to only a subset of medications with legitimate alternative(s) in a vulnerable patient population.10 In this regard, the specificity and immediacy of the drop in use of flagged medications is reassuring and suggests that local systems can be effectively tailored to meet local standards of care. Indeed, previous research suggests that drug alerts created in internally developed CPOE systems tailor-made to an individual institution or service can reduce medication errors or orders for potentially inappropriate medications.11–13
We did not observe a substantial “learning effect” - where one might hope to see a further reduction over time in the rate of ordering the potentially inappropriate medications, perhaps related to turnover of ordering housestaff yearly. On the other hand, the effect of our warning system appeared to be durable over time with no sign that users grew weary of repeated warnings.
CPOE in an Aging Population
Many clinicians have not received formal education about the unique medical needs of elderly patients and, despite the fact that more people are living longer, there are no geriatric medicine-specific performance standards for US medical students.14 This may explain why potentially inappropriate medications continue to be prescribed to hospitalized patients 65 years and older and provide a rich target for CPOE intervention.15
Understanding this limit in training, we created a CPOE warning system to decrease the use of potentially inappropriate medications in older patients. CPOE systems change the way clinicians order medications and provide new opportunities to guide behavior. While fewer than 10% of US hospitals currently use CPOE, the Institute of Medicine report calling for universal adoption of CPOE heralds an increasing reliance on this technology.16
Designing CPOE systems to shape best practice is an evolving field. Research suggests that CPOE systems without any decision support around medication ordering are associated with high rates of adverse drug events.7 Further, general drug alerts within CPOE systems are frequently overridden, up to 90% of the time.17 Initial efforts to reduce adverse drug events with CPOE systems have focused on reducing medication errors, like drug allergy and drug-drug interactions.11, 18 Our results suggest that specific drug alerts for those medications that place older patients at particular risk for adverse drug events could be a particularly attractive addition to such systems.4, 19, 20
Next Steps and Implications
Our study and some studies of outpatient drug warning systems have found a clear reduction in the use of potentially inappropriate medications.8, 9 As such, our findings – by showing that these drugs are indeed amenable to targeted change by a straightforward ordering system - provide the first necessary step in determining whether reducing use of these medication will ultimately improve patient outcomes. As yet, it is not clear if there are any differences in patient outcomes that can be attributed to this change in behavior, but our results provide optimism that this important research question can be addressed in the near future.
As CPOE is more widely adopted, it seems likely most institutions will rely upon commercially available (rather than internally developed) systems. Such systems will rarely be sufficiently malleable to allow the fine-tuned and circumscribed type of intervention that we describe here. As such, designing commercially available CPOE systems to guide clinicians at the local level to adhere to the best care is challenging. To be most effective, systems should minimize generalized warnings and, like this warning system, use focused alerts to target specific patient populations where alternate treatment exists. We encourage developers of commercial CPOE systems to build in the flexibility to implement point of care warnings appropriate to local circumstances.
Limitations
There are several limitations to our data. For lorazepam and ferrous sulfate, the warning advised a dose reduction. The warning did not advise against the use of these medications, and consistent with this advice, the rate of ordering these medications did not change after the implementation of the warning system – suggesting that the system could provide adequate specificity. However, we lack information on the dose of medication prescribed and hence cannot be certain whether or not the targeted dose reductions were achieved.
Another limitation of this study is its generalizability. Our drug warning system was utilized at an academic medical center where medical trainees or physician extenders order most medications. We do not know if a similar result would be seen in a system where attending physicians place most of the orders, or in institutions without a firmly entrenched and multipurpose CPOE system. We also lack the ability to determine if ADEs were prevented by the use of this warning system.
Similarly, we are only able to comment on medications ordered. While we recorded all orders, including PRN and standing orders, we lack the ability to ascertain the number of medications actually given to patients, as our hospital does not have an electronic medical administration record. Nonetheless, all medications actually administered at the medical center necessarily were captured as orders, so our findings accurately reflect a decline in the number of patients exposed to a subset of potentially problematic medications.
Lastly, without detailed clinical record review, we cannot determine whether or not the medications that were ordered were clinically required. One important area of future study is a better understanding of the scenarios in which it is clinically appropriate and reasonable to prescribe the Beers medications even to older adults.
In summary, we have found that a CPOE system with specific, targeted, and straightforward warnings can dramatically yet selectively reduce the prescription of potentially inappropriate medications in vulnerable hospitalized elders. Such systems can produce rapid and clinically significant change while leaving unchanged the rate of prescribing of unflagged medications. This may represent a tool for improving the safety of hospitalized older adults.
Acknowledgments
This intervention was internally funded and there was no external source of support. We would like to acknowledge Rachel Murkofsky, MD and Lisa Saubermann, Pharm D BCPS for their contributions to the development of the original alert system.
Contributor Information
Kevin A. Afonso, Email: kafonso@bidmc.harvard.edu.
Long Ngo, Email: lngo@bidmc.harvard.edu.
Kenneth J. Mukamal, Email: kmukamal@bidmc.harvard.edu.
References
- 1.Passarelli MC, Jacob-Filho W, Figueras A. Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause. Drugs Aging. 2005;22(9):767–777. doi: 10.2165/00002512-200522090-00005. [DOI] [PubMed] [Google Scholar]
- 2.Fattinger K, Roos M, Vergeres P, et al. Epidemiology of drug exposure and adverse drug reactions in two swiss departments of internal medicine. Br J Clin Pharmacol. 2000 Feb;49(2):158–167. doi: 10.1046/j.1365-2125.2000.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. Adverse Drug Events Prevention Study Group. JAMA. 1997 Jan 22–29;277(4):307–311. [PubMed] [Google Scholar]
- 4.Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003 Dec 8–22;163(22):2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 5.Onder G, Landi F, Cesari M, Gambassi G, Carbonin P, Bernabei R. Inappropriate medication use among hospitalized older adults in Italy: results from the Italian Group of Pharmacoepidemiology in the Elderly. Eur J Clin Pharmacol. 2003 Jun;59(2):157–162. doi: 10.1007/s00228-003-0600-8. [DOI] [PubMed] [Google Scholar]
- 6.Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med. 2000 Aug 1;109(2):87–94. doi: 10.1016/s0002-9343(00)00451-4. [DOI] [PubMed] [Google Scholar]
- 7.Nebeker JR, Hoffman JM, Weir CR, Bennett CL, Hurdle JF. High rates of adverse drug events in a highly computerized hospital. Arch Intern Med. 2005 May 23;165(10):1111–1116. doi: 10.1001/archinte.165.10.1111. [DOI] [PubMed] [Google Scholar]
- 8.Steele AW, Eisert S, Witter J, et al. The effect of automated alerts on provider ordering behavior in an outpatient setting. PLoS Med. 2005 Sep;2(9):e255. doi: 10.1371/journal.pmed.0020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DH, Perrin N, Feldstein A, et al. The impact of prescribing safety alerts for elderly persons in an electronic medical record: an interrupted time series evaluation. Arch Intern Med. 2006 May 22;166(10):1098–1104. doi: 10.1001/archinte.166.10.1098. [DOI] [PubMed] [Google Scholar]
- 10.Lin CP, Payne TH, Nichol WP, Hoey PJ, Anderson CL, Gennari JH. Evaluating Clinical Decision Support Systems: Monitoring CPOE Order Check Override Rates in the Department of Veterans Affairs’ Computerized Patient Record System. J Am Med Inform Assoc. 2008 Sep-Oct;15(5):620–626. doi: 10.1197/jamia.M2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bates DW, Teich JM, Lee J, et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999 Jul-Aug;6(4):313–321. doi: 10.1136/jamia.1999.00660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terrell KM, Perkins AJ, Dexter PR, Hui SL, Callahan CM, Miller DK. Computerized decision support to reduce potentially inappropriate prescribing to older emergency department patients: a randomized, controlled trial. J Am Geriatr Soc. 2009 Aug;57(8):1388–1394. doi: 10.1111/j.1532-5415.2009.02352.x. [DOI] [PubMed] [Google Scholar]
- 13.Simon SR, Smith DH, Feldstein AC, et al. Computerized prescribing alerts and group academic detailing to reduce the use of potentially inappropriate medications in older people. J Am Geriatr Soc. 2006 Jun;54(6):963–968. doi: 10.1111/j.1532-5415.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 14.Leipzig RM, Granville L, Simpson D, Anderson MB, Sauvigne K, Soriano RP. Keeping granny safe on July 1: a consensus on minimum geriatrics competencies for graduating medical students. Acad Med. 2009 May;84(5):604–610. doi: 10.1097/ACM.0b013e31819fab70. [DOI] [PubMed] [Google Scholar]
- 15.Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008 Mar;3(2):91–102. doi: 10.1002/jhm.290. [DOI] [PubMed] [Google Scholar]
- 16.Whiting SO, Gale A. Computerized physician order entry usage in North America: the doctor is in. Healthc Q. 2008;11(3):94–97. [PubMed] [Google Scholar]
- 17.van der Sijs H, Mulder A, van Gelder T, Aarts J, Berg M, Vulto A. Drug safety alert generation and overriding in a large Dutch university medical centre. Pharmacoepidemiol Drug Saf. 2009 Oct;18(10):941–947. doi: 10.1002/pds.1800. [DOI] [PubMed] [Google Scholar]
- 18.Bobb A, Gleason K, Husch M, Feinglass J, Yarnold PR, Noskin GA. The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med. 2004 Apr 12;164(7):785–792. doi: 10.1001/archinte.164.7.785. [DOI] [PubMed] [Google Scholar]
- 19.Bates DW, Miller EB, Cullen DJ, et al. Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med. 1999 Nov 22;159(21):2553–2560. doi: 10.1001/archinte.159.21.2553. [DOI] [PubMed] [Google Scholar]
- 20.Evans RS, Lloyd JF, Stoddard GJ, Nebeker JR, Samore MH. Risk factors for adverse drug events: a 10-year analysis. Ann Pharmacother. 2005 Jul-Aug;39(7–8):1161–1168. doi: 10.1345/aph.1E642. [DOI] [PubMed] [Google Scholar]